Antibiotic Hypersensitivity Mechanisms
Abstract
1. Introduction
2. Type I Reactions
2.1. Immune-Mediated IgE-Dependent Anaphylaxis
2.1.1. Histamine and Tryptase
2.1.2. Platelet Activating Factor
2.1.3. Other Mediators
2.2. Immune-Mediated IgE-Independent Anaphylaxis
2.3. Non-Immunologic Anaphylaxis
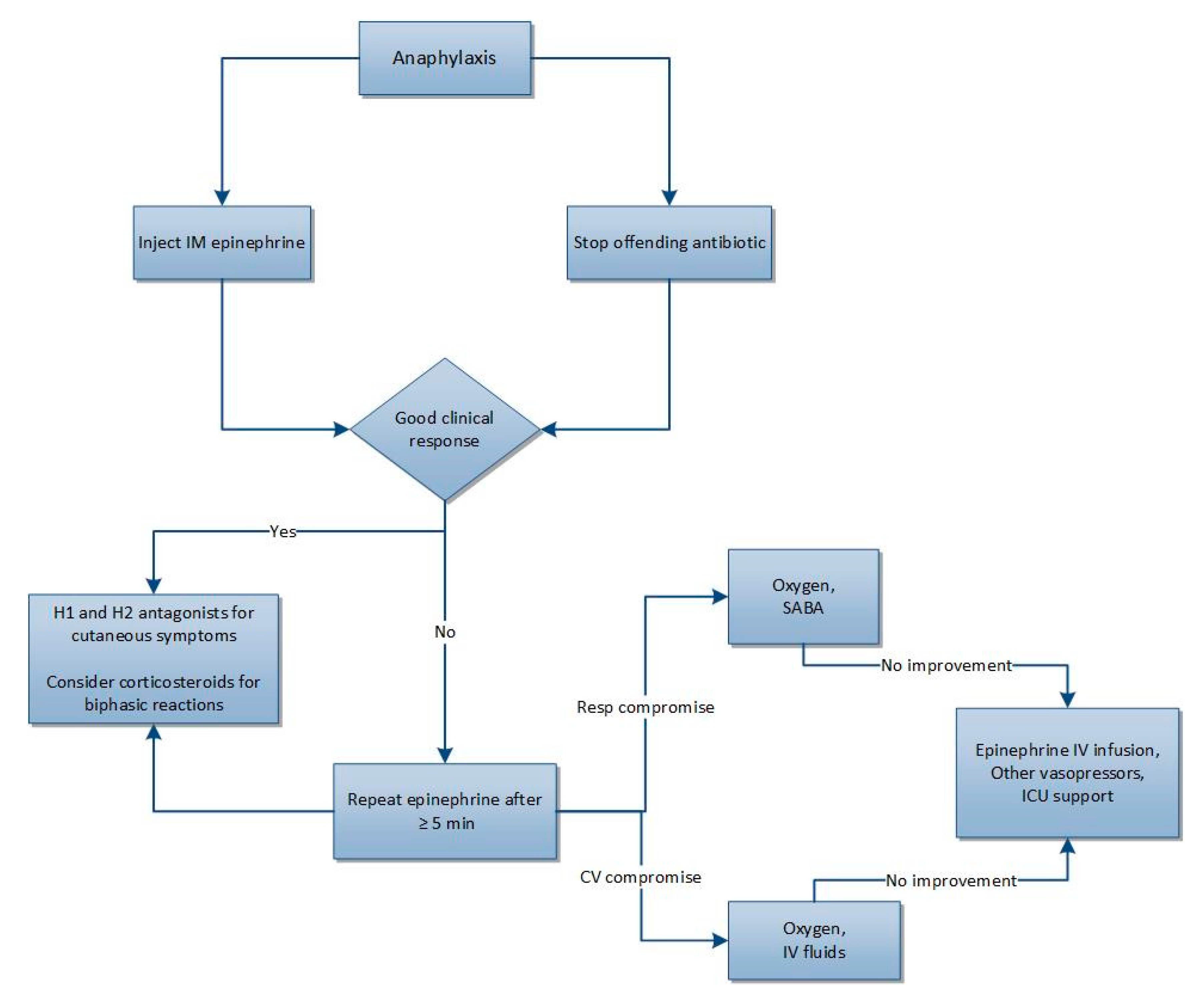
2.4. Diagnosis and Treatment
2.5. Beta-Lactam Cross-Reactivity
3. Type II Reactions
3.1. Thrombocytopenia
3.2. Neutropenia
3.3. Hemolytic Anemia
4. Type III Reactions
5. Type IV Reactions
5.1. Type IVa
5.2. Type IVb
5.3. Type IVc
5.4. Type IVd
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Demoly, P.; Adkinson, N.F.; Brockow, K.; Castells, M.; Chiriac, A.M.; Greenberger, P.A.; Khan, D.A.; Lang, D.M.; Park, H.S.; Pichler, W.; et al. International Consensus on drug allergy. Allergy 2014, 69, 420–437. [Google Scholar] [CrossRef] [PubMed]
- Coombs, P.; Gell, P.G. Classification of allergic reactions responsible for clinical hypersensitivity and disease. In Clinical Aspects of Immunology; Oxford University Press: Oxford, UK, 1968; pp. 575–596. [Google Scholar]
- Blumenthal, K.; Peter, J.; Trubiano, J.; Phillips, E.J. Antibiotic Allergy. Lancet 2019, 393, 183–198. [Google Scholar] [CrossRef]
- Uzzaman, A.; Cho, S.H. Classification of hypersensitivity reactions. Allergy Asthma Proc. 2012, 33, S96–S99. [Google Scholar] [CrossRef] [PubMed]
- Legendre, D.P.; Muzny, C.A.; Marshall, G.D.; Swiatlo, E. Antibiotic hypersensitivity reactions and approaches to desensitization. Clin. Infect. Dis. 2014, 58, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Norton, A.E.; Konvinse, K.; Phillips, E.J.; Broyles, A.D. Antibiotic allergy in pediatrics. Pediatrics 2018, 141, e20172497. [Google Scholar] [CrossRef] [PubMed]
- Joint Task Force on Practice Parameters; American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology. Drug allergy: An updated practice parameter. Ann. Allergy Asthma Immunol. 2010, 105, 259–273. [Google Scholar] [CrossRef] [PubMed]
- Aun, M.V.; Kalil, J.; Giavina-Bianchi, P. Drug-induced anaphylaxis. Immunol. Allergy Clin. N. Am. 2017, 37, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Reber, L.L.; Hernandez, J.D.; Galli, S.J. The pathophysiology of anaphylaxis. J. Allergy Clin. Immunol. 2017, 140, 335–348. [Google Scholar] [CrossRef]
- Khan, B.Q.; Kemp, S.F. Pathophysiology of anaphylaxis. Curr. Opin. Allergy Clin. Immunol. 2011, 11, 319–325. [Google Scholar] [CrossRef]
- Sala-Cunil, A.; Guilarte, M.; Cardona, V. Phenotypes, endotypes and biomarkers in anaphylaxis: Current insights. Curr. Opin. Allergy Clin. Immunol. 2018, 18, 370–376. [Google Scholar] [CrossRef]
- Sala-Cunil, A.; Cardona, V. Biomarkers of anaphylaxis, beyond tryptase. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Palgan, K.; Bartuzi, Z. Platelet activating factor in allergies. Int. J. Immunopathol. Pharmacol. 2015, 28, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Montrucchio, G.; Alloatti, G.; Camussi, G. Role of platelet-activating factor in cardiovascular pathophysiology. Physiol. Rev. 2000, 80, 1669–1699. [Google Scholar] [CrossRef] [PubMed]
- Vadas, P.; Gold, M.; Perelman, B.; Liss, G.M.; Lack, G.; Blyth, T.; Simons, F.E.; Simons, K.J.; Cass, D.; Yeung, J. Platelet-activating factor, PAF acetylhydrolase, and severe anaphylaxis. N. Engl. J. Med. 2008, 358, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Soter, N.A.; Lewis, R.A.; Corey, E.J.; Austen, K.F. Local effects of synthetic leukotrienes (LTC4, LTD4, LTE4, and LTB4) in human skin. J. Investig. Dermatol. 1983, 80, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.W.; Drazen, J.M.; Coles, N.; McFadden, E.R., Jr.; Weller, P.F.; Corey, E.J.; Lewis, R.A.; Austen, K.F. Bronchoconstrictor effects of leukotriene C in humans. Science 1982, 216, 196–198. [Google Scholar] [CrossRef] [PubMed]
- Polk, R.E. Anaphylactoid reactions to glycopeptide antibiotics. J. Antimicrob. Chemother. 1991, 27 (Suppl. B), 17–29. [Google Scholar] [CrossRef] [PubMed]
- Healy, D.P.; Sahai, J.V.; Fuller, S.H.; Polk, R.E. Vancomycin-induced histamine release and “red man syndrome”: Comparison of 1- and 2-h infusions. Antimicrob. Agents Chemother. 1990, 34, 550–554. [Google Scholar] [CrossRef]
- Subramanian, H.; Gupta, K.; Ali, H. Roles of Mas-related G protein-coupled receptor X2 on mast cell-mediated host defense, pseudoallergic drug reactions, and chronic inflammatory diseases. J. Allergy Clin. Immunol. 2016, 138, 700–710. [Google Scholar] [CrossRef]
- Muraro, A.; Roberts, G.; Worm, M.; Bilo, M.B.; Brockow, K.; Fernandez Rivas, M. Anaphylaxis: Guidelines from the European Academy of Allergy and Clinical Immunology. Allergy 2014, 69, 1026–1045. [Google Scholar] [CrossRef]
- Lieberman, P.; Nicklas, R.A.; Oppenheimer, J.; Kemp, S.F.; Lang, D.M.; Bernstein, D.I. The diagnosis and management of anaphylaxis practice parameter: 2010 update. J. Allergy Clin. Immunol. 2010, 126, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Pourmand, A.; Robinson, C.; Syed, W.; Mazer-Amirshahi, M. Biphasic anaphylaxis: A review of the literature and implications for emergency management. Am. J. Emer. Med. 2018, 36, 1480–1485. [Google Scholar] [CrossRef] [PubMed]
- Zagursky, R.; Pichichero, M. Cross-reactivity in beta-lactam allergy. J. Allergy Clin. Immunol. Pract. 2017, 6, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Terico, A.; Gallagher, J. Beta-lactam hypersensitivity and cross-reactivity. J. Pharm. Pract. 2014, 27, 530–544. [Google Scholar] [CrossRef] [PubMed]
- Har, D.; Solensky, R. Penicillin and Beta-Lactam Hypersensitivity. Immunol. Allergy Clin. N. Am. 2017, 37, 643–662. [Google Scholar] [CrossRef] [PubMed]
- Pichichero, M.; Zagursky, R. Penicillin and cephalosporin allergy. Ann. Allergy Asthma Immunol. 2014, 112, 404–412. [Google Scholar] [CrossRef]
- Stone, C.A., Jr.; Trubiano, J.; Coleman, D.T.; Rukasin, C.R.F.; Phillips, E.J. The challenge of delabeling penicillin allergy. Allergy 2019. [Google Scholar] [CrossRef]
- Lteif, L.; Eiland, L.S. The Basics of Penicillin Allergy: What A Clinician Should Know. Pharmacy 2019, 7, 94. [Google Scholar] [CrossRef]
- Chaudhry, S.B.; Veve, M.P.; Wagner, J.L. Cephalosporins: A Focus on Side Chains and β-Lactam Cross-Reactivity. Pharmacy 2019, 7, 103. [Google Scholar] [CrossRef]
- Lee, Y.; Bradley, N. Overview and Insights into Carbapenem Allergy. Pharmacy 2019, 7, 110. [Google Scholar] [CrossRef]
- Aster, R.H.; Curtis, B.R.; Mcfarland, J.G.; Bougie, D.W. Drug-induced immune thrombocytopenia: Pathogenesis, diagnosis, and management. J. Thromb. Hemost. 2009, 7, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Curtis, B.R. Drug-induced immune thrombocytopenia: Incidence, clinical features, laboratory testing, and pathogenic mechanisms. Immunohematology 2014, 30, 55–65. [Google Scholar] [PubMed]
- Adam, J.; Pichler, W.J.; Yerly, D. Delayed drug hypersensitivity: Models of T-cell stimulation. Br. J. Clin. Pharmacol. 2010, 72, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Garratty, G. Drug-induced immune hemolytic anemia. Hematol. Am. Soc. Hematol. Educ. Program 2009, 73–79. [Google Scholar] [CrossRef] [PubMed]
- George, J.N.; Aster, R.H. Drug-induced thrombocytopenia: Pathogenesis, evaluation, and management. Hematol. Am. Soc. Hematol. Educ. Program 2009, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Arnold, D.M.; Nazi, I.; Warkentin, T.E.; Smith, J.W.; Toltl, L.J.; George, J.N.; Kelton, J.G. Approach to the diagnosis and management of drug-induced immune thrombocytopenia. Transfus. Med. Rev. 2013, 27, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Pick, A.M.; Nystrom, K.K. Nonchemotherapy drug-induced neutropenia and agranulocytosis: Could medications be the culprit? J. Pharm. Pract. 2014, 27, 447–452. [Google Scholar] [CrossRef]
- Andres, E.; Zimmer, J.; Mecili, M.; Weitten, T.; Alt, M.; Maloisel, F. Clinical presentation and management of drug-induced agranulocytosis. Exp. Rev. Hematol. 2011, 4, 143–151. [Google Scholar] [CrossRef]
- Andres, E.; Mourot-Cottet, R. Non-chemotherapy drug-induced neutropenia—an update. Exp. Opin. Drug Saf. 2017, 16, 1235–1242. [Google Scholar] [CrossRef]
- Curtis, B.R. Drug-induced immune neutropenia/agranulocytosis. Immunohematology 2014, 30, 95–101. [Google Scholar]
- Garratty, G. Immune hemolytic anemia caused by drugs. Exp. Opin. Drug. Saf. 2012, 11, 635–642. [Google Scholar] [CrossRef]
- Davenport, R.D.; Judd, W.J.; Dake, L.R. Persistence of cefotetan on red blood cells. Transfusion 2004, 44, 849–852. [Google Scholar] [CrossRef]
- Posadas, S.J.; Pichler, W.J. Delayed drug hypersensitivity reactions—New concepts. Clin. Exp. Allergy 2007, 37, 989–999. [Google Scholar] [CrossRef]
- Chinen, J.; Fleisher, T.A.; Shearer, W.T. The Immune system: An overview. In Middleton’s Allergy Principles & Practice, 7th ed.; Adkinson, N.F., Jr., Bochner, B.S., Busse, W.W., Holgate, S., Simons, F.E., Lemanske, R., Eds.; Mosby: Philadelphia, PA, USA, 2009; pp. 3–17. [Google Scholar]
- Karmacharya, P.; Poudel, D.R.; Pathak, R.; Donato, A.A.; Ghimire, S.; Giri, S.; Aryal, M.R.; Bingham, C.O. Rituximab-induced serum sickness: A systematic review. Semin. Arthritis Rheum. 2015, 45, 334–340. [Google Scholar] [CrossRef]
- Grant Peter, J.; Lehloenya, R.; Dlamini, S.; Risma, K.; White, K.D.; Konvinse, K.C.; Phillips, E.J. Severe delayed cutaneous and systemic reactions to drugs: A global perspective on the science and art of current practice. J. Allergy Clin. Immunol. Pract. 2017, 5, 547–563. [Google Scholar] [CrossRef]
- Platt, R.; Dreis, M.W.; Kennedy, D.L.; Kuritsky, J.N. Serum Sickness-Like Reactions to Amoxicillin, Cefaclor, Cephalexin, and Trimethoprim-Sulfamethoxazole. J. Infect. Dis. 1988, 158, 474–477. [Google Scholar] [CrossRef]
- Kearns, G.L.; Wheeler, J.G.; Childress, S.H.; Letzig, L.G. Serum sickness-like reactions to cefaclor: Role of hepatic metabolism and individual susceptibility. J. Pediatr. 1994, 125, 805–811. [Google Scholar] [CrossRef]
- Langford, C.A. Vasculitis. J. Allergy Clin. Immunol. 2010, 125, S216–S225. [Google Scholar] [CrossRef]
- Ali, N.; Karia, N.; Goldhahn, R. Cefazolin as a cause of leukocytoclastic vasculitis. Clin. Case Rep. 2017, 5, 1051–1053. [Google Scholar] [CrossRef]
- Sáenz de Santa María Garcia, M.; Morales-Cabeza, C.; Noguerado-Mellado, B.; Rojas-Pérez-Ezquerra, P.; Zubeldia, J.M. Cutaneous leukocytoclastic vasculitis due to amoxicillin hypersensitivity. Ann. Allergy Asthma Immunol. 2016, 117, 446–447. [Google Scholar] [CrossRef]
- Vocanson, M.; Hennino, A.; Rozières, A.; Poyet, G.; Nicolas, J.F. Effector and regulatory mechanisms in allergic contact dermatitis. Allergy 2009, 64, 1699–1714. [Google Scholar] [CrossRef]
- Nguyen, H.L.; Yannias, J.A. Contact dermatitis to medications and skin products. Clin. Rev. Allergy Immunol. 2019, 56, 41–59. [Google Scholar] [CrossRef]
- Bains, S.N.; Nash, P.; Fonacier, L. Irritant contact dermatitis. Clin. Rev. Allergy Immunol. 2019, 56, 99–109. [Google Scholar] [CrossRef]
- Cacoub, P.; Musette, P.; Descamps, V.; Meyer, O.; Speirs, C.; Finzi, L.; Roujeau, J.C. The DRESS syndrome: A literature review. Am. J. Med. 2011, 124, 588–597. [Google Scholar] [CrossRef]
- Shiohara, T.; Kano, Y. Drug reaction with eosinophilia and systemic symptoms (DRESS): Incidence, pathogenesis and management. Exp. Opin. Drug Saf. 2017, 16, 139–147. [Google Scholar] [CrossRef]
- Yun, J.; Adam, J.; Yerly, D.; Pichler, W.J. Human leukocyte antigens (HLA) associated drug hypersensitivity: Consequences of drug binding to HLA. Allergy 2012, 67, 1338–1346. [Google Scholar] [CrossRef]
- Department of Health and Human Services. Panel on Antiretroviral Guidelines for Adults and Adolescents. In Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. Available online: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf (accessed on 25 July 2019).
- Zhang, F.R.; Liu, H.; Irwanto, A.; Fu, X.A.; Li, Y.; Yu, G.Q. HLA-B*13:01 and the dapsone hypersensitivity syndrome. N. Engl. J. Med. 2013, 369, 1620–1628. [Google Scholar] [CrossRef]
- Fernando, S.L. Drug-reaction eosinophilia and systemic symptoms and drug-induced hypersensitivity syndrome. Australas. J. Dermatol. 2014, 55, 15–23. [Google Scholar] [CrossRef]
- Kirchhof, M.G.; Wong, A.; Dutz, J.P. Cyclosporine Treatment of Drug-Induced Hypersensitivity Syndrome. JAMA Dermatol. 2016, 152, 1254–1257. [Google Scholar] [CrossRef]
- Frey, N.; Jossi, J.; Bodmer, M.; Bircher, A.; Jick, S.S.; Meier, C.R.; Spoendlin, J. The Epidemiology of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in the UK. J. Investig. Dermatol. 2017, 137, 1240–1247. [Google Scholar] [CrossRef]
- Yang, C.; Mosam, A.; Mankahla, A.; Dlova, N.; Saavedra, A. HIV infection predisposes skin to toxic epidermal necrolysis via depletion of skin-directed CD4⁺ T cells. J. Am. Acad. Dermatol. 2014, 70, 1096–1102. [Google Scholar] [CrossRef]
- Chung, W.H.; Hung, S.; Chen, Y.T. Human leukocyte antigens and drug hypersensitivity. Curr. Opin. Allergy Clin. Immunol. 2007, 7, 317–323. [Google Scholar] [CrossRef]
- Schneider, J.A.; Cohen, P.R. Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: A Concise Review with a Comprehensive Summary of Therapeutic Interventions Emphasizing Supportive Measures. Adv. Ther. 2017, 34, 1235–1244. [Google Scholar] [CrossRef]
- Lerch, M.; Mainetti, C.; Beretta-Piccoli, B.T.; Harr, T. Current Perspectives on Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis. Clin. Rev. Allergy Immunol. 2018, 54, 147–176. [Google Scholar] [CrossRef]
- Sekula, P.; Dunant, A.; Mockenhaupt, M.; Naldi, L.; Bouwes Bavinck, J.N.; Halevy, S. Comprehensive survival analysis of a cohort of patients with Stevens-Johnson syndrome and toxic epidermal necrolysis. J. Investig. Dermatol. 2013, 133, 1197–1204. [Google Scholar] [CrossRef]
- Roujeau, J.C.; Bastuji-Garin, S. Systematic review of treatments for Stevens-Johnson syndrome and toxic epidermal necrolysis using the SCORTEN score as a tool for evaluating mortality. Ther. Adv. Drug Saf. 2011, 2, 87–94. [Google Scholar] [CrossRef]
- Szatkowski, J.; Schwartz, R.A. Acute generalized exanthematous pustulosis (AGEP): A review and update. J. Am. Acad. Dermatol. 2015, 73, 843–848. [Google Scholar] [CrossRef]
- Fernando, S.L. Acute generalized exanthematous pustulosis. Australas. J. Dermatol. 2012, 53, 87–92. [Google Scholar] [CrossRef]
| Type | Description | Pathogenesis | Onset of Reaction | Typical Clinical Findings | Commonly Associated Antibiotics |
|---|---|---|---|---|---|
| I (Immediate) | IgE-mediated hypersensitivity | Antibiotic-specific IgE binds to Fc-epsilon-RI receptors on mast cells and basophils. Subsequent antibiotic exposure leads to mast cell and basophil degranulation | <1 h | Anaphylaxis, hives, angioedema, N/V, abdominal pain, SOB, wheezing, anxiety, confusion, chest pain, palpitations, syncope, cardiac arrest | Cephs, FQs, PCNs, |
| II (Delayed) | Antibody-mediated hypersensitivity | Antibiotic binds to WBC, RBC, or platelet and acts as antigen leading to antibody (usually IgG or complement) mediated cell destruction | 7–14 d | Hemolytic anemia, thrombocytopenia, neutropenia | Cephs, PCNs, SMX/TMP |
| III (Delayed) | Immune complex mediated hypersensitivity | Antibiotic and IgG/IgM bind to form immune complex activate complement | 7–14 d | Serum sickness *, vasculitis | Cephs (esp cefaclor), cipro, PCNs, SMX/TMP |
| IV (Delayed) | Delayed type hypersensitivity | Antigen specific T-cell activation | |||
| IVa | Monocytic inflammation (Th1 and IFN-γ) | 10–15 d | Allergic contact dermatitis | Topical neomycin, bacitracin, polymyxin | |
| IVb | Th2-mediated eosinophilic inflammation | 2–8 wk (for DRESS) | DRESS | PCNs, Cephs, Dapsone, MinocyclineSMX/TMP, Vanco | |
| IVc | CD8 T cell-mediated cytotoxicity | 4–28 d | SJS, TEN | FQs, Nevirapine, PCNs, SMX/TMP | |
| IVd | T-cell-mediated neutrophilic inflammation | 24–48 h | AGEP | Ampicillin, Antifungals, FQs, SMX/TMP |
| Organ System | Symptoms | Main Mediators |
|---|---|---|
| GI | N/V, diarrhea, abdominal pain | Histamine |
| Skin | Flushing, urticaria, itching | Histamine PAF CysLTs |
| Respiratory | Dyspnea, bronchoconstriction, stridor, wheezing, cough, angioedema | Histamine Tryptase PAF CysLTs |
| CV | Hypotension, syncope, increased vascular permeability, vasodilatation | Histamine Tryptase PAF |
| Anaphylaxis Is Highly Likely If at Least One of the Following Three Criteria Is/Are Met: |
|---|
|
|
|
| Criteria | Description |
|---|---|
| 1 | Therapy with the suspected drug preceded thrombocytopenia; Recovery was complete and sustained after drug was discontinued |
| 2 | Other drugs administered prior to thrombocytopenia were continued or reintroduced after discontinuation of the suspected drug |
| 3 | Other causes of thrombocytopenia were excluded |
| 4 | Re-exposure to the suspected drug resulted in recurrent thrombocytopenia |
| Levels of Evidence | |
| Definite | All criteria met |
| Probable | Criteria 1–3 met |
| Likely | Criterion 1 met |
| Unlikely | Criterion 1 not met |
| Name | % of BSA with Epidermal Detachment |
|---|---|
| SJS | <10 |
| SJS/TEN overlap | 10–30 |
| TEN | >30 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maker, J.H.; Stroup, C.M.; Huang, V.; James, S.F. Antibiotic Hypersensitivity Mechanisms. Pharmacy 2019, 7, 122. https://doi.org/10.3390/pharmacy7030122
Maker JH, Stroup CM, Huang V, James SF. Antibiotic Hypersensitivity Mechanisms. Pharmacy. 2019; 7(3):122. https://doi.org/10.3390/pharmacy7030122
Chicago/Turabian StyleMaker, Jenana H., Cassandra M. Stroup, Vanthida Huang, and Stephanie F. James. 2019. "Antibiotic Hypersensitivity Mechanisms" Pharmacy 7, no. 3: 122. https://doi.org/10.3390/pharmacy7030122
APA StyleMaker, J. H., Stroup, C. M., Huang, V., & James, S. F. (2019). Antibiotic Hypersensitivity Mechanisms. Pharmacy, 7(3), 122. https://doi.org/10.3390/pharmacy7030122





