Abstract
(1) Virtual reality (VR) technologies have shown significant potential for diagnosing and treating vision-related impairments. This rapid review evaluates and characterizes the existing literature on VR technologies for diagnosing and treating vision-based diseases. (2) Methods: A systematic search was conducted across Ovid MEDLINE, Ovid Embase, the Cochrane Database of Systematic Reviews (Ovid), and the Cochrane Central Register of Controlled Trials (Ovid). Abstracts were screened using Rayyan QCRI, followed by full-text screening and data extraction. Eligible studies were published in peer-reviewed journals, written in English, focused on human participants, used immersive and portable VR devices as the primary intervention, and reported on the clinical effectiveness of VR for therapeutic, diagnostic, or screening purposes for vision or auditory–visual impairments. Various study characteristics, including design and participant details, were extracted, and the MMAT assessment tool was used to evaluate study quality. (3) Results: Seventy-six studies met the inclusion criteria. Among these, sixty-four (84.2%) were non-randomized studies exploring VR’s effectiveness, while twenty-two (15.8%) were randomized-controlled trials. Of the included studies, 38.2% focused on diagnosing, 21.0% on screening, and 38.2% on treating vision impairments. Glaucoma and amblyopia were the most commonly studied visual impairments. (4) Conclusions: The use of standalone, remotely controlled VR headsets for screening and diagnosing visual diseases represents a promising advancement in ophthalmology. With ongoing technological developments, VR has the potential to revolutionize eye care by improving accessibility, efficiency, and personalization. Continued research and innovation in VR applications for vision care are expected to further enhance patient outcomes.
1. Introduction
1.1. Background
Virtual Reality (VR) technologies have emerged as increasingly valuable tools in healthcare, offering innovative applications in fields such as ophthalmology and multisensory processing [1,2,3,4]. The ability of VR to engage both visual and auditory senses, which are crucial for spatial awareness and cognitive integration, provides a unique advantage in these contexts.
VR refers to an immersive technology that simulates a computer-generated environment, allowing users to perceive themselves as active participants within this synthetic realm [5]. This immersive experience can be facilitated through a variety of display systems, including two-dimensional screens, stereoscopic 3D glasses, or more advanced head-mounted displays (HMDs), each offering varying levels of interaction and immersion [6,7].
HMDs are sophisticated VR devices that consist of a helmet or headgear fitted with screens that project images to each eye. Integrated position-tracking systems within these devices monitor the user’s gaze, enabling the real-time updating of visual content based on the viewer’s eyes/head movements (foveated rendering). This results in a seamless and interactive virtual experience, which is often described as highly immersive [8]. Over the past decade, the development of more affordable and accessible HMDs has significantly expanded their use [9,10], transforming them into essential tools in both research and clinical applications [11,12]. As a consequence, HMD-based VR has gained traction as a pivotal technology in the healthcare sector, including ophthalmology, where it supports therapeutic and diagnostic innovations [13].
Beyond the overarching advantages of telehealth that VR offers, including increased accessibility to healthcare, improved efficiency for both healthcare providers and patients, and reduced costs, there are also substantial benefits specific to the immersive nature of this technology. VR’s unique capacity to fully immerse users in an interactive virtual environment lends it ecological validity for a wide range of clinical applications. Immersive VR, when combined with advanced technologies such as inertial measurement units (IMUs) and eye-tracking cameras, provides valuable insights into physiological measures such as eye movements (e.g., saccades, pursuit, and fixation) and gaze patterns, making it an indispensable tool across multiple clinical domains [14,15].
Additionally, VR systems can incorporate sensors such as pressure and motion detectors, as well as electrodes that measure electrical fields, skin conductance, or temperature. These integrations enable healthcare providers to monitor patient states and track progress in real-time efficiently. For example, in the field of physical therapy, VR has been utilized to gamify rehabilitation exercises, thereby enhancing patient engagement, and promoting active participation in their therapeutic processes. This approach not only increases patient adherence but also facilitates the objective measurement of performance parameters, such as range of motion or motor coordination [16].
In psychology and mental health, VR has found significant clinical applications, including the treatment of pain, stress, and anxiety. By immersing patients in virtual environments designed to distract or calm them, VR can effectively alleviate psychological symptoms by shifting focus away from real-world stressors [17]. On the other hand, VR also serves as a powerful tool in exposure therapy, allowing patients to confront and process phobias or traumatic memories in a controlled and safe virtual setting. This method has been shown to increase the patient’s anxiety threshold and reduce sensitivities, offering an alternative treatment option when traditional exposure techniques are hindered by logistical challenges or patient reluctance [18,19].
Like many other areas of healthcare, ophthalmology faces significant challenges. Access to eye care, including visits to ophthalmologists and vision screenings, is a pressing public health concern, particularly for individuals in remote or underserved areas. A considerable number of visual impairments go undiagnosed each year due to insufficient screenings and underdiagnosis [20]. Socioeconomic disparities remain a major barrier to accessing eye care, as individuals from lower socioeconomic backgrounds and those without private insurance are less likely to engage in routine eye screenings [21,22]. In rural and remote communities, access to specialized ophthalmological services is particularly limited, even though teleophthalmology has alleviated some barriers by enabling remote consultations and screenings. However, physical access to specialized care continues to be a significant challenge in these regions. Additionally, immigrants and minority populations often encounter cultural and linguistic obstacles that further hinder their access to eye care services, highlighting the need for culturally sensitive health communication strategies to improve participation in vision screening programs [23].
Moreover, traditional in-office diagnostic procedures, such as standard automated perimetry, can be both costly and uncomfortable for patients, which may deter individuals from seeking necessary care or following through with recommended screenings. Studies have shown that automated perimetry, while essential for diagnosing and monitoring conditions such as glaucoma, can present financial burdens [24,25], and the testing procedure itself can be inconvenient for a significant proportion of patients [26]. These factors, including cost and perceived discomfort, have been identified as barriers to glaucoma care and follow-up, potentially leading to delays in diagnosis or treatment [27,28]. Another key limitation of existing ophthalmic tests is their lack of specificity and accuracy. Many traditional tests focus solely on one eye at a time, potentially missing discrepancies between the eyes [29] or failing to capture comprehensive visual function. Furthermore, existing tests typically do not integrate auditory stimuli, which can be crucial for assessing multisensory integration and spatial awareness [30]. These limitations highlight the need for more precise and holistic diagnostic tools in ophthalmology.
VR tools have emerged as promising technologies for the future of vision care, demonstrating significant utility in the screening, diagnosis, and treatment of various eye diseases.
1.2. Screening in Vision Care
In the context of vision screening, VR offers several advantages over traditional methods, particularly by providing an immersive environment that enhances patient engagement and reduces the impact of external distractions. VR-based screening tools are gaining attention as a potential solution for improving accessibility to vision tests, especially in underserved or remote areas. These systems can facilitate comprehensive screenings by integrating various visual stimuli, including those for visual field testing, contrast sensitivity, and peripheral vision assessments. The ability of VR to offer a more controlled and standardized testing environment, with precise control over light conditions and visual distractions, is a significant advantage over traditional methods [31,32]. Moreover, VR-based tools can provide immediate feedback to patients and clinicians, allowing for real-time adjustments and more personalized screening experiences [33]. Research has shown that VR can provide reliable results comparable to traditional vision screening techniques such as the Humphrey Field Analyzer and Octopus 900, making it a valuable tool for early detection of conditions such as glaucoma and other retinal diseases [34,35].
1.3. VR for Diagnosis
Building on its potential for screening, VR has also shown considerable promise in diagnosis as well. The immersive nature of VR headsets enables precise simulations of visual field deficits, such as those associated with glaucoma, and can be used to track the progression of visual impairments over time. Studies have demonstrated that VR-derived visual field measurements are highly correlated with those obtained using conventional diagnostic tools, such as the Octopus 900 [35], suggesting that VR could serve as an effective and cost-efficient diagnostic alternative [35,36].
1.4. VR for Treatment
In addition to its diagnostic applications, VR has also been explored as a treatment modality for various visual impairments. A systematic review of VR-based interventions for post-stroke unilateral spatial neglect reported generally positive outcomes across 10 studies, though it noted variability in methods and study quality [37]. A case report involving two patients with hemianopia following brain tumor treatment found that a home-based VR program improved visual function, including contrast sensitivity and visual field expansion [38]. A pilot study of game-based immersive VR for anisometropic amblyopia in children showed improvements in visual acuity, contrast sensitivity, and stereopsis [39].
By creating customized virtual environments for vision training, VR can help individuals improve visual acuity, depth perception, and eye-hand coordination. For instance, dichoptic VR training has been shown to significantly improve visual acuity and stereo acuity in patients with anisometropic amblyopia [40]. These findings suggest that VR has potential not only as a diagnostic tool but also as a versatile platform for personalized vision therapy and rehabilitation.
Despite a growing body of research highlighting the potential of VR in ophthalmology, to our knowledge, no comprehensive review has yet synthesized the full scope of its applications across screening, diagnostic, and therapeutic domains, particularly in relation to the diverse research designs that have explored these areas.
1.5. Objectives
This rapid review aims to synthesize the current state of peer-reviewed research on VR-based applications in vision care. Specifically, we address the following key aspects:
(A) Application Types—A comparison of VR applications used for screening, diagnosis, and intervention, with a focus on their targeted outcomes, reported validity and/or effectiveness, and the administration protocols (e.g., who administers the interventions, in what settings, duration/frequency of sessions, and integration with existing ophthalmological tools).
(B) Technical Properties—An examination of the technical specifications of the VR systems employed, including hardware (e.g., devices used) and software/content (e.g., the virtual environments or tasks utilized).
(C) Study Characteristics—An evaluation of study characteristics, including sample size, participant conditions, and the risk of bias, to assess the quality and generalizability of the findings.
2. Materials and Methods
This review was conducted according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. The review protocol was registered in PROSPERO (International Prospective Register for Systematic Reviews) with the registration number CRD42023456214.
2.1. Search Strategy
A comprehensive search strategy was developed using a combination of database-specific subject headings and text words for the main concepts of VR, select eye diseases, and auditory phenomenon. Results were limited to humans and English. Conference materials were excluded from Embase.
We searched the following databases on 14 August 2023, and 16 August 2024: Ovid MEDLINE, Ovid Embase, the Cochrane Database of Systematic Reviews (Ovid), and the Cochrane Central Register of Controlled Trials (Ovid). Full search strategies are reported in Supplementary File S1.
2.2. Study Selection
All relevant articles were uploaded into Rayyan, an online screening tool developed by Qatar Computing Research Institute (Doha, Qatar)I. To filter articles that focused on VR and visual and auditory outcomes, a dual filtration system was used to isolate articles that included (1) ‘virtual reality’ or ‘VR’ and (2) ‘vision’, ‘visual’, ‘audition’, ‘audiology’ in the abstract or title. Duplications were detected and resolved on Rayyan; if the similarity percentage was less than 98%, additional reference screening was conducted to ensure the articles were duplicates. Secondary literature was excluded using the keywords ‘meta-analysis’, ‘review’, and ‘systematic review’.
2.3. Inclusion and Exclusion Criteria
All primary research studies published in English and peer-reviewed journals up until the search date were included. There were no demographic restrictions. Studies were included if they included immersive and portable VR devices, namely head-mounted displays (HMDs), as the primary intervention. Our criteria for immersiveness are based on factors including display resolution, field of view, movement degrees of freedom, number of senses stimulated (hearing, vision, touch, and proprioception), the ability to track and update user inputs, and the ability to isolate the user from stimuli in the real world. Studies reporting outcomes that demonstrate the clinical effectiveness of VR as a therapeutic/diagnostic/screening tool for visual cognition or vision impairments with audition were included.
Studies were excluded if they were (1) conducted with animals; (2) exclusively focused on non-immersive and not portable VR devices, such as 3D glasses and cave-automatic virtual environments (CAVEs); (3) had missing data within the study or outcomes not pertaining to our clinical area of interest (i.e., vision or vision and audition); (4) secondary literature, such as reviews, meta-analyses, conference abstracts, commentaries, editorials, and full texts with no original data; or (5) full texts not accessible through the institutional library systems used within the process, including the ones provided by University Health Network, York University, McMaster University, and the University of Toronto.
The screening of the title and abstract first involved two reviewers independently screening 20 abstracts to assess inter-rater reliability. Discrepancies were resolved by consensus, and the inclusion criteria were further clarified to include studies pertaining to visual cognition or perception. The remaining abstracts were then divided in half and screened separately. Each reviewer conducted randomized cross-checks of each other’s articles to ensure agreement and reliability of screening.
2.4. Data Extraction (Coding and Categorizing Studies)
A comprehensive, full-text evaluation was performed independently by two reviewers. Qualitative and quantitative data from each article were coded and categorized into the following aspects: (1) research type and theme; (2) purpose of intervention; (3) study demographics; (4) novelty and attributes of the VR tool. Additional information, including the full coding spreadsheet, is provided in Supplementary File S2.
The conceptualization of terms was discussed during the early stages of coding and was further cross-checked by both reviewers to minimize subjective differences. For example, therapeutic interventions are defined as tools that are used to improve an existing condition, screening interventions as tools to detect whether participants have a certain condition, and diagnostic interventions as tools to confirm the condition. Supplementary File S3 provides the full definition of concepts used for the full-text screening of articles. After confirming agreement on conceptual definitions, the reviewers reverse-coded each other’s articles.
2.5. Quality Assessment
Two reviewers independently assessed the quality of each eligible study using the Mixed Methods Appraisal Tool (MMAT, Version 2018). The MMAT was chosen as it allows the critical appraisal of the methodological quality of five major research designs: qualitative research, randomized controlled trials, non-randomized studies, quantitative descriptive studies, and mixed methods studies. Each study was analyzed manually using the MMAT checklist. After selecting the most appropriate study category to appraise, responses were noted as ‘yes’, ‘no’, or ‘unclear’ according to the respective methodological criteria.
To further inform the analysis, studies were coded according to the Virtual Reality Clinical Outcomes Research Experts (VR-CORE) model as VR1, VR2, or VR3 studies [41]. VR1 studies focused on content development, VR2 studies focused on the early testing of feasibility and initial clinical efficacy, and VR3 studies were RCTs comparing clinical outcomes between intervention and control groups.
2.6. Analysis
Following data extraction, each included study was categorized according to multiple attributes: research type (e.g., randomized control trials, pilot trials, case reports, etc.), intervention purpose (screening, diagnostics, rehabilitation), clinical subspecialty/medical conditions, user population sample size and demographic, and head-mounted display device brand/model (if available).
We then conducted a descriptive analysis, calculating the frequency and proportion of studies within each subcategory (e.g., % of interventions used for rehabilitation, % classified as VR2). These proportions were determined by dividing the number of studies in each category by the total number of included studies.
Patterns across categories were qualitatively synthesized to identify trends, gaps, and opportunities in the current VR landscape for vision care.
3. Results
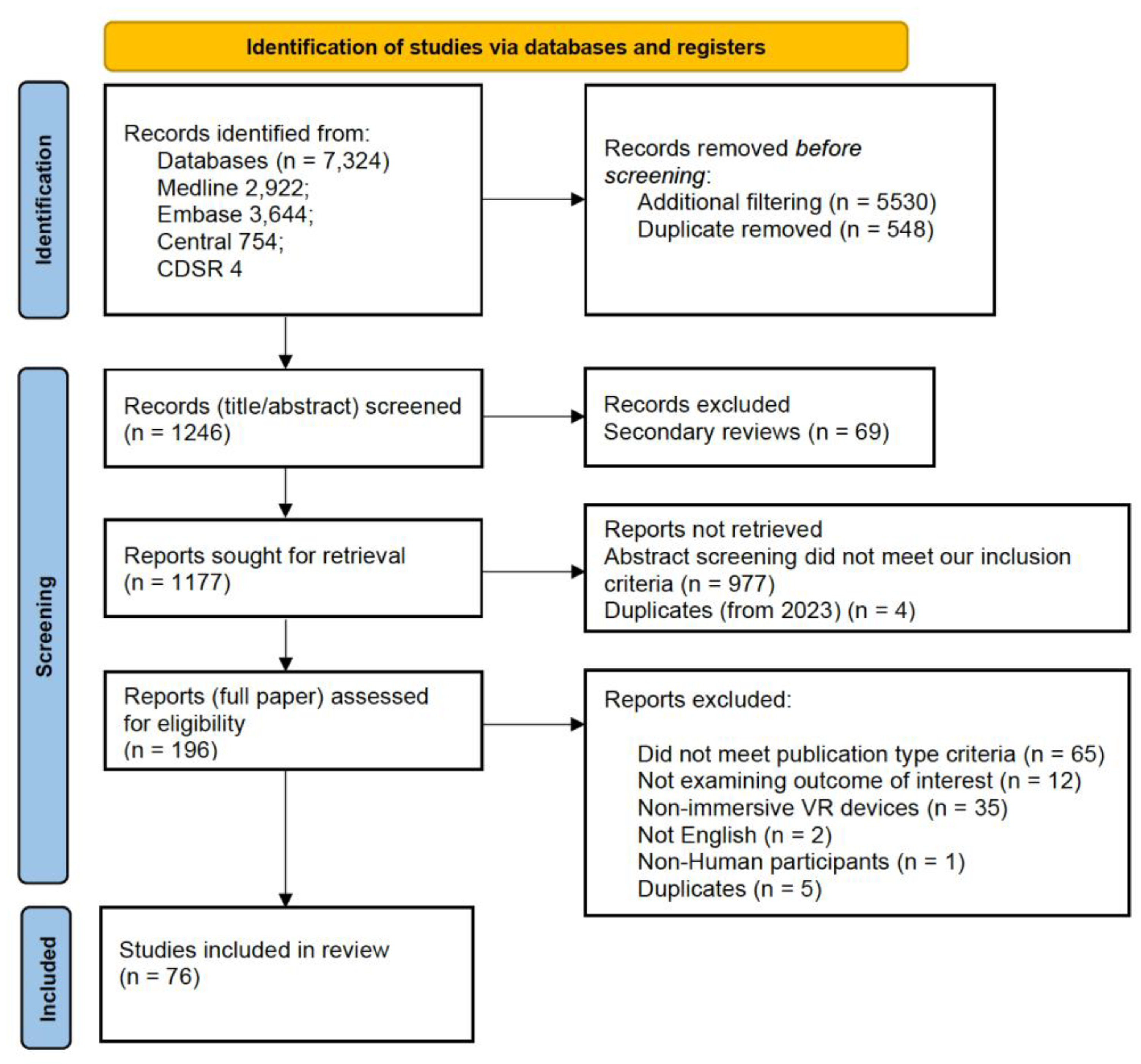
As outlined in the PRISMA diagram (Figure 1), 7324 citations were initially collected from the specified databases and processed using Rayyan QCRI. After duplicate removal and further filtration, 1246 citations proceeded to the first screening stage, where secondary reviews were excluded. Following the exclusion of 69 review articles, 1177 articles were screened at the abstract stage, resulting in the exclusion of 977 articles. A total of 196 articles underwent full-text screening, with 120 being excluded due to failure to meet one or more eligibility criteria. Ultimately, 76 publications were included for analysis (see Supplementary File S4).
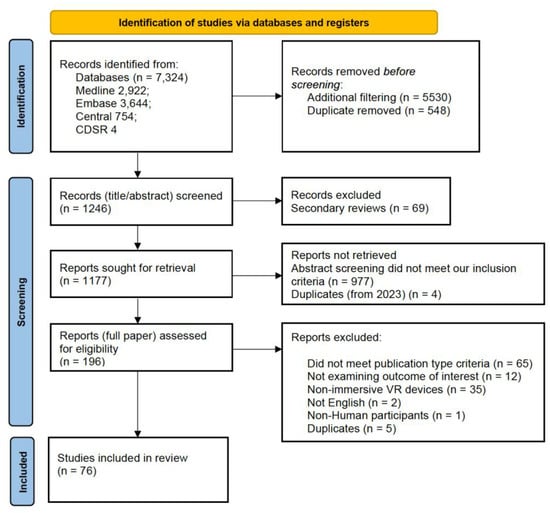
Figure 1.
PRISMA Diagram depicting the filtration process from initial retrieval to final inclusion in the qualitative synthesis.
The studies were categorized according to the VR-CORE model. Sixty-four studies (84.2%) were classified as VR2, which include non-randomized research exploring the effectiveness of VR interventions in vision care. In contrast, twelve studies (15.8%) were classified as VR3, representing randomized-controlled trials (RCTs). Table 1 provides a breakdown of the study characteristics, highlighting the distribution of research designs and interventions across the 76 publications.

Table 1.
Overview of study characteristics.
Additionally, the nature of VR interventions was analyzed to determine whether they utilized novel or existing methodologies. Of the studies reviewed, 62 (81.6%) introduced novel approaches, while 14 (18.4%) incorporated existing methodologies into VR systems. For example, the study by Chen et al. [42] employed a novel software (LUXIE) for visual field testing integrated into VR headsets, while Kim et al. [43] implemented the established King-Devick Test Chart within a VR setup.
Of the included studies, 5 (6.6%) mentioned specific regulatory compliance (e.g., FDA approval) for the HMD or associated software/devices used in their experiment. The remaining 71 (93.4%) studies did not. We further screened for references of data privacy regulations or considerations related to telehealth applications. While 10 (13.2%) studies addressed relevant data privacy matters, the remaining 66 (86.8%) did not. Regarding user experience design, 20 (26.3%) studies reported stakeholder engagement in the design or evaluation of the intervention, with usability surveys and qualitative feedback being the most prominent methods used to identify areas for improvement.
Table 2 offers an overview of participant demographics, including sample sizes and distributions across various conditions. A total of 11 medical conditions were classified as “other,” encompassing general visual impairments, retinitis pigmentosa, low vision, inherited retinal degeneration, congenital visual impairments, and ptosis. Notably, some studies simulated the effects of eye diseases on the field of vision in healthy participants. For instance, Neugebauer et al. [44] simulated retinitis pigmentosa, and Massiceti et al. [45] simulated low vision and blindness. These methodologies carry limitations, as healthy participants do not experience real-world visual field loss or develop compensatory strategies naturally.

Table 2.
Overview of study Participant characteristics.
Table 3 presents a summary of the head-mounted display (HMD) models used in the 76 studies and their publicly available technical specifications. The analysis revealed a diverse range of HMD devices, with popular models including HTC VIVE Pro Eye and Oculus Go.

Table 3.
Publicly available technical specifications and usage of HMD models across included studies. Field of view is indicated as diagonal (d), horizontal (h), and/or vertical (v). Other parameters include HMD type, display resolution per eye, refresh rate, degrees of freedom, and eye-tracking capability. Certain specifications are smartphone-based (SB), as outlined.
Of the interventions reviewed, 17 (22.4%) incorporated eye-tracking, 58 (76.3%) did not, and 1 study (1.3%) did not report this information clearly.
4. Discussion
The advancement of VR technology has opened new possibilities in ophthalmology, particularly in the screening and diagnosis of eye and visual diseases. However, as demonstrated in our study, the application of VR in vision care is still in its early stages and comes with challenges.
One of the primary obstacles in integrating VR headsets into ophthalmological practices, as suggested by our study, is the need for standardization and validation. The results from our review reveal a notable variation in study designs and sample sizes, which further complicates the process of establishing consistent and reliable VR-based diagnostic tools. Specifically, 64 studies (84.2%) were classified as VR2, representing non-randomized research that explored the effectiveness of VR interventions, while only 12 studies (15.8%) were randomized controlled trials (VR3). This disparity highlights the need for more rigorous, randomized clinical trials to establish the efficacy and reliability of VR technologies in diagnosing and monitoring eye diseases.
Additionally, the variation in population characteristics across the studies adds another layer of complexity. While some studies focused on specific visual impairments such as retinitis pigmentosa, amblyopia, or glaucoma, others simulated eye conditions in healthy participants. These differences in study populations emphasize the necessity for standardized protocols and clear guidelines for the application of VR in vision care. The diverse sample sizes and conditions examined in the reviewed studies underscore the lack of a unified approach in the field, making it difficult to draw broad conclusions about the generalizability and accuracy of VR diagnostics across different patient demographics.
Each VR system also presents its own set of specifications and performance metrics, further hindering the consistency of results. The varying devices, such as the Oculus Rift, Oculus Quest, and other head-mounted displays, differ in terms of resolution, field of view, and eye-tracking capabilities. As our study revealed, 31 studies employed tethered devices, 26 used standalone HMDs, 15 used smartphone-based systems, and 7 had unspecified connectivity. Additionally, 20 (26.3%) of the included studies utilized HMDs with eye-tracking capabilities, and 17 (22.4%) publications collected eye tracking data. This diversity in VR hardware complicates the establishment of uniform standards and protocols for clinical use. Given the sensitivity of vision-related outcomes to technical variables, future studies in vision care VR should adopt standardized reporting of system specifications—including headset model, processing power, latency, tracking fidelity, and visual display parameters—to ensure reproducibility, clinical relevance, and valid cross-study comparisons. Advancing the use of VR in ophthalmology will depend on both improved technical transparency and rigorous validation against traditional diagnostic and therapeutic tools.
Ensuring that VR technology is user-friendly and accessible is critical for its successful implementation in ophthalmology. Our study highlights the diversity in the types of VR devices used across studies, with 31 of the studies utilizing tethered headsets (e.g., Oculus Rift, Fove 0), 26 employing standalone systems (e.g., Oculus Quest, Oculus Go), 15 using smartphone-based VR devices, and 7 having unspecified HMDs. This distinction is significant in terms of usability, as tethered systems often require additional equipment such as computers or external sensors, which may complicate their use in clinical settings. On the other hand, standalone and smartphone-based devices, while potentially more user-friendly due to their portability and ease of setup, may face limitations in terms of processing power or battery life. These differences underscore the importance of the distinct needs of both healthcare providers and patients when selecting VR equipment for clinical or home-based use.
Many patients, particularly elderly individuals, or those with limited technological proficiency, may find it challenging to use VR headsets. To overcome this barrier, it is essential to design intuitive interfaces and provide comprehensive training for both patients and healthcare providers. Furthermore, addressing issues such as comfort, ease of use, and potential side effects, such as motion sickness or eye strain, is crucial for ensuring patient compliance and satisfaction. As noted in several studies, including those in our review, the use of VR technology in clinical settings often involves a learning curve, and patients may experience discomfort if the headsets are not well-fitted or if the user interface is overly complex [68,69].
In addition to usability concerns, the use of VR headsets for medical purposes necessitates the collection and processing of sensitive patient data. This introduces privacy and security risks, which were highlighted in several of the studies we reviewed. Ensuring compliance with privacy regulations such as HIPAA in the United States or GDPR in Europe is critical to safeguard patient information. Our study highlights the importance of navigating the regulatory landscape, with 15.8% of the publications identified as being jointly developed in collaboration with industry partners. These collaborations may help accelerate the development of VR systems by providing access to cutting-edge technologies and expertise, but they also raise concerns about commercialization and the potential for conflicts of interest. The implementation of robust cybersecurity measures, including data encryption, secure storage, and regular security audits, is equally essential. Clear policies and protocols must be established to govern data access and sharing, ensuring that patient data is used ethically and responsibly while maintaining trust in the system [70]. Moreover, ethical considerations such as informed consent to the collection, analysis and sharing of Personal Health Information (PHI), the potential for over-reliance on technology, and the impact on patient-provider relationships must be carefully managed to maintain trust and integrity in healthcare delivery. This includes understanding the potential biases introduced by industry collaborations and ensuring that VR-based interventions remain patient-centred.
Importantly, VR headsets must meet high technical standards to be effective in medical applications. As noted in several studies, issues such as resolution, field of view, luminance range, and tracking accuracy can affect the reliability of VR-based diagnostics. For instance, Stapelfeldt et al. [35] highlighted that the limited luminance range in their VR system prevented effective analysis of patients with advanced glaucoma. This issue, along with concerns regarding battery life, durability, and compatibility with other medical devices, underscores the need for VR headsets to meet high technical standards to be effective in medical applications. Future developments in VR for ophthalmology must prioritize these technical aspects to ensure that the technology can be used reliably for diagnostic and therapeutic purposes. The integration of artificial intelligence (AI) with VR technology has the potential to significantly enhance diagnostic accuracy and efficiency. AI algorithms can process data from VR systems to identify early signs of diseases such as glaucoma and macular degeneration. Additionally, VR headsets offer the advantage of continuous monitoring, which allows for ongoing assessment of patients’ visual health. This real-time data collection can help create more personalized treatment plans and prompt interventions when needed. As VR technology becomes more affordable, it could emerge as a cost-effective solution for large-scale vision screenings, alleviating the burden on healthcare systems and improving access to eye care services on a broader scale.
Finally, while VR technology has the potential to be a cost-effective solution in the long run, the initial investment in VR headsets and related infrastructure can be significant. Healthcare providers must consider the costs of purchasing, maintaining, and updating VR equipment. Furthermore, there may be additional costs associated with training staff and integrating VR systems into existing healthcare workflows. Securing funding and demonstrating the cost-benefit ratio of VR technology in terms of improved patient outcomes and operational efficiency will be crucial for its adoption.
4.1. Unique Contributions of This Review
In contrast to prior reviews that focus on individual clinical indications (e.g., glaucoma, strabismus, convergence insufficiency, amblyopia) [2,71,72,73], our rapid review offers a panoramic synthesis of how VR is currently being used across the full continuum of vision care, from diagnostics to rehabilitation. By applying the VR1–VR3 framework and coding each intervention’s purpose, we provide a field-level map of the current landscape, revealing not only what technologies exist but also how they are being applied and where gaps persist. This breadth of perspective—across devices, populations, and clinical goals—is not offered in any single previous review.
4.2. Limitations
While this rapid review followed established protocols to ensure methodological rigor, several limitations should be noted. First, the exclusion of conference materials from Embase may have resulted in the absence of relevant studies that were not yet fully peer-reviewed or were presented at conferences. Although the inclusion criteria were comprehensive, limiting studies to those published in English potentially excluded valuable research published in other languages. Additionally, while we aimed to capture all relevant studies, our search strategy was restricted to four databases, which might not have encompassed all existing research on VR in vision care, especially in grey literature or unpublished works. The dual filtration system for study selection was robust; however, there could have been inconsistencies in the interpretation of inclusion/exclusion criteria despite rigorous training and random cross-checks between reviewers. Moreover, the process of data extraction was highly dependent on the quality and completeness of the studies included, and missing or unclear data in some articles could have introduced bias or limited the depth of our analysis. The use of the MMAT for quality assessment, while appropriate for heterogeneous study designs, is inherently influenced by reviewer interpretation and the quality of reporting within each article. This may have introduced variability in scoring, particularly in cases where methodological details were sparse. Similarly, our application of the VR-CORE framework to classify studies into VR1, VR2, or VR3 categories was a helpful tool for mapping the maturity of interventions; however, it may have oversimplified studies with hybrid objectives or non-linear development trajectories. These conceptual tools provided structure to the review but may not fully reflect the nuances and evolving nature of VR interventions in vision care.
5. Conclusions
In conclusion, VR holds great promise for transforming the field of ophthalmology by improving the accessibility, accuracy, and efficiency of vision care. Our rapid review uniquely provides a comprehensive, field-level synthesis of VR applications across the entire vision care continuum—diagnostics to rehabilitation—using the VR1–VR3 framework to map technologies, purposes, and gaps not captured in prior, condition-specific reviews [2,71,72,73]. Although VR-based applications for vision screening, diagnosis, and treatment are still in the early stages, the potential for VR to revolutionize eye care is evident. However, to fully realize its benefits, further research is required to establish its clinical efficacy, devise standardized protocols, and refine technical aspects of the technology. Additionally, efforts to improve the user-friendliness, affordability, and ethical considerations of VR will be essential for ensuring its successful integration into healthcare systems. Moving forward, continued collaboration between healthcare providers, researchers, and technology developers will be vital to advancing VR-based solutions in vision care.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/technologies13080342/s1, File S1: Search Strategy and Terms; File S2: Coding and MMAT; File S3: Definition of Codes; File S4: List of Reviewed Articles.
Author Contributions
Conceptualization, K.M., M.W., D.T., M.R., and L.A.; methodology, K.M., M.W., D.T., A.O.-C., M.R., and L.A.; formal analysis, K.M., M.W., and D.T.; investigation, K.M., M.W. and A.O.-C.; resources, A.O.-C.; data curation, K.M., M.W., D.T., A.O.-C., and L.A.; writing—original draft preparation, K.M., M.W., and L.A.; writing—review and editing, K.M., M.W., M.R., and L.A.; visualization, K.M., M.W., D.T., M.R., and L.A.; supervision, M.R., and L.A.; project administration, D.T., M.R., and L.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All data generated or analyzed during this study are included in this article and its Supplementary Files.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ma, M.K.I.; Saha, C.; Poon, S.H.L.; Yiu, R.S.W.; Shih, K.C.; Chan, Y.K. Virtual Reality and Augmented Reality—Emerging Screening and Diagnostic Techniques in Ophthalmology: A Systematic Review. Surv. Ophthalmol. 2022, 67, 1516–1530. [Google Scholar] [CrossRef] [PubMed]
- Selvan, K.; Mina, M.; Abdelmeguid, H.; Gulsha, M.; Vincent, A.; Sarhan, A. Virtual Reality Headsets for Perimetry Testing: A Systematic Review. Eye 2024, 38, 1041–1064. [Google Scholar] [CrossRef] [PubMed]
- Babel, A.T.; Soumakieh, M.M.; Chen, A.Y.; Wong, C.; R Da Costa, D.; Almeida, D.R. Virtual Reality Visual Field Testing in Glaucoma: Benefits and Drawbacks. Clin. Ophthalmol. 2025, 19, 933–937. [Google Scholar] [CrossRef]
- Basharat, A.; Mehrabi, S.; Muñoz, J.E.; Middleton, L.E.; Cao, S.; Boger, J.; Barnett-Cowan, M. Virtual Reality as a Tool to Explore Multisensory Processing before and after Engagement in Physical Activity. Front. Aging Neurosci. 2023, 15, 1207651. [Google Scholar] [CrossRef]
- Kouijzer, M.M.T.E.; Kip, H.; Bouman, Y.H.A.; Kelders, S.M. Implementation of Virtual Reality in Healthcare: A Scoping Review on the Implementation Process of Virtual Reality in Various Healthcare Settings. Implement. Sci. Commun. 2023, 4, 67. [Google Scholar] [CrossRef]
- Rudolph, B.; Musick, G.; Wiitablake, L.; Lazar, K.B.; Mobley, C.; Boyer, D.M.; Moysey, S.; Robb, A.; Babu, S.V. Investigating the Effects of Display Fidelity of Popular Head-Mounted Displays on Spatial Updating and Learning in Virtual Reality. In Advances in Visual Computing; Bebis, G., Yin, Z., Kim, E., Bender, J., Subr, K., Kwon, B.C., Zhao, J., Kalkofen, D., Baciu, G., Eds.; Lecture Notes in Computer Science; Springer International Publishing: Cham, Switzerland, 2020; Volume 12509, pp. 666–679. ISBN 978-3-030-64555-7. [Google Scholar]
- Bailenson, J. Experience on Demand: What Virtual Reality Is, How It Works, and What It Can Do; W. W. Norton & Company: New York, NY, USA, 2018; ISBN 978-0-393-25369-6. [Google Scholar]
- Fan, X.; Jiang, X.; Deng, N. Immersive Technology: A Meta-Analysis of Augmented/Virtual Reality Applications and Their Impact on Tourism Experience. Tour. Manag. 2022, 91, 104534. [Google Scholar] [CrossRef]
- Jensen, L.; Konradsen, F. A Review of the Use of Virtual Reality Head-Mounted Displays in Education and Training. Educ. Inf. Technol. 2018, 23, 1515–1529. [Google Scholar] [CrossRef]
- Kourtesis, P.; Korre, D.; Collina, S.; Doumas, L.A.A.; MacPherson, S.E. Guidelines for the Development of Immersive Virtual Reality Software for Cognitive Neuroscience and Neuropsychology: The Development of Virtual Reality Everyday Assessment Lab (VR-EAL), a Neuropsychological Test Battery in Immersive Virtual Reality. Front. Comput. Sci. 2020, 1, 12. [Google Scholar] [CrossRef]
- Pottle, J. Virtual Reality and the Transformation of Medical Education. Future Healthc. J. 2019, 6, 181–185. [Google Scholar] [CrossRef]
- Freeman, D. 7 Virtual Reality (VR) for the Treatment of Mental Health Disorders. J. Neurol. Neurosurg. Psychiatry 2020, 91, e3. [Google Scholar] [CrossRef]
- Uimonen, J.; Villarreal, S.; Laari, S.; Arola, A.; Ijäs, P.; Salmi, J.; Hietanen, M. Virtual Reality Tasks with Eye Tracking for Mild Spatial Neglect Assessment: A Pilot Study with Acute Stroke Patients. Front. Psychol. 2024, 15, 1319944. [Google Scholar] [CrossRef]
- Zibold, R.; Sangeux, M.; Winter, R.; Visscher, R.; Cattin, P.C.; Viehweger, E. Effect of Visual Input on Gait Stability Using Immersive Virtual Reality in Children with Cerebral Palsy. Gait Posture 2024, 113, 268. [Google Scholar] [CrossRef]
- Laver, K.E.; Lange, B.; George, S.; Deutsch, J.E.; Saposnik, G.; Crotty, M. Virtual Reality for Stroke Rehabilitation. Cochrane Database Syst. Rev. 2017, 11, CD008349. [Google Scholar] [CrossRef]
- Malloy, K.M.; Milling, L.S. The Effectiveness of Virtual Reality Distraction for Pain Reduction: A Systematic Review. Clin. Psychol. Rev. 2010, 30, 1011–1018. [Google Scholar] [CrossRef]
- Carl, E.; Stein, A.T.; Levihn-Coon, A.; Pogue, J.R.; Rothbaum, B.; Emmelkamp, P.; Asmundson, G.J.G.; Carlbring, P.; Powers, M.B. Virtual Reality Exposure Therapy for Anxiety and Related Disorders: A Meta-Analysis of Randomized Controlled Trials. J. Anxiety Disord. 2019, 61, 27–36. [Google Scholar] [CrossRef]
- Javvaji, C.K.; Reddy, H.; Vagha, J.D.; Taksande, A.; Kommareddy, A.; Reddy, N.S. Immersive Innovations: Exploring the Diverse Applications of Virtual Reality (VR) in Healthcare. Cureus 2024, 16, e56137. [Google Scholar] [CrossRef] [PubMed]
- Mergen, B.; Ramsey, D.J. Underdiagnosis of Glaucoma in Patients with Exudative Age-Related Macular Degeneration. Eye 2021, 35, 3350–3357. [Google Scholar] [CrossRef] [PubMed]
- Lou, L.; Wang, J.; Xu, P.; Ye, X.; Ye, J. Socioeconomic Disparity in Global Burden of Cataract: An Analysis for 2013 with Time Trends Since 1990. Am. J. Ophthalmol. 2017, 180, 91–96. [Google Scholar] [CrossRef]
- Zhang, X.; Cotch, M.F.; Ryskulova, A.; Primo, S.A.; Nair, P.; Chou, C.-F.; Geiss, L.S.; Barker, L.E.; Elliott, A.F.; Crews, J.E.; et al. Vision Health Disparities in the United States by Race/Ethnicity, Education, and Economic Status: Findings from Two Nationally Representative Surveys. Am. J. Ophthalmol. 2012, 154, S53–S62.e1. [Google Scholar] [CrossRef]
- Sekimitsu, S.; Collins, M.E.; Zebardast, N. US Children from Non-English-Speaking Households Are Less Likely to Undergo Vision Testing. J. AAPOS 2025, 29, 104110. [Google Scholar] [CrossRef]
- Chauhan, B.C.; Garway-Heath, D.F.; Goni, F.J.; Rossetti, L.; Bengtsson, B.; Viswanathan, A.C.; Heijl, A. Practical Recommendations for Measuring Rates of Visual Field Change in Glaucoma. Br. J. Ophthalmol. 2008, 92, 569–573. [Google Scholar] [CrossRef]
- Delavar, A.; Radha Saseendrakumar, B.; Weinreb, R.N.; Baxter, S.L. Racial and Ethnic Disparities in Cost-Related Barriers to Medication Adherence Among Patients with Glaucoma Enrolled in the National Institutes of Health All of Us Research Program. JAMA Ophthalmol. 2022, 140, 354. [Google Scholar] [CrossRef] [PubMed]
- Musa, I.; Bansal, S.; Kaleem, M.A. Barriers to Care in the Treatment of Glaucoma: Socioeconomic Elements That Impact the Diagnosis, Treatment, and Outcomes in Glaucoma Patients. Curr. Ophthalmol. Rep. 2022, 10, 85–90. [Google Scholar] [CrossRef]
- Hicks, P.M.; Kang, L.; Armstrong, M.L.; Pongrac, J.R.; Stagg, B.C.; Saylor, K.M.; Newman-Casey, P.A.; Woodward, M.A. A Scoping Review of Patients’ Barriers to Eye Care for Glaucoma and Keratitis. Surv. Ophthalmol. 2023, 68, 567–577. [Google Scholar] [CrossRef]
- Newman-Casey, P.A.; Shtein, R.M.; Coleman, A.L.; Herndon, L.; Lee, P.P. Why Patients with Glaucoma Lose Vision: The Patient Perspective. J. Glaucoma 2016, 25, e668–e675. [Google Scholar] [CrossRef]
- Acuff, K.; Wu, J.; Varkhedi, V.; Baxter, S.L. Social Determinants of Health and Health Disparities in Glaucoma: A Review. Clin. Exp. Ophthalmol. 2024, 52, 276–293. [Google Scholar] [CrossRef]
- Peterson, C.L.; Yap, C.L.; Tan, T.F.; Tan, L.L.Y.; Sim, K.T.; Ong, L.; Tan, Z.K.; Tan, Y.W.; Man, R.; Fenwick, E.; et al. Monocular and Binocular Visual Function Assessments and Activities of Daily Living Performance in Age-Related Macular Degeneration. Ophthalmol. Retin. 2024, 8, 32–41. [Google Scholar] [CrossRef]
- Alwashmi, K.; Meyer, G.; Rowe, F.; Ward, R. Enhancing Learning Outcomes through Multisensory Integration: A fMRI Study of Audio-Visual Training in Virtual Reality. NeuroImage 2024, 285, 120483. [Google Scholar] [CrossRef] [PubMed]
- Groth, S.L.; Linton, E.F.; Brown, E.N.; Makadia, F.; Donahue, S.P. Evaluation of Virtual Reality Perimetry and Standard Automated Perimetry in Normal Children. Transl. Vis. Sci. Technol. 2023, 12, 6. [Google Scholar] [CrossRef] [PubMed]
- Tsapakis, S.; Papaconstantinou, D.; Diagourtas, A.; Droutsas, K.; Andreanos, K.; Moschos, M.M.; Brouzas, D. Visual Field Examination Method Using Virtual Reality Glasses Compared with the Humphrey Perimeter. Clin. Ophthalmol. 2017, 7, 1431–1443. [Google Scholar] [CrossRef]
- Wroblewski, D.; Francis, B.A.; Sadun, A.; Vakili, G.; Chopra, V. Testing of Visual Field with Virtual Reality Goggles in Manual and Visual Grasp Modes. BioMed Res. Int. 2014, 2014, 206082. [Google Scholar] [CrossRef]
- Phu, J.; Wang, H.; Kalloniatis, M. Comparing a Head-mounted Virtual Reality Perimeter and the Humphrey Field Analyzer for Visual Field Testing in Healthy and Glaucoma Patients. Ophthalmic Physiol. Opt. 2024, 44, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Stapelfeldt, J.; Kucur, Ş.S.; Huber, N.; Höhn, R.; Sznitman, R. Virtual Reality–Based and Conventional Visual Field Examination Comparison in Healthy and Glaucoma Patients. Transl. Vis. Sci. Technol. 2021, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Razeghinejad, R.; Gonzalez-Garcia, A.; Myers, J.S.; Katz, L.J. Preliminary Report on a Novel Virtual Reality Perimeter Compared with Standard Automated Perimetry. J. Glaucoma 2021, 30, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Martino Cinnera, A.; Bisirri, A.; Chioccia, I.; Leone, E.; Ciancarelli, I.; Iosa, M.; Morone, G.; Verna, V. Exploring the Potential of Immersive Virtual Reality in the Treatment of Unilateral Spatial Neglect Due to Stroke: A Comprehensive Systematic Review. Brain Sci. 2022, 12, 1589. [Google Scholar] [CrossRef]
- Daibert-Nido, M.; Pyatova, Y.; Cheung, K.; Nayomi, C.; Markowitz, S.N.; Bouffet, E.; Reber, M. Case Report: Visual Rehabilitation in Hemianopia Patients. Home-Based Visual Rehabilitation in Patients with Hemianopia Consecutive to Brain Tumor Treatment: Feasibility and Potential Effectiveness. Front. Neurol. 2021, 12, 680211. [Google Scholar] [CrossRef]
- Molina-Martín, A.; Leal-Vega, L.; De Fez, D.; Martínez-Plaza, E.; Coco-Martín, M.B.; Piñero, D.P. Amblyopia Treatment through Immersive Virtual Reality: A Preliminary Experience in Anisometropic Children. Vision 2023, 7, 42. [Google Scholar] [CrossRef]
- Žiak, P.; Holm, A.; Halička, J.; Mojžiš, P.; Piñero, D.P. Amblyopia Treatment of Adults with Dichoptic Training Using the Virtual Reality Oculus Rift Head Mounted Display: Preliminary Results. BMC Ophthalmol. 2017, 17, 105. [Google Scholar] [CrossRef]
- Birckhead, B.; Khalil, C.; Liu, X.; Conovitz, S.; Rizzo, A.; Danovitch, I.; Bullock, K.; Spiegel, B. Recommendations for Methodology of Virtual Reality Clinical Trials in Health Care by an International Working Group: Iterative Study. JMIR Ment. Health 2019, 6, e11973. [Google Scholar] [CrossRef]
- Chen, Y.-T.; Yeh, P.-H.; Cheng, Y.-C.; Su, W.-W.; Hwang, Y.-S.; Chen, H.S.-L.; Lee, Y.-S.; Shen, S.-C. Application and Validation of LUXIE: A Newly Developed Virtual Reality Perimetry Software. J. Pers. Med. 2022, 12, 1560. [Google Scholar] [CrossRef]
- Kim, J.-H.; Son, H.-J.; Lee, S.-H.; Kwon, S.-C. VR/AR Head-Mounted Display System Based Measurement and Evaluation of Dynamic Visual Acuity. J. Eye Mov. Res. 2019, 12, 1–18. [Google Scholar] [CrossRef]
- Neugebauer, A.; Stingl, K.; Ivanov, I.; Wahl, S. Influence of Systematic Gaze Patterns in Navigation and Search Tasks with Simulated Retinitis Pigmentosa. Brain Sci. 2021, 11, 223. [Google Scholar] [CrossRef]
- Massiceti, D.; Hicks, S.L.; Van Rheede, J.J. Stereosonic Vision: Exploring Visual-to-Auditory Sensory Substitution Mappings in an Immersive Virtual Reality Navigation Paradigm. PLoS ONE 2018, 13, e0199389. [Google Scholar] [CrossRef]
- Jerdan, S.W.; Grindle, M.; Van Woerden, H.C.; Kamel Boulos, M.N. Head-Mounted Virtual Reality and Mental Health: Critical Review of Current Research. JMIR Serious Games 2018, 6, e14. [Google Scholar] [CrossRef] [PubMed]
- Godinez, A.; Martín-González, S.; Ibarrondo, O.; Levi, D.M. Scaffolding Depth Cues and Perceptual Learning in VR to Train Stereovision: A Proof of Concept Pilot Study. Sci. Rep. 2021, 11, 10129. [Google Scholar] [CrossRef]
- VRcompare—The Internet’s Largest VR & AR Headset Database. Available online: https://vr-compare.com/ (accessed on 28 July 2025).
- Huygelier, H.; Schraepen, B.; Lafosse, C.; Vaes, N.; Schillebeeckx, F.; Michiels, K.; Note, E.; Vanden Abeele, V.; Van Ee, R.; Gillebert, C.R. An Immersive Virtual Reality Game to Train Spatial Attention Orientation after Stroke: A Feasibility Study. Appl. Neuropsychol. Adult 2022, 29, 915–935. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.; Aleman, E.M.; Maguire, K.H.; Nadelmann, J.; Weber, M.L.; Maguire, W.M.; Maja, A.; O’Neil, E.C.; Maguire, A.M.; Miller, A.J.; et al. Optimization and Validation of a Virtual Reality Orientation and Mobility Test for Inherited Retinal Degenerations. Transl. Vis. Sci. Technol. 2023, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- VIVE Specs & User Guide–Developer Resources. Available online: https://developer.vive.com/resources/hardware-guides/vive-specs-user-guide/ (accessed on 28 July 2025).
- Mehringer, W.; Wirth, M.; Roth, D.; Michelson, G.; Eskofier, B.M. Stereopsis Only: Validation of a Monocular Depth Cues Reduced Gamified Virtual Reality with Reaction Time Measurement. IEEE Trans. Vis. Comput. Graph. 2022, 28, 2114–2124. [Google Scholar] [CrossRef]
- Kartha, A.; Sadeghi, R.; Bradley, C.; Livingston, B.; Tran, C.; Gee, W.; Dagnelie, G. Measuring Visually Guided Motor Performance in Ultra Low Vision Using Virtual Reality. Front. Neurosci. 2023, 17, 1251935. [Google Scholar] [CrossRef]
- Neugebauer, A.; Sipatchin, A.; Stingl, K.; Ivanov, I.; Wahl, S. Influence of Open-Source Virtual-Reality Based Gaze Training on Navigation Performance in Retinitis Pigmentosa Patients in a Crossover Randomized Controlled Trial. PLoS ONE 2024, 19, e0291902. [Google Scholar] [CrossRef]
- Nascimento, E.; Silva, R.; Kim, J.A.; Li, Y.; Chen, C.; Chaudhry, A.F.; Berneshawi, A.R.; Zhang, M.; Villarreal, A.; Liu, J.; et al. Repeatability of a Virtual Reality Headset Perimeter in Glaucoma and Ocular Hypertensive Patients. Transl. Vis. Sci. Technol. 2024, 13, 14. [Google Scholar] [CrossRef]
- Olleyes_VisuALL_Brochure_V2_03JAN2023_LR. Available online: https://online.flippingbook.com/view/7883596/4/?sharedOn= (accessed on 28 July 2025).
- McLaughlin, D.E.; Savatovsky, E.J.; O’Brien, R.C.; Vanner, E.A.; Munshi, H.K.; Pham, A.H.; Grajewski, A.L. Reliability of Visual Field Testing in a Telehealth Setting Using a Head-Mounted Device: A Pilot Study. J. Glaucoma 2024, 33, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.Y.; Kim, Y.T. Understanding Virtual/Augmented Reality Devices and Their Application in Ophthalmology. J. Retin. 2024, 9, 104–111. [Google Scholar] [CrossRef]
- Soans, R.S.; Renken, R.J.; John, J.; Bhongade, A.; Raj, D.; Saxena, R.; Tandon, R.; Gandhi, T.K.; Cornelissen, F.W. Patients Prefer a Virtual Reality Approach Over a Similarly Performing Screen-Based Approach for Continuous Oculomotor-Based Screening of Glaucomatous and Neuro-Ophthalmological Visual Field Defects. Front. Neurosci. 2021, 15, 745355. [Google Scholar] [CrossRef]
- Mesfin, Y.; Kong, A.; Backus, B.T.; Deiner, M.; Ou, Y.; Oatts, J.T. Pilot Study Comparing a New Virtual Reality–Based Visual Field Test to Standard Perimetry in Children. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2024, 28, 103933. [Google Scholar] [CrossRef]
- Bradley, C.; Ahmed, I.I.K.; Samuelson, T.W.; Chaglasian, M.; Barnebey, H.; Radcliffe, N.; Bacharach, J. Validation of a Wearable Virtual Reality Perimeter for Glaucoma Staging, The NOVA Trial: Novel Virtual Reality Field Assessment. Transl. Vis. Sci. Technol. 2024, 13, 10. [Google Scholar] [CrossRef]
- Narang, P.; Agarwal, A.; Srinivasan, M.; Agarwal, A. Advanced Vision Analyzer–Virtual Reality Perimeter. Ophthalmol. Sci. 2021, 1, 100035. [Google Scholar] [CrossRef]
- Odayappan, A.; Sivakumar, P.; Kotawala, S.; Raman, R.; Nachiappan, S.; Pachiyappan, A.; Venkatesh, R. Comparison of a New Head Mount Virtual Reality Perimeter (C3 Field Analyzer) with Automated Field Analyzer in Neuro-Ophthalmic Disorders. J. Neuro-Ophthalmol. 2023, 43, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Negrillo-Cárdenas, J.; Rueda-Ruiz, A.J.; Ogayar-Anguita, C.J.; Lomas-Vega, R.; Segura-Sánchez, R.J. A System for the Measurement of the Subjective Visual Vertical Using a Virtual Reality Device. J. Med. Syst. 2018, 42, 124. [Google Scholar] [CrossRef]
- Heinzman, Z.; Linton, E.; Marín-Franch, I.; Turpin, A.; Alawa, K.; Wijayagunaratne, A.; Wall, M. Validation of the Iowa Head-Mounted Open-Source Perimeter. Transl. Vis. Sci. Technol. 2023, 12, 19. [Google Scholar] [CrossRef] [PubMed]
- Carl Zeiss VR One Plus Specifications • SizeScreens.Com. Available online: https://www.sizescreens.com/carl-zeiss-vr-one-plus-specifications/ (accessed on 28 July 2025).
- Cottingham, E.; Burgum, F.; Gosling, S.; Woods, L.; Tandon, A. Assessment of the Impact of a Head-Mounted Augmented Reality Low Vision Aid on Vision and Quality of Life in Children and Young People with Visual Impairment. Br. Ir. Orthopt. J. 2024, 20, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Astek, A.; Sparkes, V.; Sheeran, L. Exploring the Use of Immersive Virtual Reality in Adults with Chronic Primary Pain: A Scoping Review. Digit. Health 2024, 10, 20552076241254456. [Google Scholar] [CrossRef]
- Kim, E.; Shin, G. User Discomfort While Using a Virtual Reality Headset as a Personal Viewing System for Text-Intensive Office Tasks. Ergonomics 2021, 64, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Bull, S.; Roberts, N.; Parker, M. Views of Ethical Best Practices in Sharing Individual-Level Data From Medical and Public Health Research: A Systematic Scoping Review. J. Empir. Res. Hum. Res. Ethics 2015, 10, 225–238. [Google Scholar] [CrossRef]
- Shao, W.; Niu, Y.; Wang, S.; Mao, J.; Xu, H.; Wang, J.; Zhang, C.; Guo, L. Effects of Virtual Reality on the Treatment of Amblyopia in Children: A Systematic Review and Meta-Analysis. J. Pediatr. Nurs. 2023, 72, 106–112. [Google Scholar] [CrossRef]
- Islam, T.; Roy, A.D. A Virtual Approach: Systematic Review and Meta-Analysis of Virtual Reality-Based Therapies for Convergence Insufficiency. J. Optom. 2025, 18, 100540. [Google Scholar] [CrossRef]
- Hekmatjah, N.; Chibututu, C.; Han, Y.; Keenan, J.D.; Oatts, J.T. Virtual Reality Perimetry Compared to Standard Automated Perimetry in Adults with Glaucoma: A Systematic Review. PLoS ONE 2025, 20, e0318074. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).