A Comparison of Blood Plasma Small Extracellular Vesicle Enrichment Strategies for Proteomic Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Plasma Collection
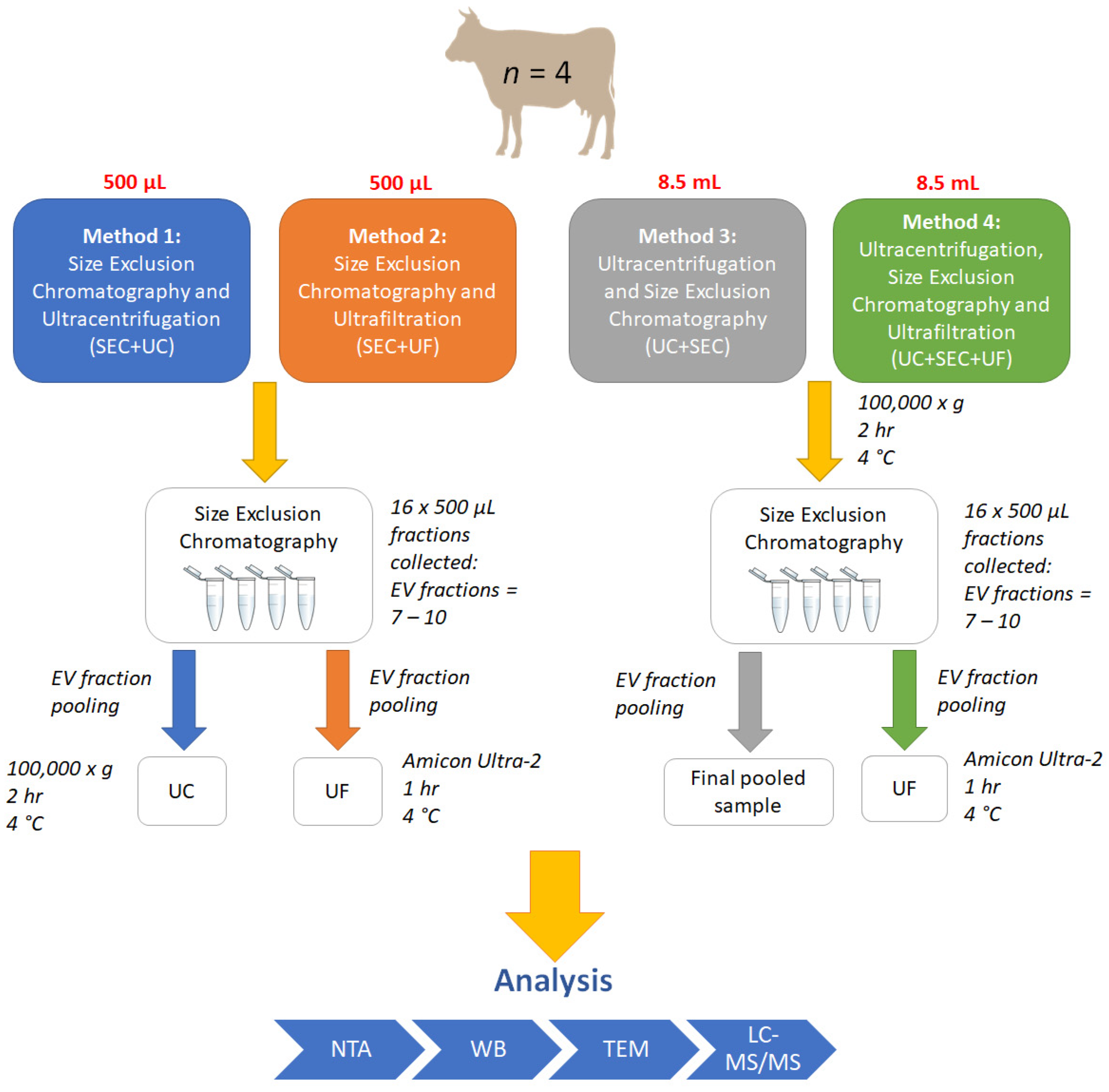
2.2. Small EV Isolation and Enrichment
2.2.1. Pre-Treatment
2.2.2. Size-Exclusion Chromatography (SEC)
2.2.3. Ultrafiltration (UF)
2.2.4. Method 1: SEC + UC
2.2.5. Method 2: SEC + UF
2.2.6. Method 3: UC + SEC
2.2.7. Method 4: UC + SEC + UF
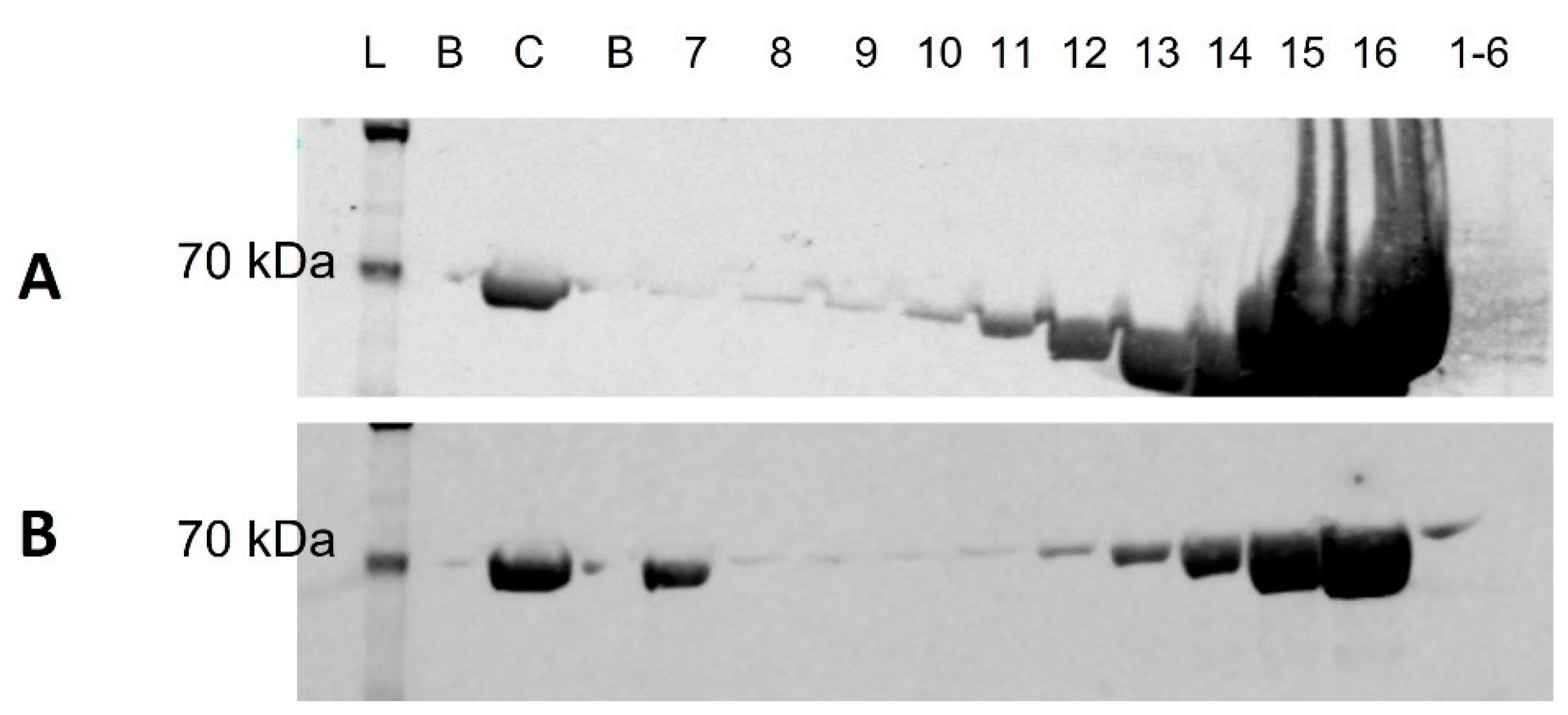
2.3. Sample Pooling and Individual SEC Fraction Analysis
2.4. Protein Quantification
2.5. Western Blot
2.6. Nanoparticle Tracking Analysis (NTA)
2.7. Statistical Analyses
2.8. Transmission Electron Microscopy (TEM)
2.9. Protein Digestion
2.10. Peptide Desalting
2.11. Peptide Assay
2.12. Mass-Spectrometry (MS)
2.13. Data-Dependent Acquisition-Mass Spetrometry (DDA-MS) Data Acquisition
2.14. Protein Identification
2.15. Gene Ontology Analysis
3. Results
3.1. Western Blot
3.2. NTA
3.2.1. Particle Concentration Profiles and Particle Yield
3.2.2. Size Distribution Profiles
3.3. Transmission Electron Microscopy (TEM)
3.4. Total Protein Quantification
3.5. Mass Spectrometry (MS)-Based Protein Identification Results
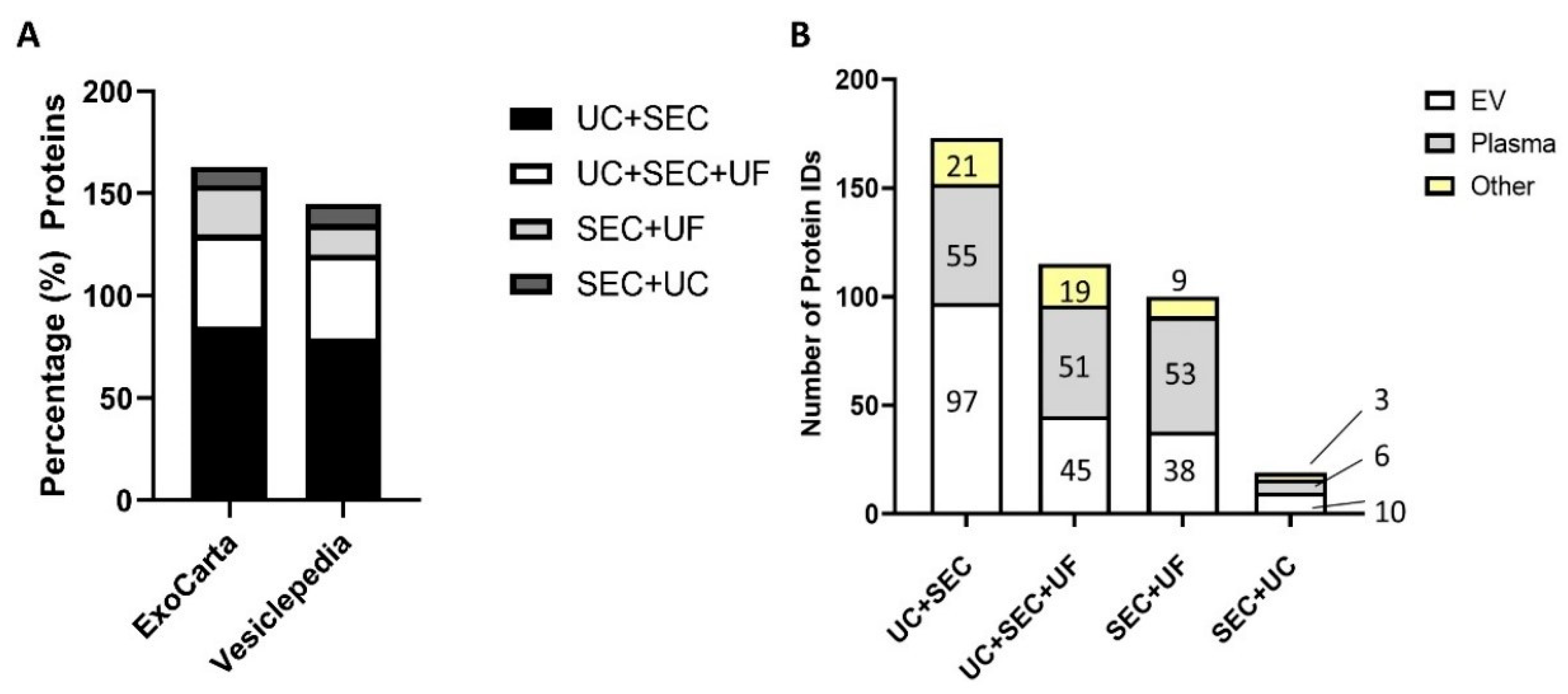
3.5.1. UC + SEC Enrichment Method Resulted in the Highest Number of Protein IDs
3.5.2. Depletion of Abundant Plasma Proteins following sEV Enrichment
4. Discussions
4.1. SEC May Lead to Variable Fractionation of sEV and Plasma Proteins
4.2. The Addition of UF Alters Size Distribution by Reducing the Mean and Mode Size of Particles
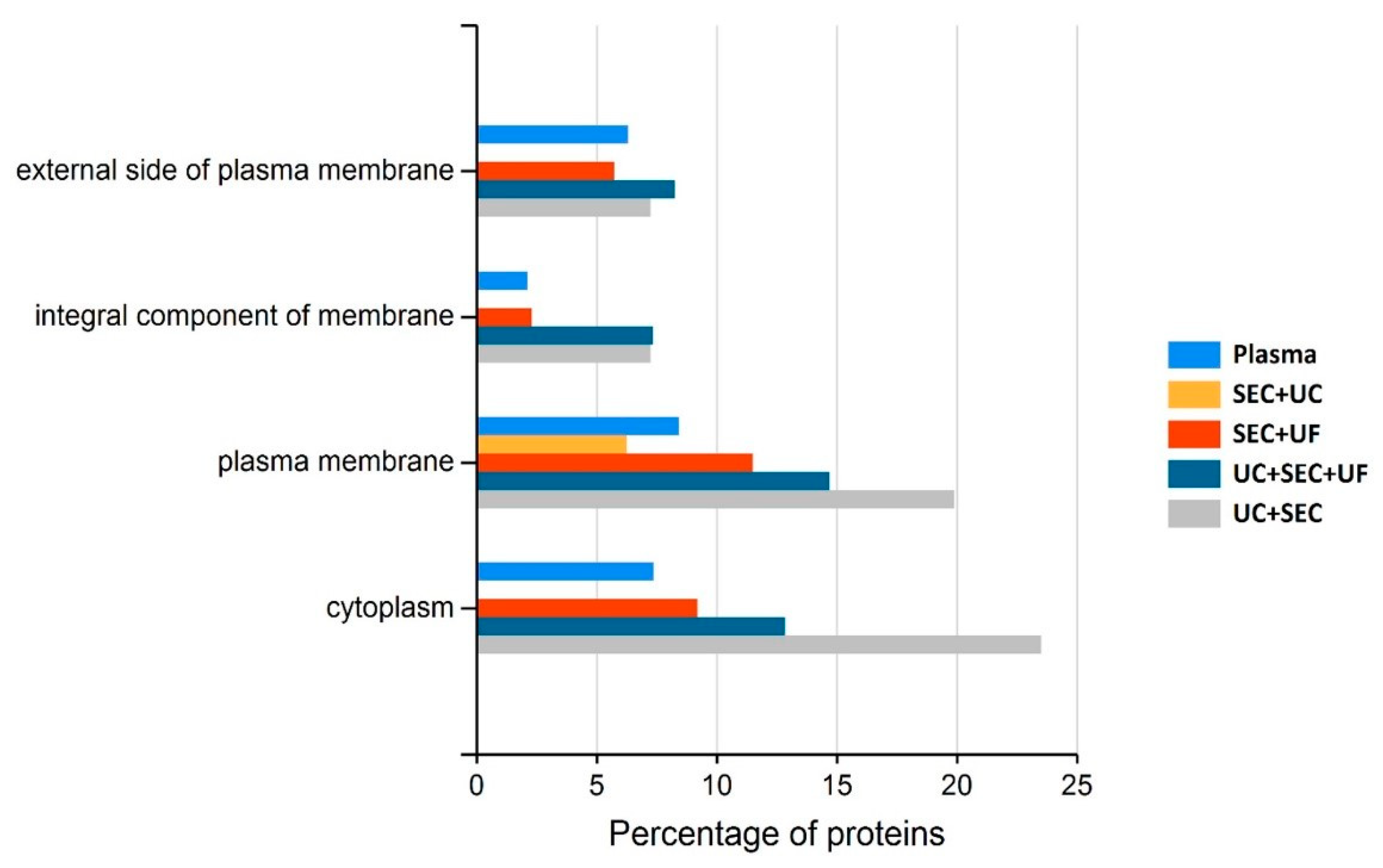
4.3. Small EV-Associated Pathways Display Variable Enrichment Dependent upon sEV Isolation Method
4.4. The Method of sEV Enrichment Has a Direct Impact on Protein ID
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BCA | Bicinchoninic acid |
| BSA | Bovine serum albumin |
| CCKR | Cholecystokinin receptors |
| DDA | Data-dependent acquisition |
| DPBS | Dulbecco’s phosphate buffered saline |
| EV | Extracellular vesicle |
| EX | Exosome |
| FASP | Filter aided sample preparation |
| FDR | False discovery rate |
| FLOT-1 | Flotillin-1 |
| ID | Identification |
| LDL | Low-density lipoprotein |
| LGALS3BP | Galectin-3 binding protein |
| MS | Mass spectrometry |
| Non-EV | Non-extracellular vesicle |
| NTA | Nanoparticle tracking analysis |
| PBS | Phosphate buffered saline |
| PBST | Phosphate buffered saline and 0.1% Tween-20 |
| PM | Plasma membrane |
| PPAI | Post-partum anoestrous interval |
| SDC | Sodium deoxycholate |
| SEC | Size-exclusion chromatography |
| sEV | Small extracellular vesicle |
| TEM | Transmission electron microscopy |
| TFA | Trifluoroacetic acid |
| TOF | Time-of-flight |
| TSG101 | Tumour susceptibility gene 101 |
| UC | Ultracentrifugation |
| UF | Ultrafiltration |
| WB | Western blot |
References
- Collins, B.C.; Hunter, C.L.; Liu, Y.; Schilling, B.; Rosenberger, G.; Bader, S.L.; Chan, D.W.; Gibson, B.W.; Gingras, A.-C.; Held, J.M.; et al. Multi-laboratory assessment of reproducibility, qualitative and quantitative performance of SWATH-mass spectrometry. Nat. Commun. 2017, 8, 291. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Record, M.; Carayon, K.; Poirot, M.; Silvente-Poirot, S. Exosomes as new vesicular lipid transporters involved in cell–cell communication and various pathophysiologies. Biochim. et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2014, 1841, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Nazimek, K.; Bryniarski, K.; Santocki, M.; Ptak, W. Exosomes as Mediators of Intercellular Communication: Clinical Implications. Pol. Arch. Intern. Med. 2015, 125, 370–380. [Google Scholar] [CrossRef]
- Dai, J.; Su, Y.; Zhong, S.; Cong, L.; Liu, B.; Yang, J.; Tao, Y.; He, Z.; Chen, C.; Jiang, Y. Exosomes: Key players in cancer and potential therapeutic strategy. Signal Transduct. Target. Ther. 2020, 5, 145. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of Exosome Composition. Cell 2019, 177, 428–445.e18. [Google Scholar] [CrossRef]
- Zhang, H.; Freitas, D.; Kim, H.S.; Fabijanic, K.; Li, Z.; Chen, H.; Mark, M.T.; Molina, H.; Martin, A.B.; Bojmar, L.; et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat. Cell Biol. 2018, 20, 332–343. [Google Scholar] [CrossRef]
- Doyle, L.; Wang, M. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef]
- Chutipongtanate, S.; Greis, K.D. Multiplex Biomarker Screening Assay for Urinary Extracellular Vesicles Study: A Targeted Label-Free Proteomic Approach. Sci. Rep. 2018, 8, 15039. [Google Scholar] [CrossRef]
- Rood, I.M.; Deegens, J.K.; Merchant, M.L.; Tamboer, W.P.; Wilkey, D.W.; Wetzels, J.F.; Klein, J.B. Comparison of three methods for isolation of urinary microvesicles to identify biomarkers of nephrotic syndrome. Kidney Int. 2010, 78, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Corbett, A.L.; Taatizadeh, E.; Tasnim, N.; Little, J.P.; Garnis, C.; Daugaard, M.; Guns, E.; Hoorfar, M.; Li, I.T. Challenges and Opportunities in Exosome Research-Perspectives from Biology, Engineering, and Cancer Therapy. APL Bioeng. 2019, 3, 011503. [Google Scholar] [CrossRef] [PubMed]
- Mashouri, L.; Yousefi, H.; Aref, A.R.; Ahadi, A.M.; Molaei, F.; Alahari, S.K. Exosomes: Composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol. Cancer 2019, 18, 75. [Google Scholar] [CrossRef]
- Rashed, M.H.; Bayraktar, E.; Helal, G.K.; Abd-Ellah, M.F.; Amero, P.; Chavez-Reyes, A.; Rodriguez-Aguayo, C. Exosomes: From Garbage Bins to Promising Therapeutic Targets. Int. J. Mol. Sci. 2017, 18, 538. [Google Scholar] [CrossRef] [PubMed]
- Konoshenko, M.Y.; Lekchnov, E.A.; Vlassov, A.V.; Laktionov, P.P. Isolation of Extracellular Vesicles: General Methodologies and Latest Trends. BioMed Res. Int. 2018, 2018, 8545347. [Google Scholar] [CrossRef] [PubMed]
- Kreimer, S.; Belov, A.M.; Ghiran, I.; Murthy, S.K.; Frank, D.A.; Ivanov, A.R. Mass-Spectrometry-Based Molecular Characterization of Extracellular Vesicles: Lipidomics and Proteomics. J. Proteome Res. 2015, 14, 2367–2384. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.A.; Zeng, X.; Todd, A.R.; Barnes, L.F.; Winstone, J.M.A.; Trinidad, J.C.; Novotny, M.V.; Jarrold, M.F.; Clemmer, D.E. Charge Detection Mass Spectrometry Measurements of Exosomes and other Extracellular Particles Enriched from Bovine Milk. Anal. Chem. 2020, 92, 3285–3292. [Google Scholar] [CrossRef]
- Mathivanan, S.; Ji, H.; Simpson, R.J. Exosomes: Extracellular organelles important in intercellular communication. J. Proteom. 2010, 73, 1907–1920. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, Q.; Cheng, L.; Wang, Y.; Li, M.; Yang, Q.; Hu, L.; Lou, D.; Li, J.; Dong, X.; et al. Exosome detection via the ultrafast-isolation system: EXODUS. Nat. Methods 2021, 18, 212–218. [Google Scholar] [CrossRef]
- Dong, L.; Zieren, R.C.; Horie, K.; Kim, C.J.; Mallick, E.; Jing, Y.; Feng, M.; Kuczler, M.D.; Green, J.; Amend, S.R.; et al. Comprehensive Evaluation of Methods for Small Extracellular Vesicles Separation from Human Plasma, Urine and Cell Culture Medium. J. Extracell. Vesicles 2020, 10, e12044. [Google Scholar] [CrossRef]
- Witwer, K.W.; Buzás, E.I.; Bemis, L.T.; Bora, A.; Lässer, C.; Lötvall, J.; Nolte-’t Hoen, E.N.; Piper, M.G.; Sivaraman, S.; Skog, J.; et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J. Extracell. Vesicles 2013, 2, 20360. [Google Scholar] [CrossRef]
- Madhavan, B.; Yue, S.; Galli, U.; Rana, S.; Gross, W.; Müller, M.; Giese, N.A.; Kalthoff, H.; Becker, T.; Büchler, M.W.; et al. Combined evaluation of a panel of protein and miRNA serum-exosome biomarkers for pancreatic cancer diagnosis increases sensitivity and specificity. Int. J. Cancer 2014, 136, 2616–2627. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.Q.; Almughlliq, F.B.; Vaswani, K.; Peiris, H.N.; Mitchell, M.D. Exosome enrichment by ultracentrifugation and size exclusion chromatography. Front. Biosci. 2018, 23, 865–874. [Google Scholar] [CrossRef]
- Sódar, B.W.; Kittel, Á.; Pálóczi, K.; Vukman, K.V.; Osteikoetxea, X.; Szabó-Taylor, K.; Németh, A.; Sperlágh, B.; Baranyai, T.; Giricz, Z.; et al. Low-density lipoprotein mimics blood plasma-derived exosomes and microvesicles during isolation and detection. Sci. Rep. 2016, 6, 24316. [Google Scholar] [CrossRef] [PubMed]
- Vergauwen, G.; Dhondt, B.; Van Deun, J.; De Smedt, E.; Berx, G.; Timmerman, E.; Gevaert, K.; Miinalainen, I.; Cocquyt, V.; Braems, G.; et al. Confounding factors of ultrafiltration and protein analysis in extracellular vesicle research. Sci. Rep. 2017, 7, 2704. [Google Scholar] [CrossRef] [PubMed]
- Diaz, G.; Bridges, C.; Lucas, M.; Cheng, Y.; Schorey, J.S.; Dobos, K.M.; Kruh-Garcia, N.A. Protein Digestion, Ultrafiltration, and Size Exclusion Chromatography to Optimize the Isolation of Exosomes from Human Blood Plasma and Serum. J. Vis. Exp. 2018, 134, e57467. [Google Scholar] [CrossRef]
- Pietrowska, M.; Funk, S.; Gawin, M.; Marczak, Ł.; Abramowicz, A.; Widłak, P.; Whiteside, T. Isolation of Exosomes for the Purpose of Protein Cargo Analysis with the Use of Mass Spectrometry. In Functional Genomics; Humana Press: New York, NY, USA, 2017; Volume 1654, pp. 291–307. [Google Scholar] [CrossRef]
- Lobb, R.j.; Becker, M.; Wen, S.W.; Wong, C.S.F.; Wiegmans, A.P.; Leimgruber, A.; Möller, A. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J. Extracell. Vesicles 2015, 4, 27031. [Google Scholar] [CrossRef]
- Stranska, R.; Gysbrechts, L.; Wouters, J.; Vermeersch, P.; Bloch, K.; Dierickx, D.; Andrei, G.; Snoeck, R. Comparison of membrane affinity-based method with size-exclusion chromatography for isolation of exosome-like vesicles from human plasma. J. Transl. Med. 2018, 16, 1. [Google Scholar] [CrossRef]
- Pietrowska, M.; Wlosowicz, A.; Gawin, M.; Widlak, P. MS-Based Proteomic Analysis of Serum and Plasma: Problem of High Abundant Components and Lights and Shadows of Albumin Removal. In Emerging Sample Treatments in Proteomics; Capelo-Martínez, J.L., Ed.; Springer: Cham, Switzerland, 2019; pp. 57–76. [Google Scholar] [CrossRef]
- Raimondo, F.; Morosi, L.; Chinello, C.; Magni, F.; Pitto, M. Advances in membranous vesicle and exosome proteomics improving biological understanding and biomarker discovery. Proteomics 2011, 11, 709–720. [Google Scholar] [CrossRef]
- Benedikter, B.J.; Bouwman, F.G.; Vajen, T.; Heinzmann, A.C.A.; Grauls, G.; Mariman, E.C.; Wouters, E.F.M.; Savelkoul, P.H.; Lopez-Iglesias, C.; Koenen, R.R.; et al. Ultrafiltration combined with size exclusion chromatography efficiently isolates extracellular vesicles from cell culture media for compositional and functional studies. Sci. Rep. 2017, 7, 15297. [Google Scholar] [CrossRef]
- Takov, K.; Yellon, D.M.; Davidson, S.M. Comparison of Small Extracellular Vesicles Isolated from Plasma by Ultracentrifugation or Size-Exclusion Chromatography: Yield, Purity and Functional Potential. J. Extracell. Vesicles 2019, 8, 1560809. [Google Scholar] [CrossRef] [PubMed]
- Almughlliq, F.; Koh, Y.Q.; Peiris, H.; Vaswani, K.; McDougall, S.; Graham, E.M.; Burke, C.R.; Arachchige, B.J.; Reed, S.; Mitchell, M.D. Proteomic content of circulating exosomes in dairy cows with or without uterine infection. Theriogenology 2018, 114, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Crookenden, M.; Walker, C.; Peiris, H.; Koh, Y.; Heiser, A.; Loor, J.; Moyes, K.; Murray, A.; Dukkipati, V.; Kay, J.; et al. Short communication: Proteins from circulating exosomes represent metabolic state in transition dairy cows. J. Dairy Sci. 2016, 99, 7661–7668. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.Q.; Peiris, H.N.; Vaswani, K.; Meier, S.; Burke, C.R.; Macdonald, K.A.; Roche, J.R.; Almughlliq, F.; Arachchige, B.J.; Reed, S.; et al. Characterization of Exosomes from Body Fluids of Dairy Cows. J. Anim. Sci. 2017, 95, 3893–3904. [Google Scholar] [CrossRef]
- Meier, S.; Fisher, B.; Eketone, K.; McNaughton, L.R.; Amer, P.R.; Beatson, P.; Bryant, J.R.; Dodds, K.G.; Spelman, R.; Roche, J.R.; et al. Calf and Heifer Development and the Onset of Puberty in Dairy Cows with Divergent Genetic Merit for Fertility. In Proceedings of the New Zealand Society of Animal Production, Online, 5 July 2017; pp. 205–210. [Google Scholar]
- Bowley, F.; Green, R.; Amer, P.; Meier, S. Novel approaches to genetic analysis of fertility traits in New Zealand dairy cattle. J. Dairy Sci. 2015, 98, 2005–2012. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.; Abeysinghe, P.; Peiris, H.; Vaswani, K.; Sadowski, P.; Cameron, N.; McGhee, N.; Logan, J.; Mitchell, M.D. Proteomic profiling of plasma-derived small extracellular vesicles: A novel tool for understanding the systemic effects of tick burden in cattle. J. Anim. Sci. 2022, 100, skac015. [Google Scholar] [CrossRef]
- Oh, J.G.; Lee, P.; Gordon, R.E.; Sahoo, S.; Kho, C.; Jeong, D. Analysis of extracellular vesicle miRNA profiles in heart failure. J. Cell. Mol. Med. 2020, 24, 7214–7227. [Google Scholar] [CrossRef]
- Yudasaka, M.; Yomogida, Y.; Zhang, M.; Nakahara, M.; Kobayashi, N.; Tanaka, T.; Okamatsu-Ogura, Y.; Saeki, K.; Kataura, H. Fasting-dependent Vascular Permeability Enhancement in Brown Adipose Tissues Evidenced by Using Carbon Nanotubes as Fluorescent Probes. Sci. Rep. 2018, 8, 14446. [Google Scholar] [CrossRef]
- Veerman, R.E.; Teeuwen, L.; Czarnewski, P.; Akpinar, G.G.; Sandberg, A.; Cao, X.; Pernemalm, M.; Orre, L.M.; Gabrielsson, S.; Eldh, M. Molecular evaluation of five different isolation methods for extracellular vesicles reveals different clinical applicability and subcellular origin. J. Extracell. Vesicles 2021, 10, e12128. [Google Scholar] [CrossRef]
- Walton, J.R.; Frey, H.A.; Vandre, D.D.; Kwiek, J.J.; Ishikawa, T.; Takizawa, T.; Robinson, J.M.; Ackerman, W.E. Expression of flotillins in the human placenta: Potential implications for placental transcytosis. Histochem. Cell Biol. 2012, 139, 487–500. [Google Scholar] [CrossRef][Green Version]
- Jung, M.K.; Mun, J.Y. Sample Preparation and Imaging of Exosomes by Transmission Electron Microscopy. J. Vis. Exp. 2018, 131, e56482. [Google Scholar] [CrossRef] [PubMed]
- Porter, J.J.; Wildsmith, J.; Melm, C.D.; Schuchard, M.D.; Ray, K.M.; Chen, D.E.; Scott, G.B. Absolute Quantification of the Lower Abundance Proteome through Immunoaffinity Depletion of the Twenty Most Abundant Proteins in Human Serum. In Proceedings of the US HUPO 2006, Long Beach, CA, USA, 28 October–1 November 2006; Sigma-Aldrich Biotechnology: St. Louis, MO, USA, 2006. [Google Scholar]
- Navarro, A.; Molins, L.; Marrades, R.M.; Moises, J.; Viñolas, N.; Morales, S.; Canals, J.; Castellano, J.J.; Ramírez, J.; Monzo, M. Exosome Analysis in Tumor-Draining Pulmonary Vein Identifies NSCLC Patients with Higher Risk of Relapse after Curative Surgery. Cancers 2019, 11, 249. [Google Scholar] [CrossRef] [PubMed]
- Sidhom, K.; Obi, P.O.; Saleem, A. A Review of Exosomal Isolation Methods: Is Size Exclusion Chromatography the Best Option? Int. J. Mol. Sci. 2020, 21, 6466. [Google Scholar] [CrossRef] [PubMed]
- Böing, A.N.; van der Pol, E.; Grootemaat, A.E.; Coumans, F.A.W.; Sturk, A.; Nieuwland, R. Single-step isolation of extracellular vesicles by size-exclusion chromatography. J. Extracell. Vesicles 2014, 3, 23430. [Google Scholar] [CrossRef] [PubMed]
- György, B.; Módos, K.; Pállinger, É.; Pálóczi, K.; Pásztói, M.; Misják, P.; Deli, M.A.; Sipos, Á.; Szalai, A.; Voszka, I.; et al. Detection and isolation of cell-derived microparticles are compromised by protein complexes resulting from shared biophysical parameters. Blood 2011, 117, e39–e48. [Google Scholar] [CrossRef]
- Bard, M.P.; Hegmans, J.P.; Hemmes, A.; Luider, T.M.; Willemsen, R.; Severijnen, L.-A.A.; Van Meerbeeck, J.P.; Burgers, S.A.; Hoogsteden, H.C.; Lambrecht, B.N. Proteomic Analysis of Exosomes Isolated from Human Malignant Pleural Effusions. Am. J. Respir. Cell Mol. Biol. 2004, 31, 114–121. [Google Scholar] [CrossRef]
- Baranyai, T.; Herczeg, K.; Onódi, Z.; Voszka, I.; Módos, K.; Marton, N.; Nagy, G.; Mäger, I.; Wood, M.J.; El Andaloussi, S.; et al. Isolation of Exosomes from Blood Plasma: Qualitative and Quantitative Comparison of Ultracentrifugation and Size Exclusion Chromatography Methods. PLoS ONE 2015, 10, e0145686. [Google Scholar] [CrossRef]
- Cvjetkovic, A.; Jang, S.C.; Konečná, B.; Höög, J.L.; Sihlbom, C.; Lässer, C.; Lötvall, J. Detailed Analysis of Protein Topology of Extracellular Vesicles–Evidence of Unconventional Membrane Protein Orientation. Sci. Rep. 2016, 6, 36338. [Google Scholar] [CrossRef]
- Abramowicz, A.; Marczak, L.; Wojakowska, A.; Zapotoczny, S.; Whiteside, T.L.; Widlak, P.; Pietrowska, M. Harmonization of exosome isolation from culture supernatants for optimized proteomics analysis. PLoS ONE 2018, 13, e0205496. [Google Scholar] [CrossRef]
- Działo, E.; Rudnik, M.; Koning, R.I.; Czepiel, M.; Tkacz, K.; Baj-Krzyworzeka, M.; Distler, O.; Siedlar, M.; Kania, G.; Błyszczuk, P. WNT3a and WNT5a Transported by Exosomes Activate WNT Signaling Pathways in Human Cardiac Fibroblasts. Int. J. Mol. Sci. 2019, 20, 1436. [Google Scholar] [CrossRef]
- Howitt, J.; Hill, A.F. Exosomes in the Pathology of Neurodegenerative Diseases. J. Biol. Chem. 2016, 291, 26589–26597. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.B.; Yan, C.; Mu, L.; Mi, Y.L.; Zhao, H.; Hu, H.; Li, X.L.; Tao, D.D.; Wu, Y.Q.; Gong, J.P.; et al. Exosomal Wnt-Induced Dedifferentiation of Colorectal Cancer Cells Contributes to Chemotherapy Resistance. Oncogene 2019, 38, 1951–1965. [Google Scholar] [CrossRef] [PubMed]
- Shimaoka, M.; Kawamoto, E.; Gaowa, A.; Okamoto, T.; Park, E. Connexins and Integrins in Exosomes. Cancers 2019, 11, 106. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.K.S.; Yue, P.Y.K.; Ip, P.P.; Huang, R.-L.; Lai, H.-C.; Cheung, A.N.Y.; Tse, K.Y.; Ngan, H.Y.S.; Wong, A.S.T. Soluble E-cadherin promotes tumor angiogenesis and localizes to exosome surface. Nat. Commun. 2018, 9, 2270. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Harris, S.L.; Levine, A.J. The Regulation of Exosome Secretion: A Novel Function of the p53 Protein. Cancer Res. 2006, 66, 4795–4801. [Google Scholar] [CrossRef]
- Conrad, K.P.; Tuna, K.M.; Mestre, C.T.; Banwatt, E.S.; Alli, A.A. Activation of multiple receptors stimulates extracellular vesicle release from trophoblast cells. Physiol. Rep. 2020, 8, e14592. [Google Scholar] [CrossRef] [PubMed]
- Vanderboom, P.M.; Dasari, S.; Ruegsegger, G.N.; Pataky, M.W.; Lucien, F.; Heppelmann, C.J.; Lanza, I.R.; Nair, K.S. A size-exclusion-based approach for purifying extracellular vesicles from human plasma. Cell Rep. Methods 2021, 1, 100055. [Google Scholar] [CrossRef]
- Sundar, I.K.; Li, D.; Rahman, I. Proteomic Analysis of Plasma-Derived Extracellular Vesicles in Smokers and Patients with Chronic Obstructive Pulmonary Disease. ACS Omega 2019, 4, 10649–10661. [Google Scholar] [CrossRef]
- Mathivanan, S.; Simpson, R.J. ExoCarta: A compendium of exosomal proteins and RNA. Proteomics 2009, 9, 4997–5000. [Google Scholar] [CrossRef]
- Vaswani, K.; Koh, Y.Q.; Almughlliq, F.; Peiris, H.; Mitchell, M.D. A method for the isolation and enrichment of purified bovine milk exosomes. Reprod. Biol. 2017, 17, 341–348. [Google Scholar] [CrossRef]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Théry, C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, E968–E977. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.Q.; Peiris, H.N.; Vaswani, K.; Reed, S.; Rice, G.E.; Salomon, C.; Mitchell, M.D. Characterization of exosomal release in bovine endometrial intercaruncular stromal cells. Reprod. Biol. Endocrinol. 2016, 14, 78. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Higginbotham, J.N.; Jeppesen, D.; Yang, Y.-P.; Li, W.; McKinley, E.T.; Graves-Deal, R.; Ping, J.; Britain, C.M.; Dorsett, K.A.; et al. Transfer of Functional Cargo in Exomeres. Cell Rep. 2019, 27, 940–954.e6. [Google Scholar] [CrossRef] [PubMed]
- Zijlstra, A.; Di Vizio, D. Size matters in nanoscale communication. Nat. Cell Biol. 2018, 20, 228–230. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Guan, S.; Wang, X.; Zhao, J.; Gao, M.; Zhang, X. Deconstruction of Heterogeneity of Size-Dependent Exosome Subpopulations from Human Urine by Profiling N-Glycoproteomics and Phosphoproteomics Simultaneously. Anal. Chem. 2020, 92, 9239–9246. [Google Scholar] [CrossRef]
- Brennan, K.; Martin, K.; Fitzgerald, S.P.; O’Sullivan, J.; Wu, Y.; Blanco, A.; Richardson, C.; Mc Gee, M.M. A comparison of methods for the isolation and separation of extracellular vesicles from protein and lipid particles in human serum. Sci. Rep. 2020, 10, 1039. [Google Scholar] [CrossRef]

| Method | UC + SEC | UC + SEC + UF | SEC + UF | SEC + UC | ||||
|---|---|---|---|---|---|---|---|---|
| FDR method | 1% FDR | 5% FDR, 2 pep | 1% FDR | 5% FDR, 2 pep | 1% FDR | 5% FDR, 2 pep | 1% FDR | 5% FDR, 2 pep |
| Number of proteins identified | 349 | 247 | 237 | 155 | 251 | 128 | 81 | 49 |
| Plasma Proteins | UC + SEC | UC + SEC + UF | SEC + UF | SEC + UC |
|---|---|---|---|---|
| Albumin | 0.16 | 0.16 | 0.31 | 0.24 |
| α1-Antitrypsin | 0.31 | 0.19 | 0.38 | 0.08 |
| IgA/IgM | 6.25 | 6.25 | 5.75 | 1.50 |
| Transferrin | 0.00 | 0.19 | 0.00 | 0.00 |
| Haptoglobin | 3.33 | 3.67 | 0.00 | 0.00 |
| α2-Macroglobulin | 1.22 | 0.84 | 1.49 | 0.18 |
| Fibrinogen | 1.86 | 1.41 | 3.24 | 0.35 |
| Complement C3 | 0.48 | 0.37 | 0.51 | 0.09 |
| α1-Acid Glycoprotein (Orosomucoid) | 0.00 | 0.00 | 0.00 | 0.00 |
| HDL (Apolipoproteins A-I) | 0.55 | 0.45 | 0.73 | 0.27 |
| HDL (Apolipoproteins A-II) | 0.50 | 0.50 | 0.50 | 0.00 |
| LDL (mainly Apolipoprotein B) | 0.29 | 0.13 | 1.95 | 0.00 |
| Hemoglobin subunit alpha | 0.88 | 0.75 | 0.00 | 0.00 |
| Hemoglobin subunit beta | 1.07 | 0.86 | 0.64 | 0.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turner, N.P.; Abeysinghe, P.; Kwan Cheung, K.A.; Vaswani, K.; Logan, J.; Sadowski, P.; Mitchell, M.D. A Comparison of Blood Plasma Small Extracellular Vesicle Enrichment Strategies for Proteomic Analysis. Proteomes 2022, 10, 19. https://doi.org/10.3390/proteomes10020019
Turner NP, Abeysinghe P, Kwan Cheung KA, Vaswani K, Logan J, Sadowski P, Mitchell MD. A Comparison of Blood Plasma Small Extracellular Vesicle Enrichment Strategies for Proteomic Analysis. Proteomes. 2022; 10(2):19. https://doi.org/10.3390/proteomes10020019
Chicago/Turabian StyleTurner, Natalie P., Pevindu Abeysinghe, Keith A. Kwan Cheung, Kanchan Vaswani, Jayden Logan, Pawel Sadowski, and Murray D. Mitchell. 2022. "A Comparison of Blood Plasma Small Extracellular Vesicle Enrichment Strategies for Proteomic Analysis" Proteomes 10, no. 2: 19. https://doi.org/10.3390/proteomes10020019
APA StyleTurner, N. P., Abeysinghe, P., Kwan Cheung, K. A., Vaswani, K., Logan, J., Sadowski, P., & Mitchell, M. D. (2022). A Comparison of Blood Plasma Small Extracellular Vesicle Enrichment Strategies for Proteomic Analysis. Proteomes, 10(2), 19. https://doi.org/10.3390/proteomes10020019







