Safety and Efficacy of Direct Oral Anticoagulants for Atrial Fibrillation in Patients with Renal Impairment
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Data Collection
2.3. Outcome
2.4. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Markides, V.; Schilling, R.J. Atrial fibrillation: classification, pathophysiology, mechanisms and drug treatment. Heart 2003, 89, 939–943. [Google Scholar] [CrossRef]
- Nayak-Rao, S.; Shenoy, M.P. Stroke in Patients with Chronic Kidney Disease...: How do we Approach and Manage it? Indian J. Nephrol. 2017, 27, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; de Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef] [PubMed]
- European Heart Rhythm, A.; European Association for Cardio-Thoracic, S.; Camm, A.J.; Kirchhof, P.; Lip, G.Y.; Schotten, U.; Savelieva, I.; Ernst, S.; Van Gelder, I.C.; Al-Attar, N.; et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur. Heart J. 2010, 31, 2369–2429. [Google Scholar] [CrossRef]
- Olesen, J.B.; Lip, G.Y.; Kamper, A.L.; Hommel, K.; Kober, L.; Lane, D.A.; Lindhardsen, J.; Gislason, G.H.; Torp-Pedersen, C. Stroke and bleeding in atrial fibrillation with chronic kidney disease. N. Engl. J. Med. 2012, 367, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Massicotte-Azarniouch, D.; Bader Eddeen, A.; Lazo-Langner, A.; Molnar, A.O.; Lam, N.N.; McCallum, M.K.; Bota, S.; Zimmerman, D.; Garg, A.X.; Harel, Z.; et al. Risk of Venous Thromboembolism in Patients by Albuminuria and Estimated GFR. Am. J. Kidney Dis. 2017, 70, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; Eikelboom, J.; Oldgren, J.; Parekh, A.; Pogue, J.; Reilly, P.A.; Themeles, E.; Varrone, J.; et al. Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2009, 361, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.R.; Mahaffey, K.W.; Garg, J.; Pan, G.; Singer, D.E.; Hacke, W.; Breithardt, G.; Halperin, J.L.; Hankey, G.J.; Piccini, J.P.; et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N. Engl. J. Med. 2011, 365, 883–891. [Google Scholar] [CrossRef]
- Granger, C.B.; Alexander, J.H.; McMurray, J.J.; Lopes, R.D.; Hylek, E.M.; Hanna, M.; Al-Khalidi, H.R.; Ansell, J.; Atar, D.; Avezum, A.; et al. Apixaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2011, 365, 981–992. [Google Scholar] [CrossRef]
- Giugliano, R.P.; Ruff, C.T.; Braunwald, E.; Murphy, S.A.; Wiviott, S.D.; Halperin, J.L.; Waldo, A.L.; Ezekowitz, M.D.; Weitz, J.I.; Spinar, J.; et al. Edoxaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2013, 369, 2093–2104. [Google Scholar] [CrossRef]
- January, C.T.; Wann, L.S.; Alpert, J.S.; Calkins, H.; Cigarroa, J.E.; Cleveland, J.C., Jr.; Conti, J.B.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014, 130, 2071–2104. [Google Scholar] [CrossRef] [PubMed]
- Dabigatran Etexilate Mesylate. Micromedex Solutions. Truven Health Analytics, I.A.A., MI. Available online: http://www.micromedexsolutions.com (accessed on 28 February 2020).
- Edoxaban. Micromedex Solutions. Truven Health Analytics, I.A.A., MI. Available online: http://www.micromedexsolutions.com. (accessed on 28 February 2020).
- Rivaroxaban. Micromedex Solutio. Truven Health Analytics ns, I.A.A., MI. Available online: http://www.micromedexsolutions.com. (accessed on 28 February 2020).
- Apixaban. Micromedex Solutions. Truven Health Analytics, I.A.A., MI. Available online: http://www.micromedexsolutions.com (accessed on 28 February 2020).
- Willett, K.C.; Morrill, A.M. Use of direct oral anticoagulants for the prevention and treatment of thromboembolic disease in patients with reduced renal function: a short review of the clinical evidence. Ther. Clin. Risk Manag. 2017, 13, 447–454. [Google Scholar] [CrossRef] [PubMed]
- January, C.T.; Wann, L.S.; Calkins, H.; Chen, L.Y.; Cigarroa, J.E.; Cleveland, J.C., Jr.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; Furie, K.L.; et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation 2019, 140, e125–e151. [Google Scholar] [CrossRef] [PubMed]
- Shroff, G.R. Renal Function in Patients With Atrial Fibrillation Receiving Anticoagulants: The Canaries in the Coal Mine. JAMA Cardiol. 2016, 1, 375–376. [Google Scholar] [CrossRef] [PubMed]
- Hijazi, Z.; Hohnloser, S.H.; Oldgren, J.; Andersson, U.; Connolly, S.J.; Eikelboom, J.W.; Ezekowitz, M.D.; Reilly, P.A.; Siegbahn, A.; Yusuf, S.; et al. Efficacy and safety of dabigatran compared with warfarin in relation to baseline renal function in patients with atrial fibrillation: a RE-LY (Randomized Evaluation of Long-term Anticoagulation Therapy) trial analysis. Circulation 2014, 129, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Center for Drug Evaluation and Research. Drug Approvals and Databases - Drug Trials Snapshot: Savaysa (edoxaban) for Prevention of Stroke in Atrial Fibrillation. U S Food and Drug Administration Home Page. Available online: https://www.fda.gov/drugs/informationondrugs/ucm428735.html (accessed on 28 February 2020).
- Koene, R.J.; Alraies, M.C.; Norby, F.L.; Soliman, E.Z.; Maheshwari, A.; Lip, G.Y.H.; Alonso, A.; Chen, L.Y. Relation of the CHA2DS2-VASc Score to Risk of Thrombotic and Embolic Stroke in Community-Dwelling Individuals Without Atrial Fibrillation (From The Atherosclerosis Risk in Communities [ARIC] Study). Am. J. Cardiol. 2019, 123, 402–408. [Google Scholar] [CrossRef]
- Lane, D.A.; Lip, G.Y. Use of the CHA(2)DS(2)-VASc and HAS-BLED scores to aid decision making for thromboprophylaxis in nonvalvular atrial fibrillation. Circulation 2012, 126, 860–865. [Google Scholar] [CrossRef]
- Stevens, P.E.; Levin, A. Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group, M. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann. Intern. Med. 2013, 158, 825–830. [Google Scholar] [CrossRef]
- Avezum, A.; Lopes, R.D.; Schulte, P.J.; Lanas, F.; Gersh, B.J.; Hanna, M.; Pais, P.; Erol, C.; Diaz, R.; Bahit, M.C.; et al. Apixaban in Comparison With Warfarin in Patients With Atrial Fibrillation and Valvular Heart Disease: Findings From the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) Trial. Circulation 2015, 132, 624–632. [Google Scholar] [CrossRef]
- Ferreira, J.P.; Girerd, N.; Pellicori, P.; Duarte, K.; Girerd, S.; Pfeffer, M.A.; McMurray, J.J.; Pitt, B.; Dickstein, K.; Jacobs, L.; et al. Renal function estimation and Cockroft-Gault formulas for predicting cardiovascular mortality in population-based, cardiovascular risk, heart failure and post-myocardial infarction cohorts: The Heart ‘OMics’ in AGEing (HOMAGE) and the high-risk myocardial infarction database initiatives. BMC Med. 2016, 14, 181. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Hohnloser, S.H.; Hijazi, Z.; Thomas, L.; Alexander, J.H.; Amerena, J.; Hanna, M.; Keltai, M.; Lanas, F.; Lopes, R.D.; Lopez-Sendon, J.; et al. Efficacy of apixaban when compared with warfarin in relation to renal function in patients with atrial fibrillation: insights from the ARISTOTLE trial. Eur. Heart J. 2012, 33, 2821–2830. [Google Scholar] [CrossRef] [PubMed]
- Sarratt, S.C.; Nesbit, R.; Moye, R. Safety Outcomes of Apixaban Compared With Warfarin in Patients With End-Stage Renal Disease. Ann. Pharmacother. 2017, 51, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Siontis, K.C.; Zhang, X.; Eckard, A.; Bhave, N.; Schaubel, D.E.; He, K.; Tilea, A.; Stack, A.G.; Balkrishnan, R.; Yao, X.; et al. Outcomes Associated With Apixaban Use in Patients With End-Stage Kidney Disease and Atrial Fibrillation in the United States. Circulation 2018, 138, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Reilly, R.F. Clinical Pharmacology of Oral Anticoagulants in Patients with Kidney Disease. Clin. J. Am. Soc. Nephrol. 2019, 14, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Ashley, J.; Sood, M.M. Novel oral anticoagulants in chronic kidney disease: ready for prime time? Curr. Opin. Nephrol. Hypertens 2018, 27, 201–208. [Google Scholar] [CrossRef]
- Aursulesei, V.; Costache, I.I. Anticoagulation in chronic kidney disease: from guidelines to clinical practice. Clin. Cardiol. 2019, 42, 774–782. [Google Scholar] [CrossRef]
- Ha, J.T.; Badve, S.V.; Jun, M. Recent evidence for direct oral anticoagulants in chronic kidney disease. Curr. Opin. Nephrol. Hypertens 2019, 28, 251–261. [Google Scholar] [CrossRef]
- By the American Geriatrics Society Beers Criteria Update Expert, P. American Geriatrics Society 2019 Updated AGS Beers Criteria(R) for Potentially Inappropriate Medication Use in Older Adults. J. Am. Geriatr. Soc. 2019, 67, 674–694. [CrossRef]
- Lauffenburger, J.C.; Farley, J.F.; Gehi, A.K.; Rhoney, D.H.; Brookhart, M.A.; Fang, G. Effectiveness and safety of dabigatran and warfarin in real-world US patients with non-valvular atrial fibrillation: A retrospective cohort study. J. Am. Heart Assoc. 2015, 4. [Google Scholar] [CrossRef]
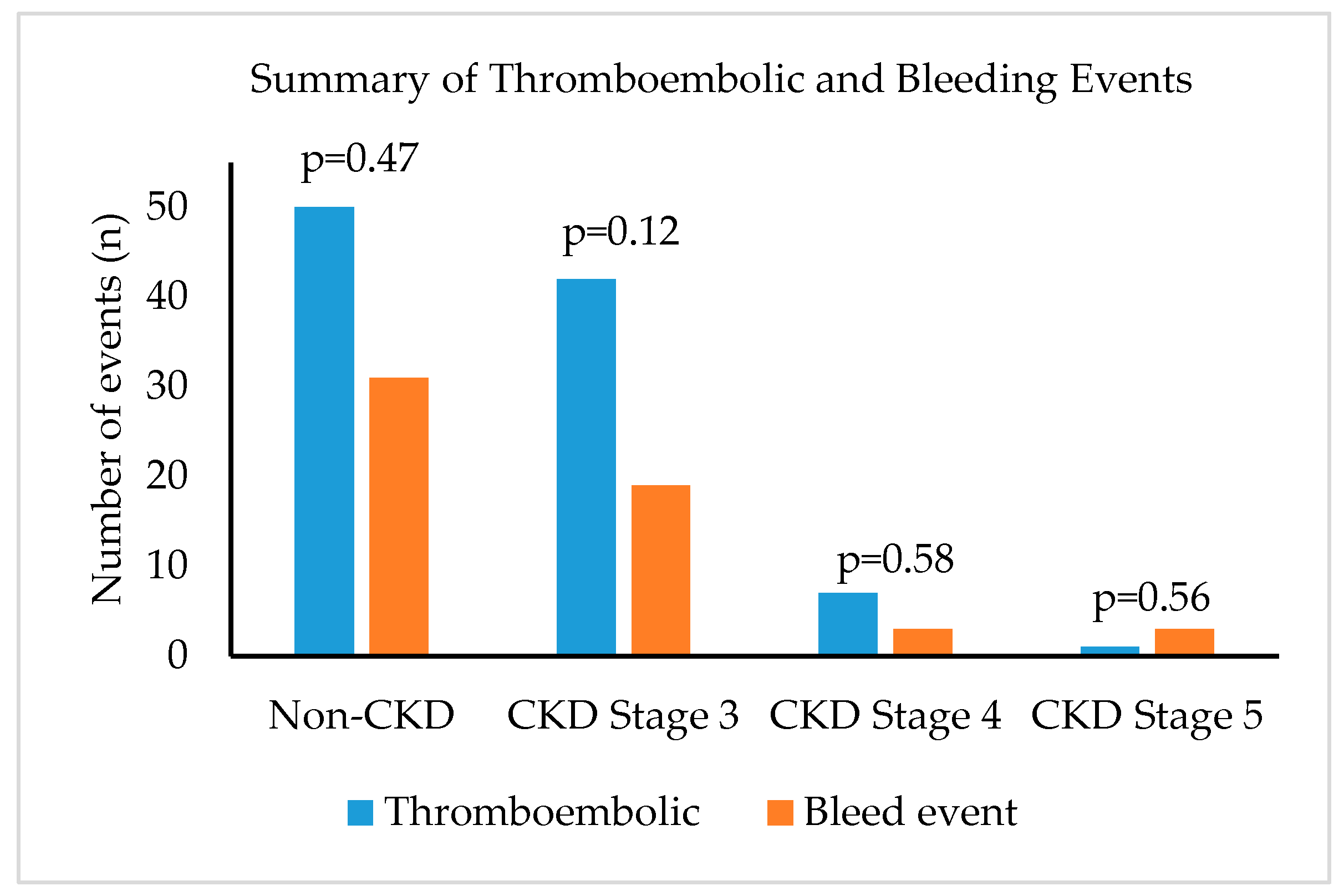
| Non-CKD (≥ 60 mL/min) n = 345 | CKD Stage 3 (30–59.9 mL/min) n = 119 | CKD Stage 4 (15–29.9 mL/min) n = 25 | CKD Stage 5 (<15 mL/min) n = 6 | p-Value | |
|---|---|---|---|---|---|
| Age, mean (SD) | 65.7 (12.4) | 79.4 (9.3) | 82.7 (9.2) | 66.5 (16) | <0.001 * |
| Males, n (%) | 231 (67) | 55 (46.2) | 10 (40) | - | <0.001 * |
| Bleeding event, n (%) | 50 (14.5) | 42 (35.3) | 7 (28) | 1 (16.7) | <0.001 * |
| Stroke event, n (%) | 31 (9) | 19 (16) | 3 (12) | 3 (5) | 0.007 * |
| Antithrombotic medication | |||||
| Apixaban, n (%) | 161 (46.7) | 63 (52.9) | 17 (68) | 4 (66.7) | 0.839 |
| Rivaroxaban, n (%) | 152 (44.1) | 43 (36) | 7 (28) | 2 (33.3) | 0.148 |
| Dabigatran, n (%) | 31 (9) | 12 (10.1) | 1 (4) | - | 0.121 |
| Edoxaban, n (%) | 1 (0.3) | - | - | - | >0.999 |
| CHA2DS2-VASc score, mean (SD) | 3.2 (1.7) | 4.6 (1.3) | 5.4 (1.5) | 5.3 (2.5) | <0.001 * |
| HAS-BLED score, median (range) | 2 (0–9) | 2 (0–6) | 3 (1–4) | 3 (2–5) | <0.001 * |
| Duration of DOAC use in months, median (range) | 13 (1–73) | 32 (3–65) | 30 (2–60) | 23.5 (3–36) | <0.001 * |
| History of anticoagulant use, n (%) | 102 (29.6) | 49 (41.2) | 12 (48) | 5 (83.3) | 0.003 * |
| Concurrent antiplatelet use, n (%) | 91 (26.4) | 40 (33.6) | 6 (24) | 5 (83.3) | 0.015 * |
| Renal Function | Anticoagulants | p-Value | Odds Ratio | 95% C.I. for Odds Ratio | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Normal kidney function | Rivaroxaban vs. Apixaban | 0.464 | 0.79 | 0.42 | 1.49 |
| Dabigatran vs. Apixaban | 0.413 | 0.59 | 0.17 | 2.09 | |
| Chronic kidney disease patients | Rivaroxaban vs. Apixaban | 0.842 | 0.93 | 0.44 | 1.97 |
| Dabigatran vs. Apixaban | 0.346 | 1.77 | 0.54 | 5.81 | |
| Renal Function | Anticoagulants | p-Value | Odds Ratio | 95% C.I. for Odds Ratio | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Normal kidney function | Rivaroxaban vs. Apixaban | 0.245 | 1.63 | 0.72 | 3.69 |
| Dabigatran vs. Apixaban | 0.998 | 0.00 | 0.00 | - | |
| Chronic kidney disease patients | Rivaroxaban vs. Apixaban | 0.961 | 0.97 | 0.30 | 3.18 |
| Dabigatran vs. Apixaban | 0.020* | 6.58 | 1.35 | 32.02 | |
| DOAC | Renal CL | Hepatic Metabolism | Dialy. | Renal Impairment Dose Adjust. (mL/min) | S/SE (Compared to Warfarin) | Major Bleeding (Compared to Warfarin) |
|---|---|---|---|---|---|---|
| Dabigatran | 80% | Metabolized by esterases | Yes | >30: 150 mg BID 15–30: 75 mg BID <15: Contraindicated | Reduced adjusted HR in CKD patients = 0.74 (95% CI 0.57 to 0.96) [35] Dabigatran 150 mg BID Overall RR [7] = 0.66 (95% CI 0.53–0.82) CrCl 30–49 mL/min = 0.85 (95% CI 0.59–1.24) | Adjusted HR in CKD patients = 1.52 (95% CI 1.27 to 1.81) [35] Dabigatran 150 mg BID Overall RR [7] = 0.93 (95% CI 0.81–1.07) CrCl 30–49 mL/min = 1.01 (95% CI 0.79–1.3) |
| Apixaban | 25% | Mainly CYP3A4 | Small | >30: 5 mg BID^ <30: 2.5 mg BID+ HD: 5 mg BID | Overall [9] = 0.79 (95% CI 0.66–0.95) CrCl 25–49 mL/min = 0.79 (95% CI 0.55–1.14) | Overall [9] = 0.69 (95% CI 0.60–0.80) CrCl 25–49 mL/min = 0.50 (95% CI 0.38–0.66) |
| Rivaroxaban | 30% | Minimal | No | >50: 20 mg QD 15–50: 15 mg QD <15: Contraindicated | Overall HR [8] = 0.79 (95% CI 0.66–0.96) CrCl 30–49 mL/min = 0.84 (95% CI 0.75–1.23) | Overall [8] = 1.04 (95% CI 0.90–1.20) CrCl 30–49 mL/min = 0.95 (95% CI 0.72–1.26) |
| Edoxaban | 50% | 10% by carboxy-esterase 1 | No | >95: FDA warning 95–50: 60 mg QD# 15–49: 30 mg QD <15: Contraindicated | High-dose edoxaban Overall RR [10] = 0.79 (95% CI 0.63–0.99 CrCl 30–49 mL/min = 0.87 (95% CI 0.72–1.04) Low-dose edoxaban Overall RR [10] = 1.07 (95% CI 0.87–1.31) CrCl 30–49 mL/min = 1.22 (not reported) | High-dose edoxaban Overall RR [10] = 0.80 (95% CI 0.71–0.91) CrCl 30–49 mL/min = 0.76 (95% CI 0.58–0.98) Low-dose edoxaban Overall RR [10] = 0.47 (95% CI 0.41–0.55) CrCl 30–49 mL/min = 0.37 (not reported) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, S.M.; Bahjri, K.; Tran, H. Safety and Efficacy of Direct Oral Anticoagulants for Atrial Fibrillation in Patients with Renal Impairment. Pharmacy 2020, 8, 30. https://doi.org/10.3390/pharmacy8010030
Jang SM, Bahjri K, Tran H. Safety and Efficacy of Direct Oral Anticoagulants for Atrial Fibrillation in Patients with Renal Impairment. Pharmacy. 2020; 8(1):30. https://doi.org/10.3390/pharmacy8010030
Chicago/Turabian StyleJang, Soo Min, Khaled Bahjri, and Huyentran Tran. 2020. "Safety and Efficacy of Direct Oral Anticoagulants for Atrial Fibrillation in Patients with Renal Impairment" Pharmacy 8, no. 1: 30. https://doi.org/10.3390/pharmacy8010030
APA StyleJang, S. M., Bahjri, K., & Tran, H. (2020). Safety and Efficacy of Direct Oral Anticoagulants for Atrial Fibrillation in Patients with Renal Impairment. Pharmacy, 8(1), 30. https://doi.org/10.3390/pharmacy8010030





