Impact of Nasal Swabs on Empiric Treatment of Respiratory Tract Infections (replace into-RTI)
Abstract
1. Introduction
2. Materials and Methods
2.1. ASP-PCRNS Bundle
2.2. Data Collection
2.3. Statistical Analysis
3. Results
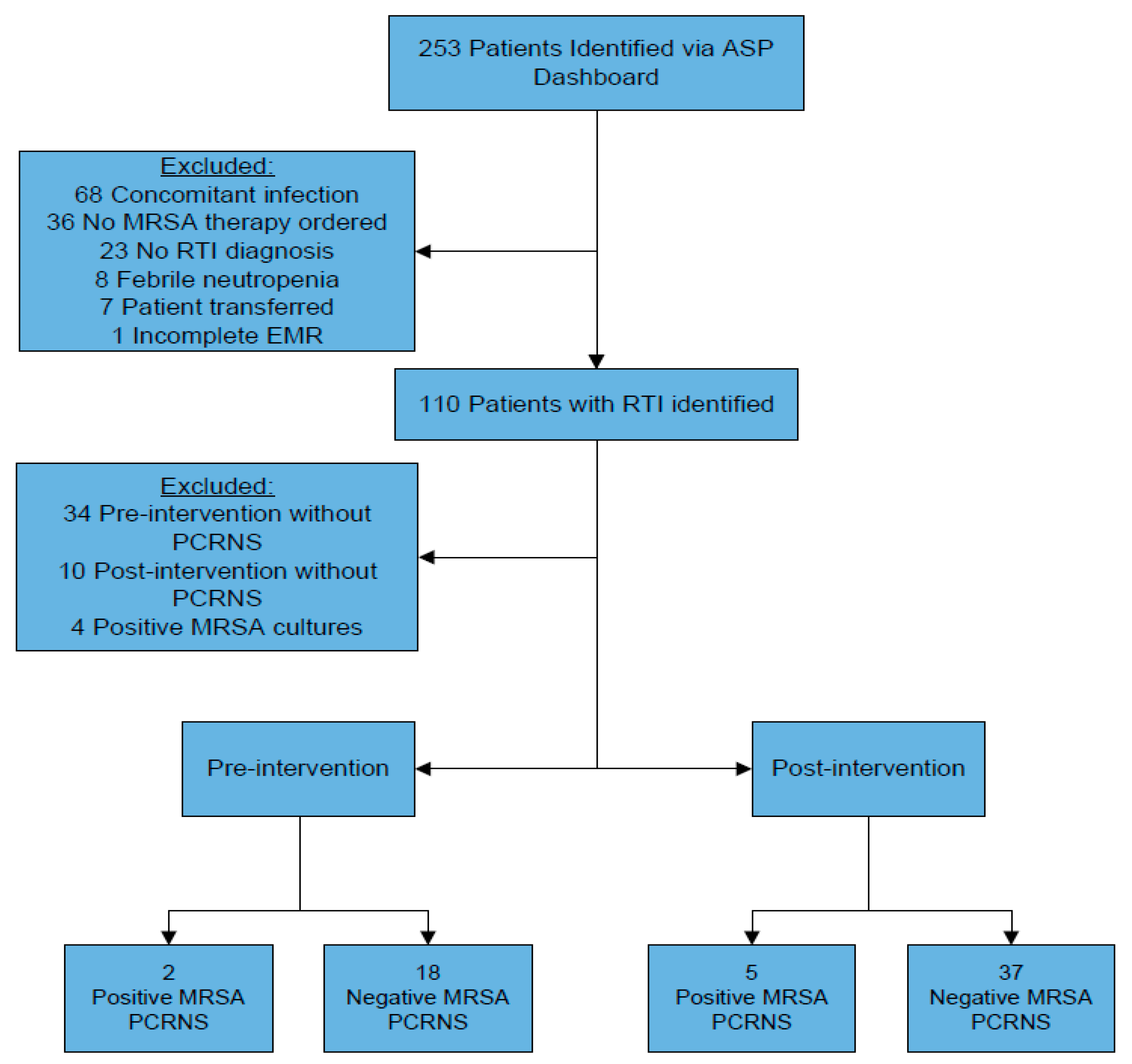
3.1. Inclusion and Exclusion
3.2. Baseline Characteristics
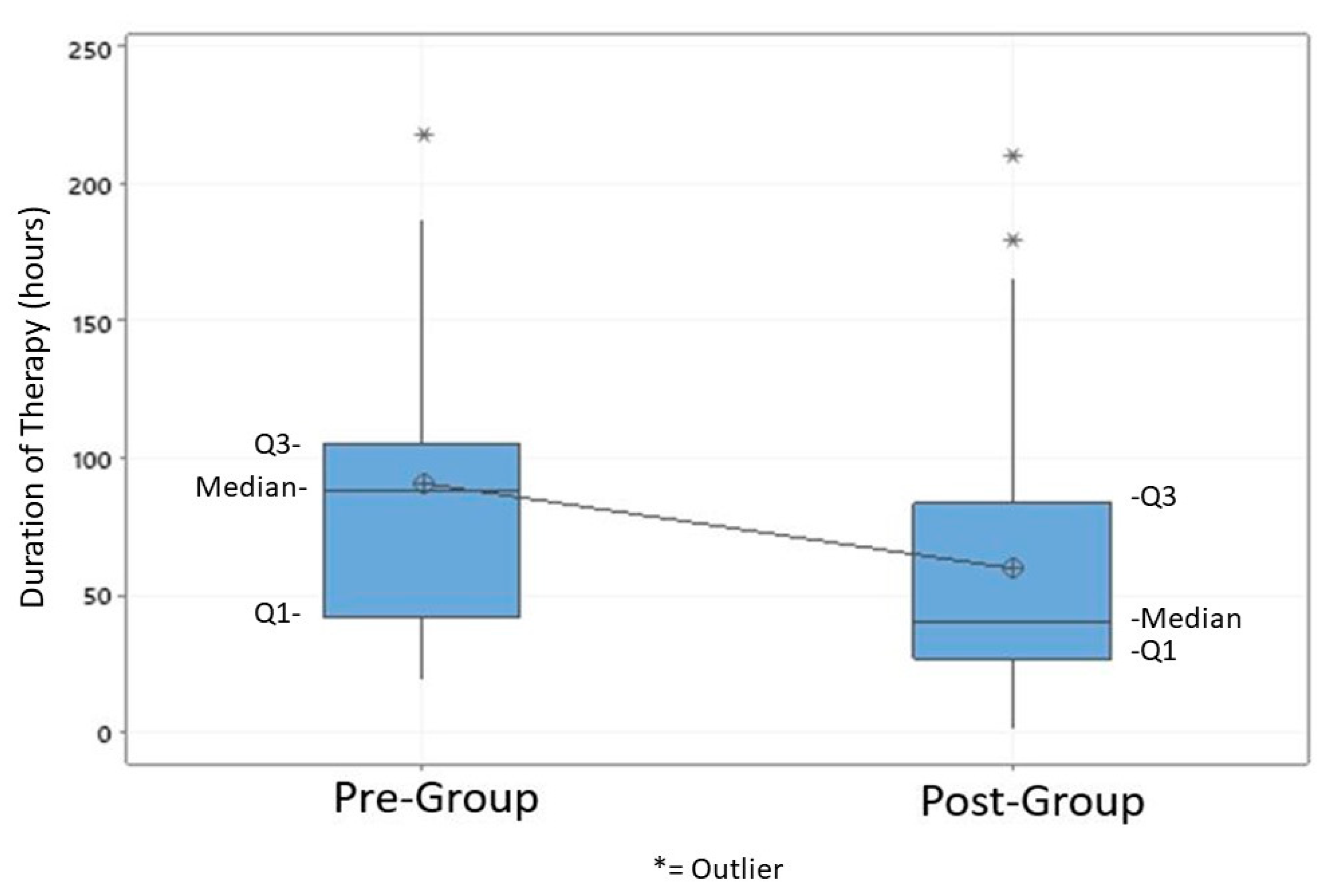
3.3. Primary and Secondary Outcomes
3.4. Negative PCRNS Outcomes
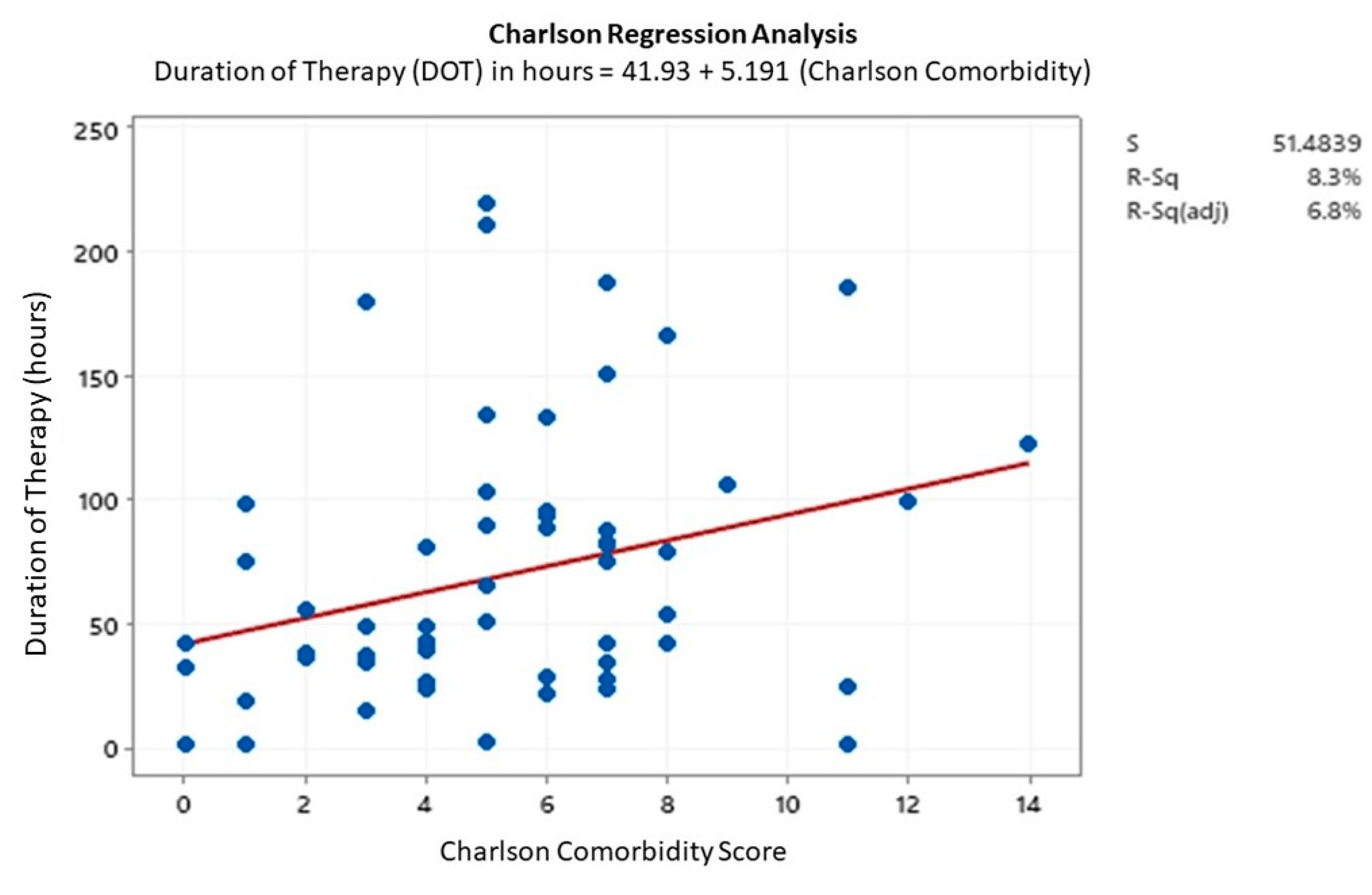
3.5. Regression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
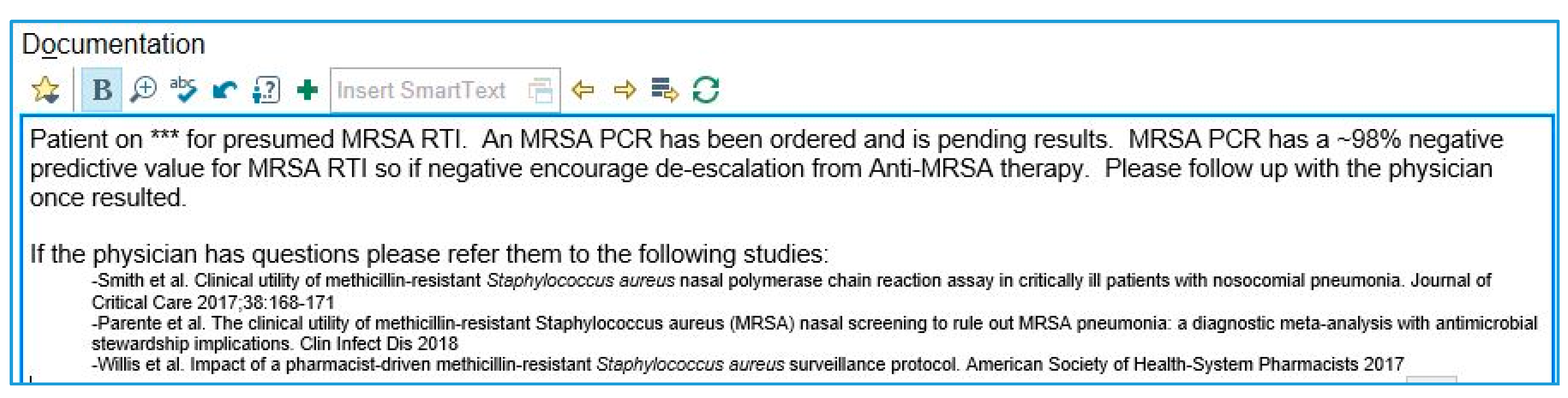

Appendix B


Appendix C
| Baseline Characteristics | Pre Group (n = 20) | Post Group (n = 42) | p-Value |
|---|---|---|---|
| Duration of therapy | |||
| Gender | Mean (Std Dev)/Median ** | Mean (Std Dev)/Median ** | |
| Male | 67.9 (30.6) | 57.8 (47.5) | 0.470 |
| Female | 94.1 ** | 35.6 ** | 0.047 |
| Age | Mean (Std Dev)/Median ** | Mean (Std Dev)/Median ** | |
| <65 years old | 65.8 ** | 36.6 ** | 0.057 |
| ≥65 years old | 106.1(62) | 74.8 (58) | 0.158 |
| Race | Mean (Std Dev)/Median ** | Mean (Std Dev)/Median ** | |
| Caucasian | 81.7 (42.8) | 67.8 (53.9) | 0.389 |
| Non-Caucasian | 98.5 ** | 34.3 ** | 0.035 |
| BMI | Mean (Std Dev)/Median ** | Mean (Std Dev)/Median ** | |
| <30 kg/m2 | 84.2 ** | 37.2 ** | 0.031 |
| ≥30 kg/m2 | 89.1 ** | 74.6 ** | 0.316 |
| Length of Stay | |||
| Gender | Mean (Std Dev)/Median ** | Mean (Std Dev)/Median ** | |
| Male | 7.8 ** | 8.5 | 0.610 |
| Female | 14.9 (7.0) | 8.3 (7.0) | 0.049 |
| Age | Median ** | Median ** | |
| <65 years old | 8.0 ** | 3.2 ** | 0.112 |
| ≥65 years old | 9.3 ** | 13.3 ** | 0.987 |
| Race | Mean (Std Dev)/Median ** | Mean (Std Dev)/Median ** | |
| Caucasian | 11.4 (7.2) | 10.3 (7.1) | 0.660 |
| Non-Caucasian | 9.4 ** | 4.1 ** | 0.193 |
| BMI | Mean (Std Dev)/Median** | Mean (Std Dev)/Median** | |
| <30 kg/m2 | 8.3 * | 5.3 * | 0.274 |
| ≥30 kg/m2 | 10.2 * | 13.6 * | 0.953 |
| Cost | |||
| Gender | Median (IQR) | Median (IQR) | |
| Male | 59.8 | 47.2 | 0.108 |
| Female | 84.5 | 46.3 | 0.052 |
| Age | Median | Median | |
| <65 years old | 57.7 | 46.6 | 0.066 |
| ≥65 years old | 82.7 | 53.3 | 0.089 |
| Race | Median ** | Median ** | |
| Caucasian | 79.2 ** | 48.4 ** | 0.092 |
| Non-Caucasian | 69.9 ** | 45.9 ** | 0.057 |
| BMI | Median ** (IQR) | Median * (IQR) | |
| <30 kg/m2 | 80.6 (48.6–101.9) | 45.3 (30.3–64.6) | 0.023 |
| ≥30 kg/m2 | 74.6 ** | 63.6 ** | 0.444 |
References
- Antibiotic Resistance Threats in the United States, 2013. Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf (accessed on 24 March 2020).
- Rubinstein, K.; Kollef, M.H.; Nathwani, D. Pneumonia caused by methicillin-resistant Staphylococcus aureus. Clin. Infect. Dis. 2008, 46, S378–S385. [Google Scholar] [CrossRef] [PubMed]
- Shorr, A.F.; Tabak, Y.P.; Gupta, V.; Johannes, R.S.; Liu, L.Z.; Kollef, M.H. Morbidity and cost burden of methicillin resistant Staphylococcus aureus in early onset ventilator associated pneumonia. Crit Care. 2006, 10, R97. [Google Scholar] [CrossRef] [PubMed]
- You, A.S.; Fukunaga, B.T.; Hanlon, A.L.; Lozano, A.J.; Goo, R.A. The Daniel K. Inouye College of Pharmacy Scripts: The effects of vancomycin use and de-escalation in patients hospitalized with pneumonia. Hawaii J. Med. Public Health 2018, 77, 261–267. [Google Scholar]
- Hazlewood, K.A.; Brouse, S.D.; Pitcher, W.D.; Hall, R.G. Vancomycin-associated nephrotoxicity: Grave concern or death by character assassination? Am. J. Med. 2010, 123, 182.e1–182.e7. [Google Scholar] [CrossRef] [PubMed]
- Kalil, A.C.; Metersky, M.L.; Klompas, M.; Muscedere, J.; Sweeney, D.A.; Palmer, L.B.; Napolitano, L.M.; O’Grady, N.P.; Bartlett, J.G.; Carratala, J.; et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 2016, 63, e61–e111. [Google Scholar] [CrossRef] [PubMed]
- Metlay, J.P.; Waterer, G.W.; Long, A.C.; Anzueto, A.; Brozek, J.; Crothers, K.; Cooley, L.A.; Dean, N.C.; Fine, M.J.; Flanders, S.A.; et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am. J. Respir. Crit. Care Med. 2019, 200, e45–e67. [Google Scholar] [CrossRef]
- Filippone, E.J.; Kraft, W.K.; Farber, J.L. The nephrotoxicity of vancomycin. Clin. Pharmacol. Ther. 2017, 102, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Cantral, D.E.; Tape, T.G.; Reed, E.C.; Spurzem, J.R.; Rennard, S.I.; Thompson, A.B. Quantitative culture of bronchoalveolar lavage fluid for the diagnosis of bacterial pneumonia. Am. J. Med. 1993, 95, 601–607. [Google Scholar] [CrossRef]
- Parente, D.M.; Cunha, C.B.; Mylonakis, E.; Timbrook, T.T. The clinical utility of methicillin-resistant Staphylococcus aureus (MRSA) nasal screening to rule out MRSA pneumonia: A diagnostic meta-analysis with antimicrobial stewardship implications. Clin. Infect. Dis. 2018, 67, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Giancola, S.E.; Nguyen, A.T. Clinical utility of a nasal swab methicillin-resistant Staphylococcus aureus polymerase chain reaction test in intensive and intermediate care unit patients with pneumonia. Diagn. Microbiol. Infect. Dis. 2016, 86, 307–310. [Google Scholar] [CrossRef]
- Dangerfield, B.; Chung, A.; Webb, B.; Seville, M.T. Predictive value of methicillin-resistant Staphylococcus aureus (MRSA) nasal swab PCR assay for MRSA pneumonia. Antimicrob. Agents. Chemother. 2014, 58, 859–864. [Google Scholar] [CrossRef]
- Smith, M.N.; Erdman, M.J.; Ferrreira, J.A.; Aldridge, P.; Jankowski, C.A. Clinical utility of methicillin-resistant Staphylococcus aureus nasal polymerase chain reaction assay in critically ill patients with nosocomial pneumonia. J. Crit. Care 2017, 38, 168–171. [Google Scholar] [CrossRef]
- Mergenhagen, K.A.; Starr, K.E.; Wattengel, B.A.; Lesse, A.J.; Sumon, Z.; Sellick, J.A. Determining the utility of methicillin-resistant Staphylococcus aureus nares screening in antimicrobial stewardship. Clin. Infect. Dis. 2019, ciz974. [Google Scholar] [CrossRef]
- Hiett, J.; Patel, R.K.; Tate, V.; Smulian, G.; Kelly, A. Using active methicillin-resistant Staphylococcus aureus surveillance nasal swabs to predict clinical respiratory culture results. Am. J. Health Syst. Pharm. 2015, 72, S20–S24. [Google Scholar] [CrossRef]
- Butler-Laporte, G.; De L’Étoile-Morel, S.; Cheng, M.P.; McDonald, E.G.; Lee, T.C. MRSA colonization status as a predictor of clinical infection: A systematic review and meta-analysis. J. Infect. 2018, 77, 489–495. [Google Scholar] [CrossRef]
- Chotiprasitsakul, D.; Tamma, P.D.; Gadala, A.; Cosgove, S.E. The role of negative methicillin-resistant Staphylococcus aureus nasal surveillance Swabs in predicting the need for empiric vancomycin therapy in intensive care unit patients. Infect. Control Hosp. Epidemiol. 2018, 39, 290–296. [Google Scholar] [CrossRef]
- Sarikonda, K.V.; Micek, S.T.; Doherty, J.A.; Reichley, R.M.; Waren, D.; Kollef, M.H. Methicillin-resistant Staphylococcus aureus nasal colonization is a poor predictor of intensive care unit-acquired methicillin-resistant Staphylococcus aureus infections requiring antibiotic treatment. Crit. Care Med. 2010, 38, 1991–1995. [Google Scholar] [CrossRef] [PubMed]
- Akrami, K.; Sweeney, D.A.; Malhotra, A. Antibiotic stewardship in the intensive care unit: Tools for de-escalation from the American Thoracic Society Meeting 2016. J. Thorac. Dis. 2016, 8, S533–S535. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Langsjoen, J.; Brady, C.; Obenauf, E.; Kellie, S. Nasal screening is useful in excluding methicillin-resistant Staphylococcus aureus in ventilator-associated pneumonia. Am. J. Infect. Control 2014, 42, 1014–1015. [Google Scholar] [CrossRef] [PubMed]
- Baby, N.; Faust, A.C.; Smith, T.; Sheperd, L.A.; Knoll, L.; Goodman, E.L. Nasal methicillin-Resistant Staphylococcus aureus (MRSA) PCR testing reduces the duration of MRSA-targeted therapy in patients with suspected MRSA pneumonia. Antimicrob. Agents Chemother. 2017, 61, e02432-16. [Google Scholar] [CrossRef]
- Willis, C.; Allen, B.; Tucker, C.; Rottman, K.; Epps, K. Impact of a pharmacist-driven methicillin-resistant Staphylococcus aureus surveillance protocol. Am. J. Health Syst. Pharm. 2017, 74, 1765–1773. [Google Scholar] [CrossRef]
- Dunaway, S.; Orwig, K.W. Evaluation of a pharmacy-driven methicillin-resistant Staphylococcus aureus surveillance protocol in pneumonia. Int. J. Clin. Pharm. 2018, 40, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Dadzie, P.; Dietrich, T.; Ashurst, J. Impact of a pharmacist-driven methicillin-resistant Staphylococcus aureus polymerase chain reaction nasal swab protocol on the de-escalation of empiric vancomycin in patients with pneumonia in a rural healthcare setting. Cureus 2019, 11, e6378. [Google Scholar] [CrossRef]
- Naughton, C.A. Drug-Induced Nephrotoxicity. Am. Fam. Physician 2008, 78, 743–750. [Google Scholar] [PubMed]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research Electronic Data Capture (REDCap)--a Metadata-Driven Methodology and Workflow Process for Providing Translational Research Informatics Support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap Consortium: Building an International Community of Software Platform Partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]
- Shenoy, E.S.; Noubary, F.; Kim, J.; Rosenberg, E.S.; Cotter, J.A.; Lee, H.; Walensky, R.P.; Hooper, D.C. Concordance of PCR and culture from nasal swabs for detection of methicillin-resistant Staphylococcus aureus in a setting of concurrent antistaphylococcal antibiotics. J. Clin. Microbiol. 2014, 52, 1235–1237. [Google Scholar] [CrossRef]
- Cano, E.L.; Haque, N.Z.; Welch, V.L.; Cely, C.M.; Peyrani, P.; Scerpella, E.G.; Ford, K.D.; Zervos, M.J.; Ramirez, J.A.; Kett, D.H. Incidence of nephrotoxicity and association with vancomycin use in intensive care unit patients with pneumonia: Retrospective analysis of the IMPACT-HAP database. Clin. Ther. 2012, 34, 149–157. [Google Scholar] [CrossRef]
- Hanrahan, T.P.; Harlow, G.; Hutchinson, J.; Dulhunty, J.L.; Whitehouse, T.; Roberts, J.A. Vancomycin-associated nephrotoxicity in the critically ill: A retrospective multivariate regression analysis. Crit. Care Med. 2014, 42, 2527–2536. [Google Scholar] [CrossRef]
- Bamgbola, O. Review of vancomycin-induced renal toxicity: An update. Ther. Adv. Endocrinol. Metab. 2016, 7, 136–147. [Google Scholar] [CrossRef]
- Cowley, M.C.; Ritchie, D.J.; Hampton, N.; Kollef, M.H.; Micek, S.T. Outcomes associated with de-escalating therapy for methicillin-resistant Staphylococcus aureus in culture-negative nosocomial pneumonia. Chest 2019, 155, 53–59. [Google Scholar] [CrossRef] [PubMed]
| Baseline Characteristics | Pre Group (n = 20) | Post Group (n = 42) | p-Value |
|---|---|---|---|
| Male, n (%) | 10 (50) | 24 (57.1) | 0.597 |
| Female, n (%) | 10 (50) | 18 (42.8) | |
| Age, mean (std) | 65.6 (19.9) | 66.9 (23.0) | 0.827 |
| Caucasian, n (%) | 13 (65) | 26 (61.9) | 0.78 |
| Asian, n (%) | 1 (5) | 1 (2.4) | |
| Black or African American, n (%) | 6 (30) | 13 (31.0) | |
| More than one race, n (%) | 0 | 1 (2.4) | |
| Unknown/not reported, n (%) | 0 | 1 (2.4) | |
| BMI, mean (std) | 26.4 (6.2) | 26.4 (6.3) | 0.983 |
| Charlson Comorbidity Index, mean (std) | 6.1 (3.5) | 5.0 (2.7) | 0.256 |
| Creatinine Clearance at Anti-MRSA start, mean (std) | 72.4 (39.8) | 66.7 (28.2) | 0.573 |
| Concomitant Nephrotoxin Usage, n (%) | 20 (100) | 41 (97.6) | − |
| Negative PCR Nasal Swabs, n (%) | 18 (90) | 37 (88.1) | 0.824 |
| PCRNS Endpoints | Pre Group (n = 20) | Post Group (n = 42) | p-Value |
|---|---|---|---|
| Duration of therapy in hours, mean (std) | 90.7 (54.4) | 59.7 (50.4) | 0.039 |
| Hospital length of stay in days, mean (std) | 10.9 (6.2) | 17.3 (48.9) | 0.407 |
| Anti-MRSA therapy restarted, n (%) | 1 (5) | 2 (4.7) | 0.967 |
| In-hospital mortality, n (%) | 2 (10) | 4 (9.5) | 0.953 |
| 30-day readmission, n (%) | 2 (10) | 2 (4.7) | 0.432 |
| Cost in US dollars, median (IQR) | 75.3 (51.7–95.4) | 51.7 (34.1–67.2) | <0.01 |
| Negative PCRNS Endpoints | Pre Group (n = 18) | Post Group (n = 37) | p-Value |
|---|---|---|---|
| Duration of therapy in hours, mean (std) | 96.2 (54.7) | 56.5 (47.7) | 0.014 |
| Cost in US dollars, median (IQR) | 79.4 (55.2–96.1) | 50.1 (33.5–64.2) | <0.01 |
| Length of stay, mean (std) | 11.1 (6.52) | 18.1 (52.1) | 0.425 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huffman, V.; Andrade, D.C.; Ham, J.; Brown, K.; Melnitsky, L.; Lopez Cohen, A.; Parmar, J. Impact of Nasal Swabs on Empiric Treatment of Respiratory Tract Infections (replace into-RTI). Pharmacy 2020, 8, 101. https://doi.org/10.3390/pharmacy8020101
Huffman V, Andrade DC, Ham J, Brown K, Melnitsky L, Lopez Cohen A, Parmar J. Impact of Nasal Swabs on Empiric Treatment of Respiratory Tract Infections (replace into-RTI). Pharmacy. 2020; 8(2):101. https://doi.org/10.3390/pharmacy8020101
Chicago/Turabian StyleHuffman, Vanessa, Diana Carolina Andrade, Jared Ham, Kyle Brown, Leonid Melnitsky, Alejandro Lopez Cohen, and Jayesh Parmar. 2020. "Impact of Nasal Swabs on Empiric Treatment of Respiratory Tract Infections (replace into-RTI)" Pharmacy 8, no. 2: 101. https://doi.org/10.3390/pharmacy8020101
APA StyleHuffman, V., Andrade, D. C., Ham, J., Brown, K., Melnitsky, L., Lopez Cohen, A., & Parmar, J. (2020). Impact of Nasal Swabs on Empiric Treatment of Respiratory Tract Infections (replace into-RTI). Pharmacy, 8(2), 101. https://doi.org/10.3390/pharmacy8020101





