Proteomes of Uropathogenic Escherichia coli Growing in Human Urine and in J82 Urinary Bladder Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Media
2.2. General Experimental Approach
2.3. Collecting Bacteria after Growth in Urine and MOPS
2.4. UPEC Infection of Bladder Cells
2.5. Isolating Bacteria from Bladder Cells
2.6. Sample Preparation and Qualitative Mass Spectrometry
2.7. Mass Spectrometry Analysis
2.8. Mass Spectrometry Data Analysis
2.9. Processing of Data
2.10. Metabolic Pathways Analysis
3. Results
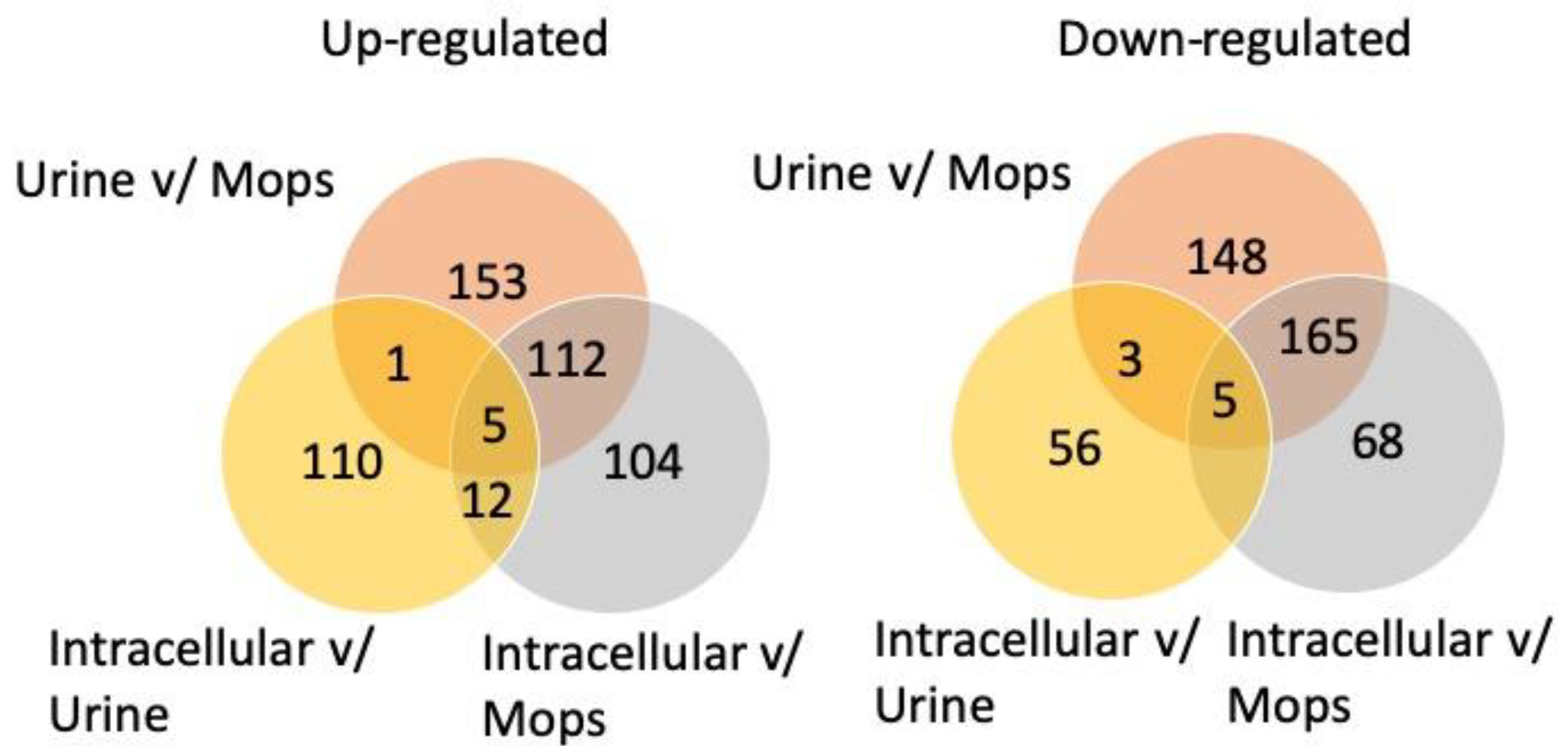
3.1. Protein Datasets of UTI89
3.2. Overrepresented Biological Processes among Upregulated Proteins
3.3. Overrepresented GO-Terms among Downregulated Proteins
3.4. Analysis of Highly Upregulated Proteins
3.5. Analysis of Highly Downregulated Proteins
3.6. Relation between Upregulation of Proteins and Attenuation Scores
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Foxman, B. Recurring urinary tract infection: Incidence and risk factors. Am. J. Public Health 1990, 80, 331–333. [Google Scholar] [CrossRef] [PubMed]
- Byron, J.K. Urinary tract infection. Vet. Clin. North Am. Small Anim. Pract. 2019, 49, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Raeispour, M.; Ranjbar, R. Antibiotic resistance, virulence factors and genotyping of uropathogenic Escherichia coli strains. Antimicrob. Resist. Infect Control 2018, 7, 118. [Google Scholar] [CrossRef] [PubMed]
- Wiles, T.J.; Kulesus, R.R.; Mulvey, M.A. Origins and virulence mechanisms of uropathogenic Escherichia coli. Exp. Mol. Pathol. 2008, 85, 11–19. [Google Scholar] [CrossRef]
- Severi, E.; Hood, D.W.; Thomas, G.H. Sialic acid utilization by bacterial pathogens. Microbiology 2007, 153, 2817–2822. [Google Scholar] [CrossRef]
- Katouli, M. Population structure of gut Escherichia coli and its role in development of extra-intestinal infections. Iran J. Microbiol. 2010, 2, 59–72. [Google Scholar]
- Conway, T.; Krogfelt, K.A.; Cohen, P.S. The life of commensal Escherichia coli in the mammalian intestine. EcoSal Plus 2004. [Google Scholar] [CrossRef]
- Gielda, L.M.; DiRita, V.J. Zinc competition among the intestinal microbiota. MBio 2012, 3, e00171-e12. [Google Scholar] [CrossRef]
- Kortman, G.A.; Raffatellu, M.; Swinkels, D.W.; Tjalsma, H. Nutritional iron turned inside out: Intestinal stress from a gut microbial perspective. FEMS Microbiol. Rev. 2014, 38, 1202–1234. [Google Scholar] [CrossRef]
- Coyte, K.Z.; Schluter, J.; Foster, K.R. The ecology of the microbiome: Networks, competition, and stability. Science 2015, 350, 663–666. [Google Scholar] [CrossRef]
- Mann, R.; Mediati, D.G.; Duggin, I.G.; Harry, E.J.; Bottomley, A.L. Metabolic adaptations of uropathogenic E. coli in the urinary tract. Front. Cell Infect. Microbiol. 2017, 7, 241. [Google Scholar] [CrossRef] [PubMed]
- Bouatra, S.; Aziat, F.; Mandal, R.; Guo, A.C.; Wilson, M.R.; Knox, C.; Bjorndahl, T.C.; Krishnamurthy, R.; Saleem, F.; Liu, P.; et al. The human urine metabolome. PLoS ONE 2013, 8, e73076. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.M.; Falkow, S. Genetic analysis of an Escherichia coli urease locus: Evidence of DNA rearrangement. J. Bacteriol. 1988, 170, 1041–1045. [Google Scholar] [CrossRef]
- Mulvey, M.A.; Lopez-Boado, Y.S.; Wilson, C.L.; Roth, R.; Parks, W.C.; Heuser, J.; Hultgren, S.J. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science 1998, 282, 1494–1497. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.J.; Mulvey, M.A.; Schilling, J.D.; Pinkner, J.S.; Hultgren, S.J. Type 1 pilus-mediated bacterial invasion of bladder epithelial cells. EMBO J. 2000, 19, 2803–2812. [Google Scholar] [CrossRef]
- Lewis, A.J.; Richards, A.C.; Mulvey, M.A. Invasion of host cells and tissues by uropathogenic bacteria. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Justice, S.S.; Hung, C.; Theriot, J.A.; Fletcher, D.A.; Anderson, G.G.; Footer, M.J.; Hultgren, S.J. Differentiation and developmental pathways of uropathogenic Escherichia coli in urinary tract pathogenesis. Proc. Natl. Acad. Sci. USA 2004, 101, 1333–1338. [Google Scholar] [CrossRef]
- Bielecki, P.; Muthukumarasamy, U.; Eckweiler, D.; Bielecka, A.; Pohl, S.; Schanz, A.; Niemeyer, U.; Oumeraci, T.; von Neuhoff, N.; Ghigo, J.M.; et al. In vivo mRNA profiling of uropathogenic Escherichia coli from diverse phylogroups reveals common and group-specific gene expression profiles. MBio 2014, 5, e01075-14. [Google Scholar] [CrossRef]
- Hagan, E.C.; Lloyd, A.L.; Rasko, D.A.; Faerber, G.J.; Mobley, H.L. Escherichia coli global gene expression in urine from women with urinary tract infection. PLoS Pathog. 2010, 6, e1001187. [Google Scholar] [CrossRef]
- Snyder, J.A.; Haugen, B.J.; Buckles, E.L.; Lockatell, C.V.; Johnson, D.E.; Donnenberg, M.S.; Welch, R.A.; Mobley, H.L. Transcriptome of uropathogenic Escherichia coli during urinary tract infection. Infect. Immun. 2004, 72, 6373–6381. [Google Scholar] [CrossRef]
- Hancock, V.; Vejborg, R.M.; Klemm, P. Functional genomics of probiotic Escherichia coli Nissle 1917 and 83972, and UPEC strain CFT073: Comparison of transcriptomes, growth and biofilm formation. Mol. Genet. Genom. 2010, 284, 437–454. [Google Scholar] [CrossRef] [PubMed]
- Alteri, C.J.; Himpsl, S.D.; Mobley, H.L. Preferential use of central metabolism in vivo reveals a nutritional basis for polymicrobial infection. PLoS Pathog. 2015, 11, e1004601. [Google Scholar] [CrossRef] [PubMed]
- Ghazalpour, A.; Bennett, B.; Petyuk, V.A.; Orozco, L.; Hagopian, R.; Mungrue, I.N.; Farber, C.R.; Sinsheimer, J.; Kang, H.M.; Furlotte, N.; et al. Comparative analysis of proteome and transcriptome variation in mouse. PLoS Genet. 2011, 7, e1001393. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Z.; Li, Y.Y.; An, T.; Huang, F.X.; Wang, M.Q.; Liu, C.X.; Mao, J.J.; Zhang, L.S. Comparative transcriptome and iTRAQ proteome analyses reveal the mechanisms of diapause in Aphidius gifuensis Ashmead (Hymenoptera: Aphidiidae). Front. Physiol. 2018, 9, 1697. [Google Scholar] [CrossRef]
- Kacírová, M.; Bober, P.; Alexovič, M.; Tomková, Z.; Tkáčiková, S.; Talian, I.; Mederová, L.; Bérešová, D.; Tóth, R.; Andrašina, I.; et al. Differential urinary proteomic analysis of endometrial cancer. Physiol. Res. 2019, 68 (Suppl. S4), S483–S490. [Google Scholar] [CrossRef]
- Alteri, C.J.; Mobley, H.L. Quantitative profile of the uropathogenic Escherichia coli outer membrane proteome during growth in human urine. Infect. Immun. 2007, 75, 2679–2688. [Google Scholar] [CrossRef]
- Alteri, C.J.; Smith, S.N.; Mobley, H.L. Fitness of Escherichia coli during urinary tract infection requires gluconeogenesis and the TCA cycle. PLoS Pathog. 2009, 5, e1000448. [Google Scholar] [CrossRef]
- Chen, S.L.; Hung, C.S.; Xu, J.; Reigstad, C.S.; Magrini, V.; Sabo, A.; Blasiar, D.; Bieri, T.; Meyer, R.R.; Ozersky, P.; et al. Identification of genes subject to positive selection in uropathogenic strains of Escherichia coli: A comparative genomics approach. Proc. Natl. Acad. Sci. USA 2006, 103, 5977–5982. [Google Scholar] [CrossRef]
- Mulvey, M.A.; Schilling, J.D.; Hultgren, J.S. Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect. Immun. 2001, 69, 4572–4579. [Google Scholar] [CrossRef]
- Neidhardt, F.C.; Bloch, P.L.; Smith, D.F. Culture medium for enterobacteria. J. Bacteriol. 1974, 119, 736–747. [Google Scholar] [CrossRef]
- Staerk, K.; Khandige, S.; Kolmos, H.J.; Møller-Jensen, J.; Andersen, T.E. Uropathogenic Escherichia coli express type 1 fimbriae only in surface adherent populations under physiological growth conditions. J. Infect. Dis. 2016, 213, 386–894. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thingholm, T.E.; Palmisano, G.; Kjeldsen, F.; Larsen, M.R. Undesirable charge-enhancement of isobaric tagged phosphopeptides leads to reduced identification efficiency. J. Proteome Res. 2010, 9, 4045–4052. [Google Scholar] [CrossRef] [PubMed]
- Perez-Riverol, Y.; Bai, J.; Bandla, C.; Hewapathirana, S.; García-Seisdedos, D.; Kamatchinathan, S.; Kundu, D.; Prakash, A.; Frericks-Zipper, A.; Eisenacher, M.; et al. The PRIDE database resources in 2022: A hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 2022, 50, D543–D552. [Google Scholar] [CrossRef] [PubMed]
- Mi, H.; Muruganujan, A.; Ebert, D.; Huang, X.; Thomas, P.D. PANTHER version 14: More genomes, a new PANTHER GO-slim and improvements in enrichment analysis tools. Nucleic Acids Res. 2019, 47, D419–D426. [Google Scholar] [CrossRef] [PubMed]
- Spurbeck, R.R.; Seth, P.C.D.; Walk, T.; Stapleton, A.E.; Hooton, T.M.; Nolan, L.K.; Kim, K.S.; Johnson, J.R.; Mobley, H.L.T. Escherichia coli isolates that carry vat, fyuA, chuA, and yfcV efficiently colonize the urinary tract. Infect. Immun. 2012, 80, 4115–4122. [Google Scholar] [CrossRef]
- Salmon, K.A.; Hung, S.P.; Steffen, N.R.; Krupp, R.; Baldi, P.; Hatfield, G.W.; Gunsalus, R.P. Global gene expression profiling in Escherichia coli K12: Effects of oxygen availability and ArcA. J. Biol. Chem. 2005, 280, 15084–15096. [Google Scholar] [CrossRef]
- Izutsu, K.; Wada, C.; Komine, Y.; Sako, T.; Ueguchi, C.; Nakura, S.; Wada, A. Escherichia coli ribosome-associated protein SRA, whose copy number increases during stationary phase. J. Bacteriol. 2001, 183, 2765–2773. [Google Scholar] [CrossRef]
- Maguire, B.A.; Wild, D.G. The roles of proteins L28 and L33 in the assembly and function of Escherichia coli ribosomes in vivo. Mol. Microbiol. 1997, 23, 237–245. [Google Scholar] [CrossRef]
- Zhou, L.; Zhao, M.; Wolf, R.Z.; Graham, D.E.; Georgiou, G. Transcriptional regulation of the Escherichia coli gene rraB, encoding a protein inhibitor of RNase, E. J. Bacteriol. 2009, 191, 6665–6674. [Google Scholar] [CrossRef]
- Gehring, A.M.; Bradley, K.A.; Walsh, C.T. Enterobactin biosynthesis in Escherichia coli: Isochorismate lyase (EntB) is a bifunctional enzyme that is phosphopantetheinylated by EntD and then acylated by EntE using ATP and 2,3-dihydroxybenzoate. Biochemical 1997, 36, 8495–8503. [Google Scholar] [CrossRef]
- Kim, H.; Chaurasia, A.K.; Kim, T.; Choi, J.; Ha, S.C.; Kim, D.; Kim, K.K. Structural and functional study of ChuY from Escherichia coli strain CFT073. Biochem. Biophys. Res. Commun. 2017, 482, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Novotny, J.R.; Reizer, J.; Esch, F.; Saier, M.H., Jr. Purification and properties of D-mannitol-1-phosphate dehydrogenase and D-glucitol-6-phosphate dehydrogenase from Escherichia coli. J. Bacteriol. 1984, 159, 986–990. [Google Scholar] [CrossRef]
- Karatza, P.; Frillingos, S. Cloning and functional characterization of two bacterial members of the NAT/NCS2 family in Escherichia coli. Mol. Membr. Biol. 2005, 22, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Cecchini, G.; Schröder, I.; Gunsalus, R.P.; Maklashina, E. Succinate dehydrogenase and fumarate reductase from Escherichia coli. Biochim. Biophys. Acta 2002, 1553, 140–157. [Google Scholar] [CrossRef]
- Tarry, M.J.; Schäfer, E.; Chen, S.; Buchanan, G.; Greene, N.P.; Lea, S.M.; Palmer, T.; Saibil, H.R.; Berks, B.C. Structural analysis of substrate binding by the TatBC component of the twin-arginine protein transport system. Proc. Natl. Acad. Sci. USA 2009, 106, 13284–13289. [Google Scholar] [CrossRef]
- Cheng, Z.F.; Deutscher, M.P. Purification and characterization of the Escherichia coli exoribonuclease RNase R. Comparison with RNase II. J. Biol. Chem. 2002, 277, 21624–21629. [Google Scholar] [CrossRef] [PubMed]
- Shea, A.E.; Marzoa, J.; Himpsl, S.D.; Smith, S.N.; Zhao, L.; Tran, L.; Mobley, H.L.T. Escherichia coli CFT073 fitness factors during urinary tract infection: Identification using an ordered transposon library. Appl. Environ. Microbiol. 2020, 86, e00691-20. [Google Scholar] [CrossRef]
- García, V.; Grønnemose, R.B.; Torres-Puig, S.; Kudirkiene, E.; Piantelli, M.; Ahmed, S.; Andersen, T.E.; Møller-Jensen, J.; Olsen, J.E.; Herrero-Fresno, A. Genome-wide analysis of fitness-factors in uropathogenic Escherichia coli during growth in laboratory media and during urinary tract infections. Microb. Genom. 2021, 7, 000719. [Google Scholar] [CrossRef]
- Willems, P.; Fels, U.; Staes, A.; Gevaert, K.; Van Damme, P. Use of hybrid data-dependent and -independent acquisition spectral libraries empowers dual-proteome profiling. J. Proteome Res. 2021, 20, 1165–1177. [Google Scholar] [CrossRef]
- Soufi, B.; Krug, K.; Harst, A.; Macek, B. Characterization of the E. coli proteome and its modifications during growth and ethanol stress. Front. Microbiol. 2015, 6, 103. [Google Scholar] [CrossRef]
- Schmidt, A.; Kochanowski, K.; Vedelaar, S.; Ahrné, E.; Volkmer, B.; Callipo, L.; Knoops, K.; Bauer, M.; Aebersold, R.; Heinemann, M. The quantitative and condition-dependent Escherichia coli proteome. Nat. Biotechnol. 2016, 34, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Reitzer, L.; Zimmern, P. Rapid growth and metabolism of uropathogenic Escherichia coli in relation to urine composition. Clin. Microbiol. Rev. 2019, 33, e00101–e00119. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, V.S.; Armbruster, C.E.; Smith, S.N.; Pirani, A.; Springman, A.C.; Walters, M.S.; Nielubowicz, G.R.; Himpsl, S.D.; Snitkin, E.S.; Mobley, H.L.T. Rapid growth of uropathogenic Escherichia coli during human urinary tract infection. MBio 2018, 9, e00186-18. [Google Scholar] [CrossRef] [PubMed]
- Gordon, D.M.; Riley, M.A. A theoretical and experimental analysis of bacterial growth in the bladder. Mol. Microbiol. 1992, 6, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Mobley, H.L. Measuring Escherichia coli gene expression during human urinary tract infections. Pathogens 2016, 5, 7. [Google Scholar] [CrossRef]
- Wurpel, D.J.; Moriel, D.G.; Totsika, M.; Easton, D.M.; Schembri, M.A. Comparative analysis of the uropathogenic Escherichia coli surface proteome by tandem mass-spectrometry of artificially induced outer membrane vesicles. J. Proteom. 2015, 115, 93–106. [Google Scholar] [CrossRef]
- Wurpel, D.J.; Totsika, M.; Allsopp, L.P.; Webb, R.I.; Moriel, D.G.; Schembri, M.A. Comparative proteomics of uropathogenic Escherichia coli during growth in human urine identify UCA-like (UCL) fimbriae as an adherence factor involved in biofilm formation and binding to uroepithelial cells. J. Proteom. 2016, 131, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Floyd, K.A.; Moore, J.L.; Eberly, A.R.; Good, J.A.; Shaffer, C.L.; Zaver, H.; Almqvist, F.; Skaar, E.P.; Caprioli, R.M.; Hadjifrangiskou, M. Adhesive fiber stratification in uropathogenic Escherichia coli biofilms unveils oxygen-mediated control of type 1 pili. PLoS Pathog. 2015, 11, e1004697. [Google Scholar] [CrossRef]
- Letoffe, S.; Delepelaire, P.; Wandersman, C. The housekeeping dipeptide permease is the Escherichia coli heme transporter and functions with two optional peptide binding proteins. Proc. Natl. Acad. Sci. USA 2006, 103, 12891–12896. [Google Scholar] [CrossRef]
- Griggs, D.W.; Tharp, B.B.; Konisky, J. Cloning and promoter identification of the iron-regulated cir gene of Escherichia coli. J. Bacteriol. 1987, 169, 5343–5352. [Google Scholar] [CrossRef]
- Madelung, M.; Kronborg, T.; Doktor, T.K.; Struve, C.; Krogfelt, K.A.; Møller-Jensen, J. DFI-seq identification of environment-specific gene expression in uropathogenic Escherichia coli. BMC Microbiol. 2017, 17, 99. [Google Scholar] [CrossRef] [PubMed]
- Vejborg, R.M.; de Evgrafov, M.R.; Phan, M.D.; Totsika, M.; Schembri, M.A.; Hancock, V. Identification of genes important for growth of asymptomatic bacteriuria with Escherichia coli in urine. Infect. Immun. 2012, 80, 3179–3188. [Google Scholar] [CrossRef]
- Hull, R.A.; Hull, S.I. Nutritional requirements for growth of uropathogenic Escherichia coli in human urine. Infect. Immun. 1997, 65, 1960–1961. [Google Scholar] [CrossRef] [PubMed]
- Preumont, A.; Snoussi, K.; Stroobant, V.; Collet, J.F.; van Schaftingen, E. Molecular identification of pseudouridine-metabolizing enzymes. J. Biol. Chem. 2008, 283, 25238–25246. [Google Scholar] [CrossRef] [PubMed]
- Plumbridge, J.; Vimr, E. Convergent pathways for utilization of the amino sugars N-acetylglucosamine, N-acetylmannosamine, and N-acetylneuraminic acid by Escherichia coli. J. Bacteriol. 1999, 181, 47–54. [Google Scholar] [CrossRef]
- Nakano, I.; Tsugawa, T.; Shinohara, R.; Watanabe, F.; Fujita, T.; Nagata, M.; Kato, T.; Himeno, Y.; Kobayashi, T.; Fujiwara, K.; et al. Urinary sorbitol measurement and the effect of an aldose reductase inhibitor on its concentration in the diabetic state. J. Diabet. Complic. 2003, 17, 337–342. [Google Scholar] [CrossRef]
- Liang, W.J.; Wilson, K.J.; Xie, H.; Knol, J.; Suzuki, S.; Rutherford, N.G.; Henderson, P.J.; Jefferson, R.A. The gusBC genes of Escherichia coli encode a glucuronide transport system. J. Bacteriol. 2005, 187, 2377–2385. [Google Scholar] [CrossRef]
- Leatham-Jensen, M.P.; Mokszycki, M.E.; Rowley, D.C.; Deering, R.; Camberg, J.L.; Sokurenko, E.V.; Tchesnokova, V.L.; Frimodt-Møller, J.; Krogfelt, K.A.; Leth Nielsen, K.; et al. Uropathogenic Escherichia coli metabolite-dependent quiescence and persistence may explain antibiotic tolerance during urinary tract infection. Msphere 2016, 1, e00055-15. [Google Scholar] [CrossRef]
- Conover, M.S.; Hadjifrangiskou, M.; Palermo, J.J.; Hibbing, M.E.; Dodson, K.W.; Hultgren, S.J. Metabolic requirements of Escherichia coli in intracellular bacterial communities during Uuinary tract infection pathogenesis. MBio 2016, 7, e00104–e00116. [Google Scholar] [CrossRef]
- Andersen-Civil, A.I.S.; Ahmed, S.; Guerra, P.R.; Andersen, T.E.; Hounmanou, Y.M.G.; Olsen, J.E.; Herrero-Fresno, A. The impact of inactivation of the purine biosynthesis genes, purN and purT, on growth and virulence in uropathogenic E. coli. Mol. Biol. Rep. 2018, 45, 2707–2716. [Google Scholar] [CrossRef]
- Thakur, A.; Mikkelsen, H.; Jungersen, G. Intracellular pathogens: Host Immunity and microbial persistence strategies. J. Immunol. Res. 2019, 2019, 1356540. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Wada, A.; Shimada, T.; Maki, Y.; Ishihama, A. Coordinated regulation of Rsd and RMF for simultaneous hibernation of transcription apparatus and translation machinery in stationary-phase Escherichia coli. Front. Genet. 2019, 10, 1153. [Google Scholar] [CrossRef] [PubMed]
- Sintsova, A.; Frick-Cheng, A.E.; Smith, S.; Pirani, A.; Subashchandrabose, S.; Snitkin, E.S.; Mobley, H. Genetically diverse uropathogenic Escherichia coli adopt a common transcriptional program in patients with UTIs. Elife 2019, 8, e49748. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, C.L.; Zhang, E.W.; Dudley, A.G.; Dixon, B.R.E.A.; Guckes, K.R.; Breland, E.J.; Floyd, K.A.; Casella, D.P.; Algood, H.M.S.; Clayton, D.B.; et al. Purine biosynthesis metabolically constrains intracellular survival of uropathogenic Escherichia coli. Infect. Immun. 2017, 85, e00471-16. [Google Scholar] [CrossRef] [PubMed]
- Ornston, M.K.; Ornston, L.N. Two forms of D-glycerate kinase in Escherichia coli. J. Bacteriol. 1969, 97, 1227–1233. [Google Scholar] [CrossRef] [PubMed]
- Hejazi, M.; Piotukh, K.; Mattow, J.; Deutzmann, R.; Volkmer-Engert, R.; Lockau, W. Isoaspartyl dipeptidase activity of plant-type asparaginases. Biochem. J. 2002, 364, 129–136. [Google Scholar] [CrossRef]
- Rothe, M.; Alpert, C.; Loh, G.; Blaut, M. Novel insights into E. coli’s hexuronate metabolism: KduI facilitates the conversion of galacturonate and glucuronate under osmotic stress conditions. PLoS ONE 2013, 8, e56906. [Google Scholar] [CrossRef]
- Bloom, F.R.; McFall, E. Isolation and characterization of D-serine deaminase constitutive mutants by utilization of D-serine as sole carbon or nitrogen source. J. Bacteriol. 1975, 121, 1078–1084. [Google Scholar] [CrossRef]
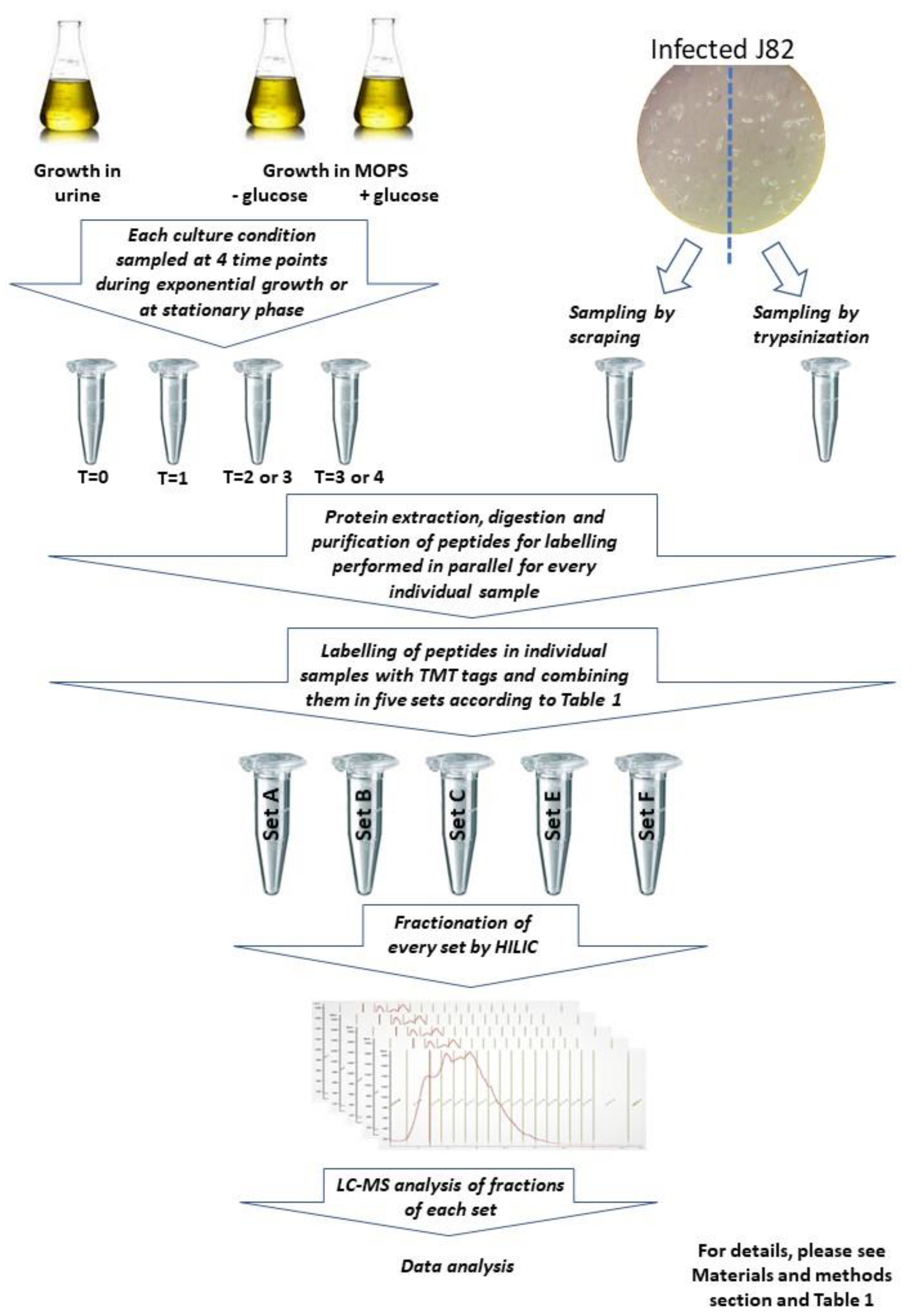
| TMT Label | Set A: Sample and Timepoint # | Set B: Sample and Timepoint # | Set C: Sample and Timepoint # | Set E: Sample and Timepoint # | Set F: Sample and Timepoint # |
|---|---|---|---|---|---|
| 126 | Urine T = 0 | Urine T = 0 | Urine T = 0 | Stationary ph. MOPS | Stationary ph. MOPS |
| 127_N | Urine T = 1 | Urine T = 1 | Urine T = 1 | Stationary ph. MOPS with glucose | Stationary ph. MOPS with glucose |
| 127_C | Urine T = 3 | Urine T = 3 | Urine T = 3 | Exponential ph. MOPS | Exponential ph. MOPS |
| 128_N | Urine T = 4 | Urine T = 4 | Urine T = 4 | Exponential ph. MOPS with glucose | Exponential ph. MOPS with glucose |
| 128_C | MOPS T = 0 | MOPS T = 0 | MOPS T = 0 | Trypsin-detached pellet, bladder cells (as in Set B) | Trypsin-detached pellet, bladder cells (as in Set C) |
| 129_N | MOPS T = 1 | MOPS T = 1 | MOPS T = 1 | Scrape-detached pellet, bladder cells (as in Set B) | Scrape-detached pellet, bladder cells (as in Set C) |
| 129_C | MOPS T = 2 | MOPS T = 2 | MOPS T = 2 | MOPS T = 2 (as in Set A) | MOPS T = 2 (as in Set C) |
| 130_N | MOPS T = 3 | MOPS T = 3 | MOPS T = 3 | MOPS T = 4 (as in Set A) | MOPS T = 4 (as in Set C) |
| 130_C | Trypsin-detached pellet, bladder cells | Trypsin-detached pellet, bladder cells | Trypsin-detached pellet, bladder cells | Urine T = 2 (as in Set A) | Urine T = 2 (as in Set C) |
| 131 | Scrape-detached pellet, bladder cells | Scrape-detached pellet, bladder cells | Scrape-detached pellet, bladder cells | Urine T = 4 (as in Set A) | Urine T = 4 (as in Set C) |
| Category (GO-Term Biological Processes) | Proteins in GO-Term a | Proteins in Proteome b | Expected Proteins c | EF d | p-Value e | FDR f |
|---|---|---|---|---|---|---|
| Analysis based on Up-regulated proteins | ||||||
| Virulence factors (incl. transport) | ||||||
| Enterobactin bio-synthesis | 8 | 6 | 0.39 | 15.3 | 2.11 × 10−5 | 1.33 × 10−3 |
| Siderophore transport | 9 | 6 | 0.44 | 13.6 | 3.39 × 10−5 | 1.84 × 10−3 |
| Heme transport | 11 | 5 | 0.54 | 9.3 | 6.05 × 10−4 | 1.55 × 10−2 |
| Amino acid biosynthesis | ||||||
| Arginine via ornithine | 8 | 8 | 0.39 | 10.2 | 1.70 × 10−3 | 3.64 × 10−2 |
| Valine | 13 | 10 | 0.64 | 15.7 | 2.67 × 10−8 | 4.22 × 10−6 |
| Isoleucine | 13 | 11 | 0.64 | 17.3 | 2.60 × 10−9 | 4.82 × 10−7 |
| Leucine | 8 | 5 | 0.39 | 12.8 | 2.00 × 10−4 | 6.93 × 10−3 |
| Tryptophan | 9 | 5 | 0.44 | 11.4 | 2.99 × 10−4 | 8.91 × 10−3 |
| Glutamine | 18 | 6 | 0.88 | 6.8 | 6.39 × 10−4 | 1.59 × 10−2 |
| Serine | 25 | 7 | 1.22 | 5.7 | 5.42 × 10−4 | 1.41 × 10−2 |
| Methionine | 16 | 6 | 0.78 | 7.7 | 3.83 × 10−4 | 1.06 × 10−2 |
| Homoserin | 9 | 4 | 0.44 | 9.1 | 2.36 × 10−3 | 4.84 × 10−2 |
| Homocystein | 6 | 4 | 0.29 | 13.6 | 7.76 × 10−4 | 1.10 × 10−2 |
| Transporters | ||||||
| L-alpha-amino acid transmembrane transport | 30 | 8 | 1.47 | 5.5 | 2.87 × 10−4 | 8.96 × 10−3 |
| Amino acid import across plasma membrane | 13 | 5 | 0.64 | 7.9 | 1.1 × 10−3 | 2.55 × 10−2 |
| D-methionine AA transport | 3 | 3 | 0.15 | 20.4 | 1.81 × 10−3 | 3.85 × 10−2 |
| L-amino acid transport | 36 | 8 | 1.76 | 4.5 | 8.17 × 10−4 | 1.94 × 10−2 |
| Others | ||||||
| Uronic acid metabolic process | 10 | 5 | 0.49 | 10.2 | 4.32 × 10−4 | 1.17 × 10−2 |
| Dicarboxylic acid metabolic process | 97 | 15 | 4.75 | 3.2 | 1.85 × 10−4 | 6.56 × 10−2 |
| Tetrahydrofolate interconvention | 6 | 4 | 0.29 | 13.6 | 7.76 × 10−4 | 1.87 × 10−2 |
| De novo IMP biosynthesis | 12 | 6 | 0.59 | 10.2 | 1.12 × 10−4 | 4.34 × 10−3 |
| Analysis based on Downregulated proteins | ||||||
| Alpha-amino acid catabolic processes | 63 | 17 | 4.32 | 3.9 | 8.41 × 10−6 | 2.65 × 10−2 |
| Category (GO-Term Biological Processes) | Proteins in GO-Term a | Proteins in Proteome b | Expected Proteins c | EF d | p-Value e | FDR f |
|---|---|---|---|---|---|---|
| Analysis based on Upregulated proteins | ||||||
| Virulence factors | ||||||
| Enterobactin biosynthesis | 8 | 6 | 0.33 | 1F | 8.41 × 10−6 | 9.83 × 10−4 |
| Amino acid biosynthesis/metabolism | ||||||
| Arginine Ornithin | 15 10 | 9 5 | 0.62 0.41 | 14.5 12.1 | 1.60 × 10−6 2.05 × 10−4 | 5.06 × 10−5 1.47 × 10−2 |
| Transporters | ||||||
| Amino acid import across plasma membrane L-alfa-amino-acid transmembrane | 13 30 | 5 7 | 0.54 1.24 | 9.3 5.6 | 5.30 × 10−4 5.23 × 10−4 | 3.56 × 10−2 3.60 × 10−2 |
| Others | ||||||
| Sulfur compound biosynthetic process | 67 | 12 | 2.78 | 4.3 | 5.43 × 10−5 | 4.28 × 10−3 |
| Analysis based on Downregulated proteins | ||||||
| Ribosome | ||||||
| Ribosomal large subunit assembly | 29 | 12 | 1.56 | 7.7 | 4.94 × 10−7 | 8.67 × 10−5 |
| Gene expression | ||||||
| Translation | 116 | 30 | 6.23 | 4.8 | 1.96 × 10−11 | 3.09 × 10−8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andersen, S.; Nawrocki, A.; Johansen, A.E.; Herrero-Fresno, A.; Menéndez, V.G.; Møller-Jensen, J.; Olsen, J.E. Proteomes of Uropathogenic Escherichia coli Growing in Human Urine and in J82 Urinary Bladder Cells. Proteomes 2022, 10, 15. https://doi.org/10.3390/proteomes10020015
Andersen S, Nawrocki A, Johansen AE, Herrero-Fresno A, Menéndez VG, Møller-Jensen J, Olsen JE. Proteomes of Uropathogenic Escherichia coli Growing in Human Urine and in J82 Urinary Bladder Cells. Proteomes. 2022; 10(2):15. https://doi.org/10.3390/proteomes10020015
Chicago/Turabian StyleAndersen, Sisse, Arkadiusz Nawrocki, Andreas Eske Johansen, Ana Herrero-Fresno, Vanesa García Menéndez, Jakob Møller-Jensen, and John Elmerdahl Olsen. 2022. "Proteomes of Uropathogenic Escherichia coli Growing in Human Urine and in J82 Urinary Bladder Cells" Proteomes 10, no. 2: 15. https://doi.org/10.3390/proteomes10020015
APA StyleAndersen, S., Nawrocki, A., Johansen, A. E., Herrero-Fresno, A., Menéndez, V. G., Møller-Jensen, J., & Olsen, J. E. (2022). Proteomes of Uropathogenic Escherichia coli Growing in Human Urine and in J82 Urinary Bladder Cells. Proteomes, 10(2), 15. https://doi.org/10.3390/proteomes10020015






