Implementation of a Pharmacist-Led Transitions of Care Program within a Primary Care Practice: A Two-Phase Pilot Study
Abstract
1. Introduction
2. Program Implementation and Evaluation
2.1. Setting
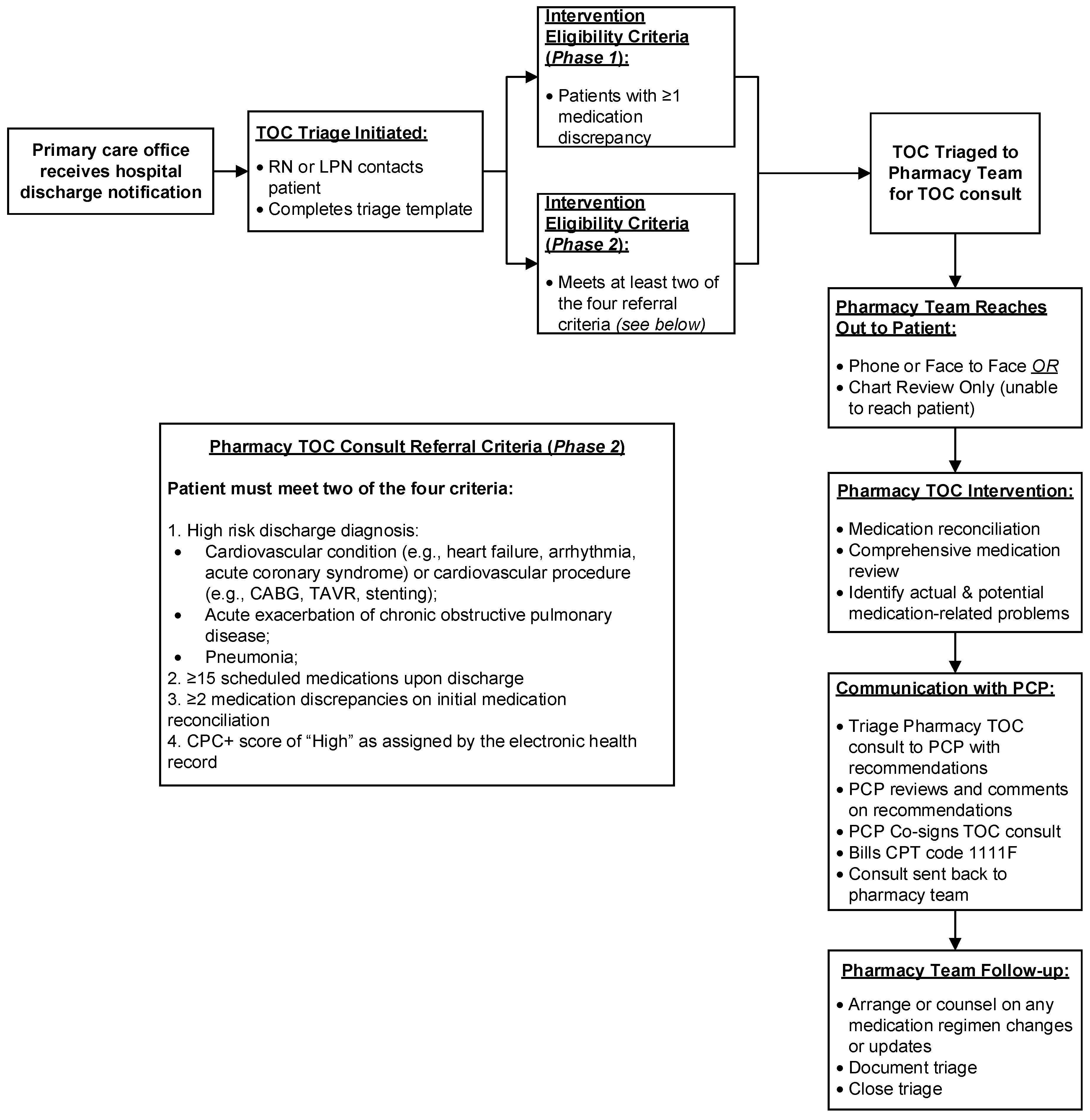
2.2. Phase 1–Implementation of Pharmacist TOC Services and Continuous Quality Improvement
- Review the participant’s medication records at the practice and on the discharge summary and reconcile any differences;
- Perform a comprehensive review to identify medication-related problems, assess medication adherence, and discuss any medication changes made during the hospital admission;
- Provide recommendations to the PCP and discuss future management plans;
- Follow-up with the participant (if needed) and counsel on any changes made to the medication regimen;
- Finalize updates within the participant’s EHR.
2.3. Phase 2–Process Refinement: Intervention Focus on High-Risk Patients
- (1)
- High-risk discharge diagnosis:
- Cardiovascular condition (e.g., heart failure, arrhythmia, acute coronary syndrome) or cardiovascular procedure (e.g., coronary artery bypass grafting, transcatheter aortic valve replacement, stenting);
- Acute exacerbation of chronic obstructive pulmonary disease;
- Pneumonia;
- (2)
- ≥15 scheduled medications upon hospital discharge;
- (3)
- ≥2 medication discrepancies on initial medication reconciliation;
- (4)
- Comprehensive primary care plus (CPC+) risk level of “high” as assigned by the EHR.
2.4. Program Evaluation
3. Clinical Results and Process Measures
3.1. Study Sample
3.2. Pharmacist TOC Interventions and Acceptance Rates
3.3. Percentage of 30-Day Hospital Readmissions
3.4. Odds Ratios of 30-day Hospital Readmissions between Intervention (Phase 1 & 2) and Usual Care Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
References
- The Joint Comission. Transitions of Care: The Need for a More Effective Approach to Continuing Patient Care. Available online: https://www.jointcommission.org/assets/1/18/Hot_Topics_Transitions_of_Care.pdf (accessed on 1 June 2019).
- Jencks, S.F.; Williams, M.V.; Coleman, E.A. Rehospitalizations among patients in the Medicare fee-for-service program. N. Engl. J. Med. 2009, 360, 1418–1428. [Google Scholar] [CrossRef] [PubMed]
- Hawes, E.M.; Maxwell, W.D.; White, S.F.; Mangun, J.; Lin, F.C. Impact of an outpatient pharmacist intervention on medication discrepancies and health care resource utilization in posthospitalization care transitions. J. Prim. Care Community Health 2014, 5, 14–18. [Google Scholar] [CrossRef] [PubMed]
- McIlvennan, C.K.; Eapen, Z.J.; Allen, L.A. Hospital readmissions reduction program. Circulation 2015, 131, 1796–1803. [Google Scholar] [CrossRef] [PubMed]
- Forster, A.J.; Murff, H.J.; Peterson, J.F.; Gandhi, T.K.; Bates, D.W. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann. Intern. Med. 2003, 138, 161–167. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, P.J.; Jacobs, M.R. Hospital admissions resulting from preventable adverse drug reactions. Ann. Pharmacother. 2002, 36, 1331–1336. [Google Scholar] [CrossRef]
- Schnipper, J.L.; Kirwin, J.L.; Cotugno, M.C.; Wahlstrom, S.A.; Brown, B.A.; Tarvin, E.; Kachalia, A.; Horng, M.; Roy, C.L.; McKean, S.C.; et al. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch. Intern. Med. 2006, 166, 565–571. [Google Scholar] [CrossRef]
- Walker, P.C.; Bernstein, S.J.; Jones, J.N.; Piersma, J.; Kim, H.W.; Regal, R.E.; Kuhn, L.; Flanders, S.A. Impact of a pharmacist-facilitated hospital discharge program: A quasi-experimental study. Arch. Intern. Med. 2009, 169, 2003–2010. [Google Scholar] [CrossRef]
- Coleman, E.A.; Smith, J.D.; Raha, D.; Min, S.J. Posthospital medication discrepancies: Prevalence and contributing factors. Arch. Intern. Med. 2005, 165, 1842–1847. [Google Scholar] [CrossRef]
- Wimmer, B.C.; Dent, E.; Bell, J.S.; Wiese, M.D.; Chapman, I.; Johnell, K.; Visvanathan, R. Medication Regimen Complexity and Unplanned Hospital Readmissions in Older People. Ann. Pharm. 2014, 48, 1120–1128. [Google Scholar] [CrossRef]
- Davies, E.C.; Green, C.F.; Mottram, D.R.; Rowe, P.H.; Pirmohamed, M. Emergency re-admissions to hospital due to adverse drug reactions within 1 year of the index admission. Br. J. Clin. Pharmacol. 2010, 70, 749–755. [Google Scholar] [CrossRef]
- Harris, C.M.; Sridharan, A.; Landis, R.; Howell, E.; Wright, S. What Happens to the Medication Regimens of Older Adults During and After an Acute Hospitalization? J. Patient Saf. 2013, 9, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Mekonnen, A.B.; McLachlan, A.J.; Brien, J.A. Pharmacy-led medication reconciliation programmes at hospital transitions: A systematic review and meta-analysis. J. Clin. Pharm. Ther. 2016, 41, 128–144. [Google Scholar] [CrossRef] [PubMed]
- Ensing, H.T.; Stuijt, C.C.; van den Bemt, B.J.; van Dooren, A.A.; Karapinar-Carkit, F.; Koster, E.S.; Bouvy, M.L. Identifying the Optimal Role for Pharmacists in Care Transitions: A Systematic Review. J. Manag. Care Spec. Pharm. 2015, 21, 614–636. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Colayco, D.; Hashimoto, J.; Komoto, K.; Gowda, C.; Wearda, B.; McCombs, J. Impact of a pharmacy-based transitional care program on hospital readmissions. Am. J. Manag. Care 2017, 23, 170–176. [Google Scholar] [PubMed]
- Slazak, E.; Cardinal, C.; Will, S.; Clark, C.M.; Daly, C.J.; Jacobs, D.M. Pharmacist-led Transitions of Care Services in Primary Care Settings: Opportunities, Experiences, and Challenges. J. Am. Pharm. Assoc. 2019. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic. Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Van Walraven, C.; Dhalla, I.A.; Bell, C.; Etchells, E.; Stiell, I.G.; Zarnke, K.; Austin, P.C.; Forster, A.J. Derivation and validation of an index to predict early death or unplanned readmission after discharge from hospital to the community. CMAJ 2010, 182, 551–557. [Google Scholar] [CrossRef]
- Fennelly, J.E.; Coe, A.B.; Kippes, K.A.; Remington, T.L.; Choe, H.M. Evaluation of Clinical Pharmacist Services in a Transitions of Care Program Provided to Patients at Highest Risk for Readmission. J. Pharm. Pract. 2018. [Google Scholar] [CrossRef]
- Proctor, E.; Silmere, H.; Raghavan, R.; Hovmand, P.; Aarons, G.; Bunger, A.; Griffey, R.; Hensley, M. Outcomes for implementation research: Conceptual distinctions, measurement challenges, and research agenda. Adm. Policy Ment. Health 2011, 38, 65–76. [Google Scholar] [CrossRef]
- Hawes, E.M.; Smith, J.N.; Pinelli, N.R.; Adams, R.; Tong, G.; Weir, S.; Gwynne, M. Accountable Care in Transitions (ACTion): A Team-Based Approach to Reducing Hospital Utilization in a Patient-Centered Medical Home. J. Pharm. Pract. 2018, 31, 175–182. [Google Scholar] [CrossRef]
- Hawes, E.M.; Pinelli, N.R.; Sanders, K.A.; Lipshutz, A.M.; Tong, G.; Sievers, L.S.; Chao, S.; Gwynne, M. Post-Hospital Discharge Care: A Retrospective Cohort Study Exploring the Value of Pharmacist-Enhanced Care and Describing Medication-Related Problems. N. C. Med. J. 2018, 79, 4–13. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kilcup, M.; Schultz, D.; Carlson, J.; Wilson, B. Postdischarge pharmacist medication reconciliation: Impact on readmission rates and financial savings. J. Am. Pharm. Assoc. 2013, 53, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Herges, J.R.; Herges, L.B.; Dierkhising, R.A.; Mara, K.C.; Davis, A.Z.; Angstman, K.B. Effect of Postdismissal Pharmacist Visits for Patients Using High-Risk Medications. Mayo Clin. Proc. Innov. Qual. Outcomes 2018, 2, 4–9. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cavanaugh, J.J.; Jones, C.D.; Embree, G.; Tsai, K.; Miller, T.; Shilliday, B.B.; McGuirt, B.; Roche, R.; Pignone, M.; DeWalt, D.A.; et al. Implementation Science Workshop: Primary care-based multidisciplinary readmission prevention program. J. Gen. Intern. Med. 2014, 29, 798–804. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhou, H.; Della, P.R.; Roberts, P.; Goh, L.; Dhaliwal, S.S. Utility of models to predict 28-day or 30-day unplanned hospital readmissions: An updated systematic review. BMJ Open 2016, 6, e011060. [Google Scholar] [CrossRef]
- Kansagara, D.; Englander, H.; Salanitro, A.; Kagen, D.; Theobald, C.; Freeman, M.; Kripalani, S. Risk prediction models for hospital readmission: A systematic review. JAMA 2011, 306, 1688–1698. [Google Scholar] [CrossRef]
- Donze, J.D.; Williams, M.V.; Robinson, E.J.; Zimlichman, E.; Aujesky, D.; Vasilevskis, E.E.; Kripalani, S.; Metlay, J.P.; Wallington, T.; Fletcher, G.S.; et al. International Validity of the HOSPITAL Score to Predict 30-Day Potentially Avoidable Hospital Readmissions. JAMA Intern. Med. 2016, 176, 496–502. [Google Scholar] [CrossRef]
| Characteristic | Control n = 118 | Phase 1 n = 101 | Phase 2 n = 37 | p |
|---|---|---|---|---|
| Age, median (IQR) | 69 (58, 78) | 68 (58, 78) | 70 (61, 81) | 0.67 |
| Gender | 0.46 | |||
| Male | 44 (37.3) | 39 (38.6) | 18 (48.7) | |
| Female | 74 (62.7) | 62 (61.4) | 19 (51.4) | |
| Race | 0.07 | |||
| White | 75 (63.6) | 53 (52.5) | 29 (78.4) | |
| Black | 40 (33.9) | 46 (45.5) | 8 (21.6) | |
| Other | 3 (2.5) | 2 (1.9) | 0 | |
| Prescription insurance | 0.96 | |||
| Medicare | 69 (58.5) | 61 (60.4) | 21 (56.8) | |
| Commercial | 37 (31.4) | 28 (27.7) | 11 (29.7) | |
| Medicaid | 12 (10.2) | 12 (11.9) | 5 (13.5) | |
| CCI score, mean (SD) | 4.2 (2.4) | 4.6 (2.8) | 5.3 (2.8) | 0.10 |
| median (IQR) | 4 (3, 6) | 4 (3, 6) | 5 (4, 7) | |
| No. medications at discharge, median (IQR) | 10 (7, 14) | 11 (8, 17) | 11 (7, 16) | 0.04 * |
| Discharged from | 0.14 | |||
| Hospital | 113 (95.8) | 90 (89.1) | 33 (89.2) | |
| Rehabilitation facility | 5 (4.2) | 11 (10.9) | 4 (10.8) | |
| Length of stay (days), Median (IQR) | 2 (1, 4) | 3 (2, 5) | 3 (2, 4) | <0.001 ^ |
| No. ED visits during previous 6 months, median (IQR) | 0 (0, 1) | 0 (0, 1) | 0 (0, 1) | 0.15 |
| Discharge diagnosis | 0.05 | |||
| CCVD or related procedure | 18 (15.3) | 22 (21.8) | 14 (37.8) | |
| COPD | 2 (1.7) | 4 (3.9) | 0 | |
| Pneumonia | 3 (2.5) | 5 (4.9) | 2 (5.4) | |
| Other | 95 (80.5) | 70 (69.3) | 21 (56.8) | |
| LACE Index | <0.0001 # | |||
| Low | 9 (7.6) | 3 (2.9) | 2 (5.4) | |
| Moderate | 79 (66.9) | 38 (37.6) | 9 (24.3) | |
| High | 30 (25.4) | 60 (59.4) | 26 (70.3) | |
| Intervention delivery | 0.11 | |||
| Face-to-face | - | 3 (2.9) | 4 (10.8) | |
| Telephonic | - | 59 (58.4) | 23 (62.2) | |
| No encounter | - | 30 (38.6) | 10 (27.0) | |
| Pharmacist intervention time, minutes, mean (SD) | - | - | 33 (9.5) | |
| Days from discharge to intervention, median (IQR) | - | 3 (2, 5) | 6 (4, 8) | <0.0001 |
| Interventions | Phase 1 n = 252 | Phase 2 n = 92 | p |
|---|---|---|---|
| Overall acceptance rate | 198 (78.6) | 75 (81.5) | 0.55 |
| Accepted Interventions | |||
| Optimize therapy | 55 (79.7) | 15 (78.9) | 0.94 |
| Recommend monitoring | 43 (87.8) | 21 (87.5) | 0.97 |
| Discontinued/hold medication | 27 (62.8) | 5 (71.4) | 0.66 |
| Initiate medication | 24 (77.4) | 8 (57.1) | 0.16 |
| Optimize dose | 13 (54.2) | 11 (84.6) | 0.06 |
| Counsel on medication and/or adherence | 22 (100) | 11 (100) | - |
| Improve medication access | 14 (100) | 4 (100) | - |
| Type of Readmission | Control n = 118 | Phase 1 n = 101 | p * | Phase 2 n = 37 | p ^ | p# |
|---|---|---|---|---|---|---|
| All-cause | 11 (9.3) | 17 (16.8) | 0.09 | 5 (13.5) | 0.46 | 0.63 |
| Clinically-related | 3 (2.5) | 9 (8.9) | 0.04 | 4 (10.8) | 0.03 | 0.73 |
| Groups | OR | 95% CI | p | aOR | 95% CI | p |
|---|---|---|---|---|---|---|
| All-cause 30-day hospital readmissions | ||||||
| Phase 1 * | 1.97 | 0.88–4.43 | 0.10 | 1.20 | 0.49–2.95 | 0.69 |
| Phase 2 * | 1.52 | 0.49–4.70 | 0.47 | 0.78 | 0.21–2.89 | 0.71 |
| Phase 2 ^ | 0.77 | 0.26–2.27 | 0.64 | 0.99 | 0.30–3.37 | 0.99 |
| Phase 1 | Ref. | - | - | Ref. | - | - |
| Clinically-related 30-day hospital readmissions | ||||||
| Phase 1 * | 3.75 | 0.99–14.2 | 0.052 | 2.87 | 0.69–11.9 | 0.15 |
| Phase 2 * | 4.65 | 0.99–21.8 | 0.051 | 4.11 | 0.52–32.3 | 0.18 |
| Phase 2 ^ | 1.24 | 0.36–4.30 | 0.73 | 1.46 | 0.37–5.76 | 0.59 |
| Phase 1 ^ | Ref. | - | - | Ref. | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slazak, E.; Shaver, A.; Clark, C.M.; Cardinal, C.; Panthapattu, M.; Prescott, W.A., Jr.; Will, S.; Jacobs, D.M. Implementation of a Pharmacist-Led Transitions of Care Program within a Primary Care Practice: A Two-Phase Pilot Study. Pharmacy 2020, 8, 4. https://doi.org/10.3390/pharmacy8010004
Slazak E, Shaver A, Clark CM, Cardinal C, Panthapattu M, Prescott WA Jr., Will S, Jacobs DM. Implementation of a Pharmacist-Led Transitions of Care Program within a Primary Care Practice: A Two-Phase Pilot Study. Pharmacy. 2020; 8(1):4. https://doi.org/10.3390/pharmacy8010004
Chicago/Turabian StyleSlazak, Erin, Amy Shaver, Collin M. Clark, Courtney Cardinal, Merin Panthapattu, William A. Prescott, Jr., Samantha Will, and David M. Jacobs. 2020. "Implementation of a Pharmacist-Led Transitions of Care Program within a Primary Care Practice: A Two-Phase Pilot Study" Pharmacy 8, no. 1: 4. https://doi.org/10.3390/pharmacy8010004
APA StyleSlazak, E., Shaver, A., Clark, C. M., Cardinal, C., Panthapattu, M., Prescott, W. A., Jr., Will, S., & Jacobs, D. M. (2020). Implementation of a Pharmacist-Led Transitions of Care Program within a Primary Care Practice: A Two-Phase Pilot Study. Pharmacy, 8(1), 4. https://doi.org/10.3390/pharmacy8010004





