Prescribing and Safety of Direct-Acting Oral Anticoagulants Compared to Warfarin in Patients with Atrial Fibrillation on Chronic Hemodialysis
Abstract
1. Introduction
2. Materials and Methods
3. Results
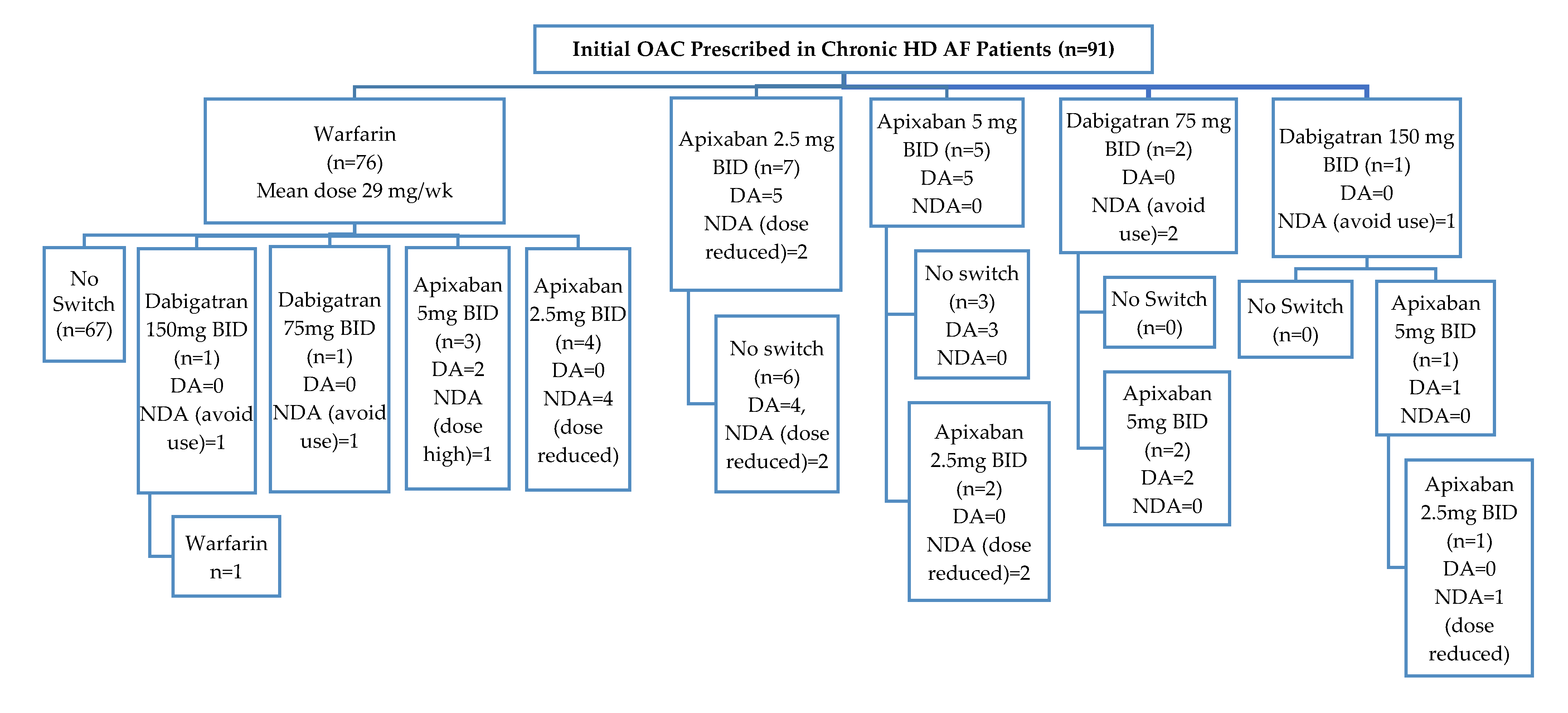
OAC Prescribing
4. Discussion
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Dabigatran | Rivaroxaban | Apixaban | Edoxaban | |
|---|---|---|---|---|
| Normal Dose | 150 mg by mouth twice daily | 20 mg by mouth daily | 5 mg by mouth twice daily | 60 mg by mouth daily |
| CrCl 15–50 mL/min | - | 15 by mouth mg daily | 2.5 mg by mouth twice daily when two of three dose reduction criteria are met: SCr ≥ 1.5 mg/dL, age ≥ 80 years, body weight ≤ 60 kg | 30 mg by mouth daily |
| CrCl 15–30 mL/min | 75 mg by mouth twice daily | - | - | |
| Hemodialysis or CrCl < 15 mL/min | No dosing recommendations | No dosing recommendations | Not recommended |
References
- CDC. Chronic Kidney Disease in the United States. Available online: https://www.cdc.gov/kidneydisease/publications-resources/2019-national-facts.html (accessed on 18 December 2019).
- CDC: Division for Heart Disease and Stroke Prevention-Atrial Fibrillation Fact Sheet. Available online: http://www.cdc.gov/dhdsp/data_statistics/fact_sheets/fs_atrial_fibrillation.htm (accessed on 18 December 2019).
- Lau, Y.C.; Proietti, M.; Guiducci, E.; Blann, A.D.; Lip, G.Y. Atrial fibrillation and thromboembolism in patients with chronic kidney disease. J. Am. Coll. Cardiol. 2016, 68, 1452–1464. [Google Scholar] [CrossRef] [PubMed]
- United States Renal Data System. 2018 USRDS Annual Data Report: Chapter 4: Cardiovascular Disease of Patients with CKD; National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2018. Available online: https://www.usrds.org/2018/view/v1_04.aspx (accessed on 18 December 2019).
- Bonde, A.N.; Lip, G.Y.; Kamper, A.L.; Hansen, P.R.; Lamberts, M.; Hommel, K.; Olesen, J.B. Net clinical benefit of anthrombotic therapy in patients with atrial fibrillation and chronic kidney disease: A nationwide observational cohort study. J. Am. Coll. Cardiol. 2014, 64, 2471–2482. [Google Scholar] [CrossRef] [PubMed]
- Olesen, J.B.; Lip, G.Y.; Kamper, A.L.; Hommel, K.; Køber, L.; Lane, D.A.; Torp-Pedersen, C. Stroke and bleeding in atrial fibrillation with chronic kidney disease. N. Engl. J. Med. 2012, 367, 625–635. [Google Scholar] [CrossRef] [PubMed]
- January, C.T.; Wann, L.S.; Alpert, J.S.; Calkins, H.; Cigarroa, J.E.; Cleveland, J.C.; Murray, K.T. 2014 AHA/ACC/HRS Guideline for the Management of Patients with Atrial Fibrillation: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2014, 64, 2246–2280. [Google Scholar] [CrossRef]
- January, C.T.; Wann, L.S.; Calkins, H.; Chen, L.Y.; Cigarroa, J.E.; Cleveland, J.C.; Heidenreich, P.A. AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients with Atrial Fibrillation. J. Am. Coll. Cardiol. 2019, 74, 104–132. [Google Scholar] [CrossRef]
- Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; Eikelboom, J.; Oldgren, J.; Parekh, A.; Wang, S. Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2009, 361, 1139–1151. [Google Scholar] [CrossRef]
- Patel, M.R.; Mahaffey, K.W.; Garg, J.; Pan, G.; Singer, D.E.; Hacke, W.; Becker, R.C. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N. Engl. J. Med. 2011, 365, 883–891. [Google Scholar] [CrossRef]
- Granger, C.B.; Alexander, J.H.; McMurray, J.J.; Lopes, R.D.; Hylek, E.M.; Hanna, M.; Bahit, M.C. Apixaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2011, 365, 981–992. [Google Scholar] [CrossRef]
- Giugliano, R.P.; Ruff, C.T.; Braunwald, E.; Murphy, S.A.; Wiviott, S.D.; Halperin, J.L.; Ruzyllo, W. Edoxaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2013, 369, 2093–2104. [Google Scholar] [CrossRef]
- You, J.J.; Singer, D.E.; Howard, P.A.; Lane, D.A.; Eckman, M.H.; Fang, M.C.; Spencer, F.A. Antithrombotic therapy for atrial fibrillation: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed.: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141, e531S–e575S. [Google Scholar] [CrossRef]
- Chan, K.E.; Edelman, E.R.; Wenger, J.B.; Thadhani, R.I.; Maddux, F.W. Dabigatran and rivaroxaban use in atrial fibrillation patients on hemodialysis. Circulation 2015, 131, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Cockroft, D.W.; Gault, M.H. Prediction of creatinine clearance from serum creatinine. Nephron 1976, 16, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Lip, G.Y.; Nieuwlaat, R.; Pisters, R.; Lane, D.A.; Crijns, H.J. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The Euro Heart Survey on Atrial Fibrillation. Chest 2010, 137, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Pisters, R.; Lane, D.A.; Nieuwlaat, R.; De Vos, C.B.; Crijns, H.J.; Lip, G.Y. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The Euro Heart Survey. Chest 2010, 138, 1093–1100. [Google Scholar] [CrossRef]
- Pradaxa; Boehringer Ingelheim: Rhein, Germany, 2010; Available online: https://docs.boehringer-ingelheim.com/Prescribing%20Information/PIs/Pradaxa/Pradaxa.pdf (accessed on 18 December 2019).
- Xarelto; Janssen Pharmaceuticals: Beerse, Belgium, 2011; Available online: http://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/XARELTO-pi.pdf (accessed on 18 December 2019).
- Savaysa; Daiichi Sankyo: Parsippany, NJ, USA, 2015; Available online: https://dsi.com/prescribing-information-portlet/getPIContent?productName=Savaysa&inline=true (accessed on 18 December 2019).
- Eliquis; Bristol-Myers Squibb: New York, NY, USA, 2012; Available online: https://packageinserts.bms.com/pi/pi_eliquis.pdf (accessed on 18 December 2019).
- Schulman, S.; Kearon, C. Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J. Thromb. Haemost. 2005, 3, 692–694. [Google Scholar]
- Siontis, K.C.; Zhang, X.; Eckard, A.; Bhave, N.; Schaubel, D.E.; He, K.; Noseworthy, P.A. Outcomes associated with apixaban use in end-stage kidney disease patients with atrial fibrillation in the united states. Circulation 2018, 138, 1519–1529. [Google Scholar] [CrossRef]
- Chan, K.E.; Giugliano, R.P.; Patel, M.R.; Abramson, S.; Jardine, M.; Zhao, S.; Piccini, J.P. Nonvitamin K anticoagulant agents in patients with advanced chronic kidney disease or on dialysis with AF. J. Am. Coll. Cardiol. 2016, 67, 2888–2899. [Google Scholar] [CrossRef]
- Lip, G.Y.; Banerjee, A.; Boriani, G.; en Chiang, C.; Fargo, R.; Freedman, B.; Patel, S. Antithrombotic therapy for atrial fibrillation: CHEST guideline and expert panel report. Chest 2018, 154, 1121–1201. [Google Scholar] [CrossRef]
- Dias, C.; Moore, K.T.; Murphy, J.; Ariyawansa, J.; Smith, W.; Mills, R.M.; Weir, M.R. Pharmacokinetics, pharmacodynamics, and safety of single-dose rivaroxaban in chronic hemodialysis. Am. J. Nephrol. 2016, 43, 229–236. [Google Scholar] [CrossRef]
- Wang, X.; Tirucherai, G.; Marbury, T.C.; Wang, J.; Chang, M.; Zhang, D.; Frost, C. Pharmacokinetics, pharmacodynamics, and safety of apixaban in subjects with end-stage renal disease on hemodialysis. J. Clin. Pharmacol. 2016, 56, 628–636. [Google Scholar] [CrossRef]
- Reed, D.; Palkimas, S.; Hockman, R.; Abraham, S.; Le, T.; Maitland, H. Safety and effectiveness of apixaban compared to warfarin in dialysis patients. Res. Pract. Thromb. Haemost. 2018, 2, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Shah, N.D.; Sangaralingham, L.R.; Gersh, B.J.; Noseworthy, P.A. Non-vitamin K antagonist oral anticoagulant dosing in patients with atrial fibrillation and renal dysfunction. J. Am. Coll. Cardiol. 2017, 69, 2279–2290. [Google Scholar] [CrossRef] [PubMed]
- Pokorney, S.D. RENal hemodialysis patients Allocated apixaban versus warfarin in atrial fibrillation (RENAL-AF) study. In Proceedings of the American Heart Association Annual Scientific Sessions (AHA 2019), Philadelphia, PA, USA, 16 November 2019; Available online: https://www.acc.org/latest-in-cardiology/clinical-trials/2019/11/15/17/29/renal-af (accessed on 18 December 2019).
- Reinecke, H.; Jürgensmeyer, S.; Engelbertz, C.; Gerss, J.; Kirchhof, P.; Breithardt, G.; Wanner, C. Design and rationale of a randomized controlled trial comparing apixaban to phenprocoumon in patients with atrial fibrillation on chronic haemodialysis: The AXADIA-AFNET 8 study. BMJ Open 2018, 8, e022690. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Overall (n = 91) | Warfarin (n = 76) | DOAC (n = 15) | p-Value |
|---|---|---|---|---|
| Age (years), mean ± SD | 69 ± 10.7 | 68.3 ± 11.0 | 73 ± 8.4 | 0.116 |
| Gender (n), %: Male | 47 (52%) | 40 (53%) | 7 (47%) | 0.673 |
| Ethnicity (n), %: Caucasian | 72 (79%) | 60 (79%) | 12 (80%) | 0.617 |
| Weight (kg), mean ± SD | 91 ± 23.5 | 90.3 ± 23.5 | 92.6± 4.3 | 0.730 |
| SCr (mg/dL), mean ± SD | 5.3 ± 2 | 5.4 ± 2.1 | 4.9 ± 1.3 | 0.447 |
| CrCl (mL/min), mean ± SD | 17.6 ± 8.5 | 17.7 ± 8.6 | 17.4 ± 8.2 | 0.903 |
| CHA2DS2VASc score, mean ± SD | 4.6 ± 1.5 | 4.6 ± 1.4 | 4.4 ± 1.9 | 0.650 |
| HAS-BLED score, mean ± SD | 3.8 ± 1.1 | 3.9 ± 1.1 | 3.6 ± 1.1 | 0.404 |
| Patient Number | OAC at Time of Bleed | Cumulative Duration of OAC | DOAC Dosed Appropriately? | HAS-BLED Score | Concurrent Antiplatelet Medication | Warfarin % Values in Subtherapeutic Range (SubR%), Therapeutic Range (TR%), Supratherapeutic Range (SupraR%) | Major Bleed: Type of Bleed, INR (If Documented), Other Comments | CRNMB/Minor Bleed: Type of Bleed, INR (If Documented), Other Comments | ||
|---|---|---|---|---|---|---|---|---|---|---|
| SubR% | TR% | SupraR% | ||||||||
| 1 | Warfarin | 6 mos | - | 4 | ASA 81 mg | 40 | 20 | 40 | GI bleed, INR 1.2 | - |
| 2 | Warfarin | 13 mos | - | 4 | None | 23 | 46 | 31 | - | GI bleed ×2 |
| 3 | Warfarin | 54 mos | - | 3 | None | 41 | 37 | 22 | - | GI bleed |
| 3 | Warfarin | 12 mos | - | 2 | None | 10 | 63 | 27 | - | GI bleed |
| 5 | Warfarin | 44 mos | - | 4 | None | 68 | 27 | 5 | - | Epistaxis ×2 |
| 6 | Warfarin | 21 mos | - | 3 | None | 32 | 32 | 36 | Hematoma arm requiring surgical intervention (Transfuse 2U PRBC), INR 5.6 | Epistaxis ×4 |
| 7 | Warfarin | 20 mos | - | 3 | None | 18 | 40 | 42 | Hematoma leg—(Transfuse 4U PRBC, Reduction Hgb ≥ 2 g/dL) GI bleed—(Transfuse 4U PRBC, Reduction Hgb ≥ 2 g/dL) Hemoptysis—(Transfuse 2U PRBC) | - |
| 8 | Warfarin | 49 mos | - | 6 | ASA 81 mg | 35 | 42 | 23 | - | Hemoptysis |
| 9 | Warfarin | 7 mos | - | 4 | None | 31 | 26 | 43 | Urologic bleed—(Transfuse 2U PRBC, Reduction Hgb ≥ 2 g/dL) | - |
| 10 | Warfarin | 5 mos | - | 2 | ASA 81 mg | 6 | 50 | 44 | - | HD access site ×2 |
| 11 | Warfarin | 48 mos | - | 4 | ASA 81 mg | 40 | 60 | 0 | - | Epistaxis |
| 12 | Warfarin | 43 mos | - | 4 | ASA 81 mg, Clopidogrel 75 mg | 50 | 50 | 0 | - | HD access site, Urologic |
| 13 | Warfarin | 8 mos | - | 3 | ASA 81 mg | 58 | 16 | 26 | - | Hemoptysis, INR 7.8 |
| 14 | Warfarin | 73 mos | - | 3 | None | 32 | 47 | 21 | GI bleed—(Transfuse 3U PRBC) | - |
| 15 | Warfarin | 9 mos | - | 2 | None | 22 | 57 | 21 | GI bleed—(Transfuse 2U PRBC), INR > 8.8 | HD access site, Hematoma |
| 16 | Warfarin | 4 mos | - | 4 | ASA 325 mg | 29 | 57 | 14 | GI bleed—(Transfuse 2U PRBC, Reduction Hgb ≥ 2 g/dL), INR 7.1 | - |
| 17 | Warfarin | 10 mos | - | 5 | ASA 81 mg | 2 | 50 | 48 | - | Epistaxis |
| 18 | Warfarin | 8 mos | - | 3 | None | 13 | 54 | 33 | GI bleed—(Transfuse 2U PRBC, Reduction Hgb ≥ 2 g/dL) | - |
| 19 | Warfarin | 1 month | - | 5 | ASA 81 mg | 36 | 24 | 40 | - | HD access site |
| Dabigatran 150 BID | 1 month | No, avoid use | See above | ASA 81 mg | - | - | - | - | GI bleed, Bruising | |
| Warfarin | 5 mos | - | See above | ASA 81 mg | See above | See above | See above | GI bleed, Death/Sepsis | - | |
| 20 | Warfarin | 52 mos | - | 3 | None | 77 | 10 | 13 | - | - |
| Dabigatran 75 BID | 1 month | No, avoid use | See above | None | - | - | - | GI bleed-(Transfuse 2U PRBC, Reduction Hgb ≥ 2 g/dL) | - | |
| 21 | Warfarin | 1 month | - | 4 | ASA 81 mg | 0 | 33 | 67 | GI bleed-(Transfuse 4U PRBC, Reduction Hgb ≥ 2 g/dL) | - |
| Apixaban 2.5 BID | 2 mos | No, dose reduced | See above | ASA 81 mg | - | - | - | - | - | |
| 22 | Warfarin | 47 mos | - | 3 | ASA 81 mg | 37 | 58 | 5 | - | HD access site ×2 |
| Apixaban 2.5 BID | 14 mos | No, dose reduced | See above | None | - | - | - | - | - | |
| 23 | Warfarin | 2 mos | - | 5 | ASA 81 mg | 67 | 33 | 0 | - | - |
| Apixaban 2.5 BID | 1 month | No, dose reduced | See above | ASA 81 mg | - | - | - | GI bleed (Reduction Hgb ≥ 2 g/dL) | - | |
| 24 | Dabigatran 150 BID | 7 days | No, avoid use | 5 | ASA 81 mg | - | - | - | - | GI bleed |
| Apixaban 5 BID | 15 days | Yes | See above | ASA 81 mg | - | - | - | GI bleed (Transfuse 2U PRBC, Reduction Hgb ≥ 2 g/dL) | - | |
| Apixaban 2.5 BID | 24 mos | No, dose reduced | See above | None | - | - | - | - | - | |
| 25 | Apixaban 2.5 BID | 9 mos | Yes | 4 | ASA 81 mg | - | - | - | - | HD access site |
| 26 | Apixaban 2.5 BID | 2 mos | Yes | 2 | None | - | - | - | - | GI bleed |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davis, E.; Darais, D.; Fuji, K.; Nekola, P.; Bashir, K. Prescribing and Safety of Direct-Acting Oral Anticoagulants Compared to Warfarin in Patients with Atrial Fibrillation on Chronic Hemodialysis. Pharmacy 2020, 8, 37. https://doi.org/10.3390/pharmacy8010037
Davis E, Darais D, Fuji K, Nekola P, Bashir K. Prescribing and Safety of Direct-Acting Oral Anticoagulants Compared to Warfarin in Patients with Atrial Fibrillation on Chronic Hemodialysis. Pharmacy. 2020; 8(1):37. https://doi.org/10.3390/pharmacy8010037
Chicago/Turabian StyleDavis, Estella, Dallin Darais, Kevin Fuji, Paige Nekola, and Khalid Bashir. 2020. "Prescribing and Safety of Direct-Acting Oral Anticoagulants Compared to Warfarin in Patients with Atrial Fibrillation on Chronic Hemodialysis" Pharmacy 8, no. 1: 37. https://doi.org/10.3390/pharmacy8010037
APA StyleDavis, E., Darais, D., Fuji, K., Nekola, P., & Bashir, K. (2020). Prescribing and Safety of Direct-Acting Oral Anticoagulants Compared to Warfarin in Patients with Atrial Fibrillation on Chronic Hemodialysis. Pharmacy, 8(1), 37. https://doi.org/10.3390/pharmacy8010037





