Application of Standardized Antimicrobial Administration Ratio as a Motivational Tool within a Multi-Hospital Healthcare System
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting
2.2. Study Design and Definitions
2.3. Motivational Tools
2.4. Targeted Stewardship Interventions
2.5. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fridkin, S.K.; Srinivasan, A. Implementing a strategy for monitoring inpatient antimicrobial use among hospitals in the United States. Clin. Infect. Dis. 2014, 58, 401–406. [Google Scholar] [CrossRef]
- Ibrahim, O.M.; Polk, R.E. Benchmarking antimicrobial drug use in hospitals. Expert Rev. Anti. Infect. Ther. 2012, 10, 445–457. [Google Scholar] [CrossRef]
- Ibrahim, O.M.; Polk, R.E. Antimicrobial use metrics and benchmarking to improve stewardship outcomes: Methodology, opportunities, and challenges. Infect. Dis. Clin. North Am. 2014, 28, 195–214. [Google Scholar] [CrossRef] [PubMed]
- Brotherton, A.L. Metrics of antimicrobial stewardship programs. Med. Clin. N. Am. 2018, 102, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.C.; Moisan, E.; Tartof, S.Y.; Nguyen, H.M.; Rieg, G.; Ramaprasad, C.; Jones, J. Benchmarking inpatient antimicrobial use: A comparison of risk-adjusted observed-to-expected ratios. Clin. Infect. Dis. 2018, 67, 1677–1685. [Google Scholar] [CrossRef] [PubMed]
- Van Santen, K.L.; Edwards, J.R.; Webb, A.K.; Pollack, L.A.; O’Leary, E.; Neuhauser, M.M.; Srinivasan, A.; Pollock, D.A. The standardized antimicrobial administration ratio: A new metric for measuring and comparing antibiotic use. Clin. Infect. Dis. 2018, 67, 179–185. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, E.N.; Edwards, J.R.; Srinivasan, A.; Neuhauser, M.M.; Webb, A.K.; Soe, M.M.; Hicks, L.A.; Wise, W.; Wu, H.; Pollock, D.A. National Healthcare Safety Network standardized antimicrobial administration ratios (SAARs): A progress report and risk modeling update using 2017 data. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Avedissian, S.N.; Rhodes, N.J.; Liu, J.; Aljefri, D.; Postelnick, M.J.; Sutton, S.H.; Zembower, T.R.; Martin, D.; Pais, G.; Cruce, C.E.; et al. Understanding the components, calculation, and impact of monthly and seasonal variation of the Standardized Antimicrobial Utilization Ratio (SAAR). Antimicrob. Agents Chemother. 2019, 63, e01780-18. [Google Scholar] [CrossRef] [PubMed]
- Al-Hasan, M.N.; Winders, H.R.; Bookstaver, P.B.; Justo, J.A. Direct measurement of performance: A new era in antimicrobial stewardship. Antibiotics 2019, 8, 127. [Google Scholar] [CrossRef] [PubMed]
- Pollack, L.A.; Srinivasan, A. Core Elements of Hospital Antibiotic Stewardship Programs from the Centers for Disease Control and Prevention. Clin. Infect. Dis. 2014, 59 (Suppl. 3), 97–100. [Google Scholar] [CrossRef] [PubMed]
- Using Telehealth to Decrease Carbapenem Use in a Critical Access Hospital—AU Option Case Examples | NHSN | CDC. Updated September 9, 2019. Available online: https://www.cdc.gov/nhsn/au-case-examples/reducing-carbapenem-use.html (accessed on 11 November 2020).
- Targeting a Reduction in Fluoroquinolone Use within a Community Hospital—AU Option Case Examples | NHSN | CDC. Updated September 9, 2019. Available online: https://www.cdc.gov/nhsn/au-case-examples/reduce-fluoroquinolone-use.html (accessed on 11 November 2020).
- O’Leary, E.N.; Van Santen, K.L.; Edwards, E.M.; Braun, D.; Buus-Frank, M.E.; Edwards, J.R.; Guzman-Cottrill, J.A.; Horbar, J.D.; Lee, G.M.; Neuhauser, M.M.; et al. Using NHSN’s antimicrobial use option to monitor and improve antibiotic stewardship in neonates. Hosp. Pediatr. 2019, 9, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Buckel, W.R.; Veillette, J.J.; Vento, T.J.; Stenehjem, E. Antimicrobial stewardship in community hospitals. Med. Clin. N. Am. 2018, 102, 913–928. [Google Scholar] [CrossRef] [PubMed]
- Griebel, M.E.; Heintz, B.; Alexander, B.; Egge, J.; Goto, M.; Livorsi, D.J. Understanding changes in the standardized antimicrobial administration ratio for total antimicrobial use after implementation of prospective audit and feedback. Infect. Control. Hosp. Epidemiol. 2018, 39, 1476–1479. [Google Scholar] [CrossRef]
- Livorsi, D.J.; O’Leary, E.; Pierce, T.; Reese, L.; Van Santen, K.L.; Pollock, D.A.; Edwards, J.R.; Srinivasan, A. A Novel Metric to Monitor the Influence of Antimicrobial Stewardship Activities. Infect. Control. Hosp. Epidemiol. 2017, 38, 721–723. [Google Scholar] [CrossRef] [PubMed]
- Mediwala, K.N.; Kohn, J.E.; Bookstaver, P.B.; Justo, J.A.; Rac, H.; Tucker, K.; Lashkova, L.; Dash, S.; Al-Hasan, M.N. Syndrome-specific versus prospective audit and feedback interventions for reducing use of broad-spectrum antimicrobial agents. Am. J. Infect. Control. 2019, 47, 1284–1289. [Google Scholar] [CrossRef] [PubMed]
- Seddon, M.M.; Bookstaver, P.B.; Justo, J.A.; Kohn, J.; Rac, H.; Haggard, E.; Mediwala, K.N.; Dash, S.; Al-Hasan, M.N. Role of early de-escalation of antimicrobial therapy on risk of Clostridioides difficile infection following Enterobacteriaceae bloodstream infections. Clin. Infect. Dis. 2019, 69, 414–420. [Google Scholar] [CrossRef]
- Al-Jaghbeer, M.J.; Justo, J.A.; Owens, W.; Kohn, J.; Bookstaver, P.B.; Hucks, J.; Al-Hasan, M.N. Risk factors for pneumonia due to beta-lactam-susceptible and beta-lactam-resistant Pseudomonas aeruginosa: A case-case-control study. Infection 2018, 46, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Hammer, K.L.; Justo, J.A.; Bookstaver, P.B.; Kohn, J.; Albrecht, H.; Al-Hasan, M.N. Differential effect of prior β-lactams and fluoroquinolones on risk of bloodstream infections secondary to Pseudomonas aeruginosa. Diagn. Microbiol. Infect. Dis. 2017, 87, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Winders, H.R.; Al-Hasan, M.N.; Jones, B.M.; Childress, D.T.; Stover, K.R.; Britt, B.B.; Chahine, E.B.; Lau, S.; Andrews, P.D.; Junco, S.J.; et al. SERGE-45 investigators. Novel method of calculating adjusted antibiotic use by microbiological burden. Infect. Control. Hosp. Epidemiol. 2021. [Google Scholar] [CrossRef] [PubMed]
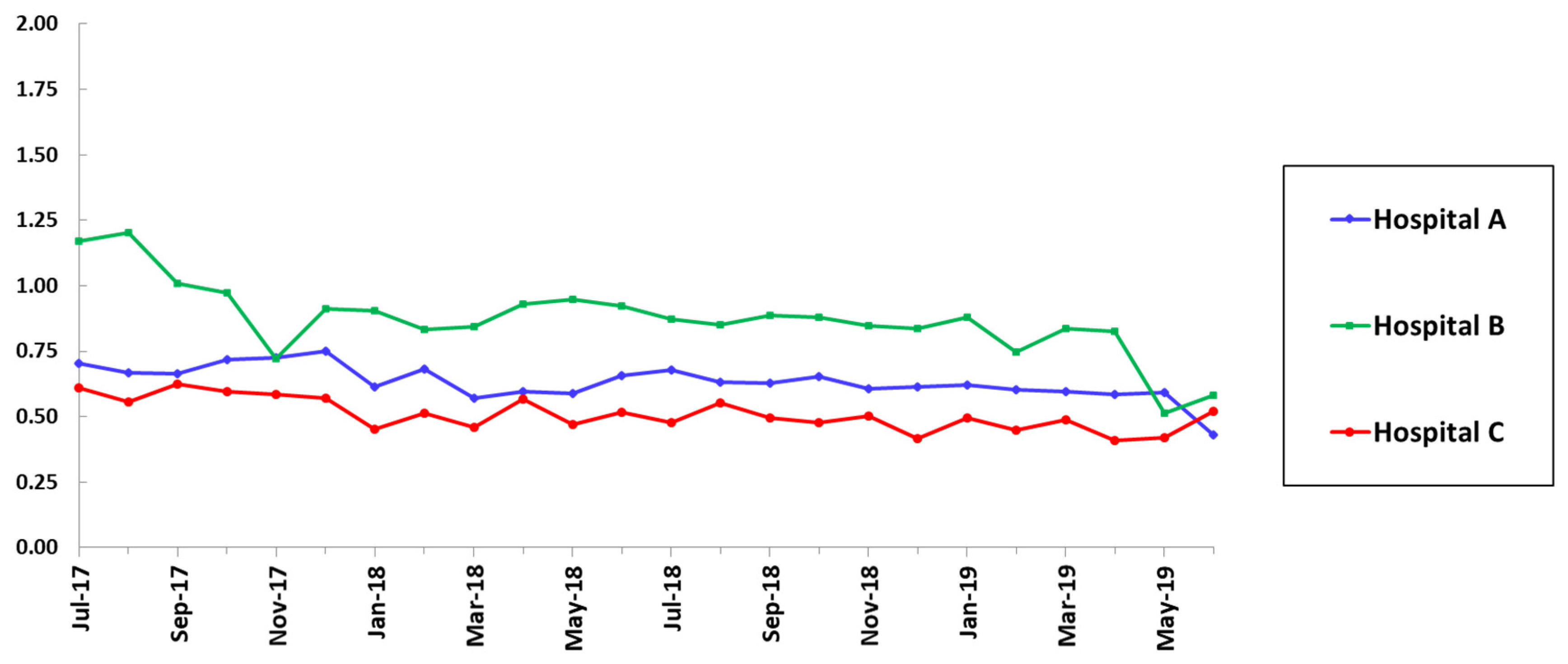

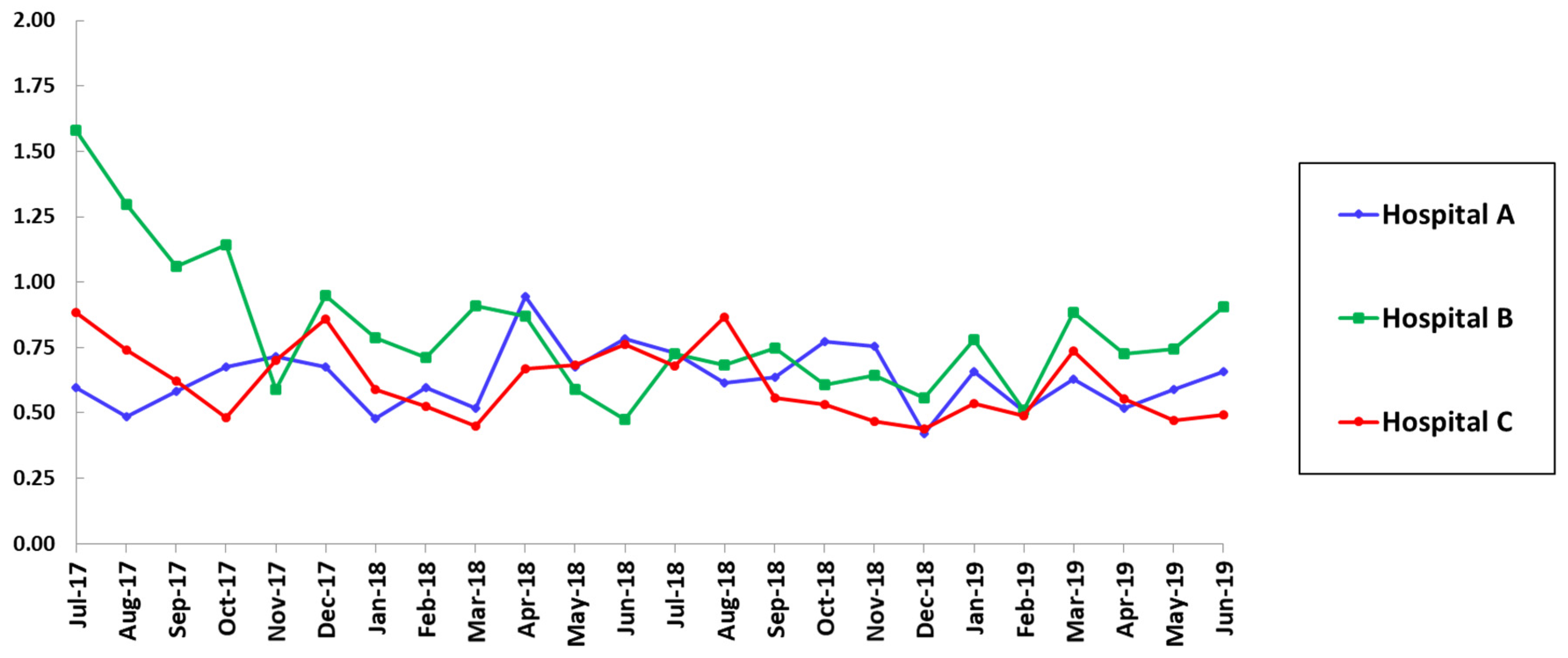
| SAAR Category | Hospital A | Hospital B | Hospital C | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | p-Value | Pre | Post | p-Value | Pre | Post | p-Value | |
| All agents, all locations | 0.69 | 0.62 | 0.06 | 1.09 | 0.83 | <0.001 | 0.60 | 0.64 | 0.69 |
| Broad-spectrum agents used for hospital-onset infections, ICU | 0.67 | 0.52 | 0.01 | 1.36 | 0.81 | <0.001 | 0.83 | 0.54 | 0.007 |
| Agents used for resistant gram-positive infections, ICU | 0.59 | 0.64 | 0.37 | 1.27 | 0.72 | <0.001 | 0.68 | 0.60 | 0.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shealy, S.; Kohn, J.; Yongue, E.; Troficanto, C.; Bookstaver, P.B.; Justo, J.A.; Winders, H.R.; Dash, S.; Al-Hasan, M.N. Application of Standardized Antimicrobial Administration Ratio as a Motivational Tool within a Multi-Hospital Healthcare System. Pharmacy 2021, 9, 32. https://doi.org/10.3390/pharmacy9010032
Shealy S, Kohn J, Yongue E, Troficanto C, Bookstaver PB, Justo JA, Winders HR, Dash S, Al-Hasan MN. Application of Standardized Antimicrobial Administration Ratio as a Motivational Tool within a Multi-Hospital Healthcare System. Pharmacy. 2021; 9(1):32. https://doi.org/10.3390/pharmacy9010032
Chicago/Turabian StyleShealy, Stephanie, Joseph Kohn, Emily Yongue, Casey Troficanto, P. Brandon Bookstaver, Julie Ann Justo, Hana R. Winders, Sangita Dash, and Majdi N. Al-Hasan. 2021. "Application of Standardized Antimicrobial Administration Ratio as a Motivational Tool within a Multi-Hospital Healthcare System" Pharmacy 9, no. 1: 32. https://doi.org/10.3390/pharmacy9010032
APA StyleShealy, S., Kohn, J., Yongue, E., Troficanto, C., Bookstaver, P. B., Justo, J. A., Winders, H. R., Dash, S., & Al-Hasan, M. N. (2021). Application of Standardized Antimicrobial Administration Ratio as a Motivational Tool within a Multi-Hospital Healthcare System. Pharmacy, 9(1), 32. https://doi.org/10.3390/pharmacy9010032







