The Mbeya Antimicrobial Stewardship Team: Implementing Antimicrobial Stewardship at a Zonal-Level Hospital in Southern Tanzania
Abstract
1. Introduction
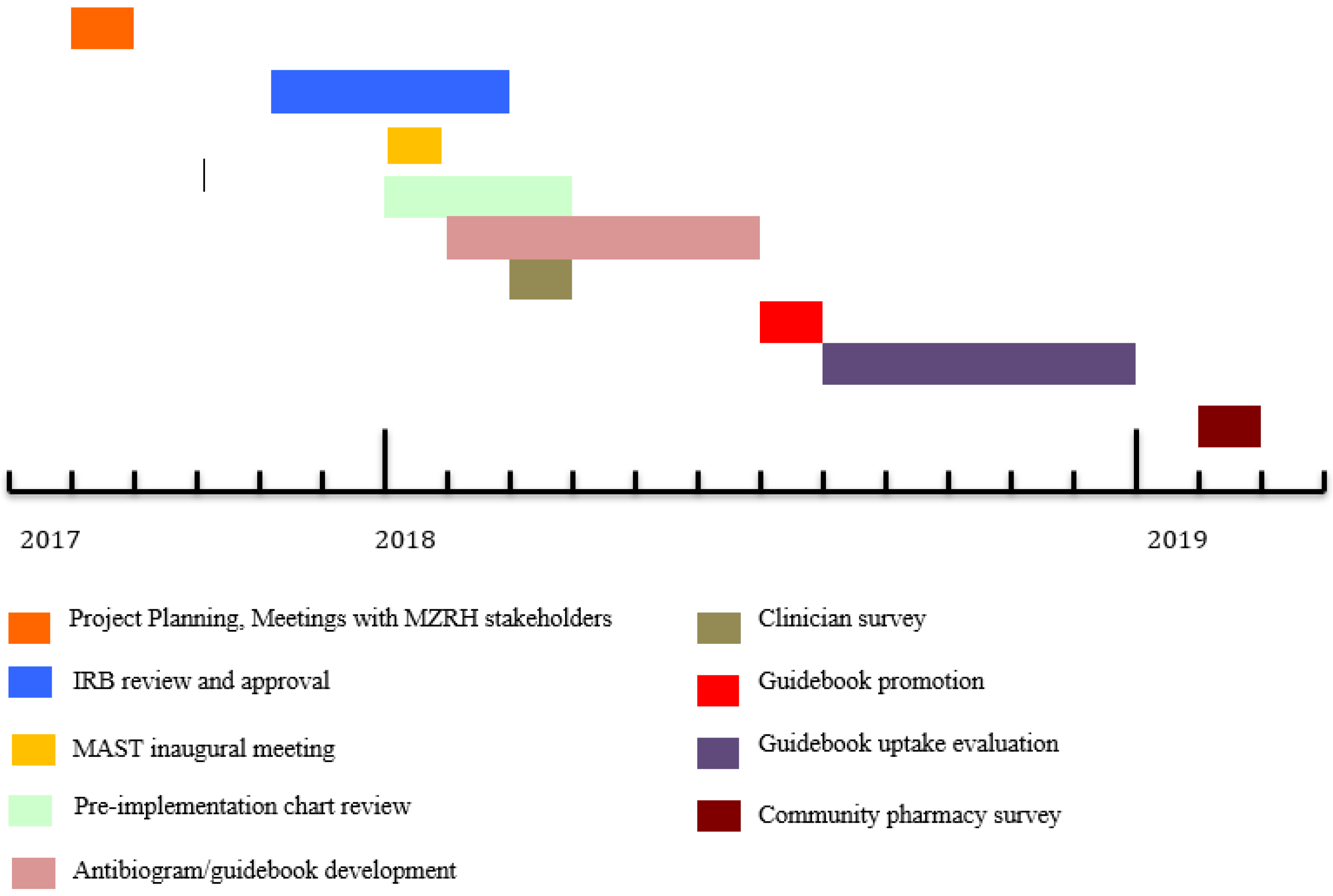
2. Materials and Methods
2.1. Partnership Development: Asset Mapping
2.2. Partnership Development: Needs Assessment
2.3. Creation of the Antimicrobial Stewardship Team
2.4. MAST: Initial Investigations
- Baseline Clinician Survey: A 16-question clinical survey regarding local AMR patterns and AMS concepts was administered to MZRH clinicians of all cadres in the departments of Internal Medicine and Pediatrics. The survey assessed clinicians’ awareness of local AMR patterns, confidence in making empirical antibiotic treatment decisions, perceived barriers to MZRH patients receiving optimal antibiotic therapy, and familiarity with the concept of AMS. Of the 16 questions, 12 were based on a 5-point Likert scale. Two were single-answer multiple choice, and 1 was a multiple choice in which more than one answer could be selected. One additional question was a ranking question in which respondents were asked to rank items in order from 1 through 6, with 1 being the most important factor and 6 being the least. The survey was anonymous, but respondents were asked to provide their designations (“intern”, “registrar”, “specialist”, etc.)
- Chart Review of Antimicrobial Prescribing Practices: A retrospective chart review assessed baseline inpatient antibiotic utilization patterns at MZRH. Beginning with 1 January 2018, the authors chronologically reviewed all adult inpatient medical records for the MZRH adult male and female medical wards until 100 charts involving antibiotic therapy were included. Data points collected included antibiotic-specific metrics, including antibiotic name, indication, days of therapy prescribed, and days of therapy administered. Other data points collected included patient demographics, infection-pertinent comorbidities, length of stay, 30-day readmission, in-house mortality, payer status, and culture utilization. Additional calculated metrics included frequency of antibiotic course completion, antibiotic use per infectious indication, and concordance with national antibiotic guidelines, specifically the Tanzania Standard Treatment Guidelines and National Essential Medicines List, 5th edition [30]. A detailed description of the methods for this investigation is published elsewhere [22].
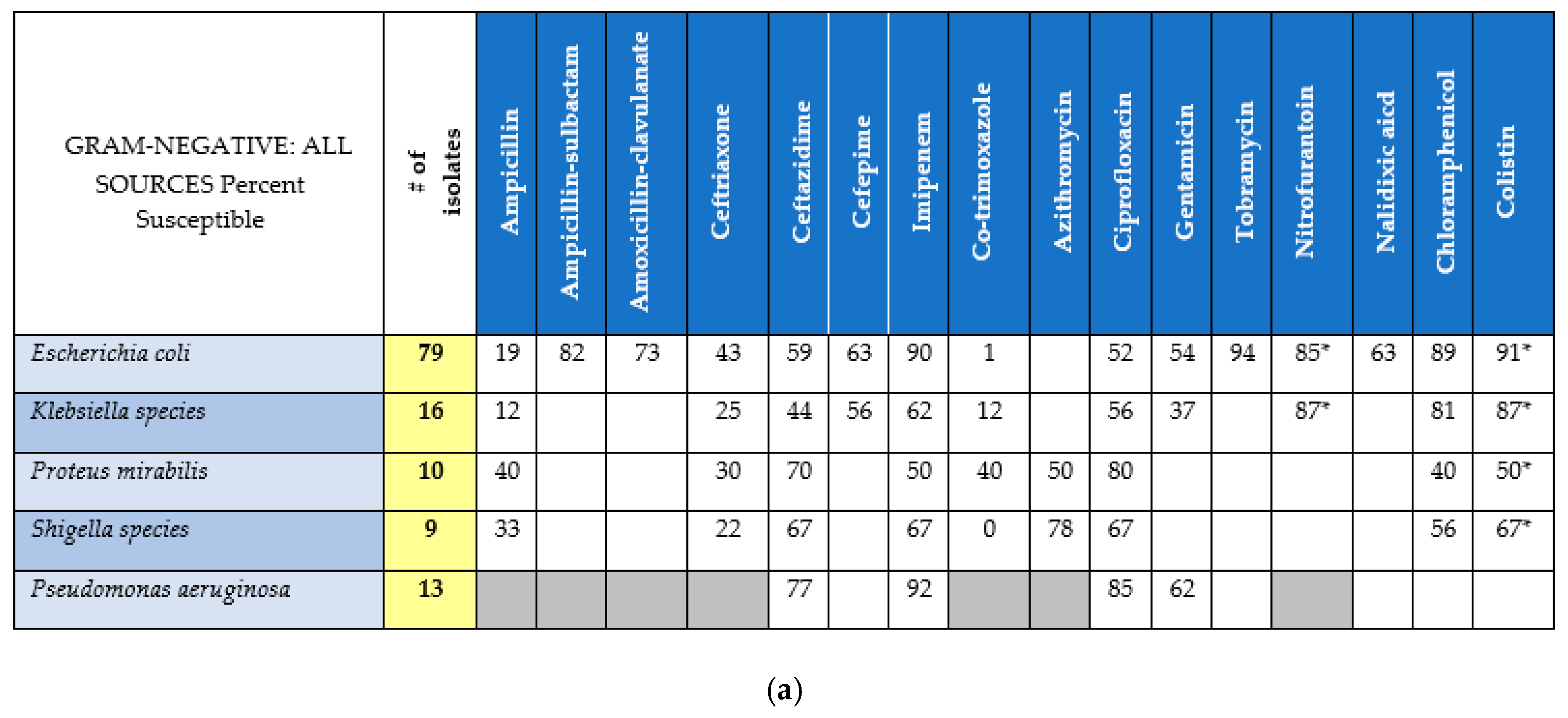
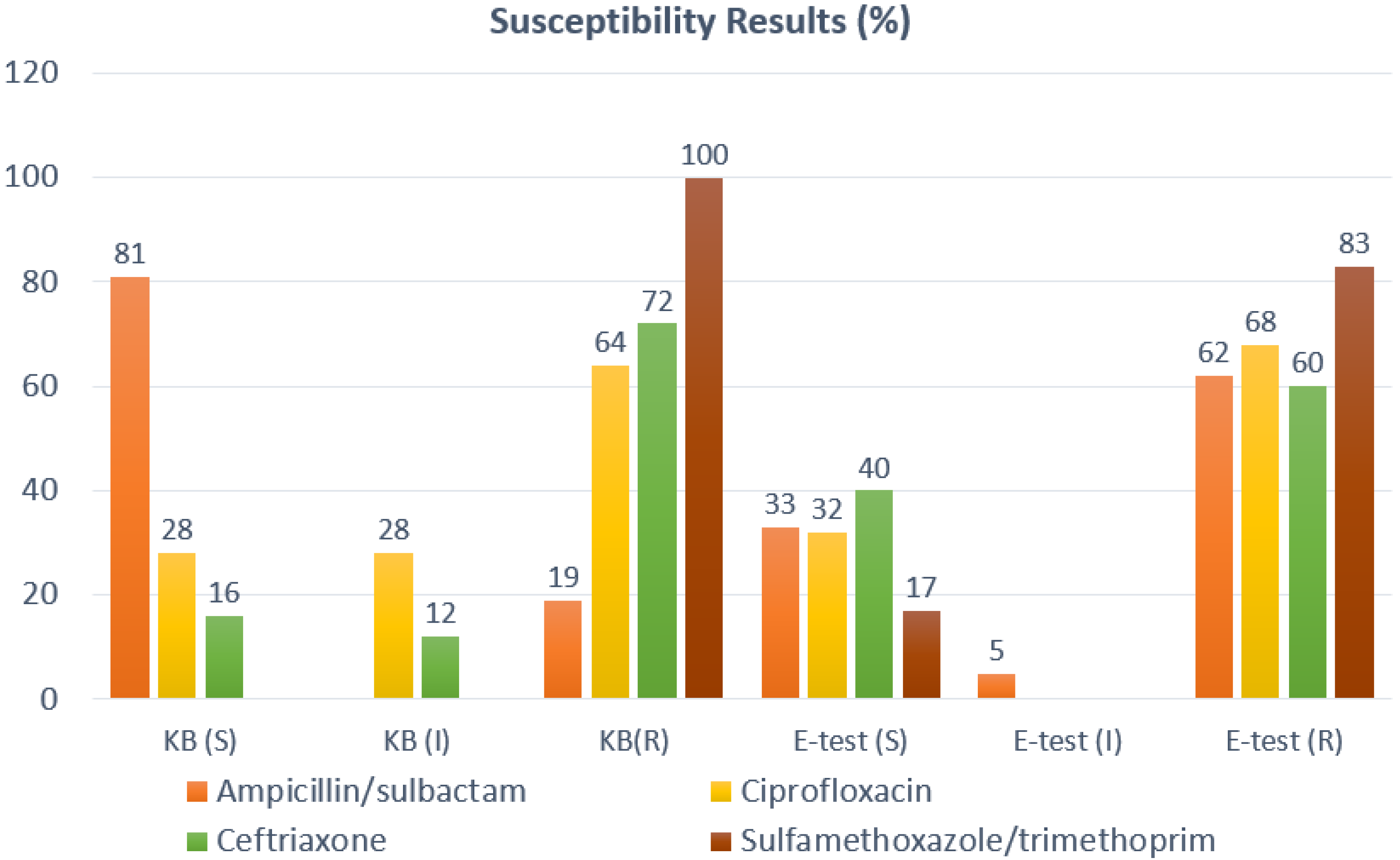
- Local Antimicrobial Resistance Patterns: To assess AMR patterns, the MAST evaluated MZRH culture and resistance data, as cataloged by the MZRH microbiology department over 2 years prior to this study’s initiation. Cultures included specimens from multiple body sites. All of the positive cultures were speciated using manual techniques, and antibiotic susceptibility was determined using Kirby–Bauer (KB) disk diffusion method. To ensure reliability of the KB methodology, a microbiologist randomly selected 25 Enterobacteriaceae isolates (comprising Escherichia coli and Klebsiella species) and performed a second independent evaluation of resistance to these agents using E-tests.
- Community pharmacy survey: Finally, we surveyed local pharmacies in the city of Mbeya to assess the availability of antibiotics without a prescription (“over-the-counter”). Two teams of 2 authors each presented to 15 different local Mbeya pharmacies complaining of nonspecific upper respiratory symptoms. The pharmacy name, location, designation of the pharmacy employee (“pharmacy technician”, “pharmacist”, etc.), antibiotics offered, and selling prices were recorded.
3. Results
3.1. Baseline Clinician Survey
3.2. Chart Review of Antimicrobial Prescribing Practices
3.3. Local Antimicrobial Resistance Patterns
3.4. Community Pharmacy Survey
4. MAST: Analysis to Implementation
Antimicrobial Guidebook
5. Discussion
5.1. Discussion of Results
5.2. Successful Strategies
5.3. Lessons Learned
5.4. Future Directions
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Harbarth, S.; Balkhy, H.H.; Goossens, H.; Jarlier, V.; Kluytmans, J.; Laxminarayan, R.; Saam, M.; van Belkum, A.; Pittet, D. Antimicrobial resistance: One world, one fight! Antimicrob. Resist. Infect. Control 2015, 4, 49. [Google Scholar] [CrossRef]
- World Health Organization. Worldwide Country Situation Analysis: Response to Antimicrobial Resistance. 2015. Available online: http://apps.who.int/iris/bitstream/10665/163468/1/9789241564946_eng.pdf?ua=1&ua=1 (accessed on 9 May 2020).
- African Countries Launch Framework to Tackle the Threat of Antibiotic Resistant Infections. African Union. Available online: https://au.int/en/pressreleases/20171107/african-countries-launch-framework-tackle-threat-antibiotic-resistant (accessed on 11 May 2020).
- Buckel, W.R.; Veillette, J.J.; Vento, T.J.; Stenehjem, E. Antimicrobial Stewardship in Community Hospitals. Med. Clin. N. Am. 2018, 102, 913–928. [Google Scholar] [CrossRef] [PubMed]
- CDC. Core Elements of Hospital Antibiotic Stewardship Programs. Antibiotic Use. Published February 7, 2020. Available online: https://www.cdc.gov/antibiotic-use/core-elements/hospital.html (accessed on 11 May 2020).
- Metronidazole Intravenous Formulation Use in In-Patients in Kapkatet District Hospital, Kenya: A Best Practice Implementation Project. Article. Nursingcenter. Available online: https://www.nursingcenter.com/journalarticle?Article_ID=3525914&Journal_ID=3425880&Issue_ID=3524729 (accessed on 11 May 2020).
- Ibrahim, R.A.; Teshal, A.M.; Dinku, S.F.; Abera, N.A.; Negeri, A.A.; Desta, F.G.; Seyum, E.T.; Gemeda, A.W.; Keficho, W.M. Antimicrobial resistance surveillance in Ethiopia: Implementation experiences and lessons learned. Afr. J. Lab. Med. 2018, 7, 770. [Google Scholar] [CrossRef] [PubMed]
- Hazim, C.; Abubeker Ibrahim, R.; Westercamp, M.; Belete, G.A.; Kibret, B.A.; Kanter, T.; Yimer, G.; Adem, T.S.; Stevenson, K.B.; Urrego, M.; et al. Establishment of a Sentinel Laboratory-Based Antimicrobial Resistance Surveillance Network in Ethiopia. Health Secur. 2018, 16 (Suppl. S1), S30–S36. [Google Scholar] [CrossRef]
- Honda, H.; Ohmagari, N.; Tokuda, Y.; Mattar, C.; Warren, D.K. Antimicrobial Stewardship in Inpatient Settings in the Asia Pacific Region: A Systematic Review and Meta-analysis. Clin. Infect. Dis. 2017, 64 (Suppl. S2), S119–S126. [Google Scholar] [CrossRef]
- Van Dijck, C.; Vlieghe, E.; Cox, J.A. Antibiotic stewardship interventions in hospitals in low-and middle-income countries: A systematic review. Bull. World Health Organ. 2018, 96, 266–280. [Google Scholar] [CrossRef]
- Junaid, E.; Jenkins, L.; Swanepoel, H.; North, Z.; Gould, T. Antimicrobial stewardship in a rural regional hospital-growing a positive culture. S. Afr. Med. J. 2018, 108, 546–550. [Google Scholar] [CrossRef]
- Cox, J.A.; Vlieghe, E.; Mendelson, M.; Wertheim, H.; Ndegwa, L.; Villegas, M.V.; Gould, I.; Hara, G.L. Antibiotic stewardship in low-and middle-income countries: The same but different? Clin. Microbiol. Infect. 2017, 23, 812–818. [Google Scholar] [CrossRef]
- Brink, A.J.; Messina, A.P.; Feldman, C.; Richards, G.A.; van den Bergh, D. From guidelines to practice: A pharmacist-driven prospective audit and feedback improvement model for peri-operative antibiotic prophylaxis in 34 South African hospitals. J. Antimicrob. Chemother. 2017, 72, 1227–1234. [Google Scholar] [CrossRef]
- Boyles, T.H.; Naicker, V.; Rawoot, N.; Raubenheimer, P.J.; Eick, B.; Mendelson, M. Sustained reduction in antibiotic consumption in a South African public sector hospital: Four year outcomes from the Groote Schuur Hospital antibiotic stewardship program. S. Afr. Med. J. 2017, 107, 115–118. [Google Scholar] [CrossRef]
- Brink, A.J.; Messina, A.P.; Feldman, C.; Richards, G.A.; Becker, P.J.; Goff, D.A.; Bauer, K.A.; Nathwani, D.; van den Bergh, D. Netcare Antimicrobial Stewardship Study Alliance Antimicrobial stewardship across 47 South African hospitals: An implementation study. Lancet. Infect. Dis. 2016, 16, 1017–1025. [Google Scholar] [CrossRef]
- Messina, A.P.; van den Bergh, D.; Goff, D.A. Antimicrobial Stewardship with Pharmacist Intervention Improves Timeliness of Antimicrobials Across Thirty-three Hospitals in South Africa. Infect. Dis Ther. 2015, 4, 5–114. [Google Scholar] [CrossRef] [PubMed]
- Boyles, T.H.; Whitelaw, A.; Bamford, C.; Moodley, M.; Bonorchis, K.; Morris, V.; Rawoot, N.; Naicker, V.; Lusakiewicz, I.; Black, J.; et al. Antibiotic stewardship ward rounds and a dedicated prescription chart reduce antibiotic consumption and pharmacy costs without affecting inpatient mortality or re-admission rates. PLoS ONE 2013, 8, e79747. [Google Scholar] [CrossRef]
- Shao, A.F.; Rambaud-Althaus, C.; Samaka, J.; Faustine, A.F.; Perri-Moore, S.; Swai, N.; Kahama-Maro, J.; Mitchell, M.; Genton, B.; D’Acremont, V. New Algorithm for Managing Childhood Illness Using Mobile Technology (ALMANACH): A Controlled Non-Inferiority Study on Clinical Outcome and Antibiotic Use in Tanzania. PLoS ONE 2015, 10, e0132316. [Google Scholar] [CrossRef] [PubMed]
- Ramsamy, Y.; Muckart, D.J.J.; Han, K.S.S. Microbiological surveillance and antimicrobial stewardship minimise the need for ultrabroad-spectrum combination therapy for treatment of nosocomial infections in a trauma intensive care unit: An audit of an evidence-based empiric antimicrobial policy. S. Afr. Med. J. 2013, 103, 371–376. [Google Scholar] [CrossRef]
- Akpan, M.R.; Isemin, N.U.; Udoh, A.E.; Ashiru-Oredope, D. Implementation of antimicrobial stewardship programmes in African countries: A systematic literature review. J. Glob. Antimicrob. Resist. 2020. [Google Scholar] [CrossRef]
- Goff, D.A.; Kullar, R.; Goldstein, E.J.C.; Gilchrist, M.; Nathwani, D.; Cheng, A.C.; Cairns, K.A.; Escandón-Vargas, K.; Villegas, M.V.; Brink, A.; et al. A global call from five countries to collaborate in antibiotic stewardship: United we succeed, divided we might fail. Lancet. Infect. Dis. 2017, 17, e56–e63. [Google Scholar] [CrossRef]
- National Bureau of Statistics-Tanzania in Figures 2018. Available online: https://www.nbs.go.tz/index.php/en/tanzania-in-figures/422-tanzania-in-figures-2018 (accessed on 11 May 2020).
- Centers of Excellence. Available online: https://www.idsociety.org/clinical-practice/antimicrobial-stewardship/centers-of-excellence/ (accessed on 11 May 2020).
- Infectious Diseases Residency (PGY-2). College of Pharmacy University of South Carolina. Available online: https://www.sc.edu/study/colleges_schools/pharmacy/pharmacy_education/residency_and_fellowship_programs/infectious_diseases_residency_pgy-2/index.php (accessed on 11 May 2020).
- Program Overview-Prisma Health–Midlands Graduate Medical Education. Available online: https://residency.palmettohealth.org/fellowships/family-medicine-global-health/program-overview (accessed on 11 May 2020).
- Moremi, N.; Claus, H.; Mshana, S.E. Antimicrobial resistance pattern: A report of microbiological cultures at a tertiary hospital in Tanzania. BMC Infect. Dis. 2016, 16, 756. [Google Scholar] [CrossRef]
- Mshana, S.E.; Matee, M.; Rweyemamu, M. Antimicrobial resistance in human and animal pathogens in Zambia, Democratic Republic of Congo, Mozambique and Tanzania: An urgent need of a sustainable surveillance system. Ann. Clin. Microbiol. Antimicrob. 2013, 12, 28. [Google Scholar] [CrossRef]
- Kumburu, H.H.; Sonda, T.; Mmbaga, B.T.; Alifrangis, M.; Lund, O.; Kibiki, G.; Aarestrup, F.M. Patterns of infections, aetiological agents and antimicrobial resistance at a tertiary care hospital in northern Tanzania. Trop. Med. Int. Health 2017, 22, 454–464. [Google Scholar] [CrossRef]
- Tanzania HIV Impact Survey (THIS) 2016–2017. Available online: https://phia.icap.columbia.edu/wp-content/uploads/2019/06/FINAL_THIS-2016-2017_Final-Report__06.21.19_for-web_TS.pdf (accessed on 11 May 2020).
- Mwalimu, H.U.A. Minister for Health, Community Development, Gender, Elderly and Children: 464. Available online: http://www.tzdpg.or.tz/fileadmin/documents/dpg_internal/dpg_working_groups_clusters/cluster_2/health/Key_Sector_Documents/Tanzania_Key_Health_Documents/STANDARD_TREATMENT_GUIDELINES__CORRECT_FINAL_USE_THIS-1.pdf (accessed on 19 June 2020).
- Haldeman, M.S.; Kishimbo, P.; Seddon, M.; Sangare, A.; Mwasomola, D.; Hall, J.; Shaffer, M.; Leclair, R.; Caulder, C.; Bookstaver, P.B.; et al. Evaluation of Antimicrobial Utilization and Concordance with National Guidelines at a Tertiary Hospital in the Southern Highlands Zone of Tanzania. Am. J. Trop. Med. Hyg. 2020, 102, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Horumpende, P.G.; Sonda, T.B.; van Zwetselaar, M.; Antony, M.L.; Tenu, F.F.; Mwanziva, C.E.; Shao, E.R.; Mshana, S.E.; Mmbaga, B.T.; Chilongola, O.J. Prescription and non-prescription antibiotic dispensing practices in part I and part II pharmacies in Moshi Municipality, Kilimanjaro Region in Tanzania: A simulated clients approach. PLoS ONE 2018, 13, e0207465. [Google Scholar] [CrossRef] [PubMed]
- Kagashe, G.A.B.; Minzi, O.; Matowe, L. An assessment of dispensing practices in private pharmacies in Dar-es-Salaam, Tanzania. Int. J. Pharm. Pract. 2011, 19, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Goff, D.A.; Bauer, K.A.; Brink, A.; Kolman, S.; Mendelson, M.; Messina, A.P.; Schellack, N.; van den Bergh, D. International Train the Trainer antibiotic stewardship program for pharmacists: Implementation, sustainability, and outcomes. J. Am. Coll. Clin. Pharm. 2020. [Google Scholar] [CrossRef]



| Question | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Q1: I believe antibiotic resistance is a problem nationally in Tanzania. | 4.5% | 2.3% | 2.3% | 36.4% | 54.5% |
| Q2: I believe antibiotic resistance is a problem locally at Mbeya Zonal Hospital. | 9.1% | 2.3% | 6.8% | 52.3% | 29.5% |
| Q5: I believe my patients receive antibiotics in a timely fashion following suspected or confirmed infectious diagnoses. | 6.7% | 15.6% | 15.6% | 33.3% | 28.9% |
| Q6: I am confident in my ability to select appropriate empirical antibiotic therapy based on a given diagnosis. | 2.2% | 4.4% | 6.7% | 33.3% | 53.5% |
| Q7: I am confident in my ability to accurately interpret culture and susceptibility reports. | 0.0% | 2.3% | 4.5% | 18.2% | 75.0% |
| Q8: I am confident in my ability to determine appropriate definitive antibiotic therapy based on available diagnostic reports. | 0.0% | 2.2% | 2.2% | 33.3% | 62.2% |
| Q9: I am confident in my ability to discuss antibiotic therapy with a patient. | 2.2% | 2.2% | 8.9% | 37.8% | 48.9% |
| Q10: I am confident in my ability to discuss or teach antibiotic therapy principles to a peer. | 4.4% | 0.0% | 8.9% | 31.1% | 55.6% |
| Q11: I am aware of antibiotic resistance patterns nationally. | 8.9% | 13.3% | 13.3% | 46.7% | 17.8% |
| Q12: I am aware of antibiotic resistance patterns at Mbeya Zonal Hospital. | 17.8% | 8.9% | 13.3% | 51.1% | 8.9% |
| Q13: I am familiar with the term antimicrobial stewardship. | 31.8% | 15.9% | 22.7% | 13.6% | 15.9% |
| Q14: I am familiar with the approximate costs of commonly used antibiotics that hospitalized patients are prescribed. | 6.7% | 8.9% | 13.3% | 55.6% | 15.6% |
| Pharmacy Number | Antibiotic Regimen | Cost (TSH) |
|---|---|---|
| 1 | Amoxicillin 500 mg 3× daily × 3 days | 2000 |
| 2 | Azithromycin 500 mg daily × 3 days | 2550 |
| 3 | Ampicillin/cloxacillin 250/250 mg 3× daily × 5 days | 2000 |
| 4 | Azithromycin 500 mg daily × 3 days | 16,400 |
| 5 | Sulfamethoxazole/Trimethoprim 2 SS tablets 2× daily × 7 days | 3000 |
| 6 | Amoxicillin/Clavulanate 625 mg 2× daily × 7 days | 32,000 |
| 7 | Amoxicillin 500 mg 3× daily × 5 days | 30,000 |
| 8 | Ampicillin/Cloxacillin 250/250 mg 3× daily × 5 days | 3000 |
| 9 | Cephalexin 250 mg 2 tablets 3× daily × 5 days | 4000 |
| 10 | Erythromycin 250 mg 2 tablets 3 × 5 days | 3000 |
| 11 | Lomefloxacin daily × 5 days | 3000 |
| 12 | Cefpodoxime 100 mg 2× daily × 5 days | 25,000 |
| 13 | Cefuroxime 250 mg BID × 5 days | 18,000 |
| 14 | None Offered | N/A |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hall, J.W.; Bouchard, J.; Bookstaver, P.B.; Haldeman, M.S.; Kishimbo, P.; Mbwanji, G.; Mwakyula, I.; Mwasomola, D.; Seddon, M.; Shaffer, M.; et al. The Mbeya Antimicrobial Stewardship Team: Implementing Antimicrobial Stewardship at a Zonal-Level Hospital in Southern Tanzania. Pharmacy 2020, 8, 107. https://doi.org/10.3390/pharmacy8020107
Hall JW, Bouchard J, Bookstaver PB, Haldeman MS, Kishimbo P, Mbwanji G, Mwakyula I, Mwasomola D, Seddon M, Shaffer M, et al. The Mbeya Antimicrobial Stewardship Team: Implementing Antimicrobial Stewardship at a Zonal-Level Hospital in Southern Tanzania. Pharmacy. 2020; 8(2):107. https://doi.org/10.3390/pharmacy8020107
Chicago/Turabian StyleHall, Jeffrey W., Jeannette Bouchard, P. Brandon Bookstaver, Matthew S. Haldeman, Peter Kishimbo, Godlove Mbwanji, Issakwisa Mwakyula, Davance Mwasomola, Megan Seddon, Mark Shaffer, and et al. 2020. "The Mbeya Antimicrobial Stewardship Team: Implementing Antimicrobial Stewardship at a Zonal-Level Hospital in Southern Tanzania" Pharmacy 8, no. 2: 107. https://doi.org/10.3390/pharmacy8020107
APA StyleHall, J. W., Bouchard, J., Bookstaver, P. B., Haldeman, M. S., Kishimbo, P., Mbwanji, G., Mwakyula, I., Mwasomola, D., Seddon, M., Shaffer, M., Shealy, S. C., & Nsojo, A. (2020). The Mbeya Antimicrobial Stewardship Team: Implementing Antimicrobial Stewardship at a Zonal-Level Hospital in Southern Tanzania. Pharmacy, 8(2), 107. https://doi.org/10.3390/pharmacy8020107






