Abstract
The increasing presence of heavy metals in aquatic environments, driven by the production of industrial waste and consumer products, poses serious environmental and health risks due to their toxicity and persistence. Copper (Cu(II)) and nickel (Ni(II)) are particularly harmful, with high concentrations linked to neurological, dermatological and carcinogenic effects. This proof-of-concept study explores the synthesis of sustainable hydrogels derived from grapefruit peel (biosorbents) for the adsorption of Cu(II) and Ni(II) from aqueous solutions. Pectin was extracted from the peels and was used to synthesize pectin-based hydrogels (PH) and pectin hydrogel metal–organic frameworks (PHM composites). The hydrogels were characterized using FT-IR, SEM, diameter size and water absorption capacity. Lyophilized hydrogels were significantly smaller than their wet counterparts, and adsorption performance was analyzed using FAAS. PHs demonstrated high Cu(II) removal efficiency, achieving 95.11% adsorption and 97.75 mg/g capacity at pH 5. PHM composites showed comparable Cu(II) adsorption with a maximum capacity of 67.53 mg/g. Notably, PHs also exhibited rapid Ni(II) adsorption, reaching 92.62% efficiency and 28.189 mg/g capacity within one minute. These findings highlight the potential of pectin-based hydrogels as an effective, low-cost and environmentally friendly method for heavy metal remediation in water.
1. Introduction
1.1. Heavy Metal Contamination in Water and Effect on Human Health
Over the past few decades, the rapid expansion of industrialization and mass production of products such as medicines, batteries, and preservatives, have become a major contribution to environmental pollution [1]. One of the more pressing environmental challenges is the contamination of water sources with heavy metals, primarily driven by industrial waste, acid rain and consumer products [2]. The introduction of heavy metals such as copper (Cu), nickel (Ni) and Zinc (Zn) into large bodies of water can lead to the contamination of groundwater, rivers and lakes [2]. At high concentrations, these heavy metals are highly toxic, resistant to environmental degradation and tend to persist in ecosystems by cycling through different oxidation states or forming organic complexes [2,3]. Their persistence in aquatic environments increases the risk of bioaccumulation within living organisms, ultimately imposing harmful effects on plants, aquatic life and human health [3]. Therefore, removal of heavy metals from water is important in preserving the environment and maintaining human health.
1.2. Copper as a Heavy Metal
Copper (Cu(II)) is the third most abundant metal ion in biological systems and plays a crucial role in biochemical reactions in the human body [4]. A deficiency of Cu(II) in the body can negatively impact enzyme activity, leading to neurological disorders [5]. Cu(II) is an essential element required by the human body in trace amounts; however, excessive exposure and accumulation of Cu(II) can lead to serious health issues, including Alzheimer’s, Wilson’s and prion diseases [4]. Cu(II) is commonly present in wastewater from sources such as electrolysis, battery disposal, metal plating and industrial catalysts [5]. To regulate its presence in drinking water, the World Health Organization (WHO) has established a maximum allowable Cu(II) concentration of 2.0 mg/L in surface and ground water used for drinking purposes [6].
1.3. Nickel as a Heavy Metal
Like Cu(II), nickel (Ni(II)) is a heavy metal with many beneficial applications in industry [7]. For instance, Ni(II) is incorporated in the production of stainless steel, metallic alloys and mineral processing, all of which is important for sustaining modern infrastructure and technological advancements [7]. On the other hand, an excess amount of Ni(II) released from Ni(II) processing can threaten to contaminate natural water systems and increase the risk of exposure of this heavy metal to living organisms [8]. Like other heavy metals, Ni(II) can bioaccumulate through aquatic systems and biomagnifies through the food chain, infiltrating living organisms and even in consumable foods [8,9]. It has been seen that when Ni(II) is present in high concentrations in the body can cause dermatitis, carcinogenesis and various infectious diseases [9]. The United States Environmental Protection Agency (USEPA) has established a maximum contaminant level of 0.2 mg/L of Ni(II) in water for consumption to help minimize health risks associated with excessive Ni(II) exposure [9].
1.4. Removal of Heavy Metals from Water Using Biosorbents
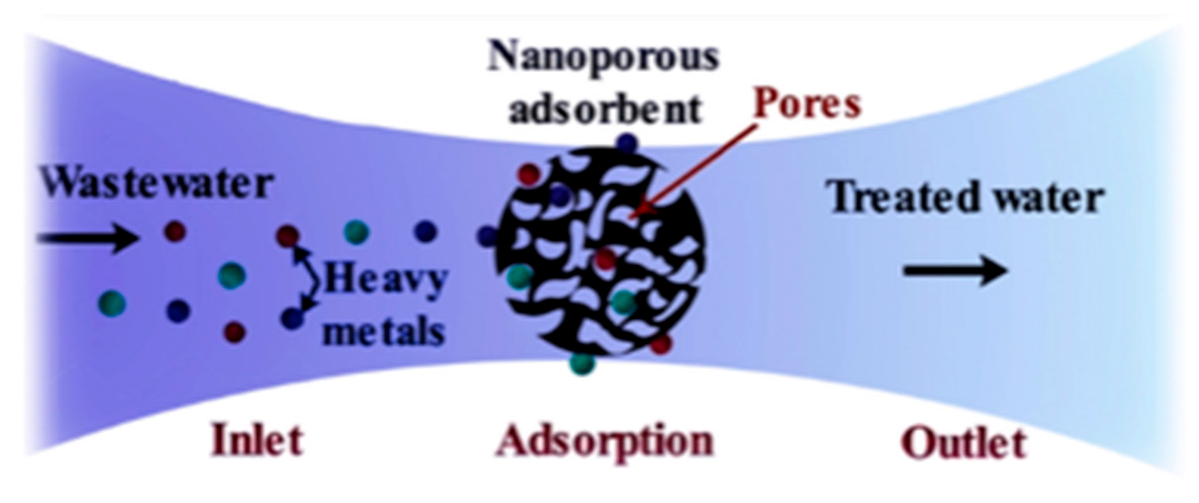
As more water sources are being contaminated by toxic heavy metals, there is a need for sustainable heavy metal removal methods [10]. The removal of heavy metals from contaminated water is a critical environmental challenge that requires efficient, cost-effective and sustainable solutions [10]. Among the available water treatment technologies, adsorption is considered one of the most efficient and practical methods due to its low cost, simplicity and effectiveness in removing heavy metal contaminants [10]. This method is particularly attractive because it is technologically feasible, allows for adsorbent regeneration and does not generate secondary waste [10]. Additionally, adsorption is highly effective even at low metal concentrations and operates with minimal energy consumption, making it a viable alternative for large scale metal removal applications [10]. Adsorption is the phenomenon in which molecules from a liquid phase (adsorbate) adhere to the surface of a solid material (adsorbent) (Figure 1) [10]. This process occurs due to the forces and functional groups present on the solid surface, which attract and retain molecules from the surrounding solution [10]. The binding mechanisms involved in adsorption include covalent bonding and Van der Waals interactions, contributing to the efficiency of metal ion removal [10].
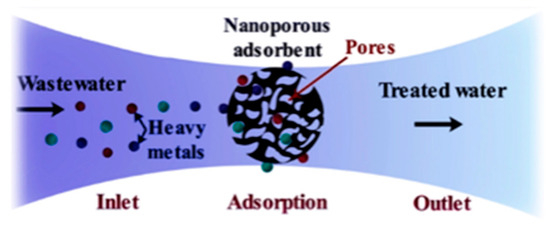
Figure 1.
Diagrammatic representation of adsorption of heavy metals from contaminated wastewater onto a porous adsorbent. The wastewater is treated and has reduced heavy metals present. The figure was adapted from Qasem et al. [11].
The adsorption process for pollutant removal consists of three primary phases [12]. Initially, pollutants diffuse from the bulk solution toward the surface of the adsorbent [12]. Once at the interface, the pollutants interact with the surface, leading to their adsorption [12]. Following this, the pollutants may penetrate further into the internal structure of the adsorbent, depending on its porosity and material properties [12]. Adsorbents used in water treatment are characterized by their high adsorption capacity and large surface area, both of which enhance their efficiency in pollutant removal [12]. Additionally, the adsorption process is reversible, allowing for the desorption of contaminants and the subsequent regeneration of the adsorbent material [12]. This reversibility ensures the sustainability of the adsorption process, enabling repeated use of the adsorbent and reducing overall material consumption in water treatment applications [12].
Over the years, various biomass-based adsorbent materials (biosorbents), such as rice husk biochar, sugar beet pulp and clay have been explored for wastewater treatment [10]. While these materials demonstrated promising adsorption properties, they also presented several limitations, including difficulty in separation from water post-treatment, high production costs and limited economic feasibility for large scale applications [10]. These drawbacks highlight the urgent need for adsorbent materials that are not only effective but also cost-efficient, biodegradable and easy to apply for practical use in water purification [10].
Hydrogels are classified as hydrophilic gels composed of chemically reactive functional groups and a physically distinct three-dimensional (3D) network [10]. This porous network structure enables hydrogels to absorb and retain large amounts of water without dissolving, which makes them highly effective for adsorption applications [10]. The hydrophilic groups present in the polymeric network contribute to the formation of a flexible and dynamic structure, which facilitates the diffusion of solutes into the gel matrix [10]. These solutes interact with functional groups along the polymeric chains, forming stable complexes [10]. Unlike traditional adsorbents, hydrogels provide a highly porous and three-dimensional environment for adsorption, which significantly enhances their efficiency in capturing heavy metals [10].
Hydrogel-based adsorbents have recently emerged as a promising alternative for heavy metal removal due to their exceptional water affinity, swelling behavior, high porosity and improved mechanical stability [10]. These properties enhance their reusability, making them a sustainable option for wastewater treatment [10]. Hydrogels have long been utilized in various fields, including agriculture, cosmetics, food additives, wound healing and drug delivery [10]. However, their application in wastewater remediation has only gained significant attention in the past decade. Given their unique properties, hydrogel-based materials represent a potential breakthrough in the development of next-generation adsorbents for the removal of toxic heavy metals from contaminated water sources [10].
The adsorption and desorption of heavy metals within hydrogel matrices are primarily influenced by surface chemistry and the presence of hydrophilic functional groups such as amine (-NH2), carboxylic acid (-COOH), hydroxyl (-OH), and sulfonic (-SO3H) groups [10]. These functional groups act as complexing agents, binding heavy metal ions and facilitating their removal from aqueous media [10]. Additionally, hydrogels can be further modified by incorporating additional functional groups or forming composite materials with natural or synthetic sources to enhance their heavy metal adsorption capacities [10].
Metal organic frameworks (MOFs) represent a specific and highly versatile class of porous adsorbent materials, and are recognized for their adjustable structures, high flexibility and diverse functional properties [13]. These characteristics make MOFs an attractive option for a wide range of adsorption-based applications, including water treatment and heavy metal removal [13]. Structurally, MOFs consist of two primary building units which include an inorganic metal-based component and an organic linker [13]. The inorganic unit may include transition metals such as manganese (Mn), iron (Fe), zinc (Zn), cobalt (Co) and copper (Cu), as well as p-block elements like gallium (Ga) and actinides such as thorium (Th) [13]. These metal ions or clusters serve as coordination centers for the formation of the MOF network [13]. The organic component typically consists of bridging ligands such as fumaric acid or trimesic acid, which contribute to the structural integrity and adsorption capabilities of the material [13].
The formation of MOFs occurs through a self-assembly process, where the coordination polymer units are influenced by several factors [13]. The coordination geometry of the inorganic unit, the flexibility of the ligand or linker and the reaction conditions such as solvent type, temperature and pH all play critical roles in determining the final structural properties of the MOF [13]. Given these adjustable characteristics, extensive research has been dedicated to expanding the potential applications of MOFs, particularly in water treatment, where their porosity and functional diversity provide significant advantages for contaminant removal [13].
In one study conducted by Mahmoud and Mohamed (2020), they synthesized a novel, pectin-based hydrogel incorporated with a MOF, and examined the adsorption of chromium (Cr(VI)) and lead (Pb(II)) ions [13]. The pectin hydrogel–metal organic framework (PHM composite) had high swelling capacity (1500%), high regenerative stability over eight adsorption and desorption cycles, and high percentage removal and adsorption capacities for both metals [13]. In another study conducted by Li et al. (2015), MOF nanoparticles (MOF-808) were successfully synthesized using microwave heating [14]. The resulting material exhibited high super acidity, making it an efficient adsorbent for arsenic (As) removal from aqueous solutions [14]. The adsorption capacity of MOF-808 was determined to be 24.83 mg/g, demonstrating its strong affinity for arsenic [14]. In addition to its high adsorption efficiency, MOF-808 displayed high stability and reusability. After undergoing five adsorption/desorption cycles, the material retained 82.10% of its initial adsorption capacity [14]. These findings suggest that MOF-808 and PHM composites can function as regenerable adsorbents for Cr(VI), Pb(II) and As removal, and are promising methods for sustainable and cost-effective water treatment applications [13,14].
1.5. Hydrogel Synthesis and Crosslinking
The synthesis of hydrogels typically requires monomers or polymers in combination with an initiator, which plays a crucial role in generating monomeric free radicals that facilitate the growth of macromolecular chains [15]. Monomers possess distinct chemical properties that enable their polymerization into macromolecular structures that ultimately form a stable polymer network [15]. A key characteristic of hydrogels is their cross-linked structure, which is achieved through the addition of a cross-linking agent [15]. The forces responsible for cross-linking primarily involve covalent bonding, although other interactions, such as electrostatic forces, hydrogen bonding, dipole-dipole interactions, and hydrophobic interactions, also contribute to the stability of the network [15]. Hydrogels are generally classified into two categories based on their cross-linking mechanisms and this includes physically or chemically cross-linked hydrogels [15]. Each type exhibits distinct structural and functional properties, which influence their performance in various applications [15].
Physically cross-linked hydrogels are formed through non-covalent interactions such as electrostatic forces, hydrogen bonding and hydrophobic interactions, which facilitate the association of oppositely charged monomers or polymers [16]. These interactions lead to the formation of multi-ion complexes, where multiple macromolecules aggregate to create a stable network [16]. The polymer chains within these hydrogels exhibit strong inter-chain interactions, contributing to a cohesive molecular structure [16]. Simultaneously, the hydrophilic nature of the polymeric network allows for significant water retention, as water molecules readily penetrate and reside within the gel structure [16]. One of the defining characteristics of physically cross-linked hydrogels is their reversible and water-sensitive nature, which results in a limited lifespan [16]. This transient stability makes them advantageous in short-term applications such as in drug release because their gelation process does not involve toxic covalent crosslinking agents [16].
Unlike physically cross-linked hydrogels, chemically cross-linked hydrogels are formed through the covalent bonding of monomers or polymers at specific sites [16]. This cross-linking process is facilitated by cross-linkers, which serve as molecular bridges between polymer chains, resulting in a stable and homogeneous network [16]. The synthesis of chemically cross-linked hydrogels can be controlled by adjusting the pH and other cross-linking parameters [16]. The presence of covalent bonds within the structure enhances the stability and durability of the chemically cross-linked hydrogels, making them well-suited for long-term applications [16]. Their robust mechanical properties and resistance to degradation further expand their potential for use in biomedical, environmental and industrial applications where sustainable solutions are needed [16].
1.6. Pectin-Based Hydrogels from Grapefruit Peel
Pectin is one of the most widely utilized hydrogel polymers in the field of heavy metal removal for water decontamination [13]. Pectin is a naturally occurring, non-toxic polysaccharide composed of linear chains of D-galacturonic acid residues with α-1,4-glycosidic linkages (Figure 2) [13]. It is commonly extracted from bio-waste materials such as grapefruit peels, orange peels and apple pomace [13]. The increasing research interest in biopolymers derived from bio-waste materials, particularly fruit peels, is driven by their non-toxic, environmentally friendly, cost-effective and abundant nature [13]. The use of sustainable and low-cost materials has the potential to contribute significantly to providing clean water solutions for the environment [13]. Citrus fruits, specifically grapefruits, are extensively produced and consumed worldwide and generate significant quantities of waste during citrus juice production, with 50–55% of this waste comprising the grapefruit peels [17]. This substantial byproduct poses environmental challenges due to improper disposal [17]. Citrus peels contain a large quantity of pectin, which is a polysaccharide present in the cell walls, intercellular spaces and middle lamella of plant cells [17]. One study determined that among various citrus peels, grapefruit peel contained the largest source of pectin (23% of dry weight), yielding the highest extraction rates [17]. Therefore, pectin and pectin-based materials from grapefruits hold great promise for applications in water treatment. Advancements can enhance the efficiency of pectin-based materials, thereby expanding their potential in environmental remediation [13]. Recent research has focused on the encapsulation of MOFs within hydrogels to improve their chemical structure and environmental applications [13]. The incorporation of MOF nanoparticles in hydrogels can enhance durability, adsorption capacity and mechanical properties [13].
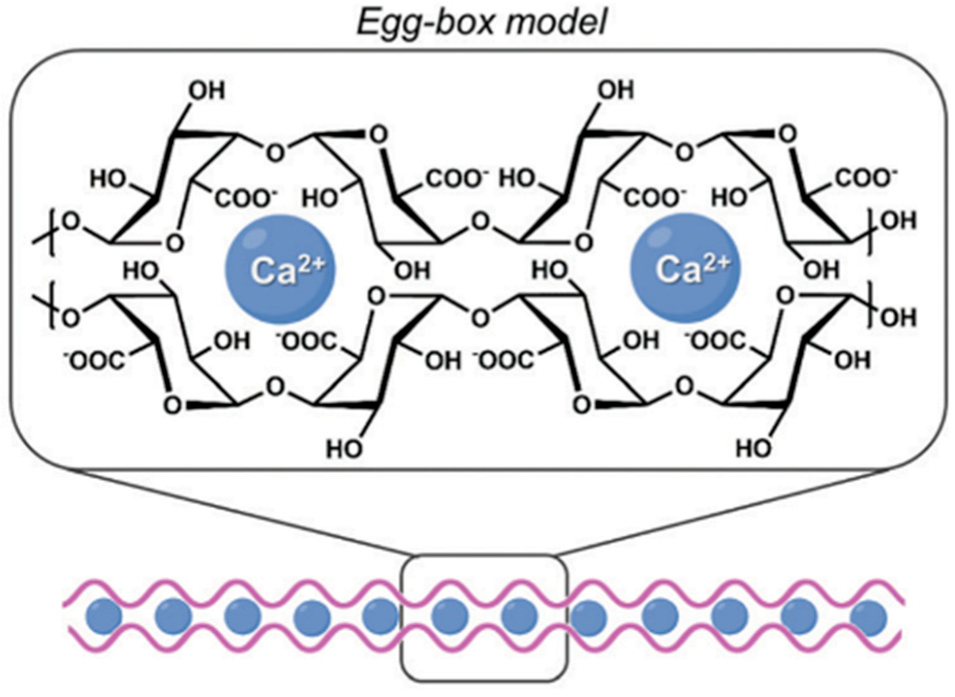
Many of the functional properties of pectin-based hydrogels (PH) are attributed to their ability to bind, assemble and form gels in the presence of divalent cations, such as calcium ions (Ca2+) [18]. The well-known “egg-box” model is a representation of the molecular assembly mechanism of polysaccharides such as pectin with divalent cations [18]. In this model, two oppositely oriented pectin sequences pair together to form a structure containing openings in which Ca2+ ions form coordination interactions which interact with pectin functional groups, including the carboxylic acid (-COOH) and hydroxyl groups (-OH) [18]. This process leads to the formation of cross-linked dimers with a geometric arrangement resembling an egg-box structure (Figure 3) [18]. In an effort to develop sustainable hydrogel composites, Mahmoud and Mohamed (2020) implemented a green synthesis approach to synthesize pectin hydrogels (PH) from mandarin peel [13]. The resulting beads were subsequently combined with MOFs to form a pectin hydrogel–MOFs (PHM composite) through cross-linking with divalent Ca2+ ions, resulting in an insoluble hydrogel network based on the egg-box model [13]. The synthesis of these hydrogels highlighted the potential of PHs as effective and environmentally sustainable methods for heavy metal adsorption and water purification applications [13].
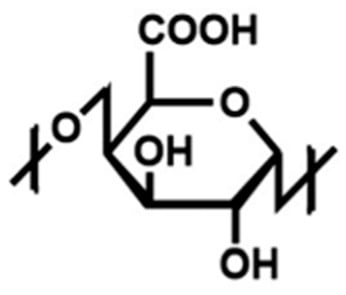
Figure 2.
The chemical structure of pectin. The figure was adapted from Tortorella et al. [19].
Figure 2.
The chemical structure of pectin. The figure was adapted from Tortorella et al. [19].
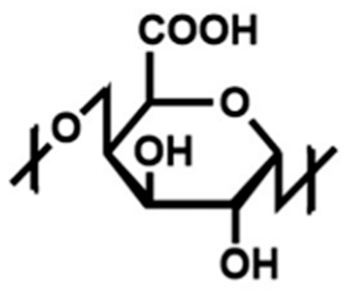
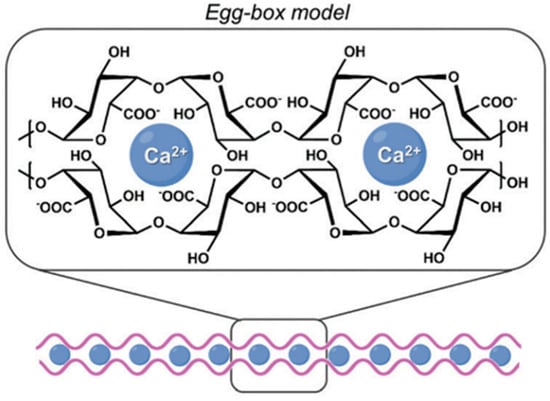
Figure 3.
Schematic diagram of the egg-box model cross-linking between pectin molecules and calcium (Ca2+) ions in pectin-based hydrogels (PH). The figure was adapted from Tortorella et al. [19].
Figure 3.
Schematic diagram of the egg-box model cross-linking between pectin molecules and calcium (Ca2+) ions in pectin-based hydrogels (PH). The figure was adapted from Tortorella et al. [19].
1.7. Flame Atomic Adsorption Spectroscopy
Flame Atomic Adsorption Spectroscopy (FAAS) is an analytical technique used to determine the concentration of over 60 elements at concentrations in the mg/L (ppm) or μg/L (ppb) range. This technique can be used in food, biological and environmental applications [20]. In this method, a sample is aspirated and atomized within a hot flame, which generates free, unexcited ground state atoms [20]. A light beam is emitted from a light source, such as a hollow cathode lamp, and is directed through the flame and into a monochromator that identifies the characteristic wavelength of the metal being analyzed [20]. The metal atoms within the flame absorb the light passing through, and the light that is not absorbed is passed to the detector which measures the amount of light absorbed [20]. Therefore, the adsorption levels directly correlate to the concentration of the metal of interest in the sample [21]. This method of quantification is highly specific because each metal has a unique adsorption wavelength [21]. Hence, FAAS will be used as the method of quantification of copper (Cu(II)) and nickel (Ni(II)) in this study. The characteristic wavelengths of absorption for Cu(II) and Ni(II) are 324.8 nm and 232 nm, respectively [22,23].
1.8. Study Aims
This study will attempt to synthesize pectin-based hydrogels (PH) and pectin hydrogel–metal organic framework (PHM composite) using grapefruit peels as a raw material by applying green chemistry principles. The extraction of pectin, hydrogel synthesis protocols and method for conducting the adsorption tests are obtained from Mahmoud and Mohamed (2020) [13]. Following the synthesis of the hydrogels, they will be characterized using Fourier Transform Infrared Spectroscopy (FTIR), Scanning Electron Microscopy (SEM), diameter size and capacity to uptake water, and the results will be compared to the literature. Finally, the adsorption of Cu(II) and Ni(II) by the pectin-based hydrogels will be investigated through batch adsorption tests. These tests include effect of contact time on heavy metal adsorption and effect of initial pH on adsorption by PHs and PHM composites. FAAS will be used to quantify the amount of Cu(II) or Ni(II) after adsorption. The findings of this study will help set the stage for future CHMD90H3 research students to further conduct detailed studies on Cu(II) and Ni(II), for instance, to explore the reusability of hydrogels or the effect of temperature on heavy metal adsorption.
2. Materials and Methods
2.1. Materials and Reagents
Organic red/pink grapefruits (product of Sunkist) were purchased from Whole Foods Market (3997 Hwy 7, Markham, ON L3R 5M6, Canada). Calcium Chloride Dihydrate (CaCl2·2H2O) powder, ACS reagent (CAS-No: 10035-04-8), 99.0–105.0%, was purchased from J. T. Baker Chemicals (Phillipsburg, NJ, USA). Hydrochloric Acid (HCl), ACS reagent (CAS-No: 7647-01-0), 37%, was purchased from Sigma Aldrich (St. Louis, MO, USA). Sodium Hydroxide (NaOH) pellets, ACS reagent (CAS-No: 1310-73-2), 99.99%, was purchased from ACP Chemicals (Zelienople, PA, USA). Methanol, gradient grade (CAS-No: 67-56-1), ≥99.9%, was purchased from Sigma Aldrich. L-(+)-Tartaric Acid, ACS reagent (CAS-No: 87-69-4), ≥99.0%, was purchased from Fisher Chemicals (Landsmeer, The Netherlands). Iron (III) Chloride (FeCl3), reagent grade (CAS-No: 7705-08-0), 97%, was purchased from Sigma Aldrich. Cupric Nitrate Trihydrate (Cu(NO3)2·3H2O), ACS reagent (CAS-No: 10031-43-3), was purchased from VWR BDH Chemicals (Leuven, Belgium). Nickel Nitrate Hexahydrate (Ni(NO3)2·6H2O), ACS reagent (CAS-No: 13478-00-7), 99%, was purchased from VWR BDH Chemicals. TraceMetal Grade Nitric Acid (HNO3), (CAS-No: 7697-37-2), 67–70%, was purchased from Fisher Chemicals. Deionized (DI)/reverse osmosis (RO) water and MilliQ water was used throughout the experiment.
2.2. Preparation of Grapefruit Peel Powder
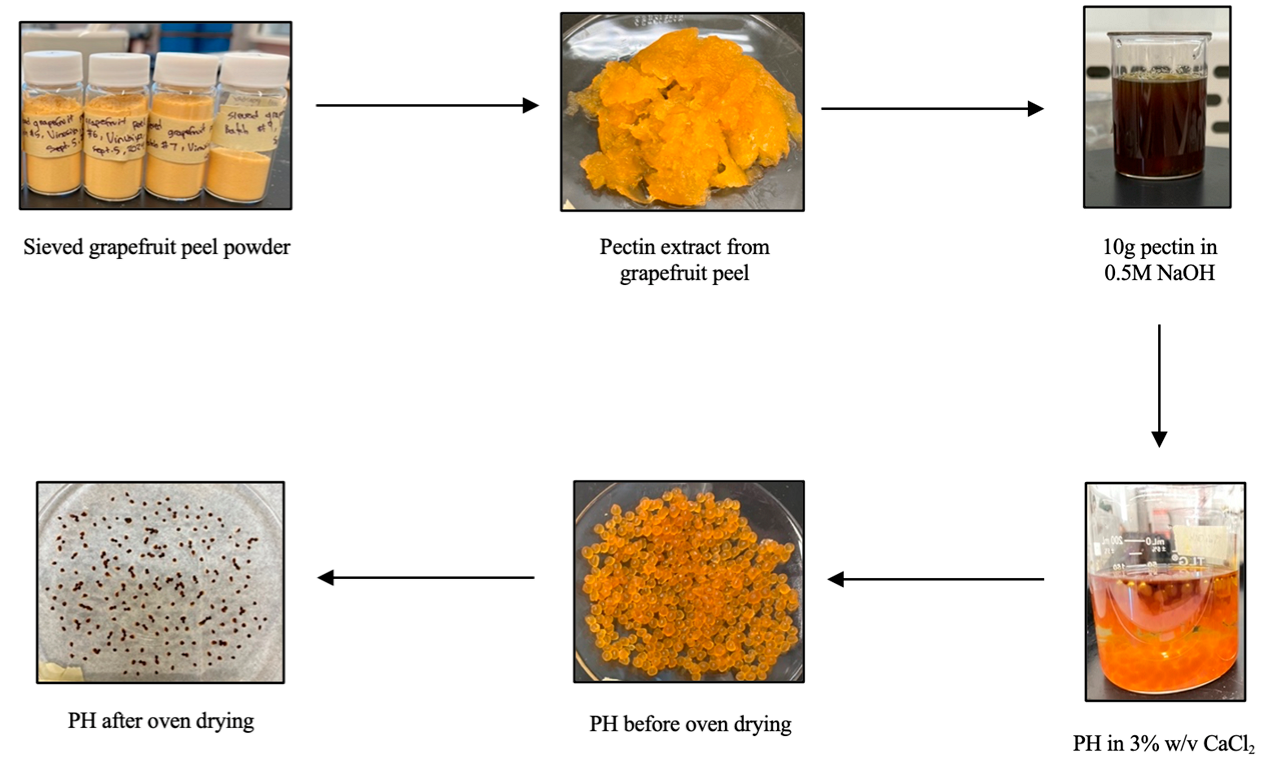
The procedure was modified from Mahmoud and Mohamed (2020) [13]. To start, a few grapefruits were peeled, and the peels were soaked in a 90 °C water bath for 5 min. The peels were then laid individually on paper towel and air-dried overnight. Afterwards, the peels were dried in a Thermo Scientific Heratherm oven (Thermo Fisher Scientific, Waltham, MA, USA) at 60 °C overnight. The dried peels were then ground in a Shardor Coffee Grinder (Kington, UK) then sieved through 100-mesh sieve to obtain a fine powder. The grapefruit peel powders were stored in 20 mL scintillation vials at −15.55 °C for further use.
2.3. Extraction of Pectin from Grapefruit Peel Powder
Slight modifications were made to the procedure written by Mahmoud and Mohamed (2020), which includes changes to the NaOH concentration, stirring time and extraction temperature [13]. Approximately 10.0 g of sieved grapefruit peel was combined with 120 mL of 0.1 M HCl solution in a 95 °C water bath and stirred well for 60 min. The resulting slurry was cooled to room temperature for 1 h, then centrifuged at 6000 rpm for 12 min. The supernatant (approximately 40 mL) was combined with three times the volume of methanol (approximately 120 mL) until precipitation occurred. The pellet was discarded, and the pectin precipitate was allowed to settle out overnight. Next, the excess methanol was discarded and the pectin precipitate was dried in an oven at 60 °C for 17 h. The dried pectin, which was brittle and dark brown, was then ground with a mortar and pestle. The pectin powder was stored in scintillation vials at −15.55 °C for further use. The percent yield was determined to be 16.82% and was calculated using Equation (1) below, where mP and mGF denote the dry mass of pectin (g) extracted and dry mass of grapefruit peel used in extraction, respectively.
2.4. Method for the Synthesis of Pectin Hydrogels (PH)
To synthesize pectin hydrogels (PH), approximately 1.70 g of pectin powder was combined with 80 mL of 0.5 M NaOH and stirred at room temperature for 1 h. The solution was incubated for 4 days at room temperature to stimulate gelatinization. Then, the solution was added dropwise with a 12 mL Fisherbrand plastic syringe (Air-Tite Luer-Lock, Virginia Beach, VA, USA) into a 200 mL solution of 3% w/v CaCl2 and left for 24 h to stabilize. Afterwards, 50 mL of methanol and 15 mL of 0.5 M NaOH was added to the beads and stirred slightly with a stir rod, then incubated at room temperature for 1 h. Subsequently, the beads were isolated by vacuum filtration using DI water to facilitate transfer and washes. The orange beads were dried in an oven at 60 °C for 24 h, lyophilized for 28 h (8 h freezing, 20 h drying), then stored in 20 mL scintillation vials at −15.55 °C for further use.
2.5. Method for the Synthesis of Pectin Hydrogel–Metal Organic Framework (PHM Composite)
To synthesize the PHM composite, approximately 1.0 g of PH was combined and stirred well with 0.1 mM FeCl3 for 30 min at room temperature. Then, 0.1 mM tartaric acid was added and the mixture was stirred for an additional 30 min. The mixture was then heated to 100 °C for 15–30 min. The prepared PHM composite was oven-dried at 60 °C for 6 h, lyophilized for 28 h (8 h freezing, 20 h drying), then stored in 20 mL scintillation vials at −15.55 °C for further use. The procedure is visualized in Figure 4, below.
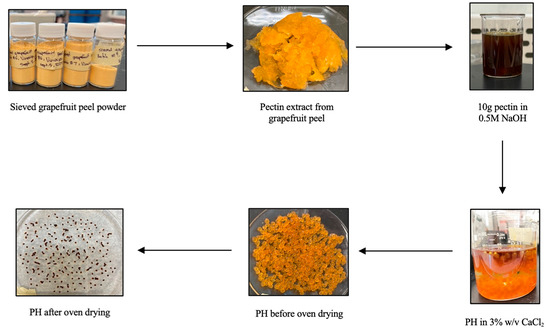
Figure 4.
Schematic diagram for the synthesis of pectin hydrogel (PH) from raw grapefruit peel.
2.6. Characterization Techniques
The techniques used to characterize the hydrogels include Fourier Transform–Infrared Radiation (FT-IR), Scanning Electron Microscopy (SEM), diameter size measurements and water adsorption capacity.
2.6.1. FT-IR
The FT-IR spectra of pectin and PH were acquired to observe which functional groups were present in each sample. A Bruker Alpha-P ATR FT-IR (Diamond ATR, Bruker, Billerica, MA, USA) was used to conduct the analysis. The PHs were ground to a fine powder using a mortar and pestle before analysis. The spectra were recreated using the Microsoft Excel program.
2.6.2. SEM
SEM analysis was conducted to observe the surface structure and cross sections of the hydrogels. SEM was conducted on PH before and after adsorption of Cu(II) and PHM composite before adsorption. The hydrogels were lyophilized prior to analysis.
2.6.3. Diameter Measurements
The diameters of PH and PHM composite were measured after the hydrogels were newly synthesized and after lyophilization. A standard 30 cm metal ruler was used. Six individual spherical hydrogels were used for each sample.
2.6.4. Water Adsorption Capacity
The capacity of the PH to absorb water was investigated. Approximately 0.020–0.030 g of dried PH was immersed in excess DI water (20 mL) in a scintillation vial and the mixture was not stirred. The beads were incubated at room temperature for 10, 60, 150 or 400 min. The beads were subsequently isolated using vacuum filtration and gently wiped with a Kimwipe to remove surface water. The mass of swollen hydrogels was determined. The swelling degree (%) was determined using Equation (2), where m0 and mt (g) denote the mass of the dry hydrogel and the swollen hydrogel, respectively. Each time trial was repeated in triplicates.
2.7. Heavy Metal Adsorption Assays
The adsorption of copper and nickel heavy metals onto PH and PHM composite was investigated under varying experimental conditions which include contact time, pH and concentration. The adsorption assays were conducted only using lyophilized hydrogels. The Cu(NO3)2·3H2O and Ni(NO3)2·6H2O standard solutions were made in 2% HNO3 (in MilliQ water) and the pH was adjusted using 30% NaOH to a pH of approximately 5.5. The pH of the standard solutions was adjusted differently for the pH assay. The pH was monitored using pH paper (Fisherbrand, pH 0.0–14.0, Waltham, MA, USA). Furthermore, each trial for all tests were performed at room temperature in triplicate, and they were diluted and filtered into 15 mL falcon tubes (VWR centrifuge tubes) with 2% HNO3 using 12 mL plastic syringes (Air-Tite Luer-Lock) and 0.22 µm syringe filters (ESBE nylon, Markham, ON, Canada). Flame Atomic Adsorption Spectroscopy (FAAS, Thermo iCE 2500 Flame Adsorption Spectrometer, Acetylene/Air, Thermo Fisher Scientific, Waltham, MA, USA) was used to measure adsorption of copper and nickel at 324.8 nm and 231.9 nm, respectively. A calibration curve was constructed for Cu(NO3)2·3H2O (R2 = 0.9998) and Ni(NO3)2·6H2O (R2 = 0.9992) using 0.2500, 0.5000, 1.0000, 2.5000 and 5.0000 ppm standard solutions, respectively. The calibration curves were reconstructed, and the adsorption data was visualized in Microsoft Excel (Version 16.100.2).
2.7.1. Effect of Contact Time on Heavy Metal Adsorption
The effect of time on the adsorption of copper and nickel heavy metals onto PH and PHM composite was investigated. Approximately 0.020–0.030 g of dried PH was immersed in 10 mL of a 50 ppm solution of Cu(NO3)2·3H2O or Ni(NO3)2·6H2O. The mixture was stirred with a stir bar in a 20 mL scintillation vial (borosilicate glass, WHAETON, Millville, NJ, USA) for 5, 10, 20, 50 or 150 min. After the stirring was completed, the mixture was decanted to a new 20 mL scintillation vial without the beads, and the solution was diluted to make a 50 mL 5 ppm solution. The diluted solutions were filtered, and the copper and nickel concentrations were analyzed by FAAS. The percentage removal (%E) of metal was calculated using equation (3) where Ci and Cf (in mg/L) denote the initial concentrations of metal ions and final concentrations after adsorption, respectively. This process was repeated for the PHM composites, and each time trial was repeated in triplicate.
2.7.2. Effect of Initial pH on Heavy Metal Adsorption
The effect of the pH of Cu(NO3)2·3H2O solution on metal adsorption by PH was investigated. Approximately 0.020–0.030 g of dried PH was immersed in 10 mL of a 50 ppm solution of Cu(NO3)2·3H2O. The 50 ppm solution was adjusted to pH 1, 3, 5 or 12 prior to the addition of the beads. The mixture was stirred with a stir bar in a 20 mL scintillation vial for 120 min. After the stirring was completed, the mixture was decanted to a new 20 mL scintillation vial without the beads, and the solution was diluted to make a 50 mL 5 ppm solution. The diluted solutions were filtered, and the copper concentration was analyzed by FAAS. The optimal pH for adsorption of copper by PH was determined using percent removal Equation (3) and adsorption capacity (qe) Equation (4), where V denotes the volume (L) and m denotes the initial mass (g) of beads used.
3. Results
3.1. Structural and Functional Characterization
The PHs were characterized using FT-IR, SEM, diameter size and water adsorption. Similarly, the PHM composites were characterized using SEM, diameter size and water adsorption.
3.1.1. FT-IR Comparison of Pectin vs. Pectin-Based Hydrogels (PH)
FT-IR is a technique that is commonly used to determine the functional groups present in the compound of a sample. In this study, the FT-IR spectra of the PHs were compared against the spectrum of the pectin that was extracted from the raw grapefruit peels to determine the similarities and differences in the functional groups present. Additionally, the spectra were used to confirm the presence of the carboxyl and hydroxyl functional groups that were previously found to interact with and trap Cu(II) and Ni(II). It was hypothesized that similar functional groups, specifically the carboxyl group and hydroxyl groups, would be found in both PHs and pectin molecules because the pectin hydrogel is formed by the cross-linking of pectin chains, which should have retained their original chemical structure [13,24].
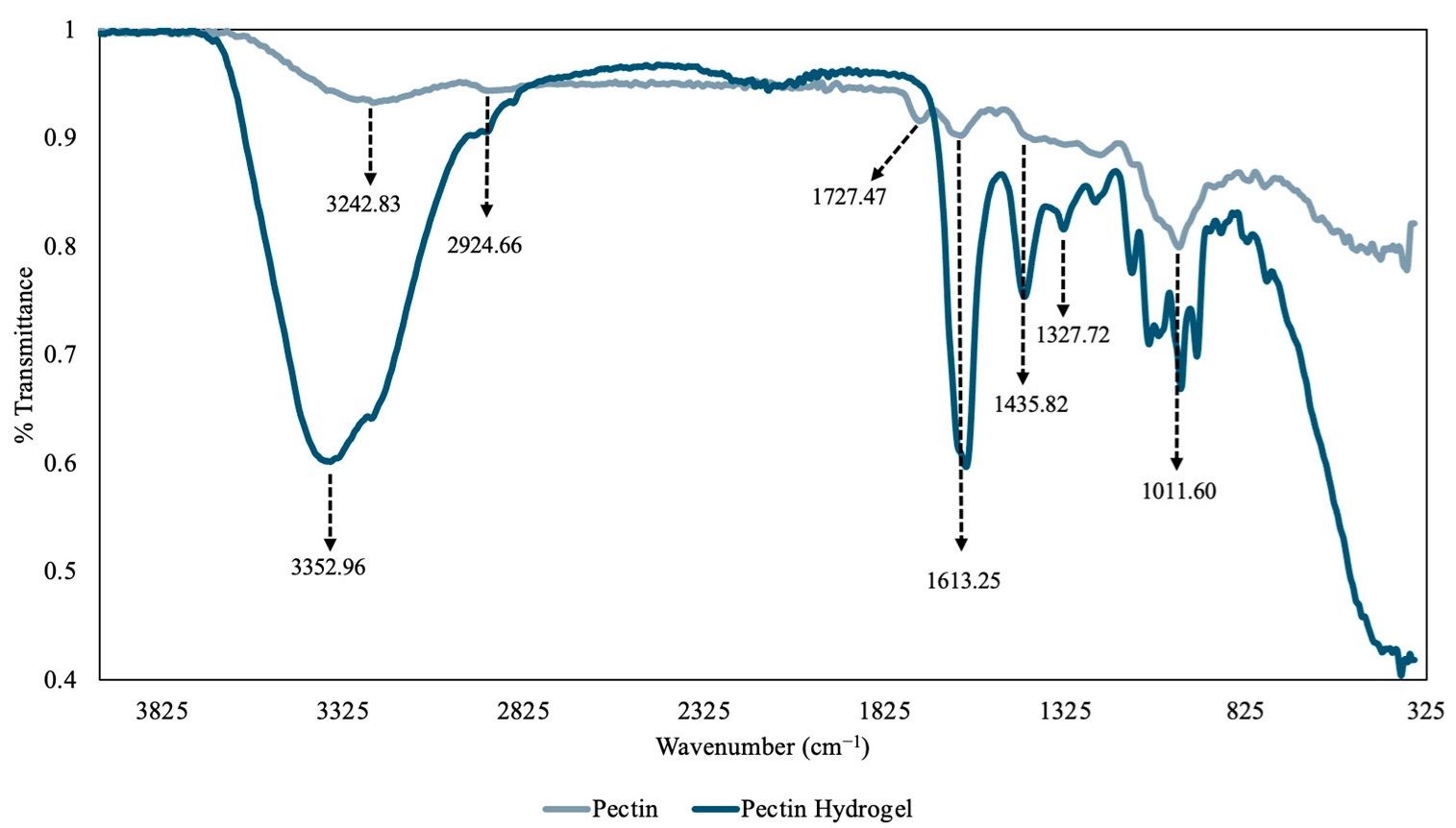
Prior to FT-IR analysis, the pectin powder and PHs were ground in a mortar and pestle to a fine powder for more accurate results. The overlapped FT-IR spectra of pectin and PH are depicted in Figure 5. The spectrum of the pectin molecule showed a broad peak at 3242.83 cm−1, which can be attributed to the presence of O-H stretching vibrations of the phenolic hydroxyl and carboxylic acid groups [25]. Additionally, the small peak at 2924.66 cm−1 can be assigned to the C-H stretches of the -CH2 groups [26]. Furthermore, the absorbance at 1727.47 cm−1 is the C=O stretch of an ester group in pectin, and the absorbance at 1613.25 cm−1 is the C=O stretching vibrations of the carboxylate ion [26]. The absorbance between 1101.60–1435.82 cm−1 are likely the C-H bending of the pyranose ring, C-O-C stretching of the glycosidic bonds, C-O stretching of the alcohol group and -CH3 bending of the methyl acetate [17]. Lastly, the peak at 1011.60 cm−1 can be attributed to the C-O stretching and bending vibrations of pyranose [26]. The IR spectrum of the PHs showed a large broad peak at 3352.96 cm−1 that can be attributed to the O-H stretching of the hydroxyl and carboxylic acid groups and indicated the presence of a large number of hydroxyl groups [26]. Similar absorbance patterns were observed in the PHs when compared to the spectrum of pectin, which aligned with the previous hypothesis and confirmed that after synthesis of the PHs, pectin retained most of its chemical structure.
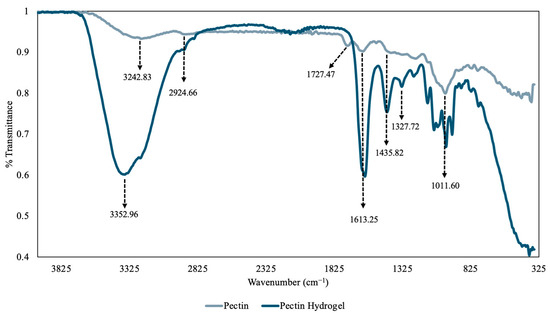
Figure 5.
The overlapped Fourier Transform–Infrared Radiation (FT-IR) spectra of pectin molecule (gray) and pectin hydrogel (PH) (dark blue).
Moreover, the IR spectrum helped reveal the functional groups that were present in the hydrogel that could coordinate and trap heavy metals such as Cu(II) and Ni(II). The hydroxyl groups on the alcohol and carboxylic acid groups are the main active sites by which the heavy metals coordinate with the hydrogel and become trapped [27]. The coordination between the hydroxyl group and heavy metals occurs primarily through electrostatic interactions, specifically the positively charged Cu(II) or Ni(II) with the negatively charged oxygen of the deprotonated hydroxyl group [27]. Therefore, the PH hydrogel with anionic groups can be used to remove heavy metal cations from contaminated water sources [27]. As a result, the PHs could be used for selective adsorption of cationic heavy metals. Furthermore, the intensity of the PH spectrum is greater than that of the pectin spectrum and this is likely due to the fact that an older batch of pectin was used to obtain the spectrum, and the pectin in the older batch may have degraded over time due to low temperature storage conditions and produced decreased signal intensity [28]. Therefore, this suggests the stability and lifetime of pectin extracted from raw grapefruit peel is relatively low.
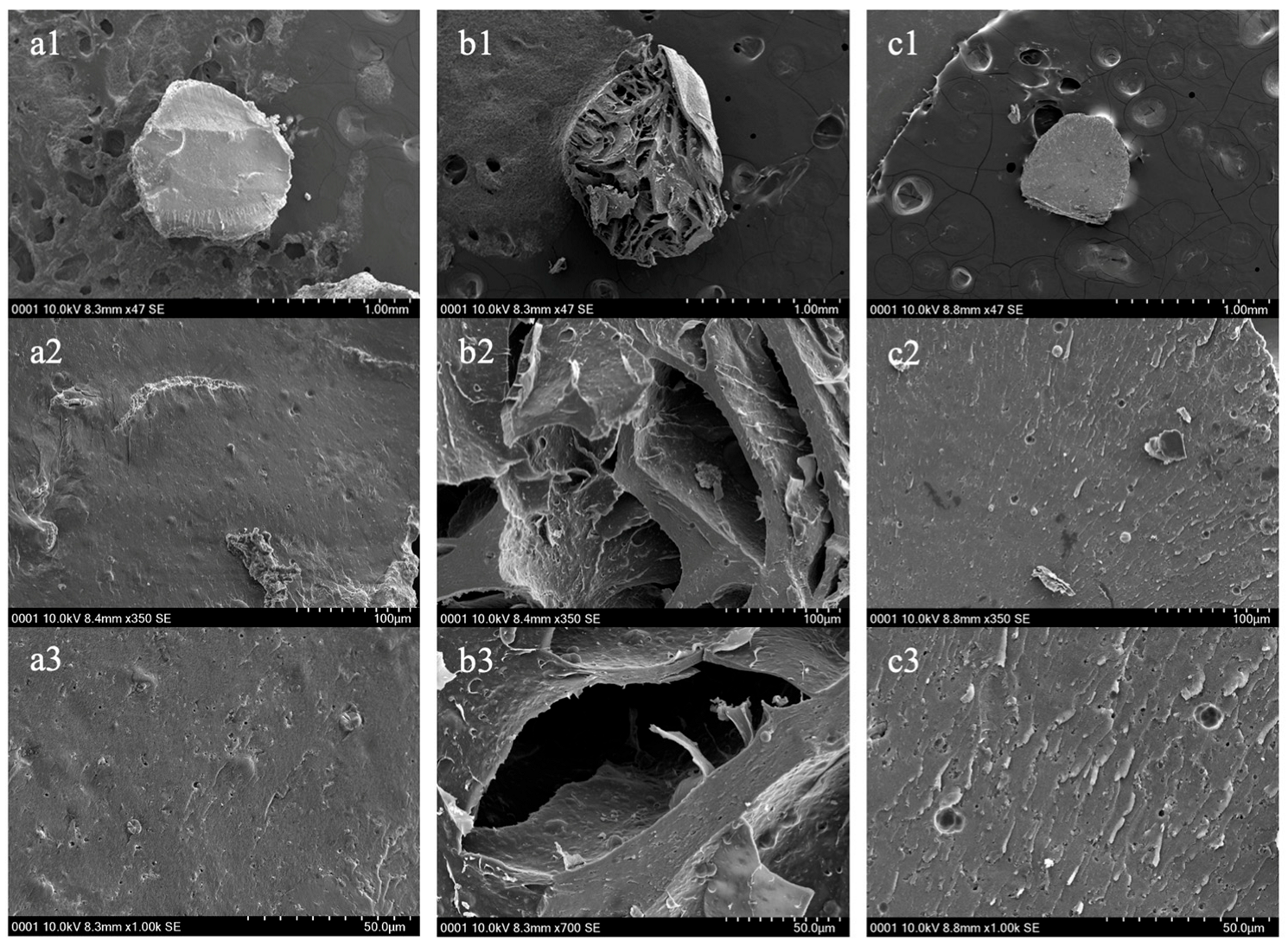
3.1.2. SEM Analysis of PH vs. PHM Composite
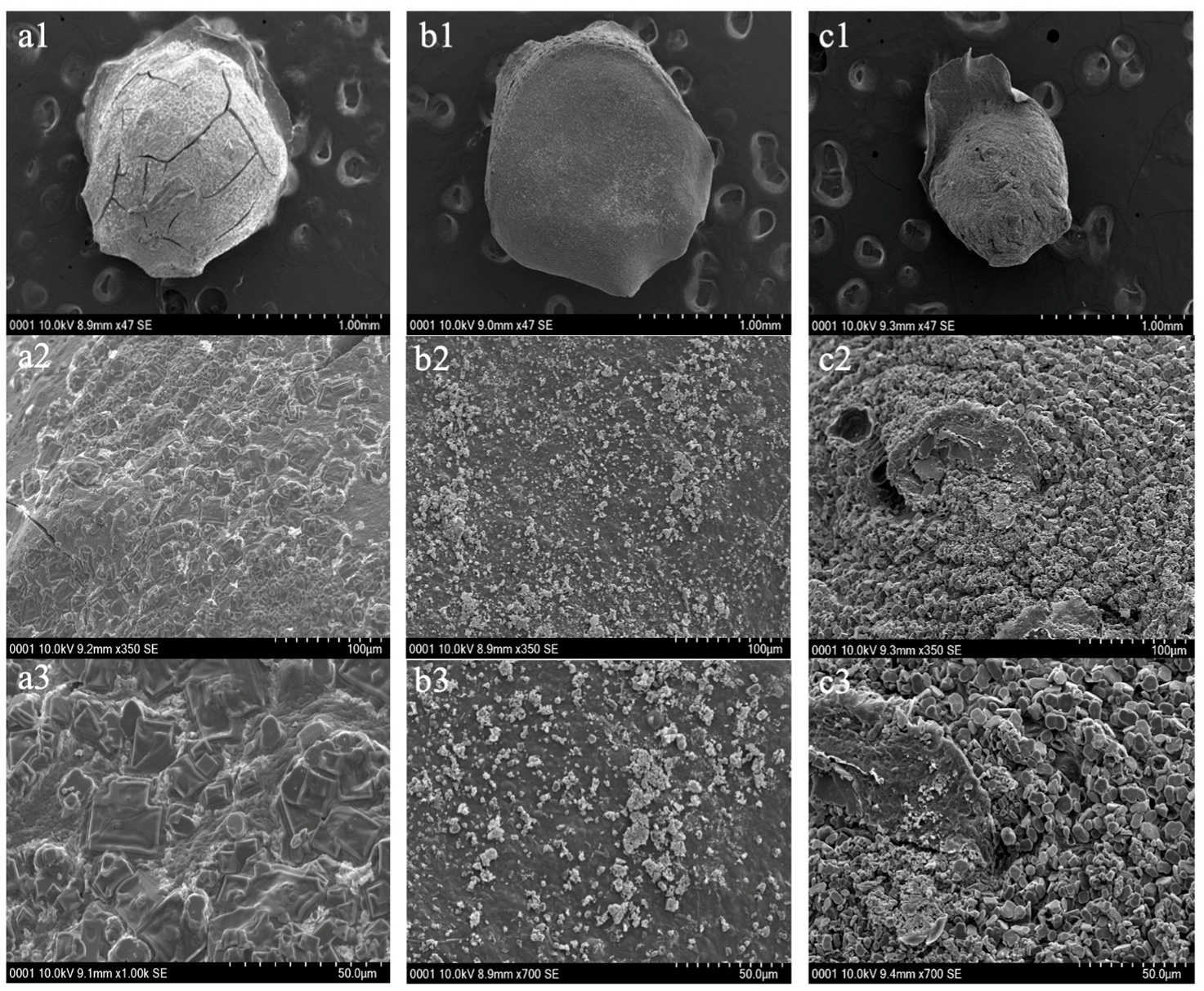
The surface structure and cross-sectional surface structures for PH before adsorption, after adsorption, and PHM composites before adsorption were visualized using SEM and are depicted in Figure 6 and Figure 7, respectively, below. The surfaces of the hydrogels were visualized at three different magnifications. The surface of PH before adsorption was seen to be more rough and irregular, as there were more surface grooves and bump-like structures (Figure 6(a1–a3)). Contrastingly, the surface of PH after adsorption of Cu(II) for ten minutes showed a smoother surface yet also irregular, with fewer surface grooves and bumps (Figure 6(b1–b3)). As previously shown by Mahmoud and Mohamed (2020), the pores of the hydrogels after adsorption likely decreased in size and appear as a smoother surface [13]. A smoother surface after adsorption indicated that the cationic heavy metal was able to occupy the active sites of the PHs through complexation interactions. It can also be noted that the PH before adsorption had a cracked surface, and this was likely due to the lyophilization process which caused the bead to shrink due to evaporation of liquids within the hydrogel. Therefore, it is highly probable that SEM preparation of the samples interfered with the size of the hydrogels, particularly due to lyophilization [29].
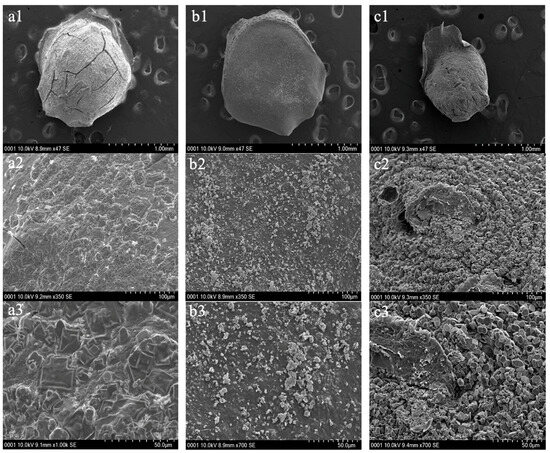
Figure 6.
Scanning Electron Microscopy (SEM) images of the surface structure of pectin hydrogel (PH) before adsorption (a1–a3), PH after adsorption (b1–b3) and pectin hydrogel–metal organic framework (PHM composites) before adsorption (c1–c3) at various magnifications (1.0 mm, 100 µm and 50.0 µm). The hydrogels were lyophilized prior to SEM analysis.
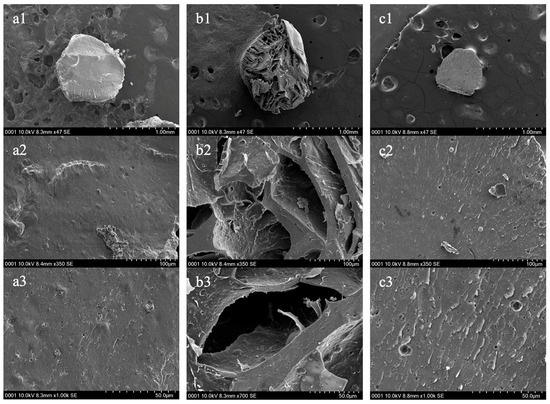
Figure 7.
Scanning Electron Microscopy (SEM) images of the cross-sectional structure of pectin hydrogel (PH) before adsorption (a1–a3), PH after adsorption (b1–b3) and pectin hydrogel–metal organic framework (PHM composites) before adsorption (c1–c3) at various magnifications (1.0 mm, 100 µm and 50.0 µm). The hydrogels were lyophilized prior to SEM analysis.
Additionally, the surface of the PHM composites before adsorption showed a rough surface pattern and displayed a rougher and bumpier surface in comparison to the PHs (Figure 6(c1–c3)). The surface pattern is less irregular than that of PHs, as it was seen that the pectin chains formed a circular ring-like shape (Figure 6(c2)). The pectin chains, however, were not as well-defined as those reported by Mahmoud and Mohamed (2020), and this may be due to differences in the degree of esterification of the pectin found in these hydrogels [13,29]. Nonetheless, a rougher surface suggests a larger surface area in which there are more binding spots available for heavy metals to be trapped upon. Furthermore, it was seen that the PHM composites were overall smaller in size when compared to the PHs.
The cross-sectional structures of the PHs and PHM composites showed similar results to the surface structures. The PHs before adsorption displayed a relatively dense and compact internal structure, with a smooth surface and minimal visible porosity (Figure 7(a1–a3)). With the assumption that the lyophilization process induced shrinkage of the hydrogel, this suggests that the initial pectin network was more expanded and maintained a uniform polymer matrix [30]. The absence of large internal cavities further indicates that the PH before adsorption had a tightly packed structure, likely contributing to its initial stability before heavy metal uptake [13]. In contrast, the PH after adsorption of Cu(II) exhibited significant structural changes, with large pores and an expanded internal network (Figure 7(b1–b3)). The appearance of more fragmented and open pores suggests that metal ion coordination led to swelling and possible mechanical stress within the hydrogel due to robust stirring during the test [31]. The presence of the pores suggests that the structural framework of the PH became more flexible after adsorption, potentially enhancing its ability to accommodate more metal binding.
Additionally, the PHM composites before adsorption demonstrated a more compact and uniform cross-sectional structure compared to PH (Figure 7(c1–c3)). The pectin network of PHM composites appeared rougher but more rigid, likely due to the incorporation of the MOF [13]. Unlike PH after adsorption, the PHM composite structure lacked large open pores, suggesting that it maintained stability before interacting with the metal cation. The reinforced structure of PHM composites may offer a more controlled adsorption process while preserving structural integrity due to the incorporation of MOFs [13].
3.1.3. Diameter Size
The average diameter size of PHs before (wet) and after (dry) lyophilization, and PHM composites after (dry) lyophilization were measured to evaluate the effect of lyophilization on hydrogel shrinkage (Table 1). The results showed a large reduction in hydrogel size (diameter) after lyophilization, which confirmed that this process may significantly impact the structural integrity of the hydrogels. The PH in its wet state had an average diameter of 4.58 ± 0.49 mm, which decreased to 1.67 ± 0.26 mm after lyophilization (Table 1). This reduction showed the effect of solvent removal from the freshly synthesized hydrogel during lyophilization, which led to shrinkage of the hydrogel matrix as the solvent molecules, which contributed to its inflated structure, were evaporated. The decrease in size suggests that the structure of PH is compacted upon drying, potentially decreasing its porosity and therefore adsorption efficiency since the lyophilized hydrogels swell less due to their more rigid structure [32].

Table 1.
Determination of the average diameter size of pectin hydrogel (PH) and pectin hydrogel–metal organic framework (PHM composite) when newly synthesized (wet) and after lyophilization (dry) (n = 6).
Furthermore, the PHM composites exhibited an even smaller average diameter compared to PH, with an average dry state size of 1.05 ± 0.12 mm (Table 1). The reduced size of PHM composites relative to the dry PHs suggests that the incorporation of the MOF altered the hydrogel’s swelling behavior, potentially leading to a denser and more rigid structure. The SEM analysis further supports this observation, as PHM composites demonstrated a more compact and less porous cross-sectional structure compared to PH. The smaller size of PHM composites may indicate stronger intermolecular interactions within the polymer network, which could contribute to its enhanced structural integrity.
Overall, these findings demonstrated that lyophilization induced hydrogel shrinkage, leading to a reduction in size due to solvent loss and structural compaction [32]. Additionally, the smaller size of PHM composites compared to PHs suggests that MOF integration influences the hydrogel’s physical properties, potentially influencing its swelling capacity and adsorption characteristics.
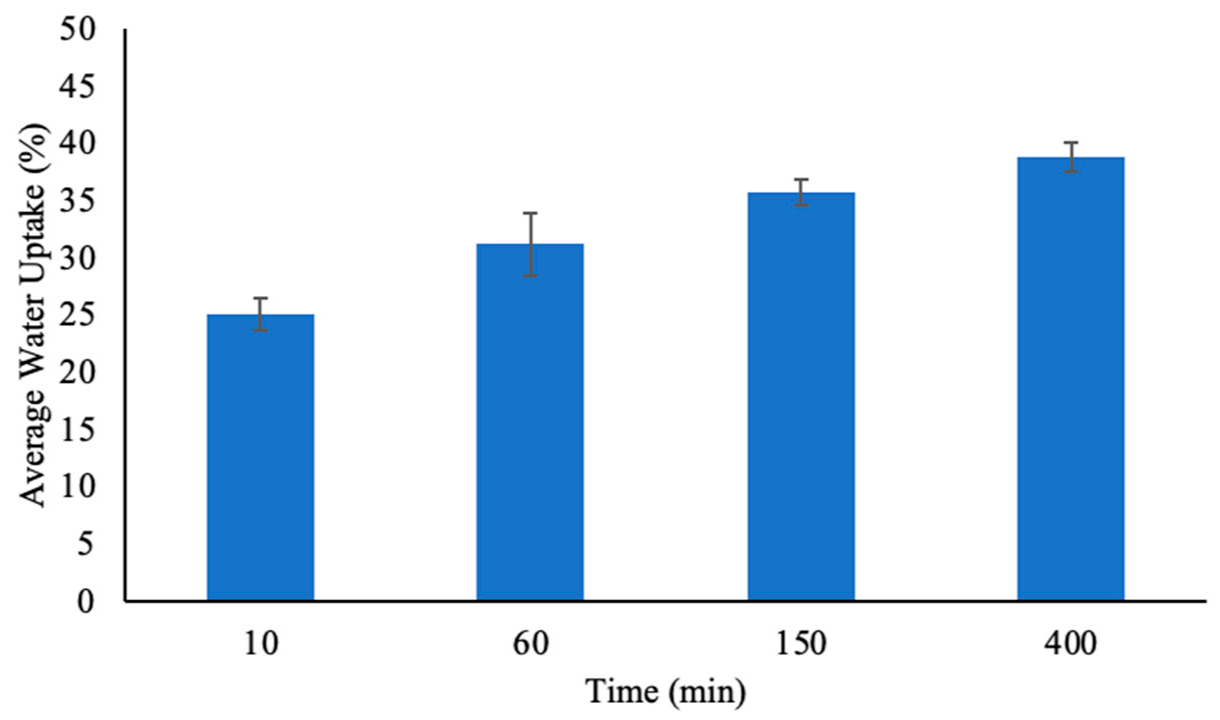
3.1.4. Effect of Time on Water Adsorption Capacity
The ability of PHs to absorb and retain water was evaluated over time, and the results are summarized in Figure 8. The percentage of water uptake by PH increased progressively with time, which indicated the hydrogel’s ability to swell as it absorbed water molecules. At 10 min, the water uptake was 25.09 ± 1.38%, which demonstrated the hydrogel’s initial rapid swelling phase from 0 min. This suggests that the structure of PH allows for immediate water diffusion into the hydrogel [32].
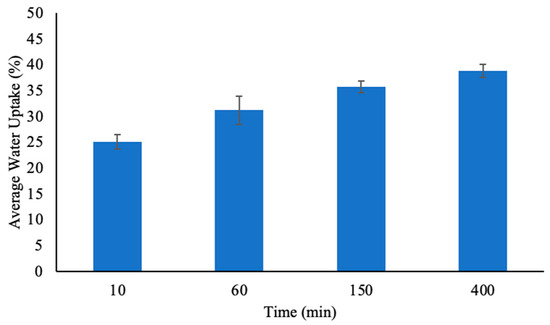
Figure 8.
The percent water uptake of pectin hydrogels (PH) placed in 20 mL of water at four different time points. The hydrogels were lyophilized prior to analysis. The error bars represent the standard deviation (n = 3).
As the contact time increased, the water uptake capacity continued to increase. By 60 min, the hydrogel absorbed 31.18 ± 2.71% of water, and at 150 min the adsorption further increased to 35.77 ± 1.13%. The relatively lower standard deviation at this stage suggests that the hydrogel structure reached a more stable swelling state, where additional water diffusion was slower but still ongoing [5]. At 400 min, PH achieved its highest recorded water uptake at 38.84 ± 1.24% which showed that the hydrogel approached its equilibrium swelling capacity. The reduced rate of water uptake at this point suggests that most of the pore volume and interaction sites within the hydrogel matrix were occupied [13].
The increase in water uptake over time aligned with the swelling behavior expected of PHs, which are highly hydrophilic due to the presence of hydroxyl and carboxylic acid functional groups that are capable of hydrogen bonding with water molecules [18]. Therefore, the more anionic groups and hydrophilic functional groups present, the easier it was for the hydrogels to swell and absorb water [31]. The data suggests that PH underwent an initial rapid swelling phase, followed by a gradual increase in water uptake until the water uptake reached equilibrium [13].
3.2. Batch Adsorption Tests for Copper and Nickel
Batch adsorption tests were conducted to determine the effect of contact time of Cu(II) and Ni(II) adsorption by PH, contact time of Cu(II) adsorption by PHM composite, and effect of initial pH of the solution for Cu(II) adsorption of PH.
3.2.1. Effect of Contact Time on Copper Adsorption by PH
The adsorption efficiency and capacity of PH for Cu(II) were assessed by measuring the percentage (%) removal of Cu(II) from the solution over time, as well as the hydrogel’s adsorption capacity (mg/g) over time (Figure 9). The results showed that PH was highly effective in removing Cu(II) from solution, with adsorption efficiencies exceeding 90% across all time points (Figure 9). This suggests that PH has a strong affinity for Cu(II) ions, likely due to the presence of functional groups such as carboxylic acid and hydroxyl groups, which facilitated electrostatic interactions and formed coordination complexes with the metal cations [31].
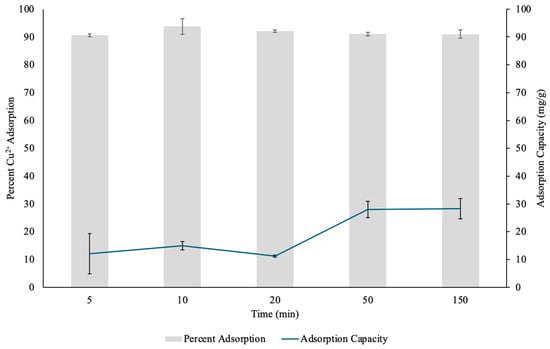
Figure 9.
The percent Copper2+ (Cu(II)) uptake and Cu(II) adsorption capacity (mg/g) determined through contact time test by pectin hydrogels (PHs) placed in 10 mL or 20 mL Cu(II) solutions over five different time points. The hydrogels were lyophilized prior to analysis. The error bars represent the standard deviation (n = 3).
At the 5 min mark, PH demonstrated rapid initial adsorption, removing 90.57% of Cu(II) from solution. This immediate uptake indicates that the hydrogel possessed a large number of available active sites on the surface of the hydrogel for metal ion binding at the start of the adsorption process. As Cu(II) diffused into the hydrogel, these sites were quickly occupied, leading to a rapid reduction in metal ion concentration in solution [31]. By 10 min, the adsorption efficiency increased to 93.72%, showing that the hydrogel continued to trap Cu(II) effectively, with further diffusion into the pectin chain network [31]. At 20 min, the percentage of Cu(II) removal slightly decreased to 92.11%, and between 50 and 150 min, the adsorption efficiency remained relatively stable at 90.94–90.99%. The small fluctuations in adsorption efficiency between 20 and 150 min suggest that PH had reached a near equilibrium state, where most of its available binding sites were occupied. The slight decrease in percentage adsorption at later time points may be attributed to minor desorption effects, competition between adsorbed Cu(II) ions, or electrostatic repulsion between Cu(II) ions which prevented additional metal ions from accessing the inner binding sites [27,31].
The adsorption capacity of PHs was calculated to determine the mass of Cu(II) absorbed per gram of hydrogel. The results showed an overall increasing trend in adsorption capacity over time, starting from 12.07 mg/g at 5 min and reaching a maximum of 28.35 mg/g at 150 min. This trend suggests that the hydrogel continued to uptake Cu(II) over time, though at a slower rate as adsorption sites became saturated [31]. At the early stages between 5 and 10 min, the hydrogel exhibited relatively lower adsorption capacities (12.07–15.01 mg/g), which corresponds to the rapid initial adsorption phase where binding sites on the surface of the hydrogel were predominantly occupied [31]. As time progressed from 20 to 150 min, the adsorption capacity increased (11.26–28.35 mg/g), which indicated that Cu(II) continued to diffuse into the hydrogel and reached the inner binding surfaces [31]. The highest adsorption capacity was recorded at 150 min, suggesting that PH retained its ability to trap Cu(II) even after prolonged exposure.
The variability in adsorption capacity across different time points may be influenced by several factors, including differences in hydrogel swelling due to different degrees of esterification of pectin, pore structure and the extent of Cu(II) diffusion into the hydrogel [31]. Pectin with a high number of carboxylic acid units offers higher adsorption capacity for Cu(II) compared to pectin with a lower number of carboxylic acid units [31]. As a result, low-esterified pectin has a larger surface area for metal binding and allows for more swelling and metal uptake [31]. Therefore, the higher adsorption capacity observed at later time points suggests that the PH was low-esterified and provided sufficient active sites for sustained metal ion retention.
The high Cu(II) removal efficiency and increasing adsorption capacity over time suggests that multiple adsorption mechanisms may be involved in PH’s interaction with metal cations. Electrostatic interactions between the negatively charged carboxylic acid groups in PH and the positively charged Cu(II) ions likely played a dominant role in the initial phase of adsorption [16,27]. Additionally, Cu(II) ions likely formed coordination complexes with hydroxyl and carboxylic acid groups of pectin within the hydrogel network, further enhancing metal cation retention [16,27]. The decrease in adsorption rate at later time points suggests that minor desorption effects and electrostatic repulsion between Cu(II) ions may play a role in restricting further Cu(II) uptake, given that the hydrogels are small [27,31]. As adsorption sites become saturated, the diffusion of metal ions into the inner layers of the hydrogel became slower, leading to an equilibrium state where adsorption and desorption of Cu(II) from solution was balanced.
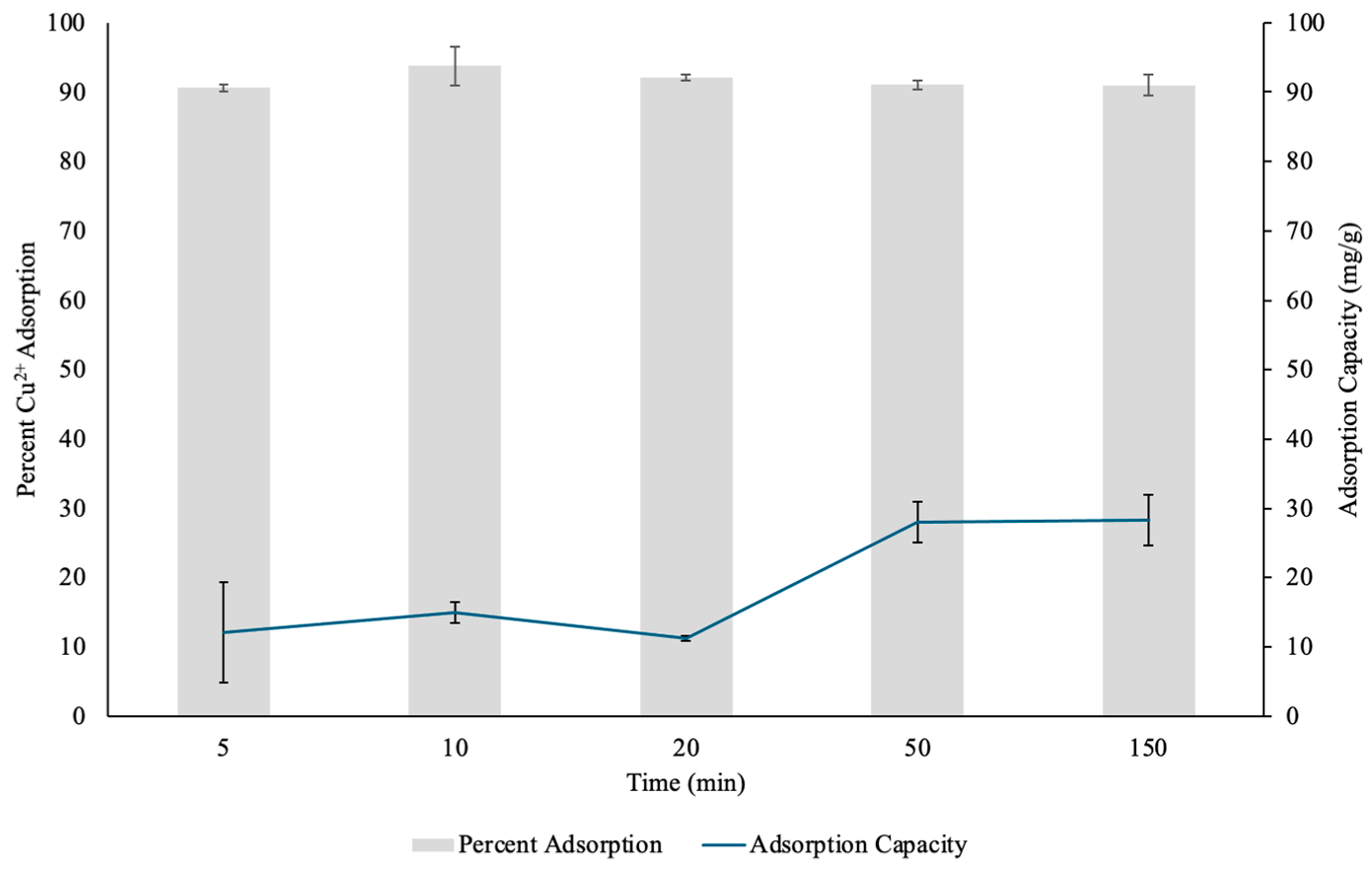
3.2.2. Effect of Contact Time on Copper Adsorption by PHM Composite
The adsorption performance of PHM composites for Cu(II) ions was similarly evaluated over a range of time points, and both the percent removal efficiency and adsorption capacity were determined (Figure 10). The data demonstrated a high initial uptake of Cu(II), with 89.88% removal within the first 5 min. The rapid uptake at the beginning suggests a strong affinity between the PHM composite and Cu(II), likely facilitated by abundant and readily available binding sites on the hydrogel surface [13]. The presence of the MOF component likely contributed to enhanced adsorption due to its high porosity and metal binding abilities, allowing for efficient Cu(II) coordination [13]. By 10 min, the adsorption efficiency increased to 92.02%, which indicated that additional Cu(II) ions continued to interact with available active sites, though the rate of adsorption appeared to slow beyond this point as active sites became more saturated.
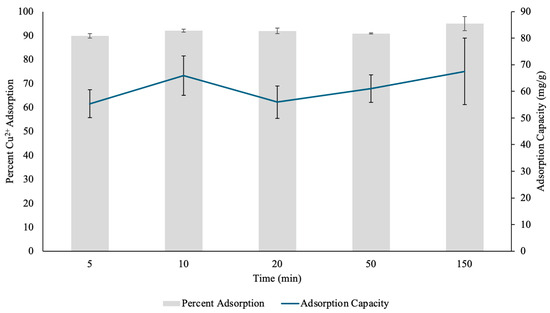
Figure 10.
The percent Copper2+ (Cu(II)) uptake and Cu(II) adsorption capacity (mg/g) determined through contact time test by pectin hydrogel–metal organic framework (PHM composites) placed in 10 mL Cu(II) solutions over five different time points. The hydrogels were lyophilized prior to analysis. The error bars represent the standard deviation (n = 3).
At 20 min, a slight reduction in percent adsorption (91.95%) compared to 10 min was observed. This minor fluctuation could be attributed to initial equilibrium adjustments, where some surface bound Cu(II) may have undergone desorption or diffusion into deeper layers of the hydrogel, similar to what was observed previously with the PHs [13]. Another possibility is the competition between ions for binding sites, which can momentarily affect the stability of adsorption before reaching equilibrium [31]. Despite this, the adsorption efficiency remained relatively stable, which further showed the hydrogel’s ability to retain Cu(II) over time. By 50 min, Cu(II) adsorption efficiency was 90.86%, suggesting that the system had reached near-saturation, with only minimal fluctuations in adsorption percentage. Interestingly, at 150 min, adsorption efficiency reached its highest level at 94.99%, which indicated continued diffusion of metal cations into the hydrogel matrix over extended exposure periods, and allowed for greater interaction between Cu(II) and the composite’s functional groups. This prolonged increase suggests that PHM composites provide a sustainable and long-lasting adsorption mechanism, which is crucial for practical wastewater treatment applications.
In terms of adsorption capacity, the data shows an initial increase over time. The highest adsorption capacity of 67.53 mg/g was observed at 150 min, while at earlier time points, the values fluctuated between 55.37 mg/g (5 min) and 65.87 mg/g (10 min). The small variations in adsorption capacity can be influenced by the Cu(II) diffusion, hydrogel swelling and initial binding site availability on the surface of the hydrogel [31]. The PHM composites, with their combination of pectin and MOF structures, showed high Cu(II) removal potential by balancing metal cation adsorption with high structural stability. The overall trend suggests that PHM composites are highly efficient in Cu(II) removal, reaching over 90% adsorption in these time conditions.
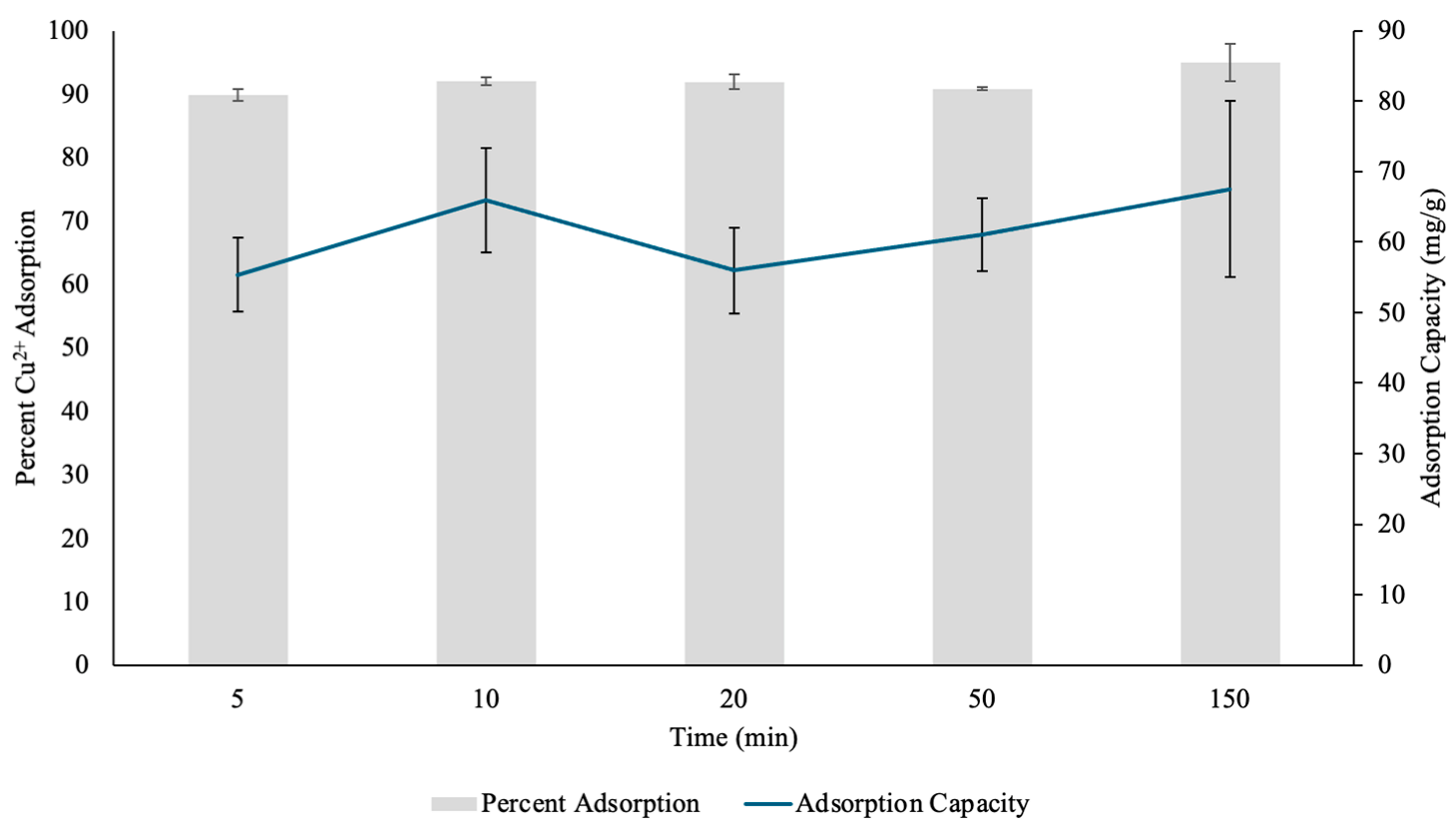
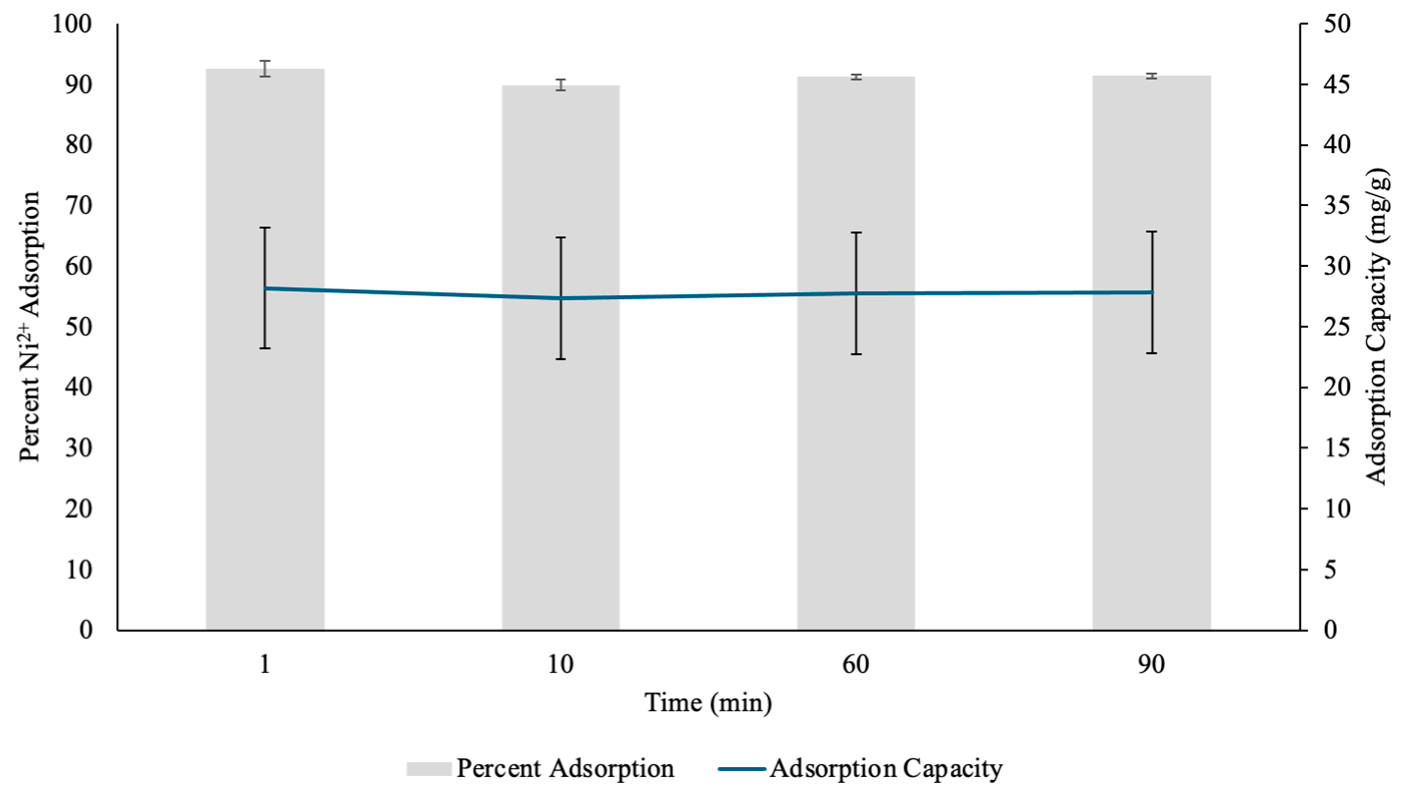
3.2.3. Effect of Contact Time on Nickel Adsorption by PH
The adsorption efficiency and capacity of PH for Ni(II) ions were analyzed over various time points, and the hydrogels showed significant metal removal performance (Figure 11). At 1 min, the hydrogel exhibited a 92.62% Ni(II) removal, suggesting a rapid initial adsorption phase on the surface of the hydrogels. This can be attributed to the abundance of active functional groups within the PH, such as carboxylic acid and hydroxyl groups, which can effectively coordinate with Ni(II) ions through electrostatic interactions and coordination complexes [31,33]. The corresponding adsorption capacity at this time point was 28.189 mg/g, reflecting the hydrogel’s ability to accommodate a high concentration of Ni(II) in a short duration. The rapid uptake showed that the majority of adsorption occurred at the hydrogel surface, where Ni(II) ions can readily interact with available binding sites [33].
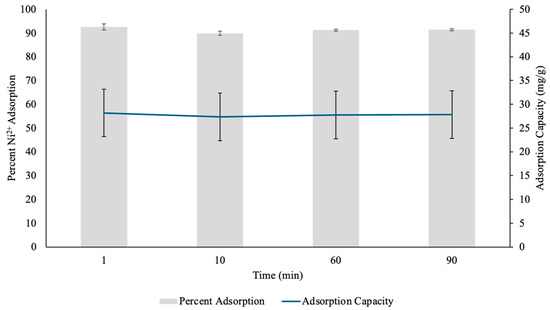
Figure 11.
The percent Nickel2+ (Ni(II)) uptake and Ni(II) adsorption capacity (mg/g) determined through contact time test by pectin hydrogels (PH) placed in 10 mL Ni(II) solutions over five different time points. The hydrogels were lyophilized prior to analysis. The error bars represent the standard deviation (n = 3).
After 10 min, the percent adsorption decreased slightly to 89.86%, while the adsorption capacity dropped to 27.349 mg/g. This decline, though minimal, suggests that as the initial surface binding sites became occupied, metal cation diffusion into the hydrogel’s internal structure became the dominant process [31,33]. The minor reduction in adsorption efficiency could be due to electrostatic repulsion between already adsorbed Ni(II) ions and incoming metal cations, limiting further adsorption on the outermost layer [34]. However, the hydrogel still demonstrated effective Ni(II) retention, suggesting that its porous structure allowed for continuous metal uptake over time.
At 60 min, the adsorption efficiency slightly increased to 91.22%, with an adsorption capacity of 27.765 mg/g. The increase indicated that the adsorption of Ni(II) was nearing equilibrium, with continuous diffusion of Ni(II) ions deeper into the layers of the hydrogel. The slight fluctuation in efficiency could be attributed to repulsion interactions between adsorbed and free metal cations, as well as gradual stabilization of the hydrogel’s binding sites [34]. The relatively stable adsorption performance at this stage suggests that the hydrogel’s internal matrix played a role in sustaining metal retention, allowing for consistent Ni(II) diffusion even after the initial surface adsorption phase.
By 90 min, the adsorption efficiency remained steady at 91.38%, with a slightly higher adsorption capacity of 27.812 mg/g. This suggests that while most of the available active sites had been occupied, slow diffusion into the deeper hydrogel layers continued, allowing for additional Ni(II) uptake. Additionally, this further showed that the adsorption of Ni(II) was nearing equilibrium. The fact that adsorption efficiency remained above 89% throughout the entire duration suggests that PH hydrogels possess a strong affinity for Ni(II) and can maintain their adsorption abilities over extended periods [13]. This stability is crucial in practical applications, as it indicates that the hydrogel can sustain high performance metal removal without rapid saturation or loss of efficiency.
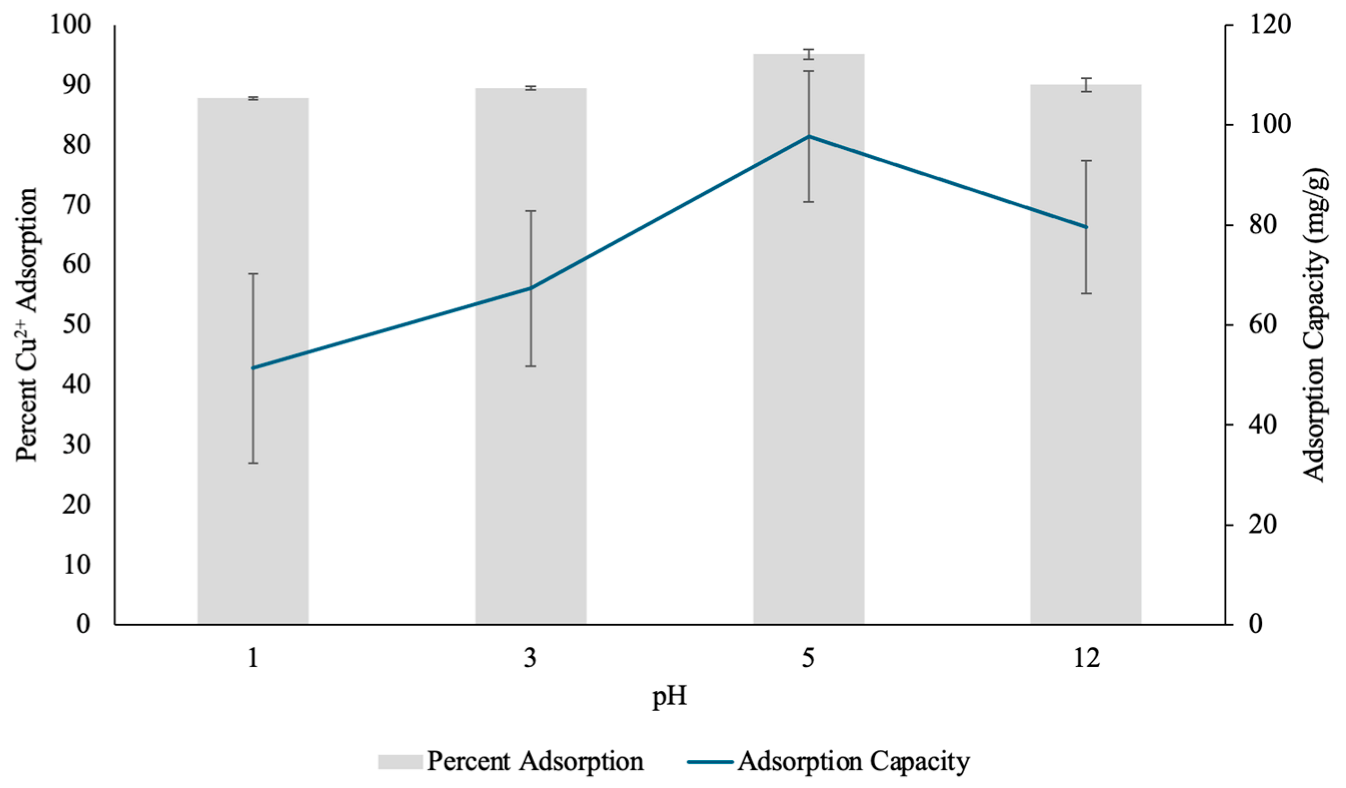
3.2.4. Effect of Initial pH on Copper Adsorption by PH
The pH of a solution plays a critical role in metal cation adsorption, as it dictates the solubility of the metal and the protonation states of the functional groups on the hydrogel [35]. To determine the optimal pH for Cu(II) removal, adsorption experiments were conducted at four different pH values (1, 3, 5 and 12), and the percentage removal efficiency and adsorption capacity of PHs were evaluated (Figure 12). The data indicated that Cu(II) adsorption is significantly influenced by pH, with a peak adsorption efficiency at pH 5.
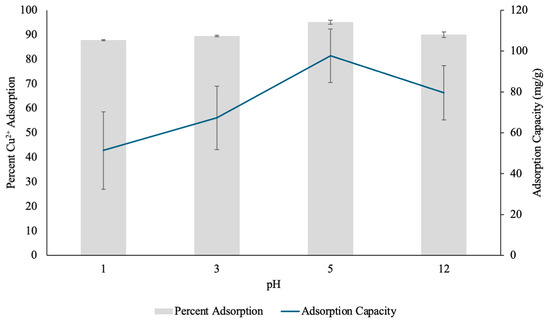
Figure 12.
The percent Copper2+ (Cu(II)) uptake and Cu(II) adsorption capacity (mg/g) determined through pH test by pectin hydrogels (PH) placed in 10 mL Cu(II) solutions over four different pH conditions. The hydrogels were lyophilized prior to analysis. The error bars represent the standard deviation (n = 3).
At pH 1, the Cu(II) removal efficiency was 87.82%, with an adsorption capacity of 51.36 mg/g. The relatively lower adsorption efficiency at this highly acidic pH can be attributed to the protonation of functional groups within the hydrogel matrix. The pectin molecules contain carboxylic acid (-COOH) and hydroxyl (-OH) functional groups, which play a key role in metal cation complexation [31]. However, at extremely low pH these groups become protonated to -COOH and -OH, respectively, because the pH of the solution is smaller than the acid dissociation constant (pKa) of D-galacturonic acid (pKaD-galacturonic acid = 3.5) [36]. Therefore, protonation of the active sites reduced their ability to coordinate with the positively charged Cu(II) ions [35]. Additionally, high concentrations of protons (H+) in the solution may adhere to the surface of the hydrogel through hydrogen bonding, and can repel the positively charged Cu(II), further limiting the hydrogel’s ability to adsorb the heavy metal [35].
At pH 3, the adsorption efficiency slightly increased to 89.55%, while the adsorption capacity rose to 67.35 mg/g. This suggests a reduction in competition between protons and Cu(II) ions for available binding sites on the surface of the hydrogel, and allowed for some metal uptake. However, at this pH, some degree of protonation still occurred because the pH was slightly lower than the pKa, which may have restricted the full complexation potential of the hydrogel [36].
The highest adsorption efficiency was observed at pH 5, with 95.11% Cu(II) removal and an adsorption capacity of 97.75 mg/g. This significant increase in adsorption performance suggests that at pH 5, the majority of the carboxylic acid and hydroxyl functional groups in the hydrogel existed in their deprotonated forms, -COO− and -O−, respectively [36]. Hence, the negatively charged adsorption sites enhanced electrostatic interactions and complexation with the free divalent Cu2+ ions at this pH and allowed for effective coordination with the hydrogel [36]. The higher adsorption capacity at this pH further confirmed that the hydrogel reaches its maximum binding efficiency when there is minimal competition for adsorption sites from protons, and optimal availability of negatively charged functional groups.
At pH 12, the adsorption efficiency slightly decreased to 90.05%, with a corresponding adsorption capacity of 79.55 mg/g. At a pH significantly higher than the pKa of D-galacturonic acid, the functional groups in the hydrogel would exist in their deprotonated forms [36]. While adsorption remained relatively high, the slight reduction suggests that at this alkaline pH, the Cu(II) ions may begin to hydrolyze and precipitate out of solution as copper hydroxide (Cu(OH)2), which reduced the amount of Cu(II) available in solution for adsorption [37]. As a result, the percent adsorption and adsorption capacity decreased. However, the hydrogel still maintained strong adsorption capabilities, indicating its structural resilience across a wide pH range.
Overall, the results showed that Cu(II) adsorption by PHs is highly pH-dependent, with the most efficient removal occurring at pH 5, where the hydrogel showed optimal functional group availability and coordination interactions. The findings by Mahmoud and Mohamed (2020) support these findings and further suggest that the initial pH of the solution is one of the most critical factors controlling heavy metal adsorption by hydrogels [13]. The findings in this study suggest that acidic conditions (pH 1–3) limit adsorption due to protonation of adsorption sites and insufficient interaction with Cu(II), while highly alkaline conditions (pH 12) may reduce adsorption due to metal precipitation.
4. Discussion
This study successfully demonstrated the synthesis, characterization and application of PHs and PHM composites derived from grapefruit peel for the removal of Cu(II) and Ni(II) from aqueous media. The results confirmed the high efficiency of these biosorbent materials and provided insights into their adsorption behaviors under varying conditions.
FT-IR analysis confirmed the retention of hydroxyl and carboxylic acid functional groups in the PHs, consistent with the expected structure of pectin following hydrogel formation. These groups are critical for metal ion coordination, as previously described, and their presence supports the mechanism of electrostatic attraction and complexation as the dominant adsorption pathway. SEM analysis further revealed notable morphological changes before and after Cu(II) adsorption. PHs displayed smoother surfaces post-adsorption, indicative of metal ion binding at active sites on the surface. In contrast, the PHM composites showed rougher, more compact structures, suggesting enhanced cross-linking and structural rigidity conferred by MOF incorporation.
The batch adsorption studies provided quantitative evidence of the hydrogels’ high metal binding capacity. PHs exhibited >90% removal efficiency for Cu(II) across all tested time points, with an optimal adsorption capacity of 97.75 mg/g at pH 5, which is supported by the deprotonation of carboxyl and hydroxyl groups. These findings are consistent with previous studies, which identified pH as a critical parameter influencing hydrogel adsorption efficiency [13,36]. PHM composites also demonstrated high Cu(II) removal (94.99%) with a maximum capacity of 67.53 mg/g.
Incorporation of the MOFs to the PHs supported the previous hypothesis that MOF integration enhanced the long-term adsorption potential, likely by increasing available surface area and introducing additional binding sites [38]. However, the nature of the PHM composites may have compromised the adsorption capacity of Cu(II) [38]. The PHM composites that were synthesized in this study for Cu(II) adsorption were used for the adsorption of chromium (Cr(IV)) and lead (Pb(II)) ions, as demonstrated by Mahmoud and Mohamed (2020) [13]. The PHM composites showed high adsorption capacity for Cr(IV) and Pb(II) at 825.97 mg/g and 913.88 mg/g, respectively [13]. The maximum capacity determined for Cu(II) in this study was 67.53 mg/g, which indicated the possibility that the PHM composite was not suitable for Cu(II) adsorption but may be more suitable for the adsorption of other heavy metals in water, such as Cr(IV) and Pb(II) [13]. Other MOF composite hydrogels should be explored for the adsorption of Cu(II) and Ni(II). For instance, Zhang et al. (2021) synthesized a zeolitic imidazolate framework-8 sodium alginate combined hydrogel material (PVA-Alg/ZIF-8) for the removal of Cu(II) from water [39]. The composite hydrogels demonstrated maximum adsorption capacity of 166.94 mg/g, which was higher than what was revealed in this study [39].
Remarkably, PHs demonstrated rapid and efficient adsorption of Ni(II) with 92.62% removal and an adsorption capacity of 28.189 mg/g achieved within one minute. This performance suggested a strong affinity between PHs and Ni(II) ions, potentially attributed to favorable interaction kinetics and high initial availability of binding sites. The sustained adsorption performance over 90 min demonstrated structural stability and diffusion-facilitated retention within the hydrogel matrix.
Comparison between the adsorption of Ni(II) and Cu(II) revealed that PHs were more efficient at removing Cu(II) from contaminated water than Ni(II). These results are supported by Sulianto et al. (2025), who further showed that Cu(II) adsorption performed better than Ni(II) under similar experimental conditions [40]. They showed that adsorption capacity was highly dependent on pH, contact time and initial ion concentration [40]. Both metal ions performed best at pH 5 for 90 min; however, Cu(II) may have performed better due to its physical properties [40,41]. For heavy metal adsorption, the process is influenced by a combination of coordination complexing and electrostatic interactions [41]. The negatively charged carboxyls facilitate the electrostatic attraction of metal cations to the hydrogel [41]. The electron-donating hydroxyl groups and dissociated carboxyl groups then serve as sites available for forming coordination bonds with metal cations [41]. While the amount and availability of the functional groups on the hydrogel influence the efficiency of coordinating with metal cations, the formation of the coordination complex, and therefore difference in adsorption behaviors, can be influenced by their physiochemical properties, such as ionic radius, molecular weight and hydration enthalpy [42,43].
Furthermore, cations with smaller radii and molecular weights, such as Ni(II) (0.69 Å), have higher hydration energies [43,44]. As a result, cations have a higher charge density and can attract more water molecules than larger cations, thus forming a large hydration shell [45]. The hydration shell acts as a barrier between the cation and the hydrogel, and some of the water molecules need to be shed in order for the cation to form a coordination complex with the active sites on the hydrogel [45]. Hence, a higher hydration energy makes it more difficult for Ni(II) to be adsorbed because it requires more energy to remove water molecules [45]. In contrast, cations with larger ionic radii and molecular weights, such as Cu(II) (0.73 Å), exhibit stronger coordination interactions with adsorbents because they interact less strongly with water and form weaker hydration shells [43,44]. Therefore, this supports the findings of this study which demonstrated higher adsorption capacities for Cu(II) than for Ni(II) when adsorption took place under the same conditions. Cu(II) was able to interact more strongly and exhibit stronger adsorption behavior because it was larger, had a smaller charge density and smaller hydration energy. Ni(II) was smaller, had a larger charge density and larger hydration energy, and as a result, adsorbed less strongly to the hydrogel.
Hydrogel morphology, particularly diameter and porosity, was shown to influence adsorption behavior. Lyophilization resulted in significant size reduction, from 4.58 mm (wet PHs) to 1.67 mm (dry PHs), with PHM composites displaying an even smaller average diameter (1.05 mm). These structural changes, combined with the observed differences in adsorption performance, suggest that hydrogel density and surface area are critical factors for optimizing metal uptake.
Sulianto et al. (2024) conducted a study where they demonstrated the use of pectin derived from orange and apple peels, and starch from banana peels in hydrogels for the adsorption of water and biodegradability [46]. They found that the extraction yield of pectin was 26% in citrus peels [46]. Furthermore, the FT-IR spectrum revealed similar functional groups present in the pectin hydrogel, namely the O-H stretching vibrations at 3300 cm−1, 2900 cm−1 of the C-H stretches and 1600–1800 cm−1 range of the C=O [46]. Additionally, the pectin hydrogels exhibited a positive relationship between time and swelling character; as contact time between hydrogel and water increased, the volume of the bead also increased [46]. The percent swelling ability of the pectin hydrogels was 40–45%, which was similar to what was observed in this study [46]. Interestingly, the combination of pectin and starch to create a fusion hydrogel performed better in terms of swelling ability, with the pectin–starch hydrogel swelling up to 300% [46]. This hydrogel needs to be further studied to understand its application in heavy metal adsorption.
In another study, Ribeiro et al. (2025) synthesized pectin-co-montmorillonite combined hydrogels as an alternative, environmentally friendly separation technique for toxic heavy metals from wastewater [47]. The morphology of the hydrogels showed porous structures, in which incorporation of more montmorillonite resulted in smaller pores due to increased cross-linking and intermolecular interactions [47]. Furthermore, Ribeiro et al. (2025) demonstrated that Cu(II) had a higher affinity for the hydrogel during the batch adsorption studies, in comparison to Ni(II) and other heavy metals which showed a lower affinity for the hydrogel [47]. A similar behavior was seen in this experiment as Cu(II) exhibited higher adsorption capacities than Ni(II).
The conventional pectin extraction methods require harsh conditions, including strong acids, high temperatures and longer heating periods which can result in degradation of the pectin molecules, equipment erosion, unsafe chemical environments and higher operating costs [48]. These drawbacks have driven the development of greener, non-conventional techniques including ultrasound-assisted, microwave-assisted, enzyme-assisted and sub-critical water extraction methods [49,50,51,52]. These methods are eco-friendly and offer significant advantages over the conventional method, including reduced energy usage, shorter extraction times and minimal solvent usage [49,50,51,52]. As a result, pectin may be extracted with improved quality and higher yields [49,50,51,52]. As sustainability becomes increasingly important, green extraction techniques present a more efficient and environmentally friendly alternative to traditional methods. The use of bio-waste-derived adsorbents, such as PHs synthesized from grapefruit peels, for the adsorption of heavy metals was shown to be beneficial in this study because of its low cost, efficiency, ability to function at different pHs and production of little to no chemical side products [53].
5. Conclusions
Collectively, these findings support the application of PHs and PHM composites as effective, sustainable biosorbents for heavy metal removal. To enhance the reproducibility and consistency of hydrogel size and shape, future experiments should consider using an automated syringe to produce uniformly sized hydrogels, which could ultimately lead to more precise adsorption measurements. A comparison study should be conducted to determine the difference in heavy metal adsorption between lyophilized and hydrated hydrogels, as this could provide further insight into which method is more feasible for real water samples. Additionally, thermodynamic, kinetic and isotherm studies should be conducted to provide deeper insights into the adsorption behavior of PHs for Cu(II) and Ni(II) adsorption, as the experimental conditions in this study focused more on time-dependent adsorption for one initial metal concentration. An additional experiment needs to be conducted to determine the optimal pH for Ni(II) adsorption by PH and PHM composites as the optimal pH was found only for Cu(II) in this study. Additionally, constraints in experimental design should be addressed to allow for continuous pH monitoring during the adsorption process. Moreover, future studies should investigate the reusability and biodegradability of PHs and PHM composites through multiple adsorption and desorption cycles to determine the adsorption efficiency when the hydrogels are reused. This would provide a better understanding of the long-term applicability of these hydrogels for heavy metal remediation. Furthermore, to improve the sustainability of hydrogel synthesis, future studies should explore the use of greener chemical substitutes, such as weaker acids and bases for pectin extraction, to reduce the environmental impact of using strong acids and bases, such as HCl and NaOH. Further modifications, such as integrating additional biopolymers or adjusting the hydrogel’s pore structure, may improve adsorption selectivity for different metal contaminants. Expanding this research to real wastewater samples and studying competitive adsorption when multiple metals are present would provide a more comprehensive understanding of hydrogels’ performance in practical settings. These studies could contribute to the development of sustainable, environmentally friendly biosorbents for water remediation applications.
Author Contributions
Conceptualization, N.T. and V.V.; investigation, V.V.; data curation, N.T. and V.V.; writing—original draft preparation, V.V.; writing—review and editing, N.T. and X.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mathew, B.B.; Jaishankar, M.; Biju, V.G.; Beeregowda, K.N. Role of Bioadsorbents in Reducing Toxic Metals. J. Toxicol. 2016, 2016, 4369604. [Google Scholar] [CrossRef]
- Ahmed, A.; Huo, A.; Ibrahim, M. Progressive Approaches in Ni(II) Contaminated Water Treatment: A Review of Adsorbent Strategies. Water Air Soil Pollut. 2024, 236, 5. [Google Scholar] [CrossRef]
- Giripunje, M.D.; Fulke, A.B.; Meshram, P.U. Remediation Techniques for Heavy-Metals Contamination in Lakes: A Mini-Review. CLEAN Soil Air Water 2015, 43, 1350–1354. [Google Scholar] [CrossRef]
- Awual, M.R. New Type Mesoporous Conjugate Material for Selective Optical Copper(II) Ions Monitoring & Removal from Polluted Waters. J. Chem. Eng. 2017, 307, 85–94. [Google Scholar] [CrossRef]
- Nie, J.; Feng, D.; Shang, J.; Nasen, B.; Jiang, T.; Liu, Y.; Hou, S. Green Composite Aerogel Based on Citrus Peel/Chitosan/Bentonite for Sustainable Removal Cu(II) from Water Matrices. Sci. Rep. 2023, 13, 15443. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, D. Safety Guidelines for Copper in Water. Am. J. Clin. Nutr. 1998, 67, 1098S–1102S. [Google Scholar] [CrossRef]
- Antić, K.; Onjia, A.; Vasiljević-Radović, D.; Veličković, Z.; Tomić, S.L. Removal of Nickel Ions from Aqueous Solutions by 2-Hydroxyethyl Acrylate/Itaconic Acid Hydrogels Optimized with Response Surface Methodology. Gels 2021, 7, 225. [Google Scholar] [CrossRef]
- Wołowicz, A.; Wawrzkiewicz, M. Screening of Ion Exchange Resins for Hazardous Ni(II) Removal from Aqueous Solutions: Kinetic and Equilibrium Batch Adsorption Method. Processes 2021, 9, 285. [Google Scholar] [CrossRef]
- Zambelli, B.; Uversky, V.N.; Ciurli, S. Nickel Impact on Human Health: An Intrinsic Disorder Perspective. Biochim. Biophys. Acta-Proteins Proteom. 2016, 1864, 1714–1731. [Google Scholar] [CrossRef]
- Darban, Z.; Shahabuddin, S.; Gaur, R.; Ahmad, I.; Sridewi, N. Hydrogel-Based Adsorbent Material for the Effective Removal of Heavy Metals from Wastewater: A Comprehensive Review. Gels 2022, 8, 263. [Google Scholar] [CrossRef]
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of Heavy Metal Ions from Wastewater: A Comprehensive and Critical Review. NPJ Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Hossain, M.S.; Hossain, M.M.; Khatun, M.K.; Hossain, K.R. Hydrogel-Based Superadsorbents for Efficient Removal of Heavy Metals in Industrial Wastewater Treatment and Environmental Conservation. EFM 2023, 2, 142–158. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Mohamed, A.K. Novel Derived Pectin Hydrogel from Mandarin Peel Based Metal-Organic Frameworks Composite for Enhanced Cr(VI) and Pb(II) Ions Removal. Int. J. Biol. Macromol. 2020, 164, 920–931. [Google Scholar] [CrossRef]
- Li, Z.-Q.; Yang, J.-C.; Sui, K.-W.; Yin, N. Facile Synthesis of Metal-Organic Framework MOF-808 for Arsenic Removal. Mater. Lett. 2015, 160, 412–414. [Google Scholar] [CrossRef]
- Bustamante-Torres, M.; Romero-Fierro, D.; Arcentales-Vera, B.; Palomino, K.; Magaña, H.; Bucio, E. Hydrogels Classification According to the Physical or Chemical Interactions and as Stimuli-Sensitive Materials. Gels 2021, 7, 182. [Google Scholar] [CrossRef]
- Said, N.S.; Olawuyi, I.F.; Lee, W.Y. Pectin Hydrogels: Gel-Forming Behaviors, Mechanisms, and Food Applications. Gels 2023, 9, 732. [Google Scholar] [CrossRef]
- Öztürk, T.; Özbek, H.N.; Koçak Yanık, D. Environmentally Friendly Approach to Pectin Extraction from Grapefruit Peel: Microwave-Assisted High-Pressure CO2/H2O. Foods 2024, 13, 476. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Y.; He, J.; Sun, C.; Lu, W.; Zhang, Y.; Fang, Y. Doubling Growth of Egg-Box Structure during Calcium-Mediated Molecular Assembly of Alginate. J. Colloid Interface Sci. 2023, 634, 747–756. [Google Scholar] [CrossRef]
- Tortorella, S.; Inzalaco, G.; Dapporto, F.; Maturi, M.; Sambri, L.; Vetri Buratti, V.; Chiariello, M.; Comes Franchini, M.; Locatelli, E. Biocompatible Pectin-Based Hybrid Hydrogels for Tissue Engineering Applications. New J. Chem. 2021, 45, 22386–22395. [Google Scholar] [CrossRef]
- Moldovan, M. Atomic Absorption Spectrometry—Flame. In Encyclopedia of Analytical Science, 3rd ed.; Worsfold, P., Poole, C., Townshend, A., Miró, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; p. 9780124095472000226. [Google Scholar] [CrossRef]
- Guerra, R. WATER ANALYSIS|Industrial Effluents. In Encyclopedia of Analytical Science, 2nd ed.; Worsfold, P., Townshend, A., Poole, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 289–299. [Google Scholar] [CrossRef]
- Bagherian, G.; Arab Chamjangali, M.; Shariati Evari, H.; Ashrafi, M. Determination of Copper(II) by Flame Atomic Absorption Spectrometry after Its Perconcentration by a Highly Selective and Environmentally Friendly Dispersive Liquid–Liquid Microextraction Technique. J. Anal. Sci. Technol. 2019, 10, 3. [Google Scholar] [CrossRef]
- Khorrami, A. Determination of Nickel in Natural Waters by FAAS after Sorption on Octadecyl Silica Membrane Disks Modified with a Recently Synthesized Schiff? Talanta 2004, 64, 13–17. [Google Scholar] [CrossRef]
- Mishra, R.K.; Majeed, A.B.A.; Banthia, A.K. Development and Characterization of Pectin/Gelatin Hydrogel Membranes for Wound Dressing. Int. J. Plast. Technol. 2011, 15, 82–95. [Google Scholar] [CrossRef]
- Güzel, M.; Akpınar, Ö. Valorisation of Fruit By-Products: Production Characterization of Pectins from Fruit Peels. FBP 2019, 115, 126–133. [Google Scholar] [CrossRef]
- Wang, W.; Ma, X.; Jiang, P.; Hu, L.; Zhi, Z.; Chen, J.; Ding, T.; Ye, X.; Liu, D. Characterization of Pectin from Grapefruit Peel: A Comparison of Ultrasound-Assisted and Conventional Heating Extractions. Food Hydrocoll. 2016, 61, 730–739. [Google Scholar] [CrossRef]
- Seida, Y.; Tokuyama, H. Hydrogel Adsorbents for the Removal of Hazardous Pollutants—Requirements and Available Functions as Adsorbent. Gels 2022, 8, 220. [Google Scholar] [CrossRef]
- Rumyanceva, O. Pectin Changes during Freezing and Storage of Plant Products. Food Process. Tech. Technol. 2024, 54, 495–507. [Google Scholar] [CrossRef]
- Marquis, M.; Davy, J.; Fang, A.; Renard, D. Microfluidics-Assisted Diffusion Self-Assembly: Toward the Control of the Shape and Size of Pectin Hydrogel Microparticles. Biomacromolecules 2014, 15, 1568–1578. [Google Scholar] [CrossRef]
- Ullrich, S.; Seyferth, S.; Lee, G. Measurement of Shrinkage and Cracking in Lyophilized Amorphous Cakes. Part IV: Effects of Freezing Protocol. Int. J. Pharm. 2015, 495, 52–57. [Google Scholar] [CrossRef]
- Shen, B.; Guo, Z.; Huang, B.; Zhang, G.; Fei, P.; Hu, S. Preparation of Hydrogels Based on Pectin with Different Esterification Degrees and Evaluation of Their Structure and Adsorption Properties. Int. J. Biol. Macromol. 2022, 202, 397–406. [Google Scholar] [CrossRef]
- Popov, S.; Paderin, N.; Chistiakova, E.; Ptashkin, D.; Markov, P.A. Effect of Cross-Linking Cations on In Vitro Biocompatibility of Apple Pectin Gel Beads. Int. J. Mol. Sci. 2022, 23, 14789. [Google Scholar] [CrossRef]
- Kulkarni, R.M.; Dhanyashree, J.K.; Varma, E.; Sirivibha, S.P. Batch and Continuous Packed Bed Column Studies on Biosorption of Nickel (II) by Sugarcane Bagasse. Results Chem. 2022, 4, 100328. [Google Scholar] [CrossRef]
- Hu, X.; Yan, L.; Wang, Y.; Xu, M. Smart and Functional Polyelectrolyte Complex Hydrogel Composed of Salecan and Chitosan Lactate as Superadsorbent for Decontamination of Nickel Ions. Int. J. Biol. Macromol. 2020, 165, 1852–1861. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Hu, L.; Hu, S.; Liu, Y. Optimized Synthesis of Novel Hydrogel for the Adsorption of Copper and Cobalt Ions in Wastewater. RSC Adv. 2019, 9, 16058–16068. [Google Scholar] [CrossRef] [PubMed]
- Opanasopit, P.; Apirakaramwong, A.; Ngawhirunpat, T.; Rojanarata, T.; Ruktanonchai, U. Development and Characterization of Pectinate Micro/Nanoparticles for Gene Delivery. AAPS Pharm. Sci. Tech. 2008, 9, 67–74. [Google Scholar] [CrossRef]
- Lin, Y.E.; Vidic, R.D.; Stout, J.E.; Yu, V.L. Negative Effect of High pH on Biocidal Efficacy of Copper and Silver Ions in Controlling Legionella Pneumophila. Appl. Environ. Microbiol. 2002, 68, 2711–2715. [Google Scholar] [CrossRef]
- Zhang, L.; Li, R.; Zheng, S.; Zhu, H.; Cao, M.; Li, M.; Hu, Y.; Long, L.; Feng, H.; Tang, C.Y. Hydrogel-Embedded Vertically Aligned Metal-Organic Framework Nanosheet Membrane for Efficient Water Harvesting. Nat. Commun. 2024, 15, 9738. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Zhang, T.; Chen, J.; Ji, W.; Wei, Y. Fabrication of an Efficient ZIF-8 Alginate Composite Hydrogel Material and Its Application to Enhanced Copper(II) Adsorption from Aqueous Solutions. New J. Chem. 2021, 45, 15876–15886. [Google Scholar] [CrossRef]
- Sulianto, A.A.; Nugroho, W.A.; Wibisono, Y.; Drannikov, A.A.; Ozaltin, K.; Safitri, A.; Azzahrah, A.R.P.; Bakshia, M.I.; Fatriasari, W.; Martino, A.D. Pectin-based Hydrogels Produced from Banana and Mango Peels as a Potential Approach to Removing Heavy Metal Ions from Contaminated Water. Appl. Sci. Eng. Prog. 2025, 18, 7717. [Google Scholar] [CrossRef]
- Li, J.; Yang, Z.; Ding, T.; Song, Y.; Li, H.; Li, D.; Chen, S.; Xu, F. The role of surface functional groups of pectin and pectin-based materials on the adsorption of heavy metal ions and dyes. Carbohydr. Polym. 2022, 276, 118789. [Google Scholar] [CrossRef]
- Lou, H.; Cao, X.; Yan, X.; Wang, L.; Chen, Z. Adsorption performance of Cd (II), Cr (III), Cu (II), Ni (II), Pb (II) and Zn (II) by aminated solution-blown polyacrylonitrile micro/nanofibers. Water Sci. Technol. 2018, 2017, 378–389. [Google Scholar] [CrossRef]
- Bohli, T.; Ouederni, A.; Villaescusa, I. Simultaneous adsorption behavior of heavy metals onto microporous olive stones activated carbon: Analysis of metal interactions. Euro-Mediterr. J. Environ. Integr. 2017, 2, 19. [Google Scholar] [CrossRef]
- Kalantari, K.; Ahmad, M.B.; Masoumi, H.R.F.; Shameli, K.; Basri, M.; Khandanlou, R. Rapid adsorption of heavy metals by Fe3O4/talc nanocomposite and optimization study using response surface methodology. Int. J. Mol. Sci. 2014, 15, 12913–12927. [Google Scholar] [CrossRef]
- Tansel, B. Significance of thermodynamic and physical characteristics on permeation of ions during membrane separation: Hydrated radius, hydration free energy and viscous effects. Sep. Purif. Technol. 2012, 86, 119–126. [Google Scholar] [CrossRef]
- Sulianto, A.A.; Adiyaksa, I.P.; Wibisono, Y.; Khan, E.; Ivanov, A.; Drannikov, A.; Ozaltin, K.; Di Martino, A. From Fruit Waste to Hydrogels for Agricultural Applications. Clean Technol. 2023, 6, 1–17. [Google Scholar] [CrossRef]
- Ribeiro, J.G.; Raimondi, R.M.; de Souza, A.G.; dos Santos Rosa, D.; Paulino, A.T. An Ecofriendly Pectin-Co-Montmorillonite Composite Hydrogel for the Separation of Contaminant Metal Ions from Water. J. Water Process Eng. 2025, 69, 106629. [Google Scholar] [CrossRef]
- Haque, S.M.; Kabir, A.; Ratemi, E.; Elzagheid, M.; Appu, S.P.; Ghani, S.S.; Sarief, A. Greener Pectin Extraction Techniques: Applications and Challenges. Separations 2025, 12, 65. [Google Scholar] [CrossRef]
- Costa, J.M.; Wang, W.; Nakasu, P.Y.S.; Hu, C.; Forster-Carneiro, T.; Hallett, J.P. Impacts of Microwaves on the Pectin Extraction from Apple Pomace: Technological Properties in Structuring of Hydrogels. Food Hydrocoll. 2025, 160, 110766. [Google Scholar] [CrossRef]
- Anoraga, S.B.; Shamsudin, R.; Hamzah, M.H.; Sharif, S.; Saputro, A.D.; Basri, M.S.M. Optimization of Subcritical Water Extraction for Pectin Extraction from Cocoa Pod Husks Using the Response Surface Methodology. Food Chem. 2024, 459, 140355. [Google Scholar] [CrossRef]
- Milošević, M.M.; Antov, M.G. Pectin from Butternut Squash (Cucurbita Moschata)–The Effect of Enzyme-Assisted Extractions on Fiber Characteristics and Properties. Food Hydrocoll. 2022, 123, 107201. [Google Scholar] [CrossRef]
- Singla, M.; Sit, N. Application of Ultrasound in Combination with Other Technologies in Food Processing: A Review. Ultrason. Sonochemistry 2021, 73, 105506. [Google Scholar] [CrossRef]
- Tariq, W.; Arslan, C.; Tayyab, N.; Rashid, H.; Nasir, A. Application of Agro-Based Adsorbent for Removal of Heavy Metals. In Emerging Techniques for Treatment of Toxic Metals from Wastewater; Ahmad, A., Kumar, R., Jawaid, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 157–182. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).