Penicillin Allergy Skin Testing in the Inpatient Setting
Abstract
1. Introduction
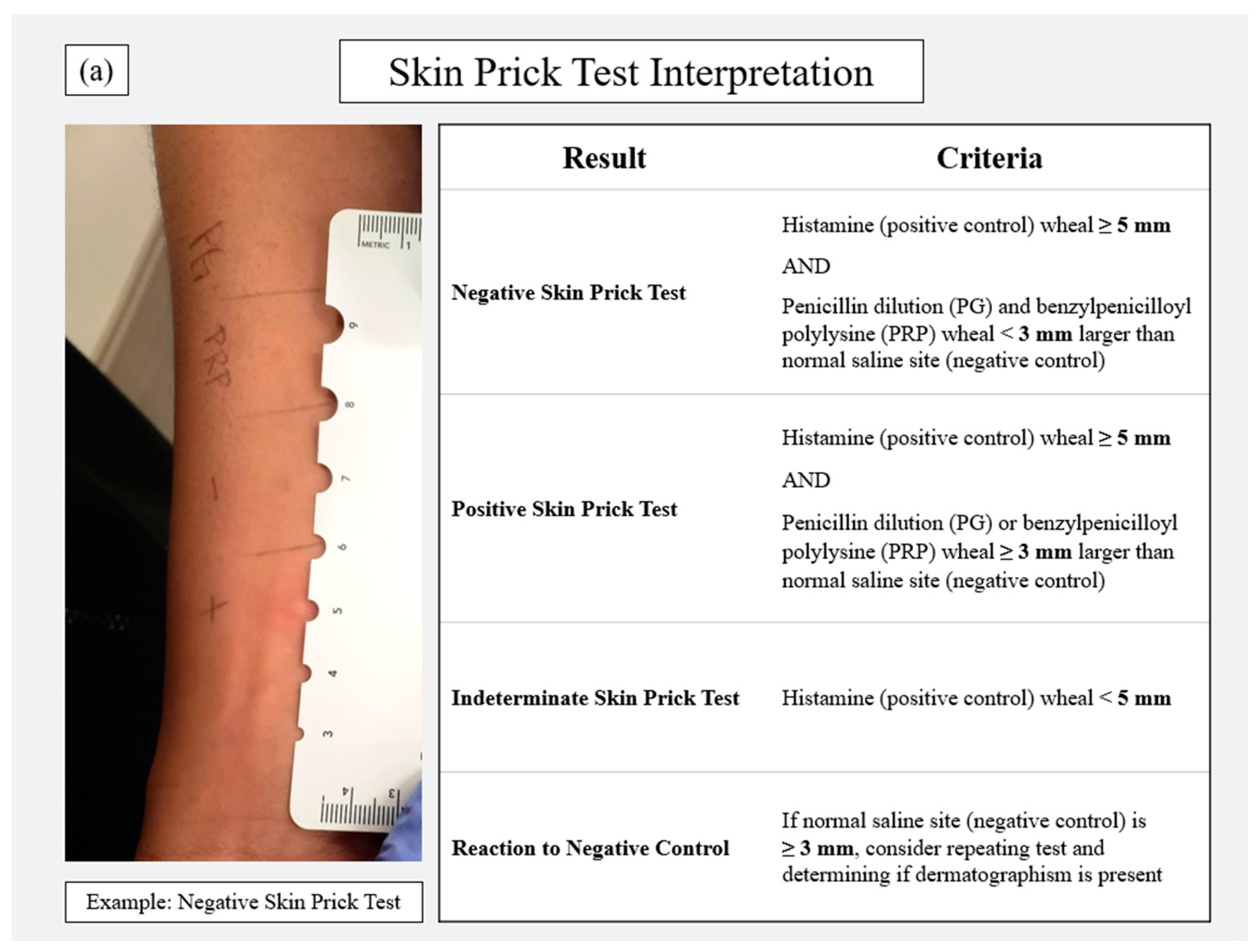
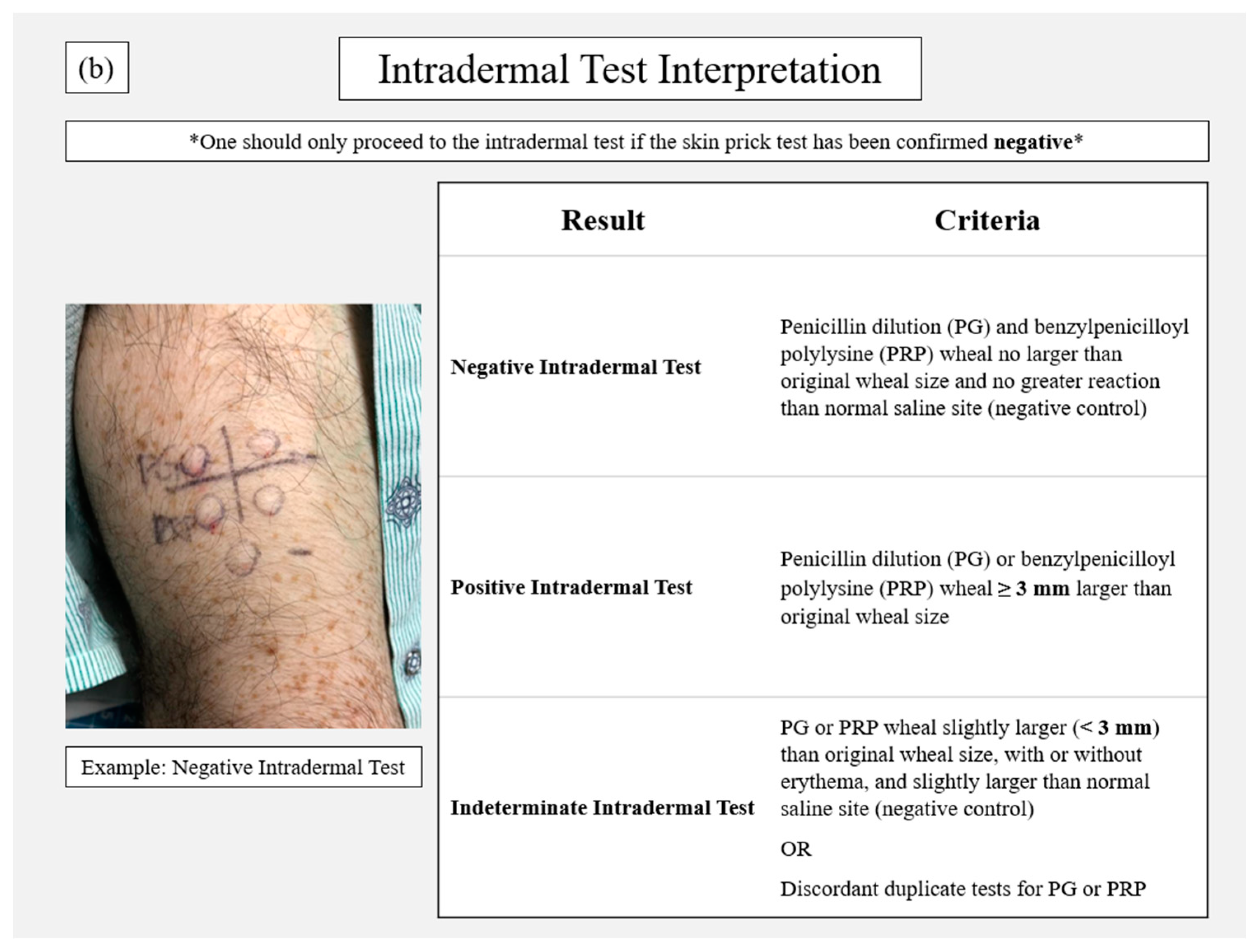
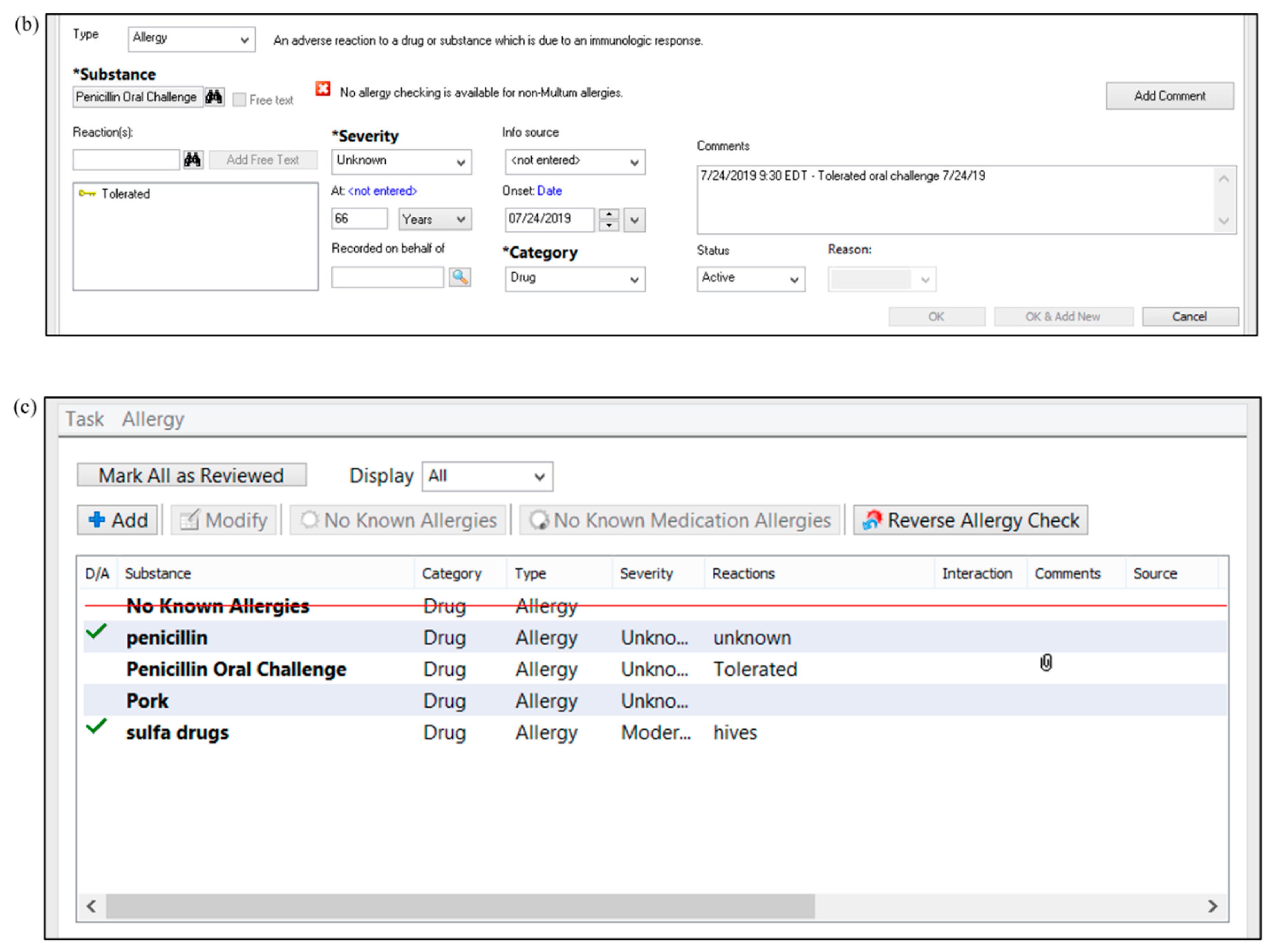
2. Penicillin Allergy Skin Testing Procedure
3. Customization by Institution
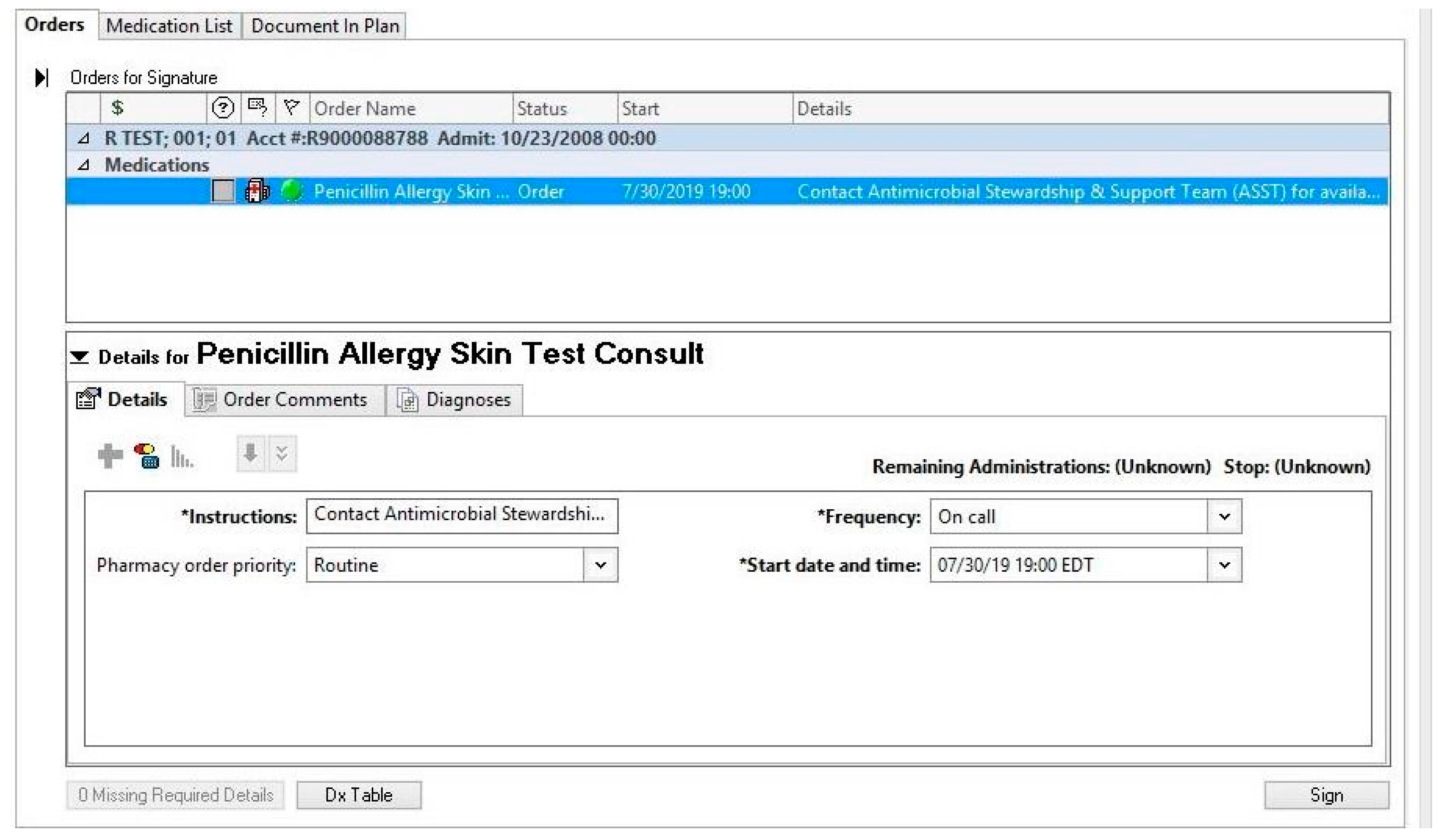
3.1. Personnel
3.2. Time
3.3. Technology
3.4. Patient Population
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhou, L.; Dhopeshwarkar, N.; Blumenthal, K.G.; Goss, F.; Topaz, M.; Slight, S.P.; Bates, D.W. Drug allergies documented in electronic health records of a large healthcare system. Allergy 2016, 71, 1305–1313. [Google Scholar] [CrossRef] [PubMed]
- Patient Trends in Hospital Inpatient Stays in the United States, 2005-2014 #225. Available online: https://www.hcup-us.ahrq.gov/reports/statbriefs/sb225-Inpatient-US-Stays-Trends.jsp?utm_source=ahrq&utm_medium=en1&utm_term=&utm_content=1&utm_campaign=ahrq_en7_18_2017 (accessed on 31 July 2019).
- Blumenthal, K.G.; Lu, N.; Zhang, Y.; Li, Y.; Walensky, R.P.; Choi, H.K. Risk of meticillin resistant Staphylococcus aureus and Clostridium difficile in patients with a documented penicillin allergy: Population based matched cohort study. BMJ 2018, 361, k2400. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, K.G.; Ryan, E.E.; Li, Y.; Lee, H.; Kuhlen, J.L.; Shenoy, E.S. The Impact of a Reported Penicillin Allergy on Surgical Site Infection Risk. Clin. Infect. Dis. 2018, 66, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Al-Hasan, M.N.; Acker, E.C.; Kohn, J.E.; Bookstaver, P.B.; Justo, J.A. Impact of Penicillin Allergy on Empirical Carbapenem Use in Gram-Negative Bloodstream Infections: An Antimicrobial Stewardship Opportunity. Pharmacotherapy 2018, 38, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Foo, H.; Chater, M.; Maley, M.; van Hal, S.J. Glycopeptide use is associated with increased mortality in Enterococcus faecalis bacteraemia. J. Antimicrob. Chemother. 2014, 69, 2252–2257. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McDanel, J.S.; Perencevich, E.N.; Diekema, D.J.; Herwaldt, L.A.; Smith, T.C.; Chrischilles, E.A.; Dawson, J.D.; Jiang, L.; Goto, M.; Schweizer, M.L. Comparative effectiveness of beta-lactams versus vancomycin for treatment of methicillin-susceptible Staphylococcus aureus bloodstream infections among 122 hospitals. Clin. Infect. Dis. 2015, 61, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Chey, W.D.; Leontiadis, G.I.; Howden, C.W.; Moss, S.F. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am. J. Gastroenterol. 2017, 112, 212–239. [Google Scholar] [CrossRef] [PubMed]
- Bland, C.M.; Bookstaver, P.B.; Griffith, N.C.; Heil, E.L.; Jones, B.M.; Ann Justo, J.; Staicu, M.L.; Torney, N.P.; Wall, G.C. A practical guide for pharmacists to successfully implement penicillin allergy skin testing. Am. J. Health-Syst. Pharm. 2019, 76, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, E.S.; Macy, E.; Rowe, T.; Blumenthal, K.G. Evaluation and Management of Penicillin Allergy: A Review. JAMA 2019, 321, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Package Insert. PRE-PEN®. Available online: https://www.penallergytest.com/implementation-2/package-inserts/ (accessed on 25 May 2019).
- Kufel, W.; Justo, J.A.; Bookstaver, P.B.; Avery, L. Penicillin allergy assessment and skin testing in the outpatient setting. Pharmacy 2019. In Press. [Google Scholar]
- Chaudhry, S.B.; Veve, M.P.; Wagner, J.L. Cephalosporins: A focus on side chains and β-lactam cross-reactivity. Pharmacy 2019, 7, 103. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Bradley, N. Overview and insights into carbapenem allergy. Pharmacy 2019, 7, 110. [Google Scholar] [CrossRef] [PubMed]
- Stover, K.R.; Barber, K.E.; Wagner, J.L. Allergic reactions and cross-reactivity potential with beta-lactamase inhibitors. Pharmacy 2019, 7, 77. [Google Scholar] [CrossRef] [PubMed]
- Wall, G.C.; Peters, L.; Leaders, C.B.; Wille, J.A. Pharmacist-managed service providing penicillin allergy skin tests. Am. J. Health-Syst. Pharm. 2004, 61, 1271–1275. [Google Scholar] [CrossRef] [PubMed]
- Adkinson, N.F., Jr.; Mendelson, L.M.; Ressler, C.; Keogh, J.C. Penicillin minor determinants: History and relevance for current diagnosis. Ann. Allergy Asthma Immunol. 2018, 121, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.B.; Redmond, A.P.; Fellner, M.J.; Voss, H.E.; Levytska, V. Penicillin allergy and the heterogenous immune responses of man to benzylpenicillin. J. Clin. Investig. 1966, 45, 1895–1906. [Google Scholar] [CrossRef] [PubMed]
- Macy, E.; Richter, P.K.; Falkoff, R.; Zeiger, R. Skin testing with penicilloate and penilloate prepared by an improved method: Amoxicillin oral challenge in patients with negative skin test responses to penicillin reagents. J. Allergy Clin. Immunol. 1997, 100, 586–591. [Google Scholar] [CrossRef]
- Sastre, J.; Quijano, L.D.; Novalbos, A.; Hernandez, G.; Cuesta, J.; de las Heras, M.; Lluch, M.; Fernandez, M. Clinical cross-reactivity between amoxicillin and cephadroxil in patients allergic to amoxicillin and with good tolerance of penicillin. Allergy 1996, 51, 383–386. [Google Scholar] [CrossRef]
- Penicillin Allergy Assessment & Skin Testing (PAAST) Certificate Program. Available online: https://www.sc.edu/study/colleges_schools/pharmacy/centers/penicillin_allergy_skin_testing_certificate_program/index.php (accessed on 25 June 2019).
- Joint Task Force on Practice Parameters; American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology. Drug Allergy: An updated practice parameter. Ann. Allergy Asthma Immunol. 2010, 105, 259–273. [Google Scholar] [CrossRef]
- Leis, J.A.; Palmay, L.; Ho, G.; Raybardhan, S.; Gill, S.; Kan, T.; Campbell, J.; Kiss, A.; McCready, J.B.; Das, P.; et al. Point-of-Care β-Lactam Allergy Skin Testing by Antimicrobial Stewardship Programs: A Pragmatic Multicenter Prospective Evaluation. Clin. Infect. Dis. 2017, 65, 1059–1065. [Google Scholar] [CrossRef]
- Sousa-Pinto, B.; Blumenthal, K.G.; Macy, E.; Bavbek, S.; Benić, M.S.; Alves-Correia, M.; Dursun, A.B.; Jerschow, E.; Kong-Cardoso, B.; Kopač, P.; et al. Diagnostic testing for penicillin allergy: A survey of practices and cost perceptions. Allergy 2019. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, K.G.; Peter, J.G.; Trubiano, J.A.; Phillips, E.J. Antibiotic allergy. Lancet Lond. Engl. 2019, 393, 183–198. [Google Scholar] [CrossRef]
- Vega, J.M.; Blanca, M.; García, J.J.; Carmona, M.J.; Miranda, A.; Pérez-Estrada, M.; Fernández, S.; Acebes, J.M.; Terrados, S. Immediate allergic reactions to amoxicillin. Allergy 1994, 49, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.M.; Avramovski, N.; Concepcion, A.M.; Crosby, J.; Bland, C.M. Clinical and Economic Outcomes of Penicillin Skin Testing as an Antimicrobial Stewardship Initiative in a Community Health System. Open Forum Infect. Dis. 2019, 6, ofz109. [Google Scholar] [CrossRef] [PubMed]
- Griffith, N.; Bookstaver, P.B. Implementation of pharmacist-administered penicillin allergy skin testing. Presented at the American College of Clinical Pharmacy Annual Meeting, Phoenix, AZ, USA, 7–10 October 2017. Abstract #455 (Poster presentation). [Google Scholar]
- Blumenthal, K.G.; Shenoy, E.S.; Wolfson, A.R.; Berkowitz, D.N.; Carballo, V.A.; Balekian, D.S.; Marquis, K.A.; Elshaboury, R.; Gandhi, R.G.; Meka, P.; et al. Addressing Inpatient Beta-Lactam Allergies: A Multihospital Implementation. J. Allergy Clin. Immunol. Pract. 2017, 5, 616–625.e7. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, K.G.; Li, Y.; Banerji, A.; Yun, B.J.; Long, A.A.; Walensky, R.P. The Cost of Penicillin Allergy Evaluation. J. Allergy Clin. Immunol. Pract. 2018, 6, 1019–1027.e2. [Google Scholar] [CrossRef] [PubMed]
- Heil, E.L.; Bork, J.T.; Schmalzle, S.A.; Kleinberg, M.; Kewalramani, A.; Gilliam, B.L.; Buchwald, U.K. Implementation of an Infectious Disease Fellow-Managed Penicillin Allergy Skin Testing Service. Open Forum Infect. Dis. 2016, 3, ofw155. [Google Scholar] [CrossRef] [PubMed]
- King, E.A.; Challa, S.; Curtin, P.; Bielory, L. Penicillin skin testing in hospitalized patients with beta-lactam allergies: Effect on antibiotic selectcion and cost. Ann. Allergy Asthma Immunol. 2016, 117, 67–71. [Google Scholar] [CrossRef]
- Mattingly, T.J., 2nd; Meninger, S.; Heil, E.L. Penicillin skin testing in methicillin-sensitive Staphylococcus aureus bacteremia: A cost-effectiveness analysis. PLoS ONE 2019, 14, e0210271. [Google Scholar] [CrossRef]


| Allergy Reconciliation b |
|
|
|
| Management |
|
|
|
|
| Baseline Assessment | |
Identify and assemble key stakeholders with interest and expertise in allergy management and antimicrobial stewardship, such as:
| ☐ |
| Identify personnel who will execute PST and assess institutional and state regulations to determine if personnel are eligible (e.g., pharmacists, nurses, physicians) | ☐ |
| Evaluate technology available to aid in PST service development (e.g., paper vs. electronic medical records, alerting system, pop-up alert capacity, documentation options) | ☐ |
| Determine time when PST can be offered (e.g., Monday–Friday, 8 a.m. to 5 p.m.) | ☐ |
| Determine target patient populations, such as those currently receiving antibiotics, or with specified infectious diseases | ☐ |
| Identify inclusion and exclusion criteria for patients and projected minimum and maximum volumes for the service | ☐ |
| Determine level of patient consent required for PST | ☐ |
| Implementation | |
| Determine training and credentialing process of testing personnel | ☐ |
| Determine alerting system to notify personnel of a potential PST (e.g., consult, page) | ☐ |
| Liaise with pharmacy staff to outline PST supply procurement, storage, preparation, and dispensing | ☐ |
| Collaborate with IT/clinical informatics to ensure electronic medical record builds for appropriate PST ordering, alerting, etc. | ☐ |
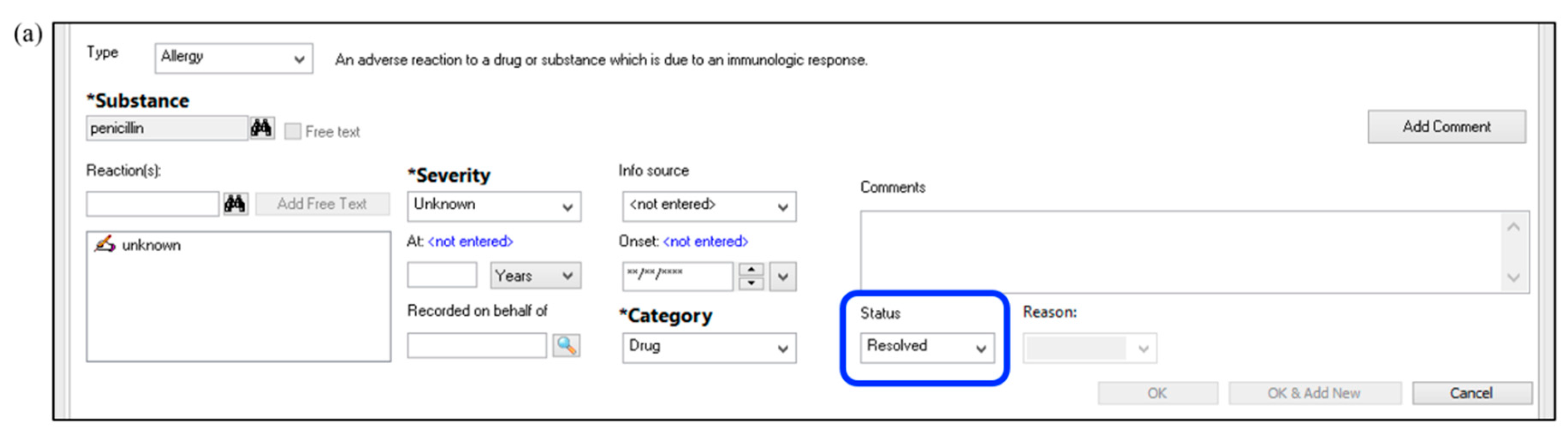
Outline documentation methods in the medical record, including updating of allergies following PST results
| ☐ |
Outline education for patients, providers, and pharmacies (both internal and external) following PST results
| ☐ |
| Determine institutional costs per PST and identify processes for billing, if possible | ☐ |
| Identify appropriate approval pathways for the PST protocol within the local institution | ☐ |
| Evaluation & Reporting | |
Determine a process for data collection, evaluation, and dissemination of PST service outcomes to:
| ☐ |
| Component | Dose/Notation | Quantity a | Comments |
|---|---|---|---|
| Drug Product | |||
| Histamine base 1 mg/mL (Histamine phosphate 2.75 mg/mL) (positive control) | 0.1 mL | 1 | For prick test |
| Sodium chloride 0.9% (negative control) | 0.15 mL | 2 | For prick and intradermal tests |
| Benzylpenicilloyl polylysine | 0.15 mL | 2 | For prick and intradermal tests |
| Penicillin dilution 5000 units/mL | 0.15 mL | 2 | For prick and intradermal tests |
| Diphenhydramine b | 50 mg IV × 1 PRN itching or severe reaction | 1 | |
| Hydrocortisone b | 50 mg IV × 1 PRN severe reaction | 1 | |
| Epinephrine 1:1000 | 0.3 mg IM × 1 PRN severe reaction | 1 | |
| Miscellaneous Component | |||
| Sterile scratch test devices (e.g., Duo-tip®) | 5 | For prick test | |
| Alcohol swabs | ~3–4 | To prep area prior to prick and intradermal tests | |
| Pen or marker | 1 | To mark test area | |
| Ruler | 1 | To measure wheal size | |
| Patient education card/document | 1 | Provided to patient |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Justo, J.A.; Kufel, W.D.; Avery, L.; Bookstaver, P.B. Penicillin Allergy Skin Testing in the Inpatient Setting. Pharmacy 2019, 7, 120. https://doi.org/10.3390/pharmacy7030120
Justo JA, Kufel WD, Avery L, Bookstaver PB. Penicillin Allergy Skin Testing in the Inpatient Setting. Pharmacy. 2019; 7(3):120. https://doi.org/10.3390/pharmacy7030120
Chicago/Turabian StyleJusto, Julie Ann, Wesley D. Kufel, Lisa Avery, and P. Brandon Bookstaver. 2019. "Penicillin Allergy Skin Testing in the Inpatient Setting" Pharmacy 7, no. 3: 120. https://doi.org/10.3390/pharmacy7030120
APA StyleJusto, J. A., Kufel, W. D., Avery, L., & Bookstaver, P. B. (2019). Penicillin Allergy Skin Testing in the Inpatient Setting. Pharmacy, 7(3), 120. https://doi.org/10.3390/pharmacy7030120






