Insights into Components of Prospective Science Teachers’ Mental Models and Their Preferred Visual Representations of Atoms
Abstract
1. Introduction
2. Literature Review
2.1. The Nature of Mental Models and Their Importance When Learning about Atomic Structure
2.2. Teaching and Learning about Atomic Structure
2.3. Research about Understanding the Structure of Atoms in the Turkish Educational Context
3. Sample and Method
3.1. Background and Sample
3.2. Data Collection
3.3. Data Analysis
4. Results
“The size of the atom is directly proportionate to its diameter. Electrons are located in orbits. If an atom has more electrons, it has more orbits. That’s why it is big. For example, an atom of hydrogen has an electron and an orbit. On the other hand, the atom of sodium has 11 electrons and three orbits.”
“Atoms can be seen by the help of a microscope. They cannot be seen by the naked eye. Since the existence of atoms is based on a scientific foundation, scientists must have seen it.”
“I think atoms can be seen because scientists propounded many atomic models. They could not have done this without seeing.”
“There are a lot of studies, models and laws related to atoms. Therefore, scientists must have seen atoms.”
“An atom cannot be seen with your eye. It cannot be seen by microscope, either. Its existence can only be accepted. For this reason, scientists form models.”
“Atoms cannot be seen by your eye. Different scientists have come up with different theories and proposed various models. The correctness of these theories has been either proved or refuted, and then new models have been developed.”
“To me, atoms can never be seen under any circumstances. I don’t think there is a scientist who has seen atoms. If anyone had seen one, Bohr, for example, wouldn’t have developed an atomic model in the way he did.”
“The texture of an atom is solid if the matter is solid; it has a soft structure if the matter is soft. For example, the atom of sodium has a soft structure. The atom of gold is more solid when compared to the atom of sodium.”
“All matter is comprised of smallest building blocks. This is an ‘atom’ in non-living things, a ‘cell’ in living things…”. “Cells, the smallest building blocks of living things, are comprised of numerous atoms formed of protein, carbohydrates and fat.”
5. Discussion
6. Conclusions
7. Limitations
Author Contributions
Funding
Conflicts of Interest
References
- Duit, R. On the role of analogies and metaphors in learning science. Sci. Educ. 1991, 75, 649–672. [Google Scholar] [CrossRef]
- Duit, R.; Treagust, D.F. Students’ conceptions and constructivist teaching approaches. In Improving Science Education; Fraser, B.J., Walberg, H.J., Eds.; The National Society for the Study of Education: Chicago, IL, USA, 1995. [Google Scholar]
- Harrison, A.G.; Treagust, D.F. Secondary students mental models of atoms and molecules: Implications for teaching science. Sci. Educ. 1996, 80, 509–534. [Google Scholar] [CrossRef]
- Taber, K.S. An alternative conceptual framework from chemistry education. Int. J. Sci. Educ. 1998, 20, 597–608. [Google Scholar] [CrossRef]
- Eilks, I. Teachers’ ways through the particulate nature of matter in lower secondary chemistry teaching: A continued change of different models vs. a coherent conceptual structure? In Concepts of Matter in Science Education; Tsaparlis, G., Sevian, H., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 213–230. [Google Scholar]
- Kiray, S.A. The pre-service science teachers’ mental models for concept of atoms and learning difficulties. Int. J. Educ. Math. Sci. Technol. 2016, 4, 147–162. [Google Scholar] [CrossRef]
- Nakiboğlu, C.; Karakoç, Ö.; Benlikaya, R. Öğretmen adaylarının atomun yapısı ile ilgili zihinsel modelleri [Prospective Teachers’ Mental Models of Atomic Sructure]. Abant İzzet Baysal Üniversitesi Eğitim Fakültesi Dergisi 2002, 2, 88–98. [Google Scholar]
- Gunstone, R.; White, R. Goals, methods and achievements of research in science education. In Improving Science Education; Millar, R., Leach, J., Osborne, J., Eds.; Open University Press: Buckingham, UK, 2000; pp. 293–307. [Google Scholar]
- Ayas, A. Kavram Öğrenimi [Concept Learning]. In Kuramdan Uygulamaya Fen ve Teknoloji Öğretimi; Çepni, S., Ed.; Pegem Yayıncılık: Ankara, Turkey, 2006; pp. 125–151. [Google Scholar]
- Vosniadou, S.; Brewer, W.F. Mental models of the earth: A study of conceptual change in childhood. Cogn. Psych. 1992, 24, 535–585. [Google Scholar] [CrossRef]
- Vosniadou, S. Capturing and modelling the process of conceptual change. Learn. Instr. 1994, 4, 45–69. [Google Scholar] [CrossRef]
- Franco, C.; Colinvaux, D. Grasping mental models. In Developing Models in Science Education; Gilbert, J.K., Boulter, C.J., Eds.; Kluwer: Dordrecht, The Netherlands, 2000; pp. 93–118. [Google Scholar]
- Coll, R.K.; Treagust, D.F. Investigation of Secondary School, Undergraduate, and Graduate Learners’ Mental Models of Ionic Bonding. J. Res. Sci. Teach. 2003, 40, 464–486. [Google Scholar] [CrossRef]
- Norman, D.N. Some observations on mental models. In Mental Models; Gentner, D., Stevens, A.L., Eds.; Lawrence Erlbaum: Hillsdale, MI, USA, 1983; pp. 7–14. [Google Scholar]
- Taber, K.S. Constructing and communicating knowledge about chemistry and chemistry education. Chem. Educ. Res. Pract. 2014, 15, 5–9. [Google Scholar] [CrossRef]
- Glynn, S.M.; Duit, R. Learning Science in the Schools: Research Reforming Practice; Lawrence Erlbaum: Mahwah, NJ, USA, 1995. [Google Scholar]
- Thiele, R.B.; Treagust, D.F. The nature and extent of analogies in secondary science textbooks. Instr. Sci. 1994, 22, 61–74. [Google Scholar] [CrossRef]
- Thiele, R.B.; Treagust, D.F. An interpretive examination of high school chemistry teachers’ analogical explanations. J. Res. Sci. Teach. 1994, 31, 227–242. [Google Scholar] [CrossRef]
- Johnstone, A.H. Why is science difficult to learn? Things are seldom what they seem. J. Comput. Assist. Learn. 1991, 7, 75–83. [Google Scholar] [CrossRef]
- Eilks, I.; Möllering, J.; Valanides, N. Seventh-grade students’ understanding of chemical reactions—Reflections from an action research interview study. Eurasia J. Math. Sci. Technol. Educ. 2007, 4, 271–286. [Google Scholar] [CrossRef]
- Eilks, I. Students’ understanding of the particulate nature of matter and some misleading illustrations from textbooks. Chem. Act. 2003, 69, 35–40. [Google Scholar]
- Harrison, A.G.; Treagust, D.F. Learning about atoms, molecules, and chemical bonds: A case study of multiple-model use in grade 11 chemistry. Sci. Educ. 2000, 84, 352–381. [Google Scholar] [CrossRef]
- Justi, R.; Gilbert, J. History and philosophy of science through models: Some challenges in the case of ‘the atom’. Int. J. Sci. Educ. 2000, 22, 993–1009. [Google Scholar] [CrossRef]
- Grosslight, L.; Unger, C.; Jay, E.; Smith, C.L. Understanding models and their use in science: Conceptions of middle and high school students and experts. J. Res. Sci. Teach. 1991, 28, 799–822. [Google Scholar] [CrossRef]
- Çökelez, A.; Dumon, A. Atom and molecule: Upper secondary school French students’ representations in long-term memory. Chem. Educ. Res. Pract. 2005, 6, 119–135. [Google Scholar] [CrossRef]
- Park, E.J.; Light, G. Identifying atomic structure as a threshold concept: Student mental models and troublesomeness. Int. J. Sci. Educ. 2009, 31, 233–258. [Google Scholar] [CrossRef]
- Papageorgiou, G.; Markos, A.; Zarkadis, N. Understanding the atom and relevant misconceptions: Students’ profiles in relation to three cognitive variables. Sci. Educ. Int. 2016, 27, 464–488. [Google Scholar]
- Papageorgiou, G.; Markos, A.; Zarkadis, N. Students’ representations of the atomic structure—The effect of some individual differences in particular task contexts. Chem. Educ. Res. Pract. 2016, 17, 209–219. [Google Scholar] [CrossRef]
- Yıldız, H.T. İlköğretim ve ortaöğretim öğrencilerinin atomun yapısı ile ilgili zihinsel modelleri [Primary and Secondary Students’ Mental Models Concerning Atomic Structure]. Master’s Thesis, Balıkesir University, Balıkesir, Turkey, 29 August 2006. [Google Scholar]
- Çökelez, A. Students’ (Grade 7–9) ideas on particle concept: Didactical transposition. Hacet. Univ. J. Educ. 2009, 36, 64–75. [Google Scholar]
- Çökelez, A.; Yalçın, S. The analysis of the mental models of students in grade-7 regarding atom concept. Element. Educ. Online 2012, 11, 452–471. [Google Scholar]
- Çökelez, A. Junior high school students’ ideas about the shape and size of the atom. Res. Sci. Educ. 2012, 42, 673–686. [Google Scholar] [CrossRef]
- Karagöz, Ö.; Sağlam-Arslan, A. İlköğretim öğrencilerinin atomun yapısına ilişkin zihinsel modellerinin analizi [Analysis of mental models of atomic structure of elementary school students]. Türk Fen Eğitimi Dergisi 2012, 9, 132–142. [Google Scholar]
- Ministry of National Education (MoNE). High School Chemistry Curriculum; MoNE: Ankara, Turkey, 2018.
- Nakiboğlu, C. Using word associations for assessing non major science students’ knowledge structure before and after general chemistry instruction: The case of atomic structure. Chem. Educ. Res. Pract. 2008, 9, 309–322. [Google Scholar] [CrossRef]
- Ministry of National Education (MoNE). High School 9th Grade Chemistry Course Book; Başak Printing: Ankara, Turkey, 2011.
- Ministry of National Education (MoNE). High School 10th Grade Chemistry Course Book; Başak Printing: Ankara, Turkey, 2012.
- Ministry of National Education (MoNE). High School 9th Grade Chemistry Course Book; Tuna Printing: Ankara, Turkey, 2015.
- Zarkadis, N.; Papageorgiou, G.; Stamovlasis, D. Studying the consistency between and within the student mental models for the atomic structure. Chem. Educ. Res. Pract. 2017, 18, 893–902. [Google Scholar] [CrossRef]
- Miles, M.B.; Huberman, A.M. Qualitative Data Analysis; Sage: Thousand Oaks, CA, USA, 1994. [Google Scholar]
- Taber, K.S. Mediating mental models of metals: Acknowledging the priority of the learner’s prior learning. Sci. Educ. 2003, 87, 732–758. [Google Scholar] [CrossRef]
- Chi, M.T.H.; Slotta, J.D.; de Leeuw, N. From things to process: A theory of conceptual change for learning science concepts. Learn. Instr. 1994, 4, 27–43. [Google Scholar] [CrossRef]
- Ben-Zvi, R.; Eylon, B.; Silberstein, J. Is an atom of copper malleable? J. Chem. Educ. 1986, 63, 64–66. [Google Scholar] [CrossRef]
- Anderson, B. Pupil’s conceptions of matter and its transformation (age 12–16). Stud. Sci. Educ. 1990, 18, 53–85. [Google Scholar] [CrossRef]
- Lee, O.; Eichinger, D.C.; Anderson, C.W.; Berkheimer, G.D.; Blakeslee, T.D. Changing middle school students’ conceptions of matter and molecules. J. Res. Sci. Teach. 1993, 30, 249–270. [Google Scholar] [CrossRef]
- Adadan, E. Model-Tabanlı Öğrenme Ortamının Kimya Öğretmen Adaylarının Maddenin Tanecikli Yapısı Kavramını ve Bilimsel Modellerin Doğasını Anlamaları Üzerine Etkisinin İncelenmesi [Investigating the Effect of Model-Based Learning Environment on Preservice Chemistry Teachers’ Understadings of the Particle Theory of Matter and the Nature of Scientific Models]. OMU J. Fac. Educ. 2014, 33, 378–403. [Google Scholar]
- Strike, K.A.; Posner, G.J. A revisionist theory of conceptual change. In Philosophy of Science, Cognitive Psychology, and Educational Theory and Practice; Duschl, R.A., Hamilton, R.J., Eds.; State University of New York Press: New York, NY, USA, 1992; pp. 147–173. [Google Scholar]
- Eilks, I.; Möllering, J.; Ralle, B. Scanning tunneling microscopy—A teaching model. Sch. Sci. Rev. 2004, 85, 17–19. [Google Scholar]
- Taber, K.S. Towards a curricular model of the nature of science. Sci. Educ. 2008, 17, 179–218. [Google Scholar] [CrossRef]
- Perry, W.G. Forms of Intellectual and Ethical Development in the College Years; Holt, Rinehart and Winston: New York, NY, USA, 1970. [Google Scholar]
- Van der Veer, R.; Valsiner, J. Understanding Vygotsky: A Quest for Synthesis; Blackwell: Oxford, UK, 1991. [Google Scholar]
- Black, P.J. Formative and summative assessment by teachers. Stud. Sci. Educ. 1993, 21, 41–97. [Google Scholar] [CrossRef]
- Gilbert, J.K.; Watts, D.M. Concepts, misconceptions and alternative conceptions: Changing perspectives in science education. Stud. Sci. Educ. 1983, 10, 61–98. [Google Scholar] [CrossRef]
- Glynn, S.M. Explaining science concepts: A teaching-with-analogies model. In The Psychology of Learning Science; Glynn, S., Yeany, R., Britton, B., Eds.; Lawrence Erlbaum: Hillsdale, MI, USA, 1991; pp. 219–240. [Google Scholar]
- Harrison, A.G.; Treagust, D.F. Teaching with analogies: A case study in grade 10 optics. J. Res. Sci. Teach. 1993, 30, 1291–1307. [Google Scholar] [CrossRef]
- Treagust, D.F. Enhancing students’ understanding of science using analogies. In Teaching and Learning in Science; Hand, B., Prain, V., Eds.; Harcourt Brace: Sydney, Australia, 1993; pp. 44–62. [Google Scholar]
- Schwarz, C.V.; Reiser, B.J.; Davis, E.A.; Kenyon, L.; Acher, A.; Fortus, D.; Shwartz, Y.; Hug, B.; Krajjcik, J. Developing a learning progression for scientific modeling: Making scientific modeling accessible and meaningful for learners. J. Res. Sci. Teach. 2009, 46, 632–654. [Google Scholar] [CrossRef]
- Harrison, A.G. Is there a scientific explanation for refraction of light? A review of textbook analogies. Austr. Sci. Teach. J. 1994, 40, 30–35. [Google Scholar]
- Bindernagel, J.A.; Eilks, I. The roadmap approach to portray and develop chemistry teachers Pedagogical Content Knowledge concerning the particulate nature of matter. Chem. Educ. Res. Pract. 2009, 9, 77–85. [Google Scholar] [CrossRef]
- Taber, K.S. Conceptual Resources for Learning Science: Issues of transience and grain-size in cognition and cognitive structure. Int. J. Sci. Educ. 2008, 30, 1027–1053. [Google Scholar] [CrossRef]
- Eilks, I. Experiences and reflections about teaching atomic structure in a jigsaw classroom in lower secondary school chemistry lessons. J. Chem. Educ. 2005, 82, 313–320. [Google Scholar] [CrossRef]
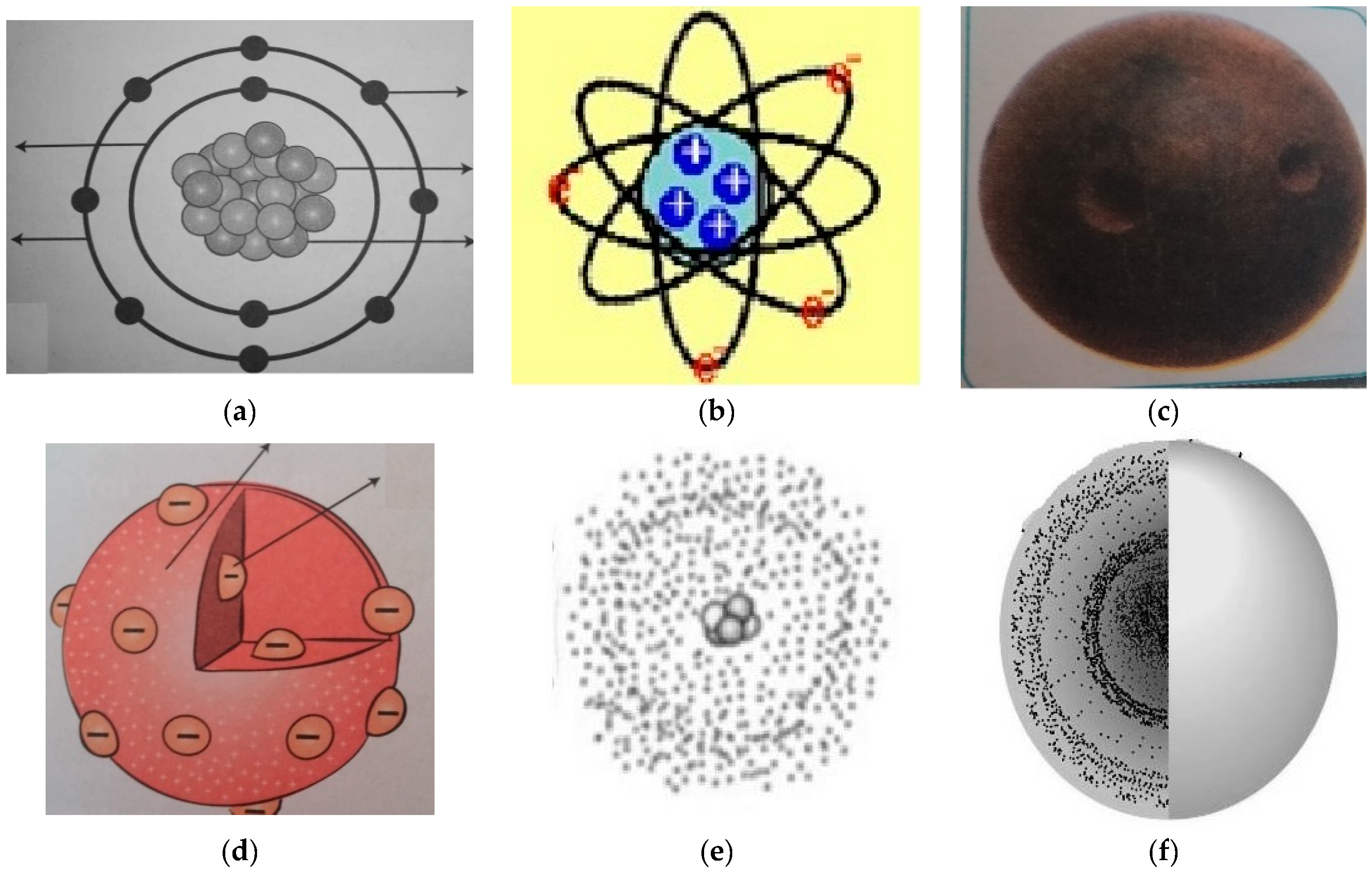
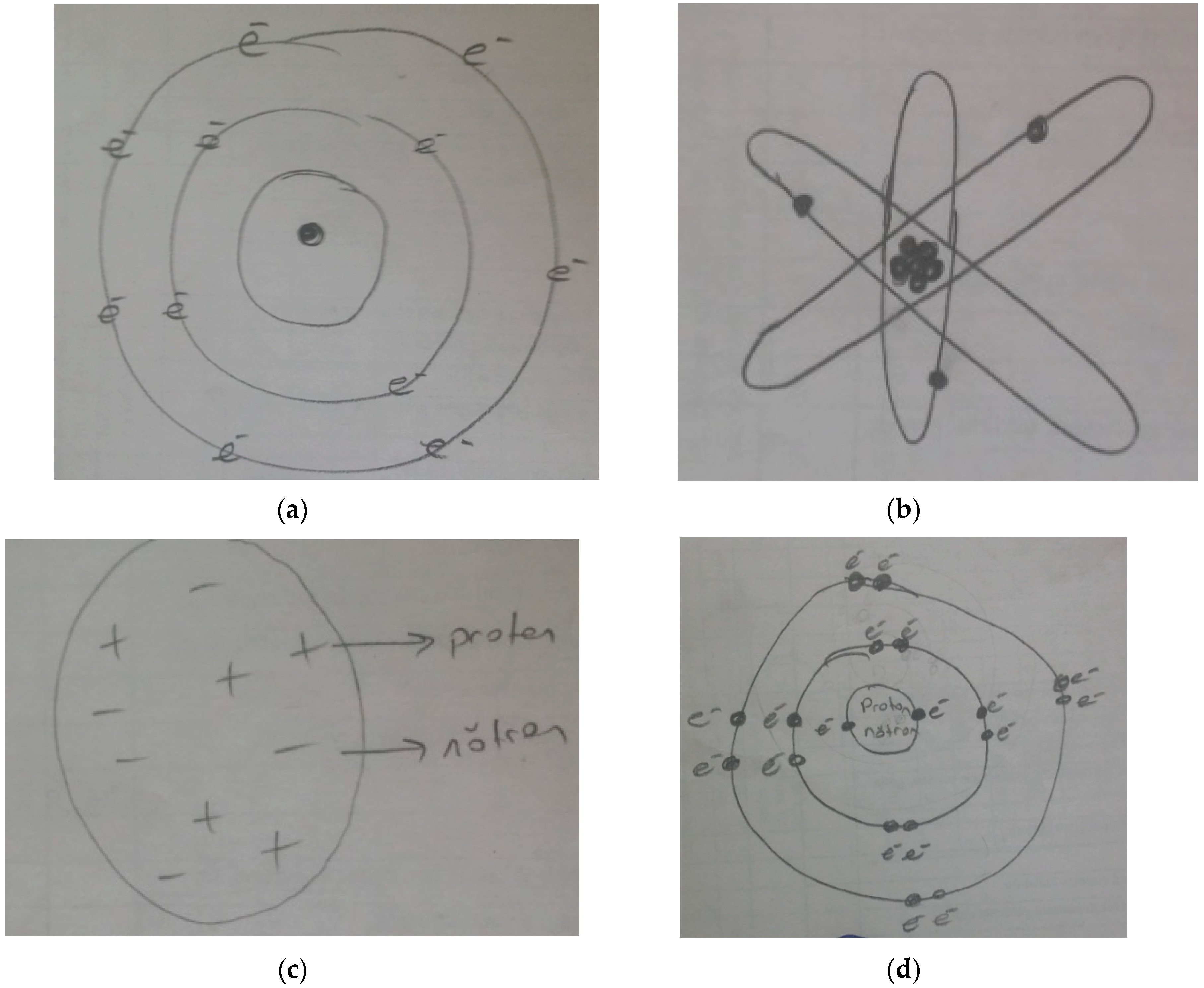
| Representation | Number of Students |
|---|---|
| General structure | |
| 2D structure | 44 |
| Explicit 3D structure | 23 |
| Nucleus-shell structure of the atom | 55 |
| Protons and neutrons | |
| Protons as particles in the nucleus | 10 |
| Label or placeholder for protons | 27 |
| Neutrons as particles in the nucleus | 9 |
| Label or placeholder for neutrons | 23 |
| Shell and electrons | |
| Presence of a shell in the atom | 59 |
| Shell only as outer boundary | 7 |
| Different shells as circles | 23 |
| Different shells as orbits | 29 |
| Explicit drawing of electrons as particles | 29 |
| Label or placeholder for electrons | 14 |
| Charges | |
| Inclusion of negative charges | 40 |
| Inclusion of positive charges | 26 |
| Negative charges connected to electrons | 33 |
| Positive charges connected to electrons | 26 |
| Most Liked (N) | Liked (N) | Not Liked (N) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Freshmen | Sophomore | Junior | Senior | Total | Freshmen | Sophomore | Junior | Senior | Total | Freshmen | Sophomore | Junior | Senior | Total | |
| 1.a | 9 | 4 | 9 | 6 | 28 | 6 | 8 | 6 | 6 | 26 | 2 | 1 | 1 | 1 | 5 |
| 1.b | 8 | 14 | 10 | 7 | 39 | 5 | 2 | 6 | 5 | 18 | 3 | - | 1 | 2 | 6 |
| 1.c | 1 | - | 1 | - | 2 | 2 | 1 | 1 | 3 | 7 | 14 | 14 | 12 | 12 | 52 |
| 1.d | 1 | 1 | 1 | 2 | 4 | 7 | 5 | 3 | 19 | 7 | 1 | 6 | 6 | 20 | |
| 1.e | 5 | 2 | 3 | 5 | 15 | 6 | 1 | 8 | 2 | 17 | 6 | 5 | 3 | 5 | 19 |
| 1.f | 2 | 2 | 2 | 1 | 7 | 8 | 3 | 4 | 5 | 20 | 4 | 3 | 7 | 5 | 19 |
| Categories | Codes/Attributes | Frequency (f) | Percentage (%) |
|---|---|---|---|
| 1.Elements | Student used the term elements for the visual given in the first question of paper-pencil test. | 22 | 32 |
| 2.Atoms | Student used the term atoms for the visual given in the first question of paper-pencil test. | 42 | 62 |
| No response | 4 | 6 | |
| 3.Composition of Matter | All substances made up of atoms | 68 | 100 |
| 4.Size of Atoms | Atom is too small to be seen | 57 | 84 |
| Other | 7 | 10 | |
| No response/Don’t know | 4 | 6 | |
| 5.Visibility of Atoms | Atom cannot be seen by naked eye; however it can only be seen by electronic or technological devices like microscope. | 44 | 65 |
| Can’t be seen | 19 | 28 | |
| Not sure | 5 | 7 | |
| 6.Texture of Atoms | Atom is solid. | 19 | 28 |
| The texture of atom changes according to the type of the material. | 22 | 32 | |
| The texture of atom changes according to the state of matter. | 5 | 7 | |
| No response/Don’t know | 12 | 18 | |
| Other | 10 | 15 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Derman, A.; Koçak, N.; Eilks, I. Insights into Components of Prospective Science Teachers’ Mental Models and Their Preferred Visual Representations of Atoms. Educ. Sci. 2019, 9, 154. https://doi.org/10.3390/educsci9020154
Derman A, Koçak N, Eilks I. Insights into Components of Prospective Science Teachers’ Mental Models and Their Preferred Visual Representations of Atoms. Education Sciences. 2019; 9(2):154. https://doi.org/10.3390/educsci9020154
Chicago/Turabian StyleDerman, Aysegül, Nuriye Koçak, and Ingo Eilks. 2019. "Insights into Components of Prospective Science Teachers’ Mental Models and Their Preferred Visual Representations of Atoms" Education Sciences 9, no. 2: 154. https://doi.org/10.3390/educsci9020154
APA StyleDerman, A., Koçak, N., & Eilks, I. (2019). Insights into Components of Prospective Science Teachers’ Mental Models and Their Preferred Visual Representations of Atoms. Education Sciences, 9(2), 154. https://doi.org/10.3390/educsci9020154






