How Do Physics Teacher Candidates Substantiate Their Knowledge? An Analytical Framework for Examining the Epistemic Dimensions of Content Knowledge in Higher Education
Abstract
1. Introduction
2. Theoretical Framework
2.1. Teachers’ Knowledge Base for Teaching
2.2. Physics Content Knowledge
2.3. Various Frameworks for Analyzing Content Knowledge
- Declarative knowledge to identify and evaluate what concepts are reasonable to apply. For example, relevant physics concepts, terms and quantities, such as electron, photon or frequency.
- Procedural knowledge to analyze the meanings and limitations of the concepts and to consider interrelations between the concepts. For example, physics relations and law, such as Compton relation or Einstein equation.
3. Aim
- How do epistemic dimensions manifest themselves in teacher candidates’ written reports analyzed with the epistemic framework?
- What kind of variation of conceptual, relational, and strategic content knowledge can be found from the written reports?
- What kinds of combinations of conceptual, relational, and strategic knowledge can be detected in teacher candidates’ written reports?
4. Methods
4.1. Context
4.2. Participants
4.3. Data Collection
“In the photoelectric effect, light’s photon is absorbed into an atom and detaches an electron from it. The classical theory had problems also to explain this experimentally observed phenomenon. According to the classical theory, light’s frequency should not matter in detaching the electron–that is, if light’s intensity is sufficiently large. Neither could the classical theory explain the photo electron’s kinetic energy’s dependence on the radiation frequency instead of its intensity.”
5. Data Analysis Framework
- Conceptual knowledge: Identification of concept(s) and their relevance to the task. The conceptual dimension (C) referred to the ability to identify and define relevant physics concepts and to reflect their applicability. Such a dimension entailed declarative knowledge about physics concepts and terms, such as “a photon” or “X-ray radiation” and about limitation(s) to the usability of the concept or term (cf. [6,7,41,42]). See detailed criteria in Table 1 and Table 2. Mathematical notations were not regarded as physics concepts unless they were clearly verbally explained, or if they had a well-known meaning in physics.
- Relational knowledge: Relations between concepts and their restrictions. Relational dimension (R) referred to the ability to identify how concepts are related to each other and what the forms and limitations are of such relations (cf. [7]). Relations and relational knowledge were essential for understanding analogies, explanations, learning concepts, proposing justifications, and problem solving [43]. See detailed criteria in Table 3 and Table 4. This dimension entailed declarative knowledge, such as “Energy can have values E = nhf, where n is an integer, h is Planck’s constant and f is radiation frequency.”
- Strategic knowledge: Knowing when, where, and how to apply the other types of knowledge by providing experimental or a modeling procedure. The strategic dimension (S) in this context meant knowledge of either an experimental or a modeling procedure, and reflection on their restrictions or limitations. Strategic dimensions thus assessed whether or not a student knows when, where, and how to apply the other two types of knowledge by providing experimental or a modeling procedure (see, e.g., [9,33,42]). Such a dimension represented the integration of declarative and procedural (i.e., knowing how) aspects of knowledge and knowing see [39,41,42]. See detailed criteria for experiments in Table 5 and Table 6, and for models in Table 7 and Table 8.
6. Data Analysis and Scoring
7. Results
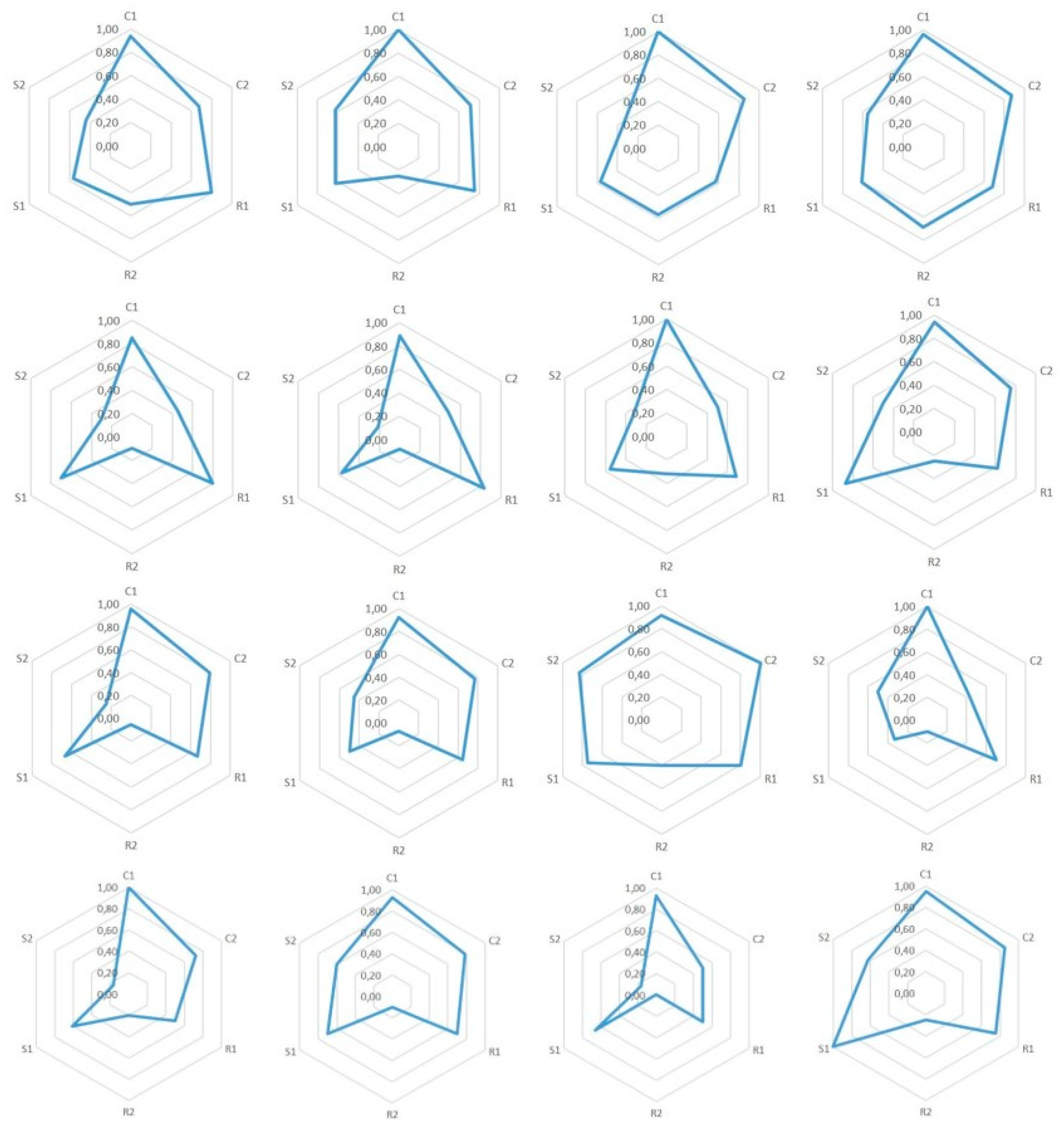
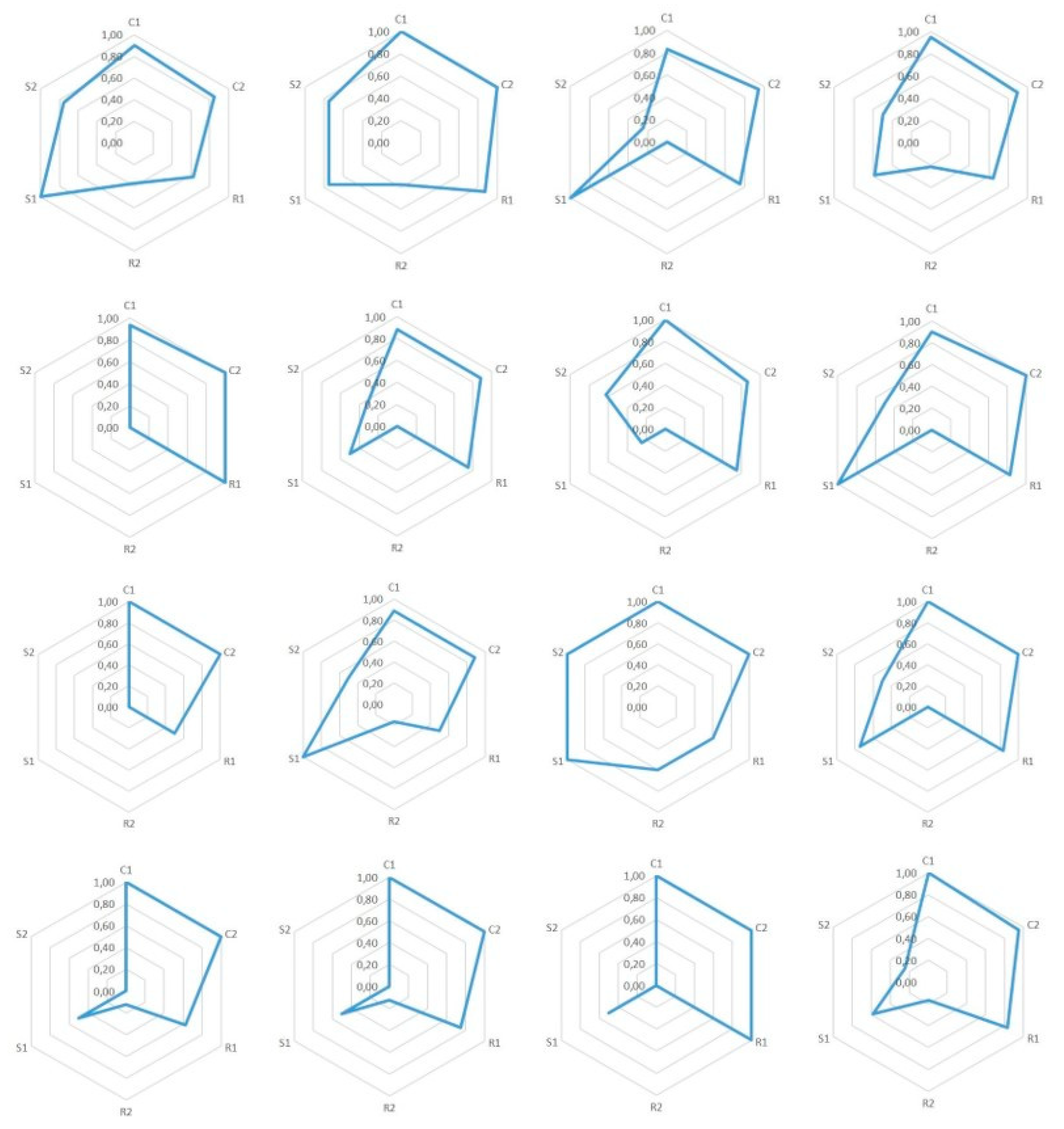
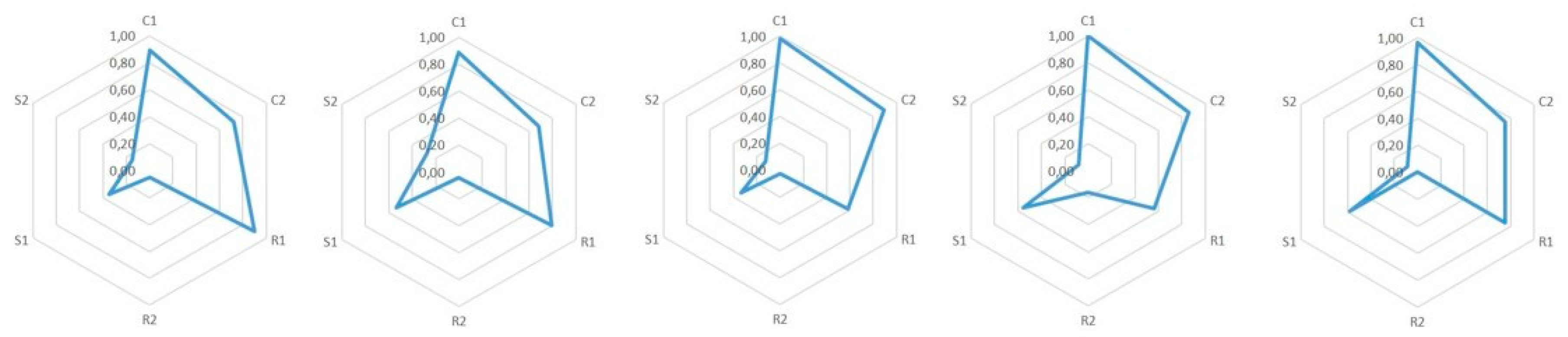
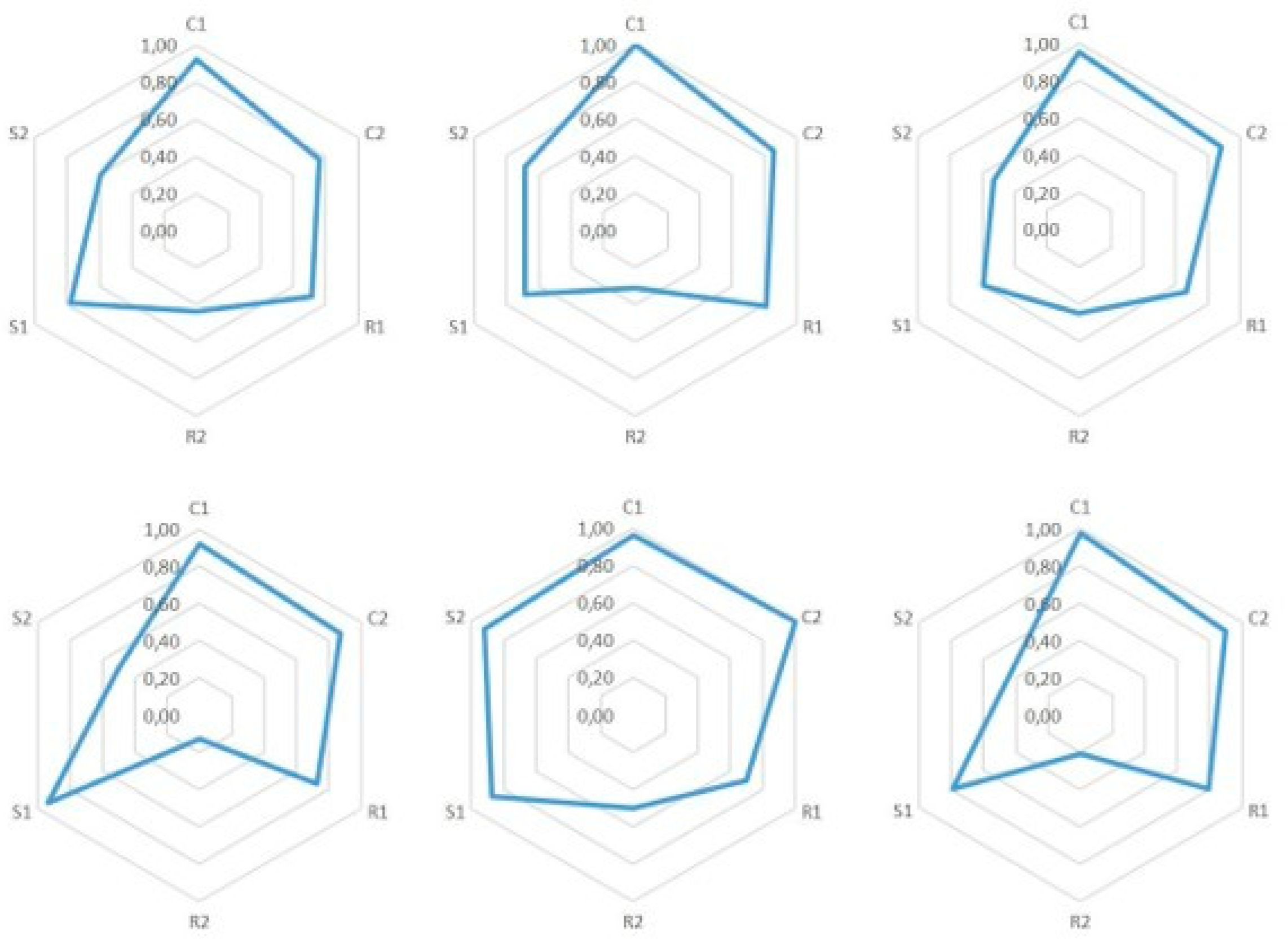
7.1. Epistemic Dimensions Manifesting most in Teacher Candidates’ Written Reports
7.2. Variation of Conceptual, Relational, and Strategic Content Knowledge
7.3. Combinations of Conceptual, Relational and Strategic Knowledge
8. Discussion and Conclusions
8.1. Discussion of Benefits and Challenges Relating to the Framework Developed and Utilized in the Study
8.2. Discussion of Findings Relating to the Framework Developed and Utilized in the Study
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Unit of Analysis | Scores | Rationale |
|---|---|---|
| 1. We can start from the fact that the classical theory couldn’t explain many phenomena, which the quantum theory later explained. The classical theory’s notions of, for example, the black body radiation and photoelectric effect were actually in contradiction with experimental results. | C1: | C1: a special concept ‘black body radiation’ |
| C2: 1 | C2: a limitation: not in reach of the classical theory | |
| R1: 0 | R1: - | |
| R2: 0 | R2: - | |
| S1: 0 | S1: - | |
| S2: 0 | S2: - | |
| 2. The classical theory was not able to explain the black body radiation’s spectrum at all frequencies. Later this phenomenon was known as ‘the ultraviolet catastrophe,’ and it was one of the first shortcomings of the classical theory. | C1: 2 | C1: a special concept ‘the black body radiation’s spectrum’ |
| C2: 1 | C2: a limitation: not in reach of the classical theory | |
| R1: 0 | R1: - | |
| R2: 0 | R2: - | |
| S1: 0 | S1: - | |
| S2: 0 | S2: - | |
| 3. Max Planck developed a law, which was able to explain the experimental results of the spectrum of black body radiation. The Planck constant was a central piece of the law, and also the assumption that the energy of the oscillators in an atom was quantized. This law was later used to derive Stefan-Boltzmann’s law by integration. | C1: 2 | C1: a special concept ‘black body radiation’ |
| C2: 2 | C2: a limitation: energy is quantized | |
| R1: 1 | R1: a physical law: the Stefan-Boltzmann’s law | |
| R2: 1 | R2: Stefan-Boltzmann’s law is derived from Planck’s law and thus has its limitations | |
| S1: 1 | S1: Stefan-Boltzmann’s law | |
| S2: 1 | S2: a limitation: energy is quantized | |
| 4. Max Planck determined a constant related to his law, the Planck constant h = 6.626 J∙s. | C1: 2 | C1: a special concept ‘the Planck constant’ |
| C2: 0 | C2: - | |
| R1: 1 | R1: a physical law: his [Planck’s] law | |
| R2: 0 | R2: - | |
| S1: 1 | S1: a physical law: his [Planck’s] law | |
| S2: 0 | S2: - | |
| 5. In the photoelectric effect, light’s photon is absorbed to an atom and detaches an electron from it. The classical theory had problems also to explain this experimentally observed phenomenon. According to the classical theory, light’s frequency should not matter in detaching the electron—that is, if light’s intensity is sufficiently large. Neither could the classical theory explain the photoelectron’s kinetic energy’s dependence of the radiation frequency instead of its intensity. | C1: 2 | C1: a special concept: ‘the photoelectric effect’ |
| C2: 2 | C2: a limitation: dependence between a photo electron’s kinetic energy and radiation frequency | |
| R1: 1 | R1: dependence between a photo electron’s kinetic energy and radiation frequency | |
| R2: 1 | R2: a limitation: not in reach of the classical theory | |
| S1: 0 | S1: - | |
| S2: 1 | S2: an experimentally observed phenomenon | |
| 6. Albert Einstein took Planck’s ideas even further and created a hypothesis that electromagnetic radiation itself was quantized. According to that hypothesis, light-quantum’s energy was E = hf, and furthermore, photoelectron’s energy was Ekin = hf-W, where W is the work needed to detach an electron. | C1: 2 | C1: a special concept ‘electromagnetic radiation’ |
| C2: 2 | C2: a limitation: electromagnetic radiation is quantized | |
| R1: 2 | R1: an exact relation: E = hf | |
| R2: 0 | R2: - | |
| S1: 2 | S1: the meaning of Einstein’s hypothesis | |
| S2: 1 | S2: a limitation: electromagnetic radiation is quantized | |
| 7. Wilhelm Hallwachs and Philipp Lenard were studying the photoelectric effect observed by Heinrich Hertz. In the experiment, a voltage is created between plate electrodes in a vacuum. Monochromatic light is aimed at the positive electrode. Light detaches electrons from the plate, which we can observe by connecting an ampere meter to the circuit. This experiment shows in practice that, no matter how high the light intensity, if its frequency is not high enough, then electrons are not detached from the plate. | C1: 2 | C1: a special concept ‘photoelectric effect’ |
| C2: 2 | C2: a limitation: the frequency needs to be sufficiently high | |
| R1: 1 | R1: aiming light at the electrode detaches electrodes and generate current | |
| R2: 1 | R2: a limitation: electrons are detached only if the frequency is high enough | |
| S1: 2 | S1: the Hallwachs-Lenard’s experiment and its arrangements | |
| S2: 1 | S2: a limitation: electrons are detached only if the frequency is high enough | |
| 8. The experiment showed in practice the meaning of light frequency to make the phenomenon happen. | C1: 2 | C1: an exact physical concept ‘frequency’ |
| C2: 1 | C2: a relevant context: the above mentioned experiment | |
| R1: 0 | R1: - | |
| R2: 0 | R2: - | |
| S1: 0 | S1: - | |
| S2: 1 | S2: a result of the experiment | |
| 9. With this experiment we can define a threshold frequency, characteristic to each metal, f = E0/h (where E0 is the minimum energy required to detach electrons), which means the minimum frequency of electromagnetic radiation that can detach electrons from metal. | C1: 2 | C1: an exact physical concept ‘frequency’ |
| C2: 2 | C2: a limitation: the relation for a threshold frequency, well explained | |
| R1: 2 | R1: an exact relation: f = E0/h | |
| R2: 0 | R2: - | |
| S1: 0 | S1: - | |
| S2: 1 | S2: a result of the experiment (a threshold frequency) | |
| 10. James Franck and Gustaf Hertz developed an experiment, which shows that electrons in an atom have quantized energy. The results of the experiment supported the atomic model created by Niels Bohr. In the experiment, there were three electrodes (cathode, anode, and a third, net-like electrode in between them) in a tube containing low-pressure mercury vapor. In addition, there was an ampere meter in the tube to measure current between the electrodes. It was observed that if the value of voltage is some integer n times 4.9 volts, the current decreased considerably. According to the quantum theory, atoms can absorb energy only in portions of a certain amount, and when electrons have enough kinetic energy, they excited mercury atoms by collisions, which led to decreased electric current. | C1: 2 | C1: a special concept ‘electrons’ |
| C2: 2 | C2: a limitation: atoms can absorb energy only in portions of a certain amount | |
| R1: 1 | R1: dependence between current and voltage | |
| R2: 1 | R2: a limitation: the current decreased considerably if the voltage equals n times 4.9 volts | |
| S1: 2 | S1: the arrangements of the Franck-Hertz experiment | |
| S2: 2 | S2: the result of the Franck-Hertz experiment and its implications | |
| 11. When a system returns from an excited state to its ground state, it releases extra energy as electromagnetic radiation, which consists of discrete spectral lines. The reason for the appearance of exact frequencies on atomic emission spectra was unclear before the development of quantum theory. | C1: 2 | C1: a special concept ‘electromagnetic radiation’ |
| C2: 1 | C2: a relevant context: creation of discrete spectra | |
| R1: 0 | R1: - | |
| R2: 0 | R2: - | |
| S1: 0 | S1: - | |
| S2: 0 | S2: - | |
| 12. Based on Planck’s hypotheses Niels Bohr created an atomic model, which explained why the spectrum is divided into lines. According to this atomic model, electrons circle the nucleus along elliptical orbits, and the electrons’ energies are quantized. However, this model still had inconsistencies. | C1: 2 | C1: a special concept ‘electrons’ |
| C2: 2 | C2: limitation: energy is quantized | |
| R1: 1 | R1: energy is quantized | |
| R2: 0 | R2: - | |
| S1: 1 | S1: Bohr’s atomic model | |
| S2: 1 | S2: energy is quantized | |
| 13. Robert Millikan also studied the photoelectric effect. He managed to measure the dependence between the stopping voltage and photon flow exactly. Moreover, he managed to calculate experimentally the value of the Planck constant. | C1: 2 | C1: an exact physical concept ‘voltage’ |
| C2: 1 | C2: a relevant context: Millikan’s study | |
| R1: 1 | R1: dependence between the stopping voltage and photon flow | |
| R2: 0 | R2: - | |
| S1: 0 | S1: - | |
| S2: 0 | S2: - | |
| 14. Owen Willans Richardson and Arthur Compton believed that the maximum energy of electrons would be constant. Millikan’s experiment showed that it was not the case. Maximum energy can be calculated by using the minimum energy to detach an electron, Ekin,max = hf-E0 | C1: 2 | C1: a special concept ‘electrons’ |
| C2: 1 | C2: a limitation: mathematical relation not explained verbally | |
| R1: 2 | R1: an exact relation: Ekin,max = hf-E0 | |
| R2: 0 | R2: - | |
| S1: 0 | S1: - | |
| S2: 0 | S2: - | |
| 15. By a stopping voltage, we mean the voltage for which between a cathode (onto which the light is focused) and anode there is no electric current (i.e., even the most energetic electrons cannot reach the anode). The stopping voltage Vstop = Ekin,max/e | C1: 2 | C1: an exact physical concept ‘voltage’ |
| C2: 1 | C2: a relevant context: the above mentioned experiment | |
| R1: 2 | R1: an exact relation: Vstop = Ekin,max/e | |
| R2: 0 | R2: - | |
| S1: 0 | S1: - | |
| S2: 0 | S2: - | |
| 16. The above listed experiments show the validity of the hypotheses. Indeed, the energy was quantized and light-quanta real. | C1: 2 | C1: a special concept ‘light-quanta’ |
| C2: 0 | C2: - | |
| R1: 0 | R1: - | |
| R2: 0 | R2: - | |
| S1: 0 | S1: - | |
| S2: 0 | S2: - | |
| 17. As the hypotheses of Einstein were proved, they could be listed as laws for the phenomena. | C1: 0 | Just an interjection. |
| C2: 0 | ||
| R1: 0 | ||
| R2: 0 | ||
| S1: 0 | ||
| S2: 0 | ||
| 18. It can also be mentioned to students that the phenomenon also works inversely (as is seen in the Franck–Hertz experiment too). An electron moving in a medium can emit its kinetic energy and this is observed as x-ray emission. | C1: 2 | C1: a special concept ‘an electron’ |
| C2: 1 | C2: a relevant context: the inverse phenomenon | |
| R1: 0 | R1: - | |
| R2: 0 | R2: - | |
| S1: 1 | S1: the Franck–Hertz experiment | |
| S2: 0 | S2: - |
References
- Ball, D.L. Bridging practices. Intertwining content and pedagogy in teaching and learning to teach. J. Teach. Educ. 2000, 51, 241–247. [Google Scholar] [CrossRef]
- Duit, R.; Schenker, H.; Höttecke, D.; Niedderer, H. Teaching Physics. In Handbook of Research on Science Education; Lederman, N.G., Abell, K.S., Eds.; Routledge: New York, NY, USA, 2014; Volume II, pp. 434–456. [Google Scholar]
- Lachner, A.; Jarodzka, H.; Nückles, M. What makes an expert teacher? Investigating teachers’ professional vision and discourse abilities. Instr. Sci. 2016, 44, 197–203. [Google Scholar] [CrossRef]
- Schulman, L.S. Those who understand: Knowledge growth in teaching. Educ. Res. 1986, 15, 4–14. [Google Scholar] [CrossRef]
- Blömeke, S.; Busse, A.; Kaiser, G.; König, J.; Suhl, U. The relation between content-specific and general teacher knowledge and skills. Teach. Teach. Educ. 2016, 56, 35–46. [Google Scholar] [CrossRef]
- Lachner, A.; Nückles, M. Bothered by abstractness or engaged by cohesion? Experts’ explanations enhance novices’ deep-learning. J. Exp. Psychol. Appl. 2015, 21, 101–115. [Google Scholar] [CrossRef]
- Lachner, A.; Nückles, M. Tell me why! Content knowledge predicts process-orientation of math researchers’ and math teachers’ explanations. Instr. Sci. 2016, 44, 221–242. [Google Scholar] [CrossRef]
- Hill, H.C.; Rowan, B.; Ball, D. Effects of teachers’ mathematical knowledge for teaching on student achievement. Am. Educ. Res. J. 2005, 42, 371–406. [Google Scholar] [CrossRef]
- Mäntylä, T.; Nousiainen, M. Consolidating Pre-service Physics Teachers’ Subject Matter Knowledge Using Didactical Reconstructions. Sci. Educ. 2014, 22, 505–525. [Google Scholar] [CrossRef]
- Nousiainen, M. Organization of physics content knowledge for teaching purposes: From knowledge justification schemes to didactical schemes. Eur. J. Sci. Math. Educ. 2017, 5, 210–221. [Google Scholar]
- Ruiz-Primo, M.; Schultz, S.E.; Li, M.; Shavelson, R.J. Comparison of the reliability and validity of scores from two concept-mapping techniques*. J. Res. Sci. Teach. 2001, 38, 260–278. [Google Scholar] [CrossRef]
- Toom, A. Learning professional competencies in teacher education and throughout the career. In The SAGE Handbook of Research on Teacher Education; Clandinin, D.J., Husu, J., Eds.; SAGE Publishers: London, UK, 2017; pp. 777–782. [Google Scholar]
- Clandinin, D.J. Personal practical knowledge: A study of teachers’ classroom images. Curric. Inq. 1985, 15, 361–385. [Google Scholar] [CrossRef]
- Darling-Hammond, L.; Bransford, J. Preparing Teachers for a Changing World: What Teachers Should Learn and Be Able to Do; Jossey-Bass: San Francisco, CA, USA, 2005. [Google Scholar]
- Elbaz, F. The teacher’s ‘practical knowledge’: Report of a case study. Curric. Inq. 1981, 11, 43–71. [Google Scholar]
- Evens, M.; Elen, J.; Larmuseau, C.; Depaepe, F. Promoting the development of teacher professional knowledge: Integrating content and pedagogy in teacher education. Teach. Teach. Educ. 2018, 75, 244–258. [Google Scholar] [CrossRef]
- Fenstermacher, G.D. The knower and the known: The nature of knowledge in research on teaching. Rev. Res. Educ. 1994, 20, 3–56. [Google Scholar] [CrossRef]
- Gitomer, D.H.; Zisk, R.C. Knowing what teachers know. Rev. Res. Educ. 2015, 39, 1–53. [Google Scholar] [CrossRef]
- Schulman, L.S. Knowledge and teaching: Foundations of the new reform. Harv. Educ. Rev. 1987, 57, 1–22. [Google Scholar] [CrossRef]
- Ball, D.L.; Thames, M.H.; Phelps, G. Content Knowledge for Teaching: What Makes It Special. J. Teach. Educ. 2008, 59, 389–407. [Google Scholar] [CrossRef]
- Ball, D.L. The mathematical understandings that prospective teachers bring to teacher education. Elem. Sch. J. 1990, 90, 449–466. [Google Scholar] [CrossRef]
- Hiebert, J.; Morris, A.K.; Berk, D.; Jansen, A. Preparing teachers to learn from teaching. J. Teach. Educ. 2007, 58, 47–61. [Google Scholar] [CrossRef]
- Nilsson, P.; van Driel, J. How will we understand what we teach?–Primary student teachers’ perceptions of their development of knowledge and attitudes towards physics. Res. Sci. Educ. 2011, 41, 541–560. [Google Scholar] [CrossRef]
- Grossman, P. The Making of a Teacher: Teacher Knowledge and Teacher Education; Teachers’ College Press: New York, NY, USA, 1990. [Google Scholar]
- Kersting, N.B.; Givvin, K.B.; Sotelo, F.L.; Stigler, J.W. Teachers’ analyses of classroom video predict student learning of mathematics: Further explorations of a novel measure of teacher knowledge. J. Teach. Educ. 2010, 61, 172–181. [Google Scholar] [CrossRef]
- Stürmer, K.; Könings, K.D.; Seidel, T. Declarative knowledge and professional vision in teacher education: Effect of courses in teaching and learning. Br. J. Educ. Psychol. 2012, 83, 467–483. [Google Scholar] [CrossRef] [PubMed]
- Blömeke, S.; Delaney, S. Assessment of teacher knowledge across countries: A review of the state of research. ZDM Math. Educ. 2012, 44, 223–247. [Google Scholar] [CrossRef]
- Blömeke, S.; Hsieh, F.; Kaiser, G.; Schimidt, W.H. (Eds.) International Perspectives on Teacher Knowledge, Beliefs and Opportunities to Learn; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar]
- Mäntylä, T.; Hämäläinen, A. Obtaining Laws Through Quantifying Experiments: Justifications of Pre-service Physics Teachers in the Case of Electric Current, Voltage and Resistance. Sci. Educ. 2015, 24, 699–723. [Google Scholar] [CrossRef]
- Sandoval, W.A.; Millwood, K.A. What can argumentation tell us about epistemology? In Argumentation in Science Education; Erduran, S., Jiménez-Aleixandre, M.P., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 71–88. [Google Scholar]
- Koponen, I.T.; Mäntylä, T. Generative role of experiments in physics and in teaching physics: A suggestion for epistemological reconstruction. Sci. Educ. 2006, 15, 31–54. [Google Scholar] [CrossRef]
- Nousiainen, M.; Koponen, I.T. Concept Maps Representing Physics Knowledge: Connecting the Structure and Content in the Context of Electricity and Magnetism. Nord. Stud. Sci. Educ. 2010, 6, 155–172. [Google Scholar] [CrossRef]
- Nousiainen, M. Coherence of pre-service physics teachers’ views of the relatedness of physics concepts. Sci. Educ. 2013, 22, 505–525. [Google Scholar] [CrossRef]
- Kosso, P. The large-scale structure of scientific method. Sci. Educ. 2009, 18, 33–42. [Google Scholar] [CrossRef]
- Sandoval, W.A.; Millwood, K.A. The Quality of Students’ Use of Evidence in Written Scientific Explanations. Cogn. Instr. 2005, 23, 23–55. [Google Scholar] [CrossRef]
- Kelly, G.J.; Takao, A. Epistemic levels in argument: An analysis of university oceanography students’ use of evidence in writing. Sci. Ed. 2002, 86, 314–342. [Google Scholar] [CrossRef]
- Kelly, J.K.; Regev, J.; Prothero, W. Analysis of Lines of Reasoning in Written Argumentation. In Argumentation in Science Education: Perspectives from Classroom-Based Research; Erduran, S., Jiménez-Aleixandre, M.P., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 137–157. [Google Scholar]
- Krathwohl, D.R. A Revision of Bloom’s Taxonomy: An Overview. Theory Pract. 2002, 41, 212–218. [Google Scholar] [CrossRef]
- Li, M.; Ruiz-Primo, M.A.; Shavelson, R.J. Towards a Science Achievement Framework: The Case of TIMSS 1999. In Contexts of Learning Mathematics and Science: Lessons Learned from TIMSS Contexts of Learning; Howie, S.J., Plomp, T., Eds.; Routledge: New York, NY, USA, 2006; pp. 291–312. [Google Scholar]
- Ruiz-Primo, M.; Shavelson, R.J. Problems and issues in the use of concept maps in science assessment. J. Res. Sci. Teach. 1996, 33, 569–600. [Google Scholar] [CrossRef]
- De Jong, T.; Ferguson-Hessler, M.G.M. Types and qualities of knowledge. Educ. Psychol. 2010, 31, 105–113. [Google Scholar] [CrossRef]
- Shavelson, R.J. Measuring College Learning Responsibly: Accountability in a New Era; Stanford University Press: Stanford, CA, USA, 2010. [Google Scholar]
- Halford, G.S.; Wilson, W.H.; Phillips, S. Relational knowledge: The foundation of higher cognition. Trends Cogn. Sci. 2010, 14, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Lindblom-Ylänne, S.; Parpala, A.; Postareff, L. Challenges in analysing change in students’ approaches to learning. In Learning Patterns in Higher Education: Dimensions and Research Perspectives; Gijbels, D., Donche, V., Richardson, J., Vermunt, J., Eds.; Routledge: New York, NY, USA, 2013; pp. 232–248. [Google Scholar]
- Berliner, D.C. Describing the Behavior and Documenting the Accomplishments of Expert Teachers. Bull. Sci. Technol. Soc. 2004, 24, 200–212. [Google Scholar] [CrossRef]
- Baartman, L.K.J.; Bastiaens, T.J.; Kirschner, P.A.; van der Vleuten, C. Evaluating assessment quality in competence-based education: A qualitative comparison of two frameworks. Educ. Res. Rev. 2007, 2, 114–129. [Google Scholar] [CrossRef]
- Hyytinen, H.; Nissinen, K.; Ursin, J.; Toom, A.; Lindblom-Ylänne, S. Problematising the equivalence of the test results of performance-based critical thinking tests for undergraduate students. Stud. Educ. Eval. 2015, 44, 1–8. [Google Scholar] [CrossRef]

| C1: Identification and Definition of Concepts | Examples |
|---|---|
| 1 score = identifies and mentions at least one general concept which is relevant in the description | light, particle, energy, radiation, spectrum |
| 2 scores = identifies and mentions at least one special concept which is relevant in the description (cf. general concept) | ultraviolet light, monochromatic light, electron, photon, kinetic energy, maximum energy, x-ray radiation, electromagnetic radiation, discrete spectrum, spectrum of light, continuous spectrum |
| or identifies an exact physical quantity | frequency, intensity, wavelength, electric current, voltage, temperature |
| or presents a specification or limitation/requirement concerning mentioned general concept | for example, a particle having a property x |
| or presents exact concepts | quantum, quantum hypothesis, Planck’s constant, black body radiation, threshold frequency, ultraviolet catastrophe, photoelectric effect |
| C2: Conditions for Using the Concept(s) | Examples |
|---|---|
| 1 score = reflects on limitations of at least one concept, some inconsistency is allowed | “Owen Willans Richardson and Arthur Compton believed that the maximum energy of electrons would be constant. Millikan’s experiment pointed out that it was not the case. Maximum energy can be calculated by using the minimum energy to detach an electron, Ekin,max = hf-E0.” “Classical theory was not able to explain the black body radiation spectrum at all frequencies. Later this phenomenon was known as ‘ultraviolet catastrophe,’ and it was one of the first shortcomings of the classical theory.” |
| or presents at least one relevant context to use the concept | “When a system returns from a state of excitation to its basic state, it releases extra energy as electromagnetic radiation, which consists of discrete spectrum lines. The reason for the appearance of exact frequencies on atom emission spectra was unclear before the development of quantum theory”. |
| 2 score = presents at least one consistent limitation to usability of the concept | “Einstein presented the idea that electromagnetic radiation is quantized. According to Einstein, light consisted of small ‘energy packages’, light quanta. We can see experimentally that electrons in metal can receive radiation energy only as packages of a certain size, quanta, the size of which was dependent only on the frequency of the radiation.” “With this experiment we can define a threshold energy, characteristic to each metal, f = E0/h (where E0 is the minimum energy required to detach electrons), which means the minimum frequency of electromagnetic radiation that can detach electrons from metal.” |
| R1: Identification and Definition of a Relation between Concepts | Examples |
|---|---|
| 1 score = identifies at least one relation between relevant concepts (quantities), relation can be inconsistent | “We perceived that if the value for voltage is some integer times 4.9 volts, the current decreased considerably.” → there is a relation between current and voltage |
| a physics law identified by name | “This law is later used to prove Stefan-Boltzmann’s law by integration.” |
| 2 scores = presents an exact mathematical relation between concepts (quantities) | “Light quantum has energy E = hf.” |
| an explicit verbal relation | “By using stopping voltage we can calculate the electron’s maximum energy which is the difference between the electron’s energy and work function.” |
| R2: Conditions for Using the Relation between Concepts | Examples |
|---|---|
| 1 score = reflects on the limitation of the relation, some inconsistency is allowed | “We perceived that if the value for voltage is some integer n times 4.9 volts, the current decreased considerably” → n is an integer “This experiment shows us in practice that, no matter how high the light intensity, if its frequency is not high enough, electrons are not detached from the plate.” |
| 2 scores = presents at least one consistent limitation to usability of the relation | “Planck was able to connect Wien’s and Rayleigh-Jeans’ radiation laws. The first one operates for short wavelengths and the second for longer ones. When merging these two laws together Planck had to assume that energy is transferred as quanta.” → quantization of energy transfer |
| S1 for Experiments: Identification and Definition of an Experiment | Examples |
|---|---|
| 1 score = identifies (by name) a relevant experiment or proposes arrangements for an experiment. Some inconsistency is allowed. | “The phenomena works also the other way around as we can see in the Franck-Hertz experiment. An electron moving in a medium can release kinetic energy and this is perceived as x-ray emission.” |
| 2 scores = identifies a relevant experiment and proposes arrangements for this experiment. Description is clear and consistent. | “Wilhelm Hallwachs and Philipp Lenard were inspecting the photoelectric effect noted by Heinrich Hertz. In the experiment, a voltage is created between plate electrodes in a vacuum. A monochromatic light is aimed at the positive electrode. Light detaches electrons from the plate, which we can perceive by connecting an ampere meter to the circuit.” |
| S2: The Results and Meaning of the Experiment | Examples |
|---|---|
| 1 score = presents the result of the experiment OR explains the meaning or relevance of the experiment. Some inconsistency is allowed. | “The experiment showed us the meaning of light frequency to make the phenomenon happen.” |
| 2 scores = presents the result of the experiment AND explains the meaning or relevance of the experiment. Description is clear and consistent. | “James Franck and Gustaf Hertz developed an experiment which points out that electrons in an atom have quantized energy. The results of the experiment supported the atomic model created by Niels Bohr. In the experiment, there were three electrodes (cathode, anode and a third, netlike electrode in between them) in a tube containing low-pressure mercury gas. In addition, there was an ammeter in the tube to measure current between the electrodes. We perceived that if the value for voltage is some integer n times 4.9 volts, the current decreased considerably. According to quantum theory, atoms can absorb energy only in portions of a certain amount, and when electrons have enough kinetic energy, they excited mercury atoms by collations, which led to decreased electric current.” |
| S1 for Models: Identification and Definition of a Model | Examples |
|---|---|
| 1 score = identifies (by name) at least one relevant model | “Based on Planck’s hypothesis Niels Bohr created an atomic model, which explained why the spectrum is divided into lines. According to this atomic model, electrons circulate the nucleus along elliptical orbits, and the electrons’ energy is quantized. However, this model still had inconsistencies.” |
| 2 scores = explains the meaning of the model | “According to that hypothesis, light quantum’s energy was E = hf, and furthermore, photoelectron’s energy was Ekin = hf-W, where W is the work done to detach an electron” → consequences of the hypothesis |
| S2: The Applicability and the Relevance of the Model | Examples |
|---|---|
| 1 score = presents limitations or assumptions that are needed in the model. Some inconsistency is allowed. | “Based on Planck’s hypothesis Niels Bohr created an atomic model, which explained why the spectrum is divided into lines. According to this atomic model, electrons circulate the nucleus along elliptical orbits, and the electrons’ energy is quantized. However, this model still had inconsistencies.” → quantization of energy |
| 2 scores = reflects on the relevance of the model, where the model leads. | “Albert Einstein took Planck’s ideas [Planck’s law] even further and created a hypothesis that electromagnetic radiation itself was quantized.” → Planck’s law is used to derive a new hypothesis |
| Task 1 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | M1 | SD1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C1 | 32 | 14 | 20 | 23 | 17 | 39 | 34 | 15 | 23 | 24 | 11 | 14 | 18 | 13 | 13 | 19 | 20.6 | 8.3 |
| C2 | 23 | 10 | 17 | 21 | 9 | 21 | 17 | 12 | 19 | 20 | 12 | 6 | 13 | 11 | 8 | 17 | 14.8 | 5.3 |
| R1 | 8 | 6 | 8 | 11 | 8 | 10 | 15 | 5 | 12 | 9 | 8 | 7 | 5 | 7 | 4 | 6 | 8.1 | 2.9 |
| R2 | 5 | 2 | 1 | 4 | 1 | 1 | 7 | 2 | 1 | 1 | 4 | 1 | 2 | 1 | 0 | 2 | 2.2 | 1.9 |
| S1 | 5 | 2 | 1 | 4 | 1 | 1 | 7 | 2 | 1 | 1 | 4 | 1 | 2 | 1 | 0 | 2 | 2.2 | 1.9 |
| S2 | 7 | 5 | 5 | 10 | 3 | 3 | 6 | 4 | 3 | 9 | 10 | 6 | 3 | 6 | 1 | 5 | 5.4 | 2.6 |
| Task 2 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | M2 | SD2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C1 | 18 | 14 | 15 | 19 | 15 | 23 | 22 | 9 | 18 | 16 | 12 | 10 | 14 | 12 | 10 | 20 | 15.4 | 4.3 |
| C2 | 17 | 14 | 17 | 18 | 16 | 23 | 19 | 10 | 18 | 16 | 12 | 10 | 14 | 12 | 10 | 19 | 15.3 | 3.8 |
| R1 | 5 | 7 | 6 | 9 | 6 | 6 | 9 | 5 | 5 | 3 | 6 | 5 | 5 | 6 | 2 | 10 | 5.9 | 2.0 |
| R2 | 3 | 3 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 1 | 6 | 0 | 1 | 1 | 0 | 2 | 1.3 | 1.7 |
| S1 | 4 | 3 | 4 | 7 | 0 | 3 | 2 | 2 | 0 | 2 | 4 | 3 | 1 | 2 | 1 | 7 | 2.8 | 2.1 |
| S2 | 3 | 3 | 1 | 6 | 0 | 2 | 5 | 1 | 0 | 1 | 4 | 2 | 0 | 0 | 0 | 3 | 1.9 | 1.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nousiainen, M.; Hyytinen, H.; Palmgren, E.; Toom, A. How Do Physics Teacher Candidates Substantiate Their Knowledge? An Analytical Framework for Examining the Epistemic Dimensions of Content Knowledge in Higher Education. Educ. Sci. 2019, 9, 120. https://doi.org/10.3390/educsci9020120
Nousiainen M, Hyytinen H, Palmgren E, Toom A. How Do Physics Teacher Candidates Substantiate Their Knowledge? An Analytical Framework for Examining the Epistemic Dimensions of Content Knowledge in Higher Education. Education Sciences. 2019; 9(2):120. https://doi.org/10.3390/educsci9020120
Chicago/Turabian StyleNousiainen, Maija, Heidi Hyytinen, Elina Palmgren, and Auli Toom. 2019. "How Do Physics Teacher Candidates Substantiate Their Knowledge? An Analytical Framework for Examining the Epistemic Dimensions of Content Knowledge in Higher Education" Education Sciences 9, no. 2: 120. https://doi.org/10.3390/educsci9020120
APA StyleNousiainen, M., Hyytinen, H., Palmgren, E., & Toom, A. (2019). How Do Physics Teacher Candidates Substantiate Their Knowledge? An Analytical Framework for Examining the Epistemic Dimensions of Content Knowledge in Higher Education. Education Sciences, 9(2), 120. https://doi.org/10.3390/educsci9020120





