Abstract
The COVID-19 pandemic ushered in an unprecedented period of both crisis and innovation in higher education. The shift to an online learning environment was particularly problematic for courses in which students learn disciplinary practices. Scientific practice requires hands-on training and collaborative engagement with instructors and peers, dimensions of the learning environment that were challenging to recreate online. Here, we describe the resulting instructional innovations and challenges experienced in shifting multiple undergraduate- and graduate-level molecular bioscience labs, including Genetics, Cell Biology, Bioinformatics, and Advanced Microscopy, to an online learning environment. Instructors pursued novel approaches, techniques, and at-home lab tools with varying success. Many innovations were retained after the transition back to an in-person learning environment because they uniquely supported previously overlooked aspects of student learning. Consistent with other reports, we found that marginalized students pursuing science were disproportionately burdened by COVID-19 and the shift to an online learning environment. A description of what worked for online learning, what didn’t, and what is worth holding onto in the future is valuable for constructing learning environments that effectively support learners in their disciplinary practice.
Keywords:
molecular bioscience; genetics; cell biology; microscopy; bioinformatics; pandemic; online; learning environment; DIPLE 1. Introduction
As with most campuses, the University of Wisconsin-La Crosse (UWL) was forced to quickly transition to online learning in the spring of 2020 due to the COVID-19 pandemic [1,2,3,4]. Following our spring break in March of 2020, the whole campus rapidly changed to online learning after an in-person start. Attempts were made to go back to in-person learning in the fall of 2020, but spiking COVID-19 cases again forced a return to mostly online in spring of 2021. UWL’s College of Science and Health has a solid reputation for extensive upper-level laboratory and disciplinary training that uniquely prepares students for careers in the health professions, scientific research, and biotechnology fields. Beyond exposing students to both fundamental and innovative scientific technologies, faculty value student inquiry and experimentation as crucial to developing disciplinary skills and critical thinking.
Most lab-intensive curricula focus on students learning by doing, making mistakes, troubleshooting, and analyzing results. Students tend to value hands-on labs over cookbook labs and as such, see online labs as an obstacle to learning [3,5,6]. Hands-on learning and inquiry-based approaches are particularly beneficial for boosting achievement, attitudes, and scientific interest in underrepresented students [7,8,9]. During the pandemic, instructors teaching in the molecular biosciences found it possible to meet most lab learning objectives through a combination of high-quality videos demonstrating techniques, online simulations, and providing real or simulated data for student analysis, but most approaches fell short of teaching students how to physically perform techniques and engaging them in genuine research experiences [1,2,3,4,5]. A major criticism of online courses, particularly massive open online courses (MOOCs), is the absence of hands-on activities. While it has been recently shown that integration of at-home lab kits into online classrooms can significantly enhance student learning and self-efficacy [10,11,12], the development of at-home lab kits for upper-level college science labs is still emerging.
In scientific teaching that emphasizes inquiry and research, interactions with instructors and peers are particularly valuable in helping students to construct meaning from their ideas and observations while holding each other accountable to disciplinary norms [13,14,15]. One-on-one discussions with an instructor and just-in-time teaching are examples of classroom interactions associated with students’ perception of a collaborative learning environment and development of self-efficacy and self-determination [14]. Therefore, the shift to online learning, which produced the physical separation of instructors and learners, jeopardized multiple key aspects of the learning environment. Some have found success in restoring the level of interpersonal interactions necessary for student learning in course-embedded research experiences (CUREs) using video conferencing. Weekly one-on-one video conferences with an instructor who was physically carrying out experiments in collaboration with the remote student were successful in recreating a CURE in microbiology [16]. Generally, by integrating digital social platforms, synchronous teaching, and active learning activities, it has been possible to support instructor–student and student–student interactions [17]. However, whether engaging in a synchronous lecture via Zoom, accessing a video, or participating in a small group discussion, remote learning during the pandemic necessarily required internet and internet-connecting devices, as well as proficiency in their use. An online survey of 2913 undergraduate college students from 30 U.S. universities found that the effectiveness of online learning was highly correlated with a student’s previous experience with online learning, reliable access to the internet and devices, and the extent of financial hardship experienced during the pandemic [18]. Together, pandemic-related restrictions on the use of the physical labs, the loss of in-person student engagement opportunities, and challenges with technology have the propensity to deepen STEM achievement gaps if left unmitigated.
With these challenges in mind, we chose to offer some lab-based courses as fully online and others as hybrids, and to keep some labs in-person (with adjustments to class size, masking, and distancing). Here, we describe our experience transforming several courses in the molecular biosciences, including Genetics, Cell Biology, Bioinformatics, and Advanced Microscopy, from an in-person to an online learning environment.
2. Materials and Methods
Design: Procedures were developed by the multiple authors alone or as a course-specific group in response to the COVID-19 switch to online learning. The goal was to maintain as many hands-on and critical thinking activities as possible in an online environment.
Participants: Study populations were randomly enrolled undergraduate students in their second, third, or fourth year at UWL.
Ethical Information: Some research (J.W., J.K., and A.S.) was partially funded by a UWL online education grant. All studies were approved by the Institutional Review Board of the University of Wisconsin-La Crosse.
3. Results
3.1. Genetics Lab
Genetics (Bio 306) is a 4-credit sophomore and junior level class required for all Biology majors and frequently taken by allied health students. The class comprises three hours of lecture and two hours of lab per week. A primary learning objective of the class is to develop students’ ability to analyze data and diagnose the underlying causes of experimental deviations from expected Mendelian ratios. A laboratory component that addresses this learning objective includes asking students to carry out a semester-long dihybrid cross in Drosophila. Comparing student work produced during online and in-person semesters suggests that learning may be best supported by a combination of hands-on and virtual exercises.
During a typical in-person semester, students are guided through the mechanics of working with the flies, predicting F2 phenotypic ratios, and comparing their expected versus observed results with a Chi-square test. Since flies that express mutant phenotypes are at a survival disadvantage relative to their wild-type siblings, rejection of the null hypothesis is a frequent outcome of the experiment. Additionally, some combinations of mutant alleles exhibit unexpected results such as epistasis. Therefore, many students are faced with the problem of explaining unexpected experimental results. Anecdotally, Genetics laboratory instructors at UWL frequently report that even when students are encouraged to consider biological explanations for unexpected results, they nevertheless typically attribute unexpected results to errors executing the experiment.
During the Fall 2020 semester, the Genetics lab was carried out as a series of virtual online experiments. To simulate the fly cross experiment, instructors wrote an R program to generate realistic F2 cross data, taking the underlying biology into account. The program randomly selects two mutant strains for each student, but with certain constraints (for example, avoiding two mutations that would not be distinguishable visually, such as two similarly-colored eye color mutations or a wing morphology mutation and a wing presence/absence mutation). Importantly, the program was designed to generate realistic F2 data. Each offspring is simulated separately by drawing gametes randomly from the F1 parents, taking linkage and reduced survival of single and double mutant flies into account. The program outputs a set of simulated F2 data for the student, and a Chi-square test (comparing observed results to those expected based on the expected Mendelian ratios) for the instructor to use in grading the Chi-square test that the student performs in their report. Details of the simulation will be provided in a separate publication (Osmundson and Yu, in preparation). Because the program takes reduced survival of mutant flies into account, many students received data sets that, when correctly analyzed, led to a rejection of the null hypothesis.
The students knew that the data that they had received had been generated by a computer program, removing technical errors executing the experiment as a potential explanation for unexpected results. We were curious to see whether this would spur students to consider potential biological explanations for unexpected results. Informally, this prediction appears to be supported. Evaluation of 20 student experiment write-ups of simulation-generated data in which the null hypothesis was rejected revealed that only five of the 20 attributed the unexpected results primarily to an error in experimental technique. Eleven of the remaining 15 narratives attempted to identify a biological explanation for the unexpected results.
In fall 2021, in-person laboratory classes resumed, and the students again carried out the dihybrid cross and generated their own data. A reading of 18 write-ups from fall 2021 real-life experiments in which the null hypothesis was rejected showed that 14 of these students attributed their unexpected results primarily to errors executing the experiment, with only four considering possible biological factors as the most likely explanation.
However, it appears that other goals of the fly project were less well-served by the simulated fly cross project and the online lab. For example, a comparison of fly project papers written during the online and in-person semesters revealed that a greater proportion of the fly papers from the online semester included substantial misconceptions about the nature of genes and alleles (15/37, or 40.5%) as opposed to papers written during the in-person semester (6/38, or 15.8%). We speculate that the reduced amount of time students spent engaging with lab instructors during the online lab resulted in reduced opportunities for instructors to provide just-in-time corrections of misconceptions.
Informally comparing the virtual with the in-person fly lab, it appears that students were more open to considering potential biological explanations for unexpected results when the results had been generated by a simulation. This suggests to us that the fly cross simulation may serve a valuable function even during an in-person semester as an introductory exercise to analysis of the in-person experiment. This insight is a “silver lining” to the COVID-19 pandemic, as the fly cross simulation would not have been developed without the need for an online lab. However, the decline in indicators of other learning outcomes serves as a caution that an online Genetics lab may not be equivalent to an in-person lab for supporting all learning outcomes. We further note that there are hands-on skills typically taught in Genetics lab, such as pipetting, that cannot be taught in a virtual setting. So, although a valuable pedagogical innovation and insight resulted from the switch to online labs, it appears that an entirely virtual Genetics laboratory experience is not interchangeable with an in-person laboratory experience.
To facilitate Genetics’ student participation in virtual lecture, the course was “flipped” during the Fall 2020 semester. Students watched asynchronous lecture videos prior to scheduled synchronous meetings online. The synchronous online class meetings were used for problem solving activities, which were also recorded. Anecdotally, the students appeared to appreciate the “flipped” format for the synchronous class, as it offered a good balance of flexibility and an opportunity for interaction with the instructor. However, group problem solving activities in the online “flipped” classroom were not successful. Students appeared to not engage well with virtual group activities in the context of the online platform and virtual synchronous group activities were discontinued at mid-semester. This may be mitigated by increased synchronous virtual instruction [4]. However, effective individualized virtual instruction is limited by class size even more than in-person instruction. Reliance on synchronous virtual instruction excludes or disproportionately burdens students with poor technological resources [18].
When Genetics lecture returned to in-person instruction during the Fall 2021 semester, the “flipped” format was retained. Students watched lectures online and in-person classes were devoted to problem solving activities. The students carried out these activities in groups, which were assigned based on a survey of students’ preferred group interaction styles. In this way, the asynchronous lecture modules developed for online instruction during the pandemic facilitated far greater opportunities for peer and instructor interaction with the return to in-person Genetics lecture classes.
3.2. Cell Biology Lab
Cell Biology (Bio 315) is an upper-level course in the biology core curriculum that serves students in their junior or senior year. This 4-credit course comprises approximately three hours of lecture and one 3-h laboratory each week. The laboratory component is subdivided into three multi-week project-based modules with 20 students per lab section arranged into groups of 3–4 students [19].
Historically, cell- and molecular-based lab techniques have aided students in securing jobs in industry and acceptance into graduate school programs. In fall 2020, when nearly all courses were taught entirely online, Cell Biology instructors petitioned administration to retain an in-person component to Cell Biology Lab to provide valuable hands-on learning with pandemic safeguards. To accomplish this lofty goal, we devised a hybrid, cohort laboratory model that blended development of hands-on skills in-person with presentation of experimental design and biochemical principles through recorded lectures. Despite some apprehension about the spread of the coronavirus, students appreciated the opportunity for skills-based learning and to see classmates and instructors face to face.
For safety, in-person learning required a smaller cohort size in the lab, and thus less hands-on time for students to complete the labs (90 min rather than 3 h). To make the most of the time available, we provided online tools, mini-lecture recordings, and additional pre-lab assignments such that students were well-prepared when beginning their hands-on work (basically a “flipped” laboratory). These assignments turned out to be valuable tools and some continued to be used in the regular-density labs in fall 2021. Instructors have continued to ask students to carry out example calculations for enzyme activity and protein concentration, an activity requiring students to discriminate between good and bad digital microscopy images, and an activity focused on best practices for preparing and displaying their images.
Cell Biology Lab was originally designed for extensive group work, which some students thrive upon and others struggle with. Data from group members are shared to make a complete data set used for the creation of the module’s product (written report, poster, or oral presentation). When taught entirely face-to-face, all group members are in the same lab section and work together each week to complete tasks. Most students contribute to positive group dynamics and share work equitably, as reflected in peer- and self-evaluations at end of semester. In the hybrid, cohort laboratory model, one or two students from cohort A were grouped with one or two students from cohort B. Group members were introduced to each other virtually by the instructor via the online learning management system (LMS). From there, students were expected to devise plans to share data and complete the work. Often group members in different cohorts never met each other in person. Though we provided the students with online resources through the LMS suite of tools, it was often found that students used their own preferred online tools (e.g., text messages, Snapchat, Instagram, etc.) to communicate. Though this made it more difficult for instructors to keep track of the group interactions, this may have led to greater levels of interaction among the students. Through peer- and self-evaluations, it was evident that students were generally positive about their in-person partner and critical of their virtual group members. Slow internet connections and inexperience with some forms of technology hindered teamwork. As instructors, we have learned that a more proactive approach to building team dynamics might be helpful in future semesters.
3.3. Bioinformatics
Biological research is increasingly dependent on analyzing large genomic, proteomic, and structural databases. Our capstone Bioinformatics course (Bio 440) is normally taught in a computer lab where the instructor is present to answer questions and give introductory lectures while students work with online programs and databases. We have integrated bioinformatics into several courses earlier in the curriculum, so students have experience with some of the programs [20]. Assignments include labs, in-class and take-home exams, and final group presentations in front of the class. The course focuses on analyzing data and using bioinformatics tools to answer biological questions and does not teach students how to do programming [21]. The course has modules on databases, phylogenetics, genomics and transcriptomics, and proteomics, very similar to many bioinformatics courses on other campuses [22]. We use all online free databases and programs to increase accessibility in and out of the classroom [23].
During the pandemic, the class was adapted to be taught either hybrid or all online. Lectures were replaced with asynchronous recorded presentations. Instructions were included for students to download and install software for programs like the structural visualization tool PyMOL (pymol.org, accessed on 17 March 2022). Exams and review sessions were moved online. In spite of these adaptations, this class was probably the least impacted by the pandemic because the students could download the software or use online programs to analyze sequences and molecular models. Others have noted that students prefer a virtual bioinformatics lab format [24]. However, we did observe that some students struggled more with the software when learning online. Some students had slow data connections from their homes, which made learning and interaction with the instructors quite difficult. Lack of live interactions and failure to reach the online tools and databases, along with ease of hiding behind a screen, led to increased problems for struggling students. Instructors also found it much more difficult to assist students with problems through the use of multiple email exchanges rather than more straightforward, in-person instruction with the student sitting at a computer. This only exacerbated the problems for students with poor connections or weak computer skills. In the end, despite this course being the least potentially affected by online approaches, the instructors agreed to return to in-person instruction once that again became possible.
3.4. Advanced Microscopy and Biological Imaging
Advanced Microscopy and Biological Imaging (Bio 449/549) is an upper-level biology elective that serves senior undergraduates and graduate students, typically 24 students per semester. Students majoring in biology, microbiology, and biochemistry take this course to strengthen their research skills in preparation for graduate school or a career in biotech. The normal structure of the course includes two hours of lecture and two hours of lab per week. The first half of the 16-week semester focuses on concepts and principles of use for brightfield, phase contrast, differential interference contrast, fluorescence, and electron microscopy. The second half of the semester focuses on course-embedded research projects posed by other faculty members or external clients. Every student partnership works on a different project. Projects vary broadly in their focus and have included electron microscopic characterization of bee anatomy, immunofluorescence of differentiating muscle stem cells, immunohistochemistry of mouse spleen sections, protein localization, and protein–protein interactions. Each student works collaboratively with the instructor, client, and a peer to complete the research and present the results as a research paper containing a portfolio of curated images acquired during the semester.
In fall 2020, many students left campus to live at home when UWL shifted to online learning. The microscopy facility could not accommodate more than one or two individuals at a time given COVID-19 safety concerns, meaning that it would not be possible to train 24 students in person on fluorescence and electron microscopes. Instructors decided that it was crucial to maintain some hands-on microscopy training and the course-embedded research project. An internal Curricular Redesign Grant supported the development of a version of Advanced Microscopy that could be pursued entirely online.
Innovation springs readily from challenges, and here it took the form of a novel smartphone microscope with research-grade resolution. DIPLE (Smart Micro Optics, Genova, Italy) is a compact and portable box containing a light source, a stage for samples/microscope slides, and three objective lenses with the following specifications: the red objective lens has 35× magnification, 3-micrometer resolution, and 1.5 mm working distance; the grey objective lens has 75× magnification, 1-micrometer resolution, and 0.6 mm working distance; and the black objective lens has 150× magnification, 0.7-micrometer resolution, and 0.3 mm working distance [25]. Every student received their own DIPLE in order to pursue their research projects at home, relieving all pressure on space in the existing microscopy suite. The instructor demonstrated how to use the DIPLE via a high-quality document camera and video conferencing.
Some students partnered with local agencies and businesses to apply their microscopy skills using the DIPLE. For example, several students partnered with Mississippi Valley Conservancy (La Crosse, Wisconsin) to document life within their nature preserves for the purpose of educational outreach and research. Some worked with a kombucha company to quantify yeast and bacterial growth during fermentation. Others used the pandemic and their DIPLE as an opportunity to venture outdoors and simply explore the microscopic world. One student produced a successful prototype for polarization microscopy based on his DIPLE.
Students thrived learning the basics of light microscopy using DIPLE microscopes, as evidenced by the quality of their image portfolios (Figure 1). Students felt that the DIPLE allowed them to experience the microscopic world outside of the laboratory, which instilled in them a genuine appreciation for the technique and the insight it provides in a variety of settings. Students did initially struggle to learn and use CellProfiler image analysis software (cellprofiler.org, accessed on 17 March 2022) online. However, instructors quickly learned how to remotely operate student computers via video conferencing in order to demonstrate and troubleshoot issues directly and in real-time, just as they would in person.
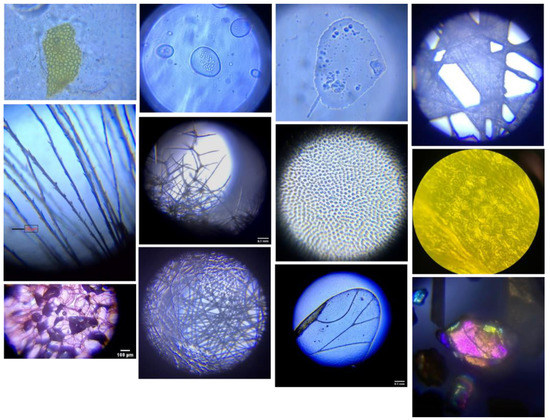
Figure 1.
Example images from student microscopy portfolios. Row 1: Tomato skin in ketchup, fungal cell, cheek cell, dryer sheet. Row 2: Dandelion, mullein leaf, ladybug wing, fern stomata. Row 3: Honeycrisp apple cells, N95 mask, insect wing, and sand particle.
Graduate students generally worked on their thesis projects using whatever microscope they needed, as graduate students were allowed to continue working in our fluorescence and electron microscopy facilities. Graduate students and interested undergraduates were trained by their instructor on the microscope they wanted to use via video conferencing, which was highly successful. The DIPLE microscopes were an excellent hands-on alternative to virtual labs or simulations for introducing the fundamentals of light microscopy and image analysis.
It was necessary to use a different approach for teaching advanced microscopy techniques. For live-cell fluorescence microscopy, confocal fluorescence microscopy, and scanning electron microscopy, we turned to biotech companies from around the world who were able to demonstrate their microscopes via video conferencing. This approach is similar in concept to a remote microscopy experience using an automated live-cell imaging system for undergraduates in a Cell Biology Lab [26], except that we relied on companies to create the experience rather than purchasing these very expensive, high-end instruments for our labs. At one point, students remotely operated a confocal fluorescence microscope located in San Francisco from their homes in Wisconsin. Several students later applied for internships at these companies and were hired, so the connections made during the pandemic played out beautifully in both the short and long term. We had been blind to such valuable connections pre-COVID-19, but will now continue to integrate these remote demonstrations into future courses.
4. Discussion
To retain as much of the hands-on lab training as possible, even in an online learning environment, our faculty pursued instructional innovations with varying degrees of success. Some of these tools, techniques, and approaches were discarded with the return to in-person classes, but most of them were retained, in whole or part, because they had the potential to improve the students’ experience of the in-person learning environment as well. Others have also found that while virtual labs alone cannot adequately substitute for in-person experiences, virtual tools, in combination with physical experiences, can be particularly effective [11,27,28]. The DIPLE microscopes were a striking example of an instructional innovation that provided hands-on learning in conjunction with virtual instruction that was retained for in-person instruction to relieve pressure on microscopy facilities and to aid students doing field research. Relationships with biotech companies that greatly expanded the boundaries of the traditional classroom and facilitated relationships between students and potential employers were also retained. Building on the success of the fly cross simulation in encouraging students to consider biological explanations for unexpected results, a simulation-based exercise will now precede the students’ analysis of their own data, allowing them practice before their own experiments. The “flipped” format for Genetics shows how an asynchronous lecture can free more in-person class time for problem solving and result in a more interactive classroom experience. The hybrid approach to teaching Cell Biology was particularly successful in supporting student development of practical lab skills, critical thinking skills, and proficiency with lab calculations; in comparison to a fully online model, the hybrid model is more effective in ensuring that practical lab skills develop [2]. Pre-lab activities, including calculations with simulated data, will be retained to prime students’ understanding before they carry out actual experiments.
Many of our students were ill-equipped to meet the demands of a virtual learning environment because of a combination of inexperience and disproportionately distributed pandemic-related burdens. Students with reliable computers, cameras, and internet access had a distinct advantage because they could reliably access and use course materials on their own schedule and participate in video conferences with their instructor and peers. Learning for marginalized students was particularly impacted by the virtual learning environment; these students struggled with reliably accessing technology, finding spaces within their homes to study, balancing family responsibilities with school, and building relationships with the instructor and peers [18,29]. A complicating factor unique to UWL is that many of our students in the molecular biosciences are pursuing careers in the health professions, and thus worked extensive shifts as EMTs, CNAs, and technicians in COVID-19 testing labs during the pandemic, compounding their stress and distraction. However, these students are juniors and seniors who had a couple of years of college experience and were generally more resilient to the shift to online learning than first-year students or sophomores. Instructors found it useful to use course management software to see how much time individual students engaged with the platform, which makes it possible to directly reach out to struggling students. Providing an asynchronous alternative to live virtual activities was also important. Genetics students were more successful when lectures were offered asynchronously and problem solving occurred in live virtual interaction. Microscopy students thrived on synchronous virtual interaction, but the instructor did have to directly intervene to solve technology issues on occasion. While Bioinformatics was not as impacted because it was very computer intensive, some students did struggle without in-person feedback from instructors.
Understandably, most of our faculty knew little about how to effectively shape an online learning environment in ways that supported student learning. While there has been ample study of how to support online learners in introductory level courses, little has been published on how to support disciplinary practices online [15]. Challenges go far beyond ease and comfort with the technologies that support online learning. Teaching strategies that include student inquiry and experimentation require a highly responsive environment that supports engagement and collaboration. Instructors must be able to promptly offer verbal or written support to students as they navigate creative scientific processes. Peer interactions, too, are valuable as students grapple with forming hypotheses, designing experiments, and interpreting data. Tools and techniques that facilitate this level of engagement and collaboration online have not been well described for courses in the molecular biosciences. In Genetics, there was evidence that students who participated in a completely online lab missed critical opportunities for just-in-time learning that would have normally corrected misperceptions. A virtual Genetics lab experience in which synchronous instruction was provided [4] reported a subjective perception of fewer conceptual errors, which is in agreement with our perceptions that just-in-time interaction is important for robust student understanding of key concepts. Students in Cell Biology Lab continued to work successfully with partners and within teams in a hybrid setting, but students chose to connect via their own social media tools rather than instructor-provided tools. Microscopy students attended synchronous lectures via video conferencing in which the instructor demonstrated how to use microscopes and even remote-operated student computers to troubleshoot technology issues. In conclusion, while the pandemic-induced virtual teaching had some negative short-term effects, in the long run, it spurred some teaching innovations that will improve instruction as we go back to in-person instruction.
Author Contributions
Conceptualization, investigation, and analysis, A.Y., J.W., T.O., A.S., S.C. and J.K.; writing—original draft preparation, A.Y., J.W., T.O., A.S., S.C. and J.K.; writing—review and editing, J.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of the University of Wisconsin-La Crosse (protocol code 19-ML-456 and date of approval of 6 August 2018).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Acknowledgments
The University of Wisconsin College of Science and Health funded many of the instructional innovations described in this work.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Chandrasekaran, A.R. Transitioning Undergraduate Research from Wet Lab to the Virtual in the Wake of a Pandemic. Biochem. Mol. Biol. Educ. 2020, 48, 436–438. [Google Scholar] [CrossRef]
- Delgado, T.; Bhark, S.J.; Donahue, J. Pandemic Teaching: Creating and Teaching Cell Biology Labs Online during COVID-19. Biochem. Mol. Biol. Educ. 2021, 49, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.L.; Rowland-Goldsmith, M. Student Perceptions of an Inquiry-Based Molecular Biology Lecture and Lab Following a Mid-Semester Transition to Online Teaching. Biochem. Mol. Biol. Educ. 2021, 49, 15–25. [Google Scholar] [CrossRef]
- Zhou, C. Lessons from the Unexpected Adoption of Online Teaching for an Undergraduate Genetics Course with Lab Classes. Biochem. Mol. Biol. Educ. 2020, 48, 460–463. [Google Scholar] [CrossRef]
- Brockman, R.M.; Taylor, J.M.; Segars, L.W.; Selke, V.; Taylor, T.A.H. Student Perceptions of Online and in-Person Microbiology Laboratory Experiences in Undergraduate Medical Education. Med. Educ. Online 2020, 25, 1710324. [Google Scholar] [CrossRef]
- Bachhawat, A.K.; Pandit, S.B.; Banerjee, I.; Anand, S.; Sarkar, R.; Mrigwani, A.; Mishra, S.K. An Inquiry-Based Approach in Large Undergraduate Labs: Learning, by Doing It the Wrong Way. Biochem. Mol. Biol. Educ. 2020, 48, 227–235. [Google Scholar] [CrossRef]
- Kanter, D.E.; Konstantopoulos, S. The Impact of a Project-Based Science Curriculum on Minority Student Achievement, Attitudes, and Careers: The Effects of Teacher Content and Pedagogical Content Knowledge and Inquiry-Based Practices. Sci. Educ. 2010, 94, 855–887. [Google Scholar] [CrossRef]
- Haak, D.C.; HilleRisLambers, J.; Pitre, E.; Freeman, S. Increased Structure and Active Learning Reduce the Achievement Gap in Introductory Biology. Science 2011, 332, 1213–1216. [Google Scholar] [CrossRef]
- Cervantes, B.; Hemmer, L.; Kouzekanani, K. The Impact of Project-Based Learning on Minority Student Achievement: Implications for School Redesign. Educ. Leadersh. Rev. Dr. Res. 2015, 2, 50–66. [Google Scholar]
- DeBoer, J.; Haney, C.; Atiq, S.Z.; Smith, C.; Cox, D. Hands-on Engagement Online: Using a Randomised Control Trial to Estimate the Impact of an at-Home Lab Kit on Student Attitudes and Achievement in a MOOC. Eur. J. Eng. Educ. 2019, 44, 234–252. [Google Scholar] [CrossRef]
- Hanzlick-Burton, C.; Ciric, J.; Diaz-Rios, M.; Colgan, W., 3rd; Gage, G.J. Developing and Implementing Low-Cost Remote Laboratories for Undergraduate Biology and Neuroscience Courses. J. Undergrad. Neurosci. Educ. 2020, 19, A118–A123. [Google Scholar] [PubMed]
- Jawad, M.N.; Bhattacharjee, A.; Lehmann, R.; Busza, A.; Perez-Pinera, P.; Jensen, K. Remote Laboratory Exercise to Develop Micropipetting Skills. J. Microbiol. Biol. Educ. 2021, 22, ev22i1.2399. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Han, X.; Yang, J.; Cheng, J. Examining the Necessary Condition for Engagement in an Online Learning Environment Based on Learning Analytics Approach: The Role of the Instructor. Internet High. Educ. 2015, 24, 26–34. [Google Scholar] [CrossRef]
- Esparza, D.; Wagler, A.E.; Olimpo, J.T. Characterization of Instructor and Student Behaviors in Cure and Non-Cure Learning Environments: Impacts on Student Motivation, Science Identity Development, and Perceptions of the Laboratory Experience. CBE—Life Sci. Educ. 2020, 19, ar10. [Google Scholar] [CrossRef] [PubMed]
- Dede, C.; Ketelhut, D.J.; Whitehouse, P.; Breit, L.; McCloskey, E.M. A Research Agenda for Online Teacher Professional Development. J. Teach. Educ. 2009, 60, 8–19. [Google Scholar] [CrossRef]
- Smith, M.A.V. Cure in Antibiotic Discovery Using a Combination of in-Person, Hands-on Laboratory Activities and Remote, Mentor-Type Experiences During COVID-19. J. Microbiol. Biol. Educ. 2021, 22, ev22i1.2461. [Google Scholar] [CrossRef]
- Ahshan, R. A Framework of Implementing Strategies for Active Student Engagement in Remote/Online Teaching and Learning During the Covid-19 Pandemic. Educ. Sci. 2021, 11, 483. [Google Scholar] [CrossRef]
- Katz, V.S.; Jordan, A.B.; Ognyanova, K. Digital Inequality, Faculty Communication, and Remote Learning Experiences during the COVID-19 Pandemic: A Survey of U.S. Undergraduates. PLoS ONE 2021, 16, e0246641. [Google Scholar] [CrossRef]
- Howard, D.R.; Miskowski, J.A. Using a Module-Based Laboratory to Incorporate Inquiry into a Large Cell Biology Course. Cell Biol. Educ. 2005, 4, 249–260. [Google Scholar] [CrossRef][Green Version]
- Cooper, S. Integrating Bioinformatics into Undergraduate Courses. Biochem. Mol. Biol. Educ. 2008, 29, 167–168. [Google Scholar] [CrossRef]
- Taylor, M.D.; Mendenhall, B.; Woods, C.S.; Rasband, M.E.; Vallejo, M.C.; Bailey, E.G.; Payne, S.H. Online Tools for Teaching Cancer Bioinformatics. J. Microbiol. Biol. Educ. 2021, 22, e00167-21. [Google Scholar] [CrossRef] [PubMed]
- Magana, A.J.; Taleyarkhan, M.; Alvarado, D.R.; Kane, M.; Springer, J.; Clase, K. A Survey of Scholarly Literature Describing the Field of Bioinformatics Education and Bioinformatics Educational Research. CBE Life Sci. Educ. 2014, 13, 607–623. [Google Scholar] [CrossRef]
- Weaver, T.; Cooper, S. Exploring Protein Function and Evolution Using Free Online Bioinformatics Tools. Biochem. Mol. Biol. Educ. 2005, 33, 319–322. [Google Scholar] [CrossRef]
- Weisman, D. Incorporating a Collaborative Web-Based Virtual Laboratory in an Undergraduate Bioinformatics Course. Biochem. Mol. Biol. Educ. 2010, 38, 4–9. [Google Scholar] [CrossRef]
- Cesaretti, M.; Gal, J.; Bouveyron, C.; Diaspro, A.; Fontas, E.; Antonini, A.; Anty, R.; Iannelli, A.; Patouraux, S. Accurate Assessment of Nonalcoholic Fatty Liver Disease Lesions in Liver Allograft Biopsies by a Smartphone Platform: A Proof of Concept. Microsc. Res. Tech. 2020, 83, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.R. Cutting-Edge Microscopy Systems as Remote Teaching and Research Tools for Undergraduate Students. J. Microbiol. Biol. Educ. 2021, 22, ev22i1.2495. [Google Scholar] [CrossRef] [PubMed]
- Beltz, D.; Desharnais, R.; Narguizian, P.; Son, J. Comparing Physical, Virtual, and Hybrid Flipped Labs for General Education Biology. Online Learn. 2016, 20, 228–243. [Google Scholar]
- Sypsas, A.; Kalles, D. Virtual Laboratories in Biology, Biotechnology and Chemistry Education: A Literature Review. In Proceedings of the PCI ’18: 22nd Pan-Hellenic Conference on Informatics, Athens, Greece, 29 November–1 December 2018; pp. 70–75. [Google Scholar]
- Kimble-Hill, A.C.; Rivera-Figueroa, A.; Chan, B.C.; Lawal, W.A.; Gonzalez, S.; Adams, M.R.; Heard, G.L.; Gazley, J.L.; Fiore-Walker, B. Insights Gained into Marginalized Students Access Challenges During the COVID-19 Academic Response. J. Chem. Educ. 2020, 97, 3391–3395. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).