Monoclonal Antibodies as a Breakthrough in Personalised Leukaemia Therapy: What Pharmacists and Doctors Should Know
Abstract
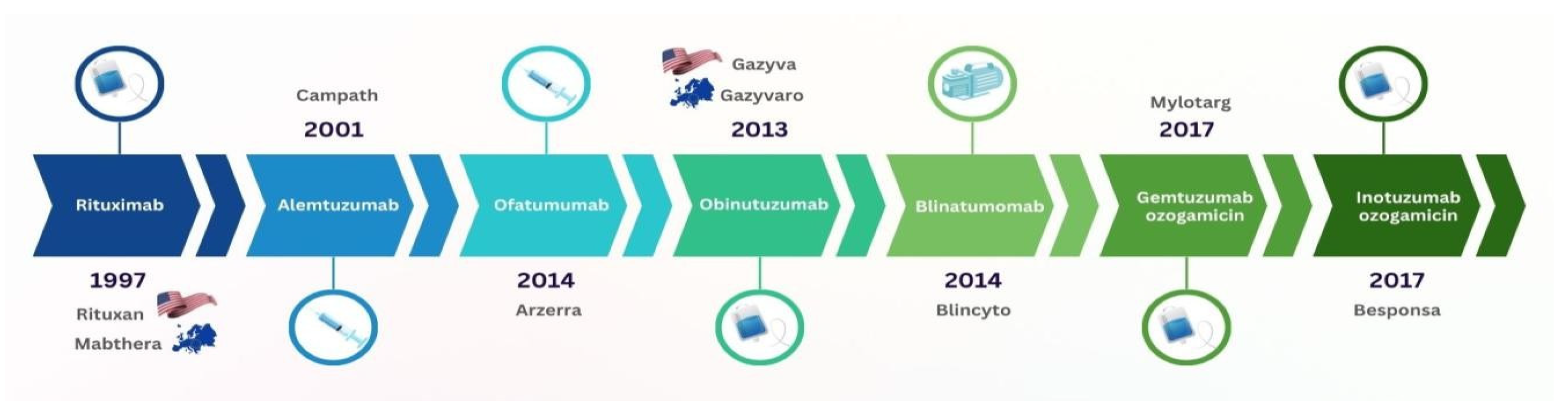
1. Introduction
2. Materials and Methods
3. Results and Discussion
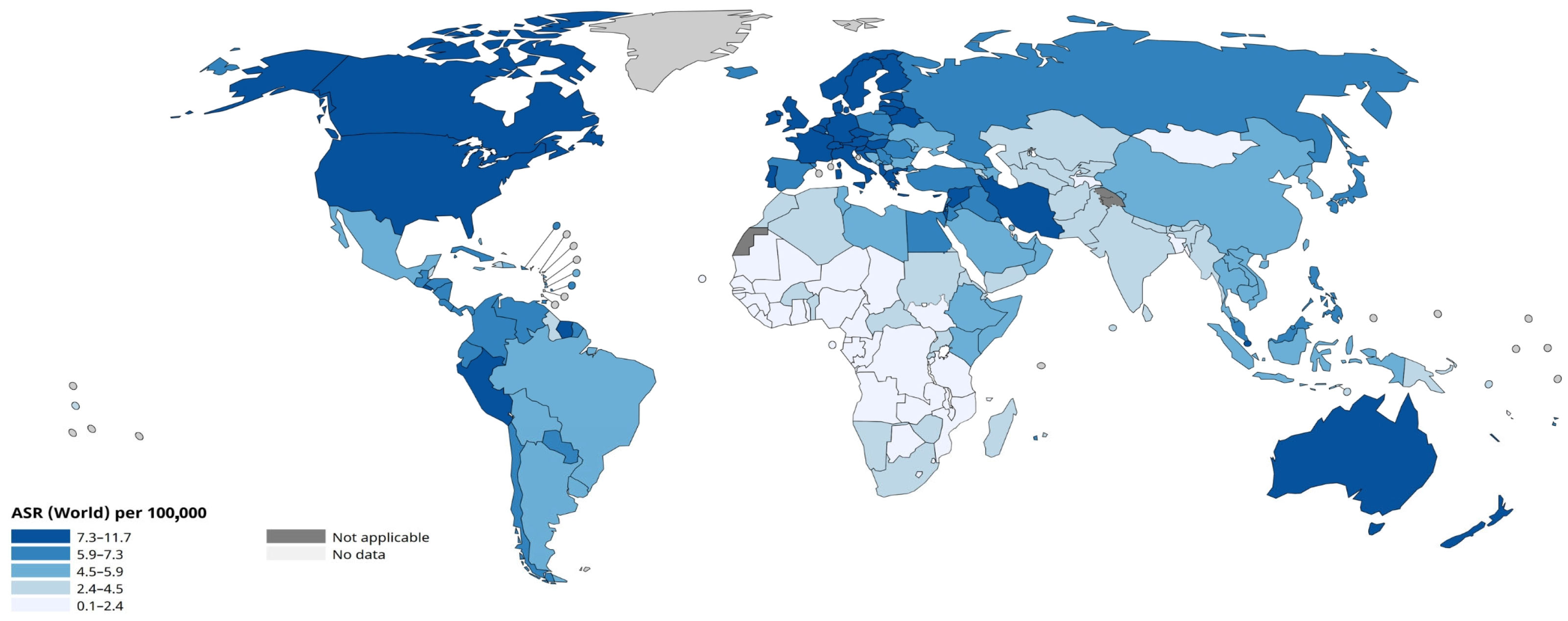
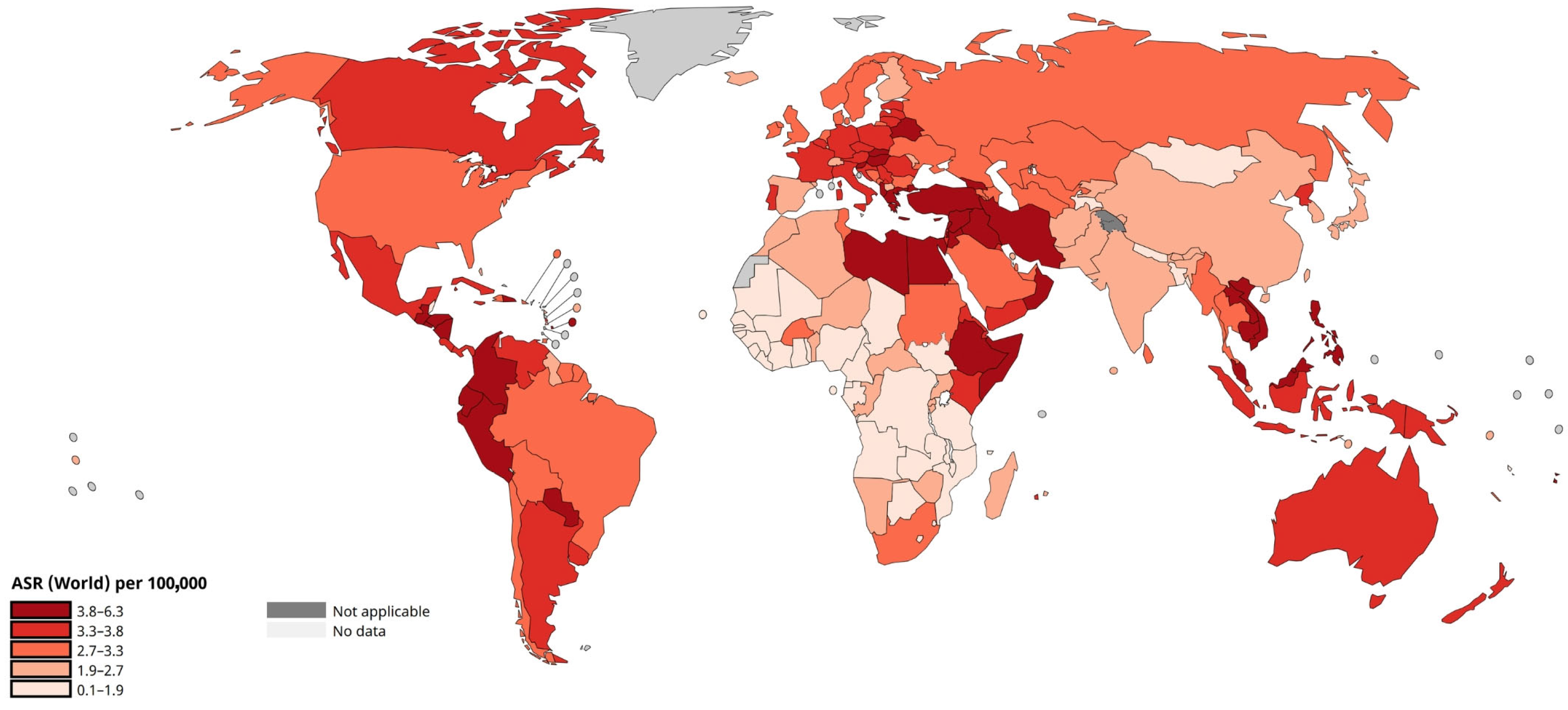
3.1. Introduction to Leukaemia Therapy
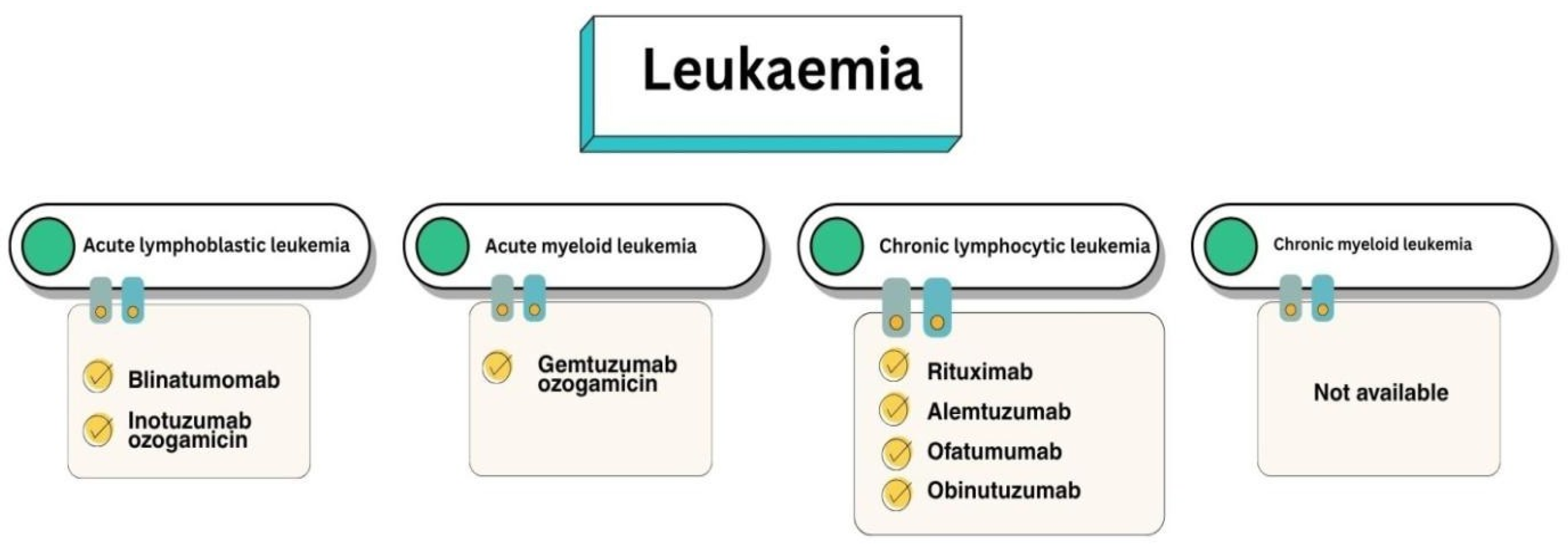
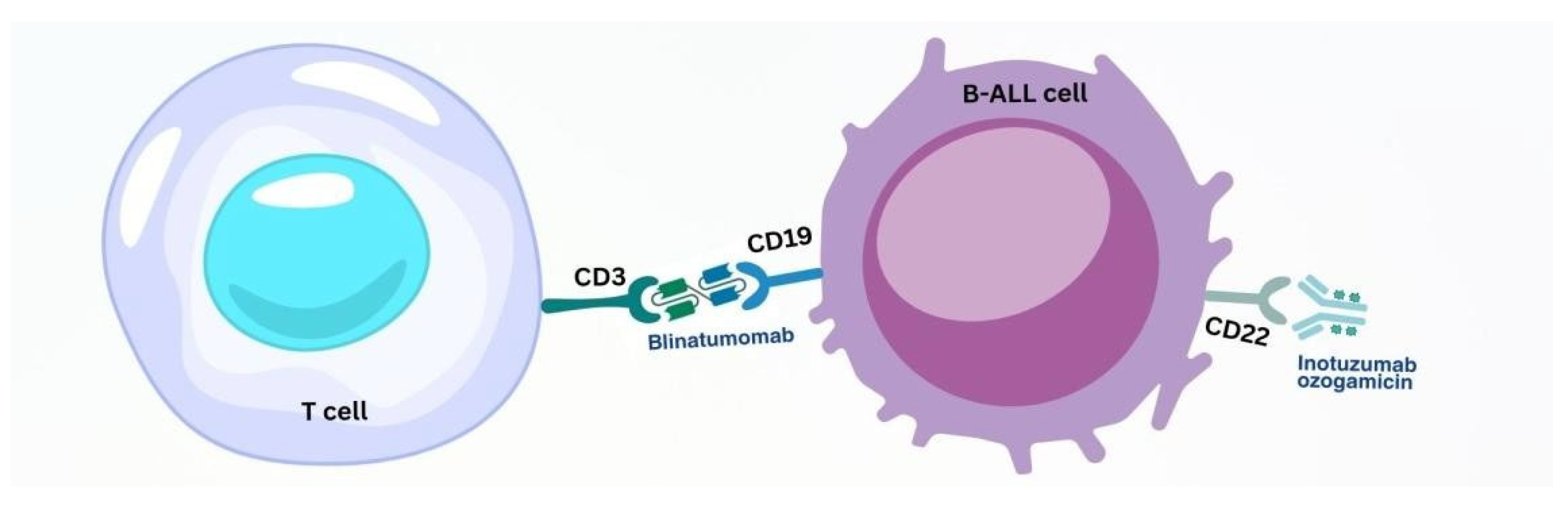
3.2. Targets of Immunobiological Therapy for B-ALL
3.2.1. BsAb Therapy of B-ALL
3.2.2. Anti-CD22 Therapy for B-ALL
- (1)
- The recombinant humanised immunoglobulin class G subtype 4 (IgG4) kappa antibody inotuzumab, specific to human CD22;
- (2)
- N-acetyl-gamma-calicheamicin, which causes double-stranded DNA breaks;
- (3)
- An acid-cleavable linker composed of the condensation product of 4-(4′-acetylphenoxy)-butanoic acid and 3-methyl-3-mercaptobutane hydrazide that covalently attaches N-acetyl-gamma-calicheamicin to inotuzumab [86].
3.3. Targets of Immunobiological Therapy for AML
3.4. Targets of Immunobiological Therapy for CLL
3.4.1. Anti-CD20 Therapy for CLL
3.4.2. Anti-CD52 Therapy for CLL
3.5. Chronic Myeloid Leukaemia
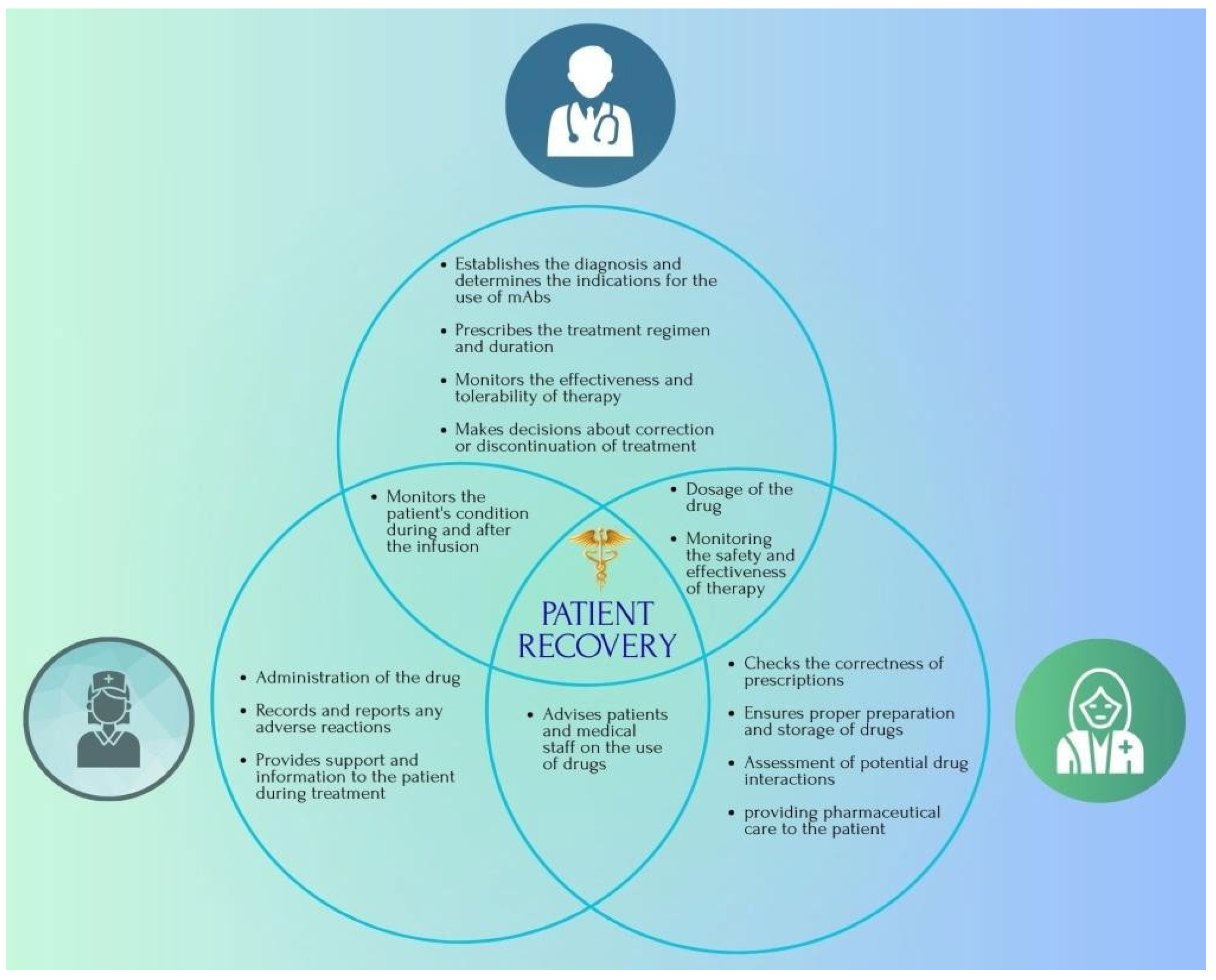
3.6. The Role of the Pharmacist in the Treatment of Various Types of Leukaemia with mAbs-Based Drugs
4. Conclusions
5. Limitations and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALL | Acute lymphocytic leukaemia |
| AML | Acute myeloid leukaemia |
| B-ALL | B-lineage acute lymphocytic leukaemia |
| B-CLL | B-cell chronic lymphocytic leukaemia |
| BsAb | Bispecific antibody |
| CLL | Chronic lymphocytic leukaemia |
| CML | Chronic myeloid leukaemia |
| CRS | Cytokine release syndrome |
| FDA | U.S. Food and Drug Administration |
| GO | Gemtuzumab ozogamicin |
| ICER | Institute for Clinical and Economic Review |
| ILD | Interstitial lung disease |
| InO | Inotuzumab ozogamicin |
| NICE | National Institute for Health and Care Excellence |
| mAbs | Monoclonal antibodies |
| MRD | Minimal residual disease |
| Ph | Philadelphia |
| PML | Progressive multifocal leukaemia |
| R/R | Relapsed or refractory |
| TLS | Tumour lysis syndrome |
| TT | Targeted therapy |
| VOD | Veno-occlusive liver disease |
| WHO | World Health Organisation |
References
- Ali, S.R.; Nigam, M.; Mishra, A.P. Monoclonal Antibodies and Cancer. In Biotechnology and Cancer Therapeutics; Springer: Singapore, 2025. [Google Scholar] [CrossRef]
- Justiz-Vaillant, A.; Pandit, B.R.; Unakal, C.; Vuma, S.; Akpaka, P.E. A Comprehensive Review About the Use of Monoclonal Antibodies in Cancer Therapy. Antibodies 2025, 14, 35. [Google Scholar] [CrossRef]
- Kumar, M.; Jalota, A.; Sahu, S.K.; Haque, S. Therapeutic Antibodies for the Prevention and Treatment of Cancer. J. Biomed. Sci. 2024, 31, 6. [Google Scholar] [CrossRef]
- Delgado, M.; Garcia-Sanz, J.A. Therapeutic Monoclonal Antibodies against Cancer: Present and Future. Cells 2023, 12, 2837. [Google Scholar] [CrossRef]
- World Health Organization. Clinical Research Landscape of Monoclonal Antibodies: Report on 2014–2023 Period; WHO: Geneva, Switzerland, 2024. [Google Scholar] [CrossRef]
- Broer, L.N.; Knapen, D.G.; de Groot, D.A.; Mol, P.G.M.; Kosterink, J.G.W.; de Vries, E.G.E.; Lub-de Hooge, M.N. Monoclonal Antibody Biosimilars for Cancer Treatment. iScience 2024, 27, 110115. [Google Scholar] [CrossRef]
- Malhotra, S.; Cameron, A.I.; Gotham, D.; Burrone, E.; Gardner, P.J.; Loynachan, C.; Morin, S.; Scott, C.P.; Pérez-Casas, C. Novel Approaches to Enable Equitable Access to Monoclonal Antibodies in Low- and Middle-Income Countries. PLOS Glob. Public Health 2024, 4, 0003418. [Google Scholar] [CrossRef] [PubMed]
- Gieber, L.; Muturi-Kioi, V.; Malhotra, S.; Sitani, A. Clinical and Regulatory Challenges and Opportunities for Monoclonal Antibodies in Low- and Middle-Income Countries: Lessons from COVID-19 and Beyond. Pharm. Med. 2023, 37, 203–214. [Google Scholar] [CrossRef]
- Huang, J.; Chan, S.C.; Ngai, C.H.; Lok, V.; Zhang, L.; Lucero-Prisno, D.E., 3rd; Xu, W.; Zheng, Z.J.; Elcarte, E.; Withers, M.; et al. Disease Burden, Risk Factors, and Trends of Leukaemia: A Global Analysis. Front. Oncol. 2022, 12, 904292. [Google Scholar] [CrossRef]
- Du, M.; Chen, W.; Liu, K.; Wang, L.; Hu, Y.; Mao, Y.; Sun, X.; Luo, Y.; Shi, J.; Shao, K.; et al. The Global Burden of Leukemia and Its Attributable Factors in 204 Countries and Territories: Findings from the Global Burden of Disease 2019 Study and Projections to 2030. J. Oncol. 2022, 2022, 1612702. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, P.; Grillo-Lopez, A.J.; Link, B.K.; Levy, R.; Czuczman, M.S.; Williams, M.E.; Heyman, M.R.; Bence-Bruckler, I.; White, C.A.; Cabanillas, F.; et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: Half of patients respond to a four-dose treatment program. J. Clin. Oncol. 1998, 16, 2825–2833. [Google Scholar] [CrossRef]
- Lu, R.M.; Hwang, Y.C.; Liu, I.J.; Lee, C.C.; Tsai, H.S.; Li, H.-J.; Wu, H.-C. Development of therapeutic antibodies for the treatment of diseases. J. Biomed. Sci. 2020, 27, 1. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. Cancer Today: GLOBOCAN 2020. Global Cancer Observatory 2022. Available online: https://gco.iarc.who.int/today/en/dataviz/maps-heatmap?mode=population&cancers=36 (accessed on 14 October 2025).
- Lin, Q.; Mao, L.; Shao, L.; Li, Z.; Qingmei, H.; Honghu, Z.; Jie, J.; Liangshun, Y. Global, regional, and national burden of chronic myeloid leukemia, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Front. Oncol. 2020, 10, 580759. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. Cancer Today: GLOBOCAN—Maps-heatmap (Cancers = 36, Types = 1). Global Cancer Observatory 2022. Available online: https://gco.iarc.who.int/today/en/dataviz/maps-heatmap?mode=population&cancers=36&types=1 (accessed on 14 October 2025).
- Fentie, A.M.; Huluka, S.A.; Gebremariam, G.T.; Gebretekle, G.B.; Abebe, E.; Fenta, T.G. Impact of Pharmacist-Led Interventions on Medication-Related Problems among Patients Treated for Cancer: A Systematic Review and Meta-Analysis of Randomized Control Trials. Res. Soc. Adm. Pharm. 2024, 20, 487–497. [Google Scholar] [CrossRef]
- Staynova, R.; Gavazova, E.; Kafalova, D. Clinical Pharmacist-Led Interventions for Improving Breast Cancer Management—A Scoping Review. Curr. Oncol. 2024, 31, 4178–4191. [Google Scholar] [CrossRef]
- Shah, M.; Rajha, E.; DiNardo, C.; Muckey, E.; Wierda, W.G.; Yeung, S.J. Adverse events of novel therapies for hematologic malignancies: What emergency physicians should know. Ann. Emerg. Med. 2020, 75, 264–286. [Google Scholar] [CrossRef]
- Conde-Royo, D.; Juárez-Salcedo, L.M.; Dalia, S. Management of adverse effects of new monoclonal antibody treatments in acute lymphoblastic leukaemia. Drugs Context 2020, 9, 2020-7-2. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M. Problems Facing Monoclonal Antibody Technology. Highlights Sci. Eng. Technol. 2024, 102, 550–554. [Google Scholar] [CrossRef]
- Cai, J.; Li, M.X.; Lu, S.; Shen, D.; Xie, W.; Zhu, J.-J.; Jiang, G.-J.; Lu, C.-X. Use of failure mode and effect analysis to improve the monoclonal antibody drugs management process in pharmacy intravenous admixture services. Sci. Rep. 2025, 15, 4653. [Google Scholar] [CrossRef] [PubMed]
- Prašnikar, M.; Bjelošević Žiberna, M.; Gosenca Matjaž, M.; Ahlin Grabnar, P. Novel strategies in systemic and local administration of therapeutic monoclonal antibodies. Int. J. Pharm. 2024, 667 Pt A, 124877. [Google Scholar] [CrossRef]
- Amgen, Inc. Blincyto® (Blinatumomab) for Injection, Prescribing Information. U.S. Food and Drug Administration 2024. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/125557Orig1s028Correctedlbl.pdf (accessed on 14 June 2024).
- Pfizer, Inc. Besponsa™ (Inotuzumab Ozogamicin) for Injection, Prescribing Information. U.S. Food and Drug Administration 2017. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761040s000lbl.pdf (accessed on 5 October 2025).
- Pfizer, Inc. Mylotarg™ (Gemtuzumab Ozogamicin) for Injection, Prescribing Information. U.S. Food and Drug Administration 2020. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761060lbl.pdf (accessed on 5 October 2025).
- Novartis Pharmaceuticals Corporation. ARZERRA® (Ofatumumab) Injection, Prescribing Information. U.S. Food and Drug Administration 2016. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/125326s062lbl.pdf (accessed on 5 October 2025).
- Genentech, Inc. Gazyva® (Obinutuzumab) Injection, Prescribing Information. U.S. Food and Drug Administration 2020. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2025/125486s037s038lbl.pdf (accessed on 5 October 2025).
- Genzyme Corporation. Campath® (Alemtuzumab) Injection, Prescribing Information. U.S. Food and Drug Administration 2023. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/103948s5171lbl.pdf (accessed on 5 October 2025).
- Genentech, Inc.; Biogen, Inc. Rituxan® (Rituximab) Injection, Prescribing Information. U.S. Food and Drug Administration 2012. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/103705s5367s5388lbl.pdf (accessed on 5 October 2025).
- Short, N.J.; Jabbour, E.; Jain, N.; Kantarjian, H. Inotuzumab ozogamicin for the treatment of adult acute lymphoblastic leukaemia: Past progress, current research and future directions. J. Hematol. Oncol. 2024, 17, 32. [Google Scholar] [CrossRef] [PubMed]
- Chennamadhavuni, A.; Iyengar, V.; Mukkamalla, S.K.R.; Shimanovsky, A. Leukaemia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; Araujo, I.B.O.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 2022, 36, 1720–1748. [Google Scholar] [CrossRef]
- Yun, J. Reclassification of Acute Myeloid Leukemia According to the 2022 World Health Organization Classification and the International Consensus Classification Using Open-Source Data. Ann. Lab. Med. 2025, 45, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Saleem, S.; Amin, J.; Sharif, M.; Mallah, G.A.; Kadry, S.; Gandomi, A.H. Leukaemia segmentation and classification: A comprehensive survey. Comput. Biol. Med. 2022, 150, 106135. [Google Scholar] [CrossRef]
- Pourmontaseri, H.; Habibzadeh, N.; Entezari, S.; Samadian, F.; Kiyani, S.; Taheri, M.; Ahmadi, A.; Fallahi, M.S.; Sheikhzadeh, F.; Ansari, A.; et al. Monoclonal antibodies for the treatment of acute lymphocytic leukemia: A literature review. Hum. Antibodies 2022, 30, 117–130. [Google Scholar] [CrossRef]
- Abuasab, T.; Rowe, J.; Tvito, A. Emerging Monoclonal Antibody Therapy for the Treatment of Acute Lymphoblastic Leukemia. Biologics 2021, 15, 419–431. [Google Scholar] [CrossRef]
- Jabbour, E.; O’Brien, S.; Ravandi, F.; Kantarjian, H. Monoclonal antibodies in acute lymphoblastic leukemia. Blood 2015, 125, 4010–4016. [Google Scholar] [CrossRef]
- Molica, M.; Perrone, S.; Andriola, C.; Rossi, M. Immunotherapy with Monoclonal Antibodies for Acute Myeloid Leukaemia: A Work in Progress. Cancers 2023, 15, 5060. [Google Scholar] [CrossRef] [PubMed]
- Soleimani Samarkhazan, H.; Noormohamadi, H.; Shafiei, F.S.; Taghinejad, Z.; Maleknia, M.; Raoufi, A.; Nouri, S.; Mohammadi, M.H. Targeting acute myeloid leukemia through antibody engineering: Innovations in immunotherapy and combination regimens. Clin. Exp. Med. 2025, 25, 215. [Google Scholar] [CrossRef]
- Isidori, A.; Cerchione, C.; Daver, N.; DiNardo, C.; Garcia-Manero, G.; Konopleva, M.; Jabbour, E.; Ravandi, F.; Kadia, T.; de la Fuente Burguera, A.; et al. Immunotherapy in Acute Myeloid Leukemia: Where We Stand. Front. Oncol. 2021, 11, 656218. [Google Scholar] [CrossRef] [PubMed]
- Fathi, A.T. Monoclonal Antibodies in Acute Myeloid Leukemia—Are We There Yet? Cancer J. 2022, 28, 37–42. [Google Scholar] [CrossRef]
- Welch, B.M.; Parikh, S.A.; Kay, N.E.; Medina, K.L. Profound phenotypic deficiencies in mature blood and bone marrow progenitor dendritic cells in chronic lymphocytic leukemia patients. Leukemia 2025, 39, 1915–1927. [Google Scholar] [CrossRef]
- Wainman, L.M.; Khan, W.A.; Kaur, P. Chronic Lymphocytic Leukemia: Current Knowledge and Future Advances in Cytogenomic Testing. In Advancements in Cancer Research; Sergi, C.M., Ed.; Exon Publications: Brisbane, Australia, 2023; Chapter 6. [Google Scholar] [CrossRef]
- Perutelli, F.; Jones, R.; Griggio, V.; Vitale, C.; Coscia, M. Immunotherapeutic Strategies in Chronic Lymphocytic Leukemia: Advances and Challenges. Front. Oncol. 2022, 12, 837531. [Google Scholar] [CrossRef]
- Kipps, T.J.; Choi, M.Y. Targeted Therapy in Chronic Lymphocytic Leukemia. Cancer J. 2019, 25, 378–385. [Google Scholar] [CrossRef]
- Patel, K.; Pagel, J.M. Current and future treatment strategies in chronic lymphocytic leukemia. J. Hematol. Oncol. 2021, 14, 69. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, E.; Kantarjian, H. Chronic myeloid leukemia: 2025 update on diagnosis, therapy, and monitoring. Am. J. Hematol. 2024, 99, 2191–2212. [Google Scholar] [CrossRef]
- Kumar, R.; Krause, D.S. Recent advances in understanding chronic myeloid leukemia: Where do we stand? Fac. Rev. 2021, 10, 35. [Google Scholar] [CrossRef]
- Bignold, L.P. (Ed.) Clinical features of tumours (Chapter 9). In Principles of Tumours; Academic Press: Cambridge, MA, USA, 2020; Volume 2, pp. 263–277. [Google Scholar]
- Puckett, Y.; Chan, O. Acute Lymphocytic Leukaemia; StatPearls Publishing: Treasure Island, FL, USA, 2025.
- Yin, H.; Wang, J.; Tan, Y.; Jiang, M.; Zhang, H.; Meng, G. Transcription factor abnormalities in B-ALL leukemogenesis and treatment. Trends Cancer 2023, 9, 855–870. [Google Scholar] [CrossRef] [PubMed]
- Aureli, A.; Marziani, B.; Venditti, A.; Sconocchia, T.; Sconocchia, G. Acute Lymphoblastic Leukaemia Immunotherapy Treatment: Now, Next, and Beyond. Cancers 2023, 15, 3346. [Google Scholar] [CrossRef] [PubMed]
- Boissel, N.; Chiaretti, S.; Papayannidis, C.; Ribera, J.M.; Bassan, R.; Sokolov, A.N.; Alam, N.; Brescianini, A.; Pezzani, I.; Kreuzbauer, G.; et al. Real-world use of blinatumomab in adult patients with B-cell acute lymphoblastic leukemia in clinical practice: Results from the NEUF study. Blood Cancer J. 2023, 13, 2. [Google Scholar] [CrossRef]
- Mirfakhraie, R.; Kuhestani Dehaghi, B.; Dehghani Ghorbi, M.; Ghaffari-Nazari, H.; Mohammadian, M.; Salimi, M.; Tavakoli Ardakani, M.; Parkhideh, S. All about blinatumomab: The bispecific T cell engager immunotherapy for B cell acute lymphoblastic leukemia. Hematol. Transfus. Cell Ther. 2024, 46, 101246. [Google Scholar] [CrossRef]
- Saygin, C.; Cannova, J.; Stock, W.; Muffly, L. Measurable residual disease in acute lymphoblastic leukaemia: Methods and clinical context in adult patients. Blood 2022, 107, 2783. [Google Scholar]
- Litzow, M.R.; Sun, Z.; Mattison, R.J.; Paietta, E.M.; Roberts, K.G.; Zhang, Y.; Racevskis, J.; Lazarus, H.M.; Rowe, J.M.; Arber, D.A.; et al. Blinatumomab for MRD-Negative Acute Lymphoblastic Leukemia in Adults. N. Engl. J. Med. 2024, 391, 320–333. [Google Scholar] [CrossRef]
- Attarbaschi, A.; Poyer, F. Blinatumomab is changing the standard of care paradigms of newly diagnosed and relapsed pediatric B-cell acute lymphoblastic leukemia. Memo 2025, 18, 104–107. [Google Scholar] [CrossRef]
- Jabbour, E.; Lussana, F.; Martínez-Sánchez, P.; Torrent, A.; Rifón, J.J.; Agrawal, V.; Tormo, M.; Cassaday, R.D.; Cluzeau, T.; Huguet, F.; et al. Subcutaneous blinatumomab in adults with relapsed or refractory B-cell acute lymphoblastic leukaemia: Post-hoc safety and activity analysis from a multicentre, single-arm, phase 1/2 trial. Lancet Haematol. 2025, 12, e529–e541. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, E.; Zugmaier, G.; Agrawal, V.; Martínez-Sánchez, P.; Rifón Roca, J.J.; Cassaday, R.D.; Böll, B.; Rijneveld, A.; Abdul-Hay, M.; Huguet, F.; et al. Single agent subcutaneous blinatumomab for advanced acute lymphoblastic leukemia. Am. J. Hematol. 2024, 99, 586–595. [Google Scholar] [CrossRef]
- Pourhassan, H.; Agrawal, V.; Pullarkat, V.; Aldoss, I. Positioning blinatumomab in the frontline of adult B-cell acute lymphoblastic leukemia treatment. Front. Oncol. 2023, 13, 1237031. [Google Scholar] [CrossRef]
- Ouyang, M.; Zhang, Y.; Shi, L.; Liu, J.; Wang, C.; Wang, F.; Zhang, Y.; Li, Y.; Zhang, M.X.; Hu, W.Q.; et al. Clinical study of blinatumomab in the treatment of adult relapsed/refractory Ph-negative acute B-lymphoblastic leukemia. PubMed 2023, 31, 1352–1357. [Google Scholar]
- DuVall, A.S.; Jori Sheade, J.; Anderson, D.; Yates, S.J.; Stock, W. Updates in the Management of Relapsed and Refractory Acute Lymphoblastic Leukaemia: An Urgent Plea for New Treatments Is Being Answered! JCO Oncol. Pract. 2022, 18, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.Y.; Muffly, L.S.; Stock, W. Philadelphia Chromosome-Negative B-Cell Acute Lymphoblastic Leukaemia in Adolescents and Young Adults. JCO Oncol. Pract. 2020, 16, 231–238. [Google Scholar] [CrossRef]
- Marrapodi, M.M.; Mascolo, A.; di Mauro, G.; Mondillo, G.; Pota, E.; Rossi, F. The safety of blinatumomab in pediatric patients with acute lymphoblastic leukemia: A systematic review and meta-analysis. Front. Pediatr. 2022, 10, 929122. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.S.; Schiller, G.; Benjamin, R.; Jia, C.; Zhang, A.; Zhu, M.; Zimmerman, Z.; Topp, M.S. Neurologic adverse events in patients with relapsed/refractory acute lymphoblastic leukemia treated with blinatumomab: Management and mitigating factors. Ann. Hematol. 2019, 98, 159–167. [Google Scholar] [CrossRef]
- Gao, W.; Yu, J.; Sun, Y.; Song, Z.; Liu, X.; Han, X.; Li, L.; Qiu, L.; Zhou, S.; Qian, Z.; et al. Adverse events in the nervous system associated with blinatumomab: A real-world study. BMC Med. 2025, 23, 72. [Google Scholar] [CrossRef]
- Queudeville, M.; Schlegel, P.; Heinz, A.T.; Lenz, T.; Döring, M.; Holzer, U.; Hartmann, U.; Kreyenberg, H. Blinatumomab in pediatric patients with relapsed/refractory B-cell precursor acute lymphoblastic leukemia. Eur. J. Haematol. 2021, 106, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Ojemolon, P.E.; Kalidindi, S.; Ahlborn, T.A.; Aihie, O.P.; Awoyomi, M.I. Cytokine Release Syndrome Following Blinatumomab Therapy. Cureus 2022, 14, e21583. [Google Scholar] [CrossRef] [PubMed]
- Tannous, A.; Gupta, A.; Alnaber, Y.; Gadhiya, D.; Singh, A.; Adla Jala, S.R.; Sakthivel, H.; Ramphul, K.; Chennapragada, S.S. Cytokine release syndrome among acute lymphocytic leukemia adults undergoing treatment with blinatumomab in the United States. JCO 2025, 43, e18518. [Google Scholar] [CrossRef]
- Jain, T.; Litzow, M.R. No free rides: Management of toxicities of novel immunotherapies in ALL, including financial. Blood Adv. 2018, 2, 3393–3403. [Google Scholar] [CrossRef]
- Maschmeyer, G.; De Greef, J.; Mellinghoff, S.C.; Nosari, A.; Thiebaut-Bertrand, A.; Bergeron, A.; Franquet, T.; Blijlevens, N.M.A.; Maertens, J.A. Infections associated with immunotherapeutic and molecular targeted agents in hematology and oncology. A position paper by the European Conference on Infections in Leukemia (ECIL). Leukemia 2019, 33, 844–862. [Google Scholar] [CrossRef]
- Quattrone, M.; Di Pilla, A.; Pagano, L.; Fianchi, L. Infectious complications during monoclonal antibodies treatments and cell therapies in Acute Lymphoblastic Leukemia. Clin. Exp. Med. 2023, 23, 1823–1833. [Google Scholar] [CrossRef]
- So, W.; Pandya, S.; Quilitz, R.; Shah, B.; Greene, J.N. Infectious Risks and Complications in Adult Leukemic Patients Receiving Blinatumomab. Mediterr. J. Hematol. Infect. Dis. 2018, 10, e2018029. [Google Scholar] [CrossRef]
- Amgen. Blincyto (Blinatumomab) Injection, for Intravenous Use [Prescribing Information]. 2024. Available online: www.accessdata.fda.gov/drugsatfda_docs/label/2024/125557s030lbl.pdf (accessed on 1 May 2025).
- Caillon, M.; Brethon, B.; van Beurden-Tan, C.; Supiot, R.; Le Mezo, A.; Chauny, J.V.; Majer, I.; Petit, A. Cost-Effectiveness of Blinatumomab in Pediatric Patients with High-Risk First-Relapse B-Cell Precursor Acute Lymphoblastic Leukemia in France. PharmacoEconomics Open 2023, 7, 639–653. [Google Scholar] [CrossRef]
- Ozeki, S.K.; Moriwaki, K.; Morimoto, K.; Shimozuma, K. EE289 Cost-Effectiveness Analysis of Blinatumomab for Advanced Acute Lymphoblastic Leukemia in Japan. Value Health 2024, 27, S111. [Google Scholar] [CrossRef]
- Diaz Martinez, J.P.; de Maraumont, T.A.; Camacho, L.M.; Garcia, L. Cost-effectiveness of blinatumomab for the treatment of B-precursor acute lymphoblastic leukemia pediatric patients with high-risk first-relapse in Mexico. Leuk. Res. 2024, 145, 107560. [Google Scholar] [CrossRef] [PubMed]
- Mizuguchi, T. Mathematical Optimization of Blinatumomab Preparation Method Simplifies Dosing and Administration. Blood 2024, 144 (Suppl. S1), 7624. [Google Scholar] [CrossRef]
- Marini, B.L.; Wechter, A.R.; Burke, P.W.; Bixby, D.; Perissinotti, A.J. Minimizing waste during preparation of blinatumomab infusions. Am. J. Health Syst. Pharm. 2016, 73, 19–20. [Google Scholar] [CrossRef]
- Cirino, M.; Provasi, R.; Cebulec, I.; Palmieri, C.; Schincariol, P.; Zanon, D. Pediatric blinatumomab preparation: Risk assessment on SmPC for software compliance. J. Oncol. Pharm. Pract. 2020, 27, 1674–1683. [Google Scholar] [CrossRef]
- Martínez Sánchez, P.; Zugmaier, G.; Lussana, F.; Berthon, C.; Kadu, P.; Katlinskaya, Y.; Wong, H.; Jabbour, E.; Rifon Roca, J.J.; Huguet, F.; et al. Safety and Pharmacokinetics of Subcutaneous Blinatumomab for the Treatment of Adults with Relapsed or Refractory B-Cell Precursor Acute Lymphoblastic Leukemia: Results from a Phase 1b Study. Blood 2022, 140, 6122–6124. [Google Scholar] [CrossRef]
- Zugmaier, G.; Subklewe, M.; Locatelli, F. Immunotherapy with blinatumomab in B-cell acute lymphoblastic leukemia: A narrative review of efficacy, toxicity, and patient management in relapse and consolidation. Ann. Blood 2025, 10, 9. [Google Scholar] [CrossRef]
- Seago, K.; Gallagher, C.M.; Yingling, S.K.; Cumpston, A. Financial Impact and Utilization of a Pharmacist-Driven Home Infusion Pathway for Blinatumomab in Patients Requiring Short-Term Inpatient Monitoring after Outpatient Initiation of Blinatumomab. J. Hematol. Oncol. Pharm. 2025, 15, 121–126. [Google Scholar]
- Bojilova-Dor, L.; Pinkney, K.; Cauff, B.; Kramer, D.; Schaefer, A.M.; Ballestas, C.; Diaz, M.H.; Stevens, J.S.; Grunwald, H.; Siryk, A.; et al. Successful Outpatient Administration of Blinatumomab Infusion in Adults with B-Cell Acute Lymphoblastic Leukemia. Blood 2021, 138 (Suppl. S1), 4028. [Google Scholar] [CrossRef]
- Takano, M.; Inoue, M.; Ikeda, Y.; Kage, H.; Inokawa, T.; Nakadate, K.; Yasu, T.; Tsuda, Y.; Goto, K. SEM Observation of the Filter after Administration of Blinatumomab: A Possibility of Leakage during Home Administration Using a Portable Infusion Pump. Int. J. Mol. Sci. 2023, 24, 5729. [Google Scholar] [CrossRef]
- Pfizer. Besponsa (Inotuzumab Ozogamicin) for Injection, for Intravenous Use [Prescribing Information]. 2024. Available online: https://www.pfizermedical.com/besponsa (accessed on 1 August 2025).
- Shor, B.; Gerber, H.P.; Sapra, P. Preclinical and clinical development of inotuzumab-ozogamicin in hematological malignancies. Mol Immunol. 2015, 67, 107–116. [Google Scholar] [CrossRef]
- Kirchhoff, H.; Karsli, U.; Schoenherr, C.; Battmer, K.; Erschow, S.; Talbot, S.R.; Steinemann, D.; Heuser, M.; Heidenreich, O.; Hilfiker-Kleiner, D.; et al. Venetoclax and dexamethasone synergize with inotuzumab ozogamicin-induced DNA damage signaling in B-lineage ALL. Blood 2021, 137, 2657–2661. [Google Scholar] [CrossRef]
- Zhao, Y.; Short, N.J.; Kantarjian, H.M.; Chang, T.C.; Ghate, P.S.; Qu, C.; Macaron, W.; Jain, N.; Thakral, B.; Phillips, A.H.; et al. Genomic determinants of response and resistance to inotuzumab ozogamicin in B-cell ALL. Blood 2024, 144, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Pfizer Inc. Besponsa (Inotuzumab Ozogamicin) for Injection, for Intravenous Use [Prescribing Information]. March 2024. Available online: https://labeling.pfizer.com/ShowLabeling.aspx?id=13312 (accessed on 1 August 2025).
- Kebriaei, P.; van Besien, K.; Cutler, C.; de Lima, L.; Giralt, S.; ∙Lee, S.; Marks, D.; Merchant, A.; Stock, W.; Stelljes, M. Management of Important Adverse Events Associated with Inotuzumab Ozogamicin in Patients with Relapsed or Refractory Acute Lymphoblastic Leukemia. Clin. Lymphoma Myeloma Leuk. 2017, 17, S261. [Google Scholar] [CrossRef]
- Kebriaei, P.; Cutler, C.; de Lima, M.; Giralt, S.; Lee, S.J.; Marks, D.; Merchant, A.; Stock, W.; van Besien, K.; Stelljes, M. Management of important adverse events associated with inotuzumab ozogamicin: Expert panel review. Bone Marrow Transplant. 2018, 53, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Papayannidis, C.; Petracci, E.; Zappasodi, P.; Fracchiolla, N.; Ciceri, F.; Sartor, C.; Roncoroni, E.; Di Raimondo, F.; Mattei, D.; Giannini, M.B.; et al. INO-CD22: A Multicenter, Real-World Study of Inotuzumab Ozogamicin Safety and Effectiveness in Adult Patients with Relapsed/Refractory Acute Lymphoblastic Leukemia. Cancer 2025, 131, 35820. [Google Scholar] [CrossRef]
- Kantarjian, H.M.; DeAngelo, D.J.; Advani, A.S.; Stelljes, M.; Kebriaei, P.; Cassaday, R.D.; Merchant, A.A.; Fujishima, N.; Uchida, T.; Calbacho, M.; et al. Hepatic adverse event profile of inotuzumab ozogamicin in adult patients with relapsed or refractory acute lymphoblastic leukaemia: Results from the open-label, randomised, phase 3 INO-VATE study. Lancet Haematol. 2017, 4, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Kantarjian, H.M.; DeAngelo, D.J.; Stelljes, M.; Liedtke, M.; Stock, W.; Gökbuget, N.; O’Brien, S.M.; Jabbour, E.; Wang, T.; White, J.L.; et al. Inotuzumab Ozogamicin versus Standard of Care in Relapsed or Refractory Acute Lymphoblastic Leukemia: Final Report and Long-Term Survival Follow-Up from the Randomized, Phase III INO-VATE Study. Cancer 2019, 125, 2474–2487. [Google Scholar] [CrossRef]
- Wells, K.N.; Martino, J.G. Evaluation of the inpatient use of inotuzumab ozogamicin and creation of appropriate use guidelines. J. Oncol. Pharm. Pract. 2023. [Google Scholar] [CrossRef]
- Radhakrishnan, V.S.; Modak, K.; Bhave, S.J.; Kumar, J.; Roychowdhury, M.; Ghosh, M.; Parihar, M.; Arora, N.; Mishra, D.K.; Nair, R.; et al. Inotuzumab Ozogamicin Monotherapy as an Outpatient Salvage Treatment in Relapsed Refractory B-Cell Acute Lymphoblastic Leukemia: Compassionate Access. Indian J. Med. Paediatr. Oncol. 2021, 42, 199–203. [Google Scholar] [CrossRef]
- Cox, E.; Wade, R.; Peron, M.; Dietz, K.C.; Eastwood, A.; Palmer, S.; Griffin, S. The Clinical and Cost Effectiveness of Inotuzumab Ozogamicin for the Treatment of Adult Relapsed or Refractory B-Cell Acute Lymphoblastic Leukaemia: An Evidence Review Group Evaluation of a NICE Single Technology Appraisal. Pharmacoeconomics 2019, 37, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Delea, T.E.; Zhang, X.; Amdahl, J.; Boyko, D.; Dirnberger, F.; Campioni, M.; Cong, Z. Cost Effectiveness of Blinatumomab versus Inotuzumab Ozogamicin in Adult Patients with Relapsed or Refractory B-Cell Precursor Acute Lymphoblastic Leukemia in the United States. Pharmacoeconomics 2019, 37, 1177–1193. [Google Scholar] [CrossRef]
- Djambazov, S.; Slavchev, G.; Encheva-Malinova, M.; Varbanova, V.; Velchev, M.; Raduilov, B.; Vekov, T. PCN142—Cost-Effectiveness Analysis of Inotuzumab Ozogamicin for the Treatment of Adults with Relapsed or Refractory B-Cell Precursor Acute Lymphoblastic Leukemia in Bulgaria. Value Health 2018, 21 (Suppl. S3), S38. [Google Scholar] [CrossRef]
- van Oostrum, I.; Russell-Smith, T.A.; Jakobsson, M.; Torup Østby, J.; Heeg, B. Cost-Effectiveness of Inotuzumab Ozogamicin Compared to Standard of Care Chemotherapy for Treating Relapsed or Refractory Acute Lymphoblastic Leukaemia Patients in Norway and Sweden. Pharmacoecon Open 2022, 6, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.Y.; Chen, H.Y.; Chen, T.Y.; Li, S.S.; Fang, W.T.; Wen, Y.C.; Lo, Y.W.; Ou, H.T. Cost-utility analysis of inotuzumab ozogamicin for relapsed or refractory B cell acute lymphoblastic leukemia from the perspective of Taiwan’s health care system. Eur. J. Health Econ. 2020, 21, 1105–1116. [Google Scholar] [CrossRef] [PubMed]
- Russell-Smith, A.; Murphy, L.; Nguyen, A.; Blauer-Peterson, C.; Terpenning, M.; Cao, F.; Li, S.; Bancroft, T.; Webb, N.; Dorman, S.; et al. Real-world Use of Inotuzumab Ozogamicin Is Associated with Lower Health Care Costs Than Blinatumomab in Patients with Acute Lymphoblastic Leukemia in the First Relapsed/Refractory Setting. J. Comp. Eff. Res. 2024, 13, e230142. [Google Scholar] [CrossRef]
- Juliusson, G.; Antunovic, P.; Derolf, A.; Lehmann, S.; Möllgård, L.; Stockelberg, D.; Tidefelt, U.; Wahlin, A.; Höglund, M. Age and Acute Myeloid Leukaemia: Real World Data on Decision to Treat and Outcomes From the Swedish Acute Leukaemia Registry. Blood 2009, 113, 4179–4187. [Google Scholar] [CrossRef]
- Sasaki, K.; Ravandi, F.; Kadia, T.M.; DiNardo, C.D.; Short, N.J.; Borthakur, G.; Jabbour, E.; Kantarjian, H.M. De Novo Acute Myeloid Leukaemia: A Population-Based Study of Outcome in the United States Based on the Surveillance, Epidemiology, and End Results (SEER) Database, 1980 to 2017. Cancer 2021, 127, 2049–2061. [Google Scholar] [CrossRef]
- Strickland, S.A.; Vey, N. Diagnosis and treatment of therapy-related acute myeloid leukaemia. Crit. Rev. Oncol./Hematol. 2022, 171, 103607. [Google Scholar] [CrossRef]
- Li, M.; Zhao, X. Leukocyte immunoglobulin-like receptor B4 (LILRB4) in acute myeloid leukaemia: From prognostic biomarker to immunotherapeutic target. Chin. Med. J. 2024, 22, 2697–2711. [Google Scholar] [CrossRef]
- Jiang, Q.; Patel, B.; Jin, X.; Di Grandi, D.; Bortell, E.; Czapkowski, B.; Lerch, T.F.; Meyer, D.; Patel, S.; Pegg, J.; et al. Structural Characterization of the Aggregates of Gemtuzumab Ozogamicin. ACS Omega 2019, 4, 6468–6475. [Google Scholar] [CrossRef]
- Pfizer Inc. MYLOTARG (Gemtuzumab Ozogamicin) for Injection, for Intravenous Use [Prescribing Information]. Available online: https://www.pfizermedical.com/patient/mylotarg (accessed on 23 March 2024).
- Baron, J.; Wang, E.S. Gemtuzumab ozogamicin for the treatment of acute myeloid leukaemia. Expert Rev. Clin. Pharmacol. 2018, 11, 549–559. [Google Scholar] [CrossRef]
- Lambert, J.; Pautas, C.; Terré, C.; Raffoux, E.; Turlure, P.; Caillot, D.; Legrand, O.; Thomas, X.; Gardin, C.; Gogat-Marchant, K.; et al. Gemtuzumab ozogamicin for de novo acute myeloid leukaemia: Final efficacy and safety updates from the open-label, phase III ALFA-0701 trial. Haematologica 2019, 104, 113–119. [Google Scholar] [CrossRef]
- Swaminathan, M.; Cortes, J.E. Update on the role of gemtuzumab-ozogamicin in the treatment of acute myeloid leukaemia. Ther. Adv. Hematol. 2023, 14, 20406207231154708. [Google Scholar] [CrossRef]
- Cortes, J.E.; de Lima, M.; Dombret, H.; Estey, E.H.; Giralt, S.A.; Montesinos, P.; Röllig, C.; Venditti, A.; Wang, E.S. Prevention, recognition, and management of adverse events associated with gemtuzumab ozogamicin use in acute myeloid leukaemia. J. Hematol. Oncol. 2020, 13, 137. [Google Scholar] [CrossRef]
- Dargenio, M.; Buquicchio, C.; Pastore, D.; Aprile, L.; Ciuffreda, L.; Greco, G.; Delia, M.; Federico, V.; Fina, M.P.; Seripa, D.; et al. Real-World Efficacy and Safety of Gemtuzumab Ozogamycin (GO) and 3 + 7 Regimen in Fit Newly Diagnosed Acute Myeloid Leukemia (AML) Patients. Leukemia Res. Rep. 2025, 24, 100525. [Google Scholar] [CrossRef]
- Corbacioglu, S.; Jabbour, E.J.; Mohty, M. Risk Factors for Development of and Progression of Hepatic Veno-Occlusive Disease/Sinusoidal Obstruction Syndrome. Biol. Blood Marrow Transplant. 2019, 25, 1271–1280. [Google Scholar] [CrossRef]
- Knabe, R.; Muller, P.; Schliemann, C.; Hanoun, M.; Unglaub, J.M.; Weinbergerová, B.; Mayer, J.; Krekeler, C.; Krause, S.W.; Kaufmann, M.; et al. Gastrointestinal Toxicity of Gemtuzumab Ozogamicin: Real-Life Data from the AMLCG, SAL, and CELL Study Groups. Blood Adv. 2025, 9, 3336–3339. [Google Scholar] [CrossRef]
- EMA. MYLOTARG (Gemtuzumab Ozogamicin)—European Medicines Agency. Product Information. September 2017. Available online: https://www.ema.europa.eu/en/documents/product-information/mylotarg-epar-product-information_en.pdf (accessed on 21 August 2025).
- Russell-Smith, T.A.; Brockbank, J.; Mamolo, C.; Knight, C. Cost Effectiveness of Gemtuzumab Ozogamicin in the First-Line Treatment of Acute Myeloid Leukaemia in the UK. PharmacoEconomics Open 2021, 5, 677–691. [Google Scholar] [CrossRef]
- Mamolo, C.; Welch, V.; Walter, R.B.; Cappelleri, J.C.; Brockbank, J.; Cawson, M.; Knight, C.; Wilson, M. Budget Impact Analysis of Gemtuzumab Ozogamicin for the Treatment of CD33-Positive Acute Myeloid Leukemia. Pharmacoeconomics 2021, 39, 121–131. [Google Scholar] [CrossRef]
- Mareque, M.; Montesinos, P.; Font, P.; Guinea, J.M.; de la Fuente, A.; Soto, J.; Oyagüez, I.; Brockbank, J.; Iglesias, T.; Llinares, J.; et al. Cost-Effectiveness Analysis of Gemtuzumab Ozogamicin for First-Line Treatment of Patients with CD33-Positive Acute Myeloid Leukaemia in Spain. Clinicoecon. Outcomes Res. 2021, 13, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Cairoli, R.; Furneri, G.; Di Virgilio, R.; Veggia, B.; Ferrara, F. Cost-effectiveness analysis of gemtuzumab ozogamicin for the treatment of de novo CD33-positive Acute Myeloid Leukaemia (AML) in Italy. BMC Health Serv. Res. 2023, 23, 36. [Google Scholar] [CrossRef]
- Hallek, M. Chronic Lymphocytic Leukaemia: 2025 Update on the Epidemiology, Pathogenesis, Diagnosis, and Therapy. Am. J. Hematol. 2025, 100, 450–480. [Google Scholar] [CrossRef]
- Casan, J.M.L.; Wong, J.; Northcott, M.J.; Opat, S. Anti-CD20 monoclonal antibodies: Reviewing a revolution. Hum. Vaccines Immunother. 2018, 14, 2820–2841. [Google Scholar] [CrossRef] [PubMed]
- Kikushige, Y. Pathogenesis of chronic lymphocytic leukaemia and the development of novel therapeutic strategies. J. Clin. Exp. Hematopathol. 2020, 60, 146–158. [Google Scholar] [CrossRef]
- James, D.F.; Kipps, T.J. Rituximab in chronic lymphocytic leukaemia. Adv. Ther. 2011, 28, 534–554. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kasi, P.M.; Tawbi, H.A.; Oddis, C.V.; Kulkarni, H.S. Clinical review: Serious adverse events associated with the use of rituximab—A critical care perspective. Crit Care 2012, 16, 231. [Google Scholar] [CrossRef] [PubMed]
- Ostensen, M.; Lockshin, M.; Doria, A.; Valesini, G.; Meroni, P.; Gordon, C.; Brucato, A.; Tincani, A. Update on safety during pregnancy of biological agents and some immunosuppressive anti-rheumatic drugs. Rheumatology 2008, 47, 28–31. [Google Scholar] [CrossRef][Green Version]
- Cohen, S.B.; Emery, P.; Greenwald, M.W.; Dougados, M.; Furie, R.A.; Genovese, M.C.; Keystone, E.C.; Loveless, J.E.; Burmester, G.R.; Cravets, M.W.; et al. Rituximab for rheumatoid arthritis refractory to anti-tumour necrosis factor therapy: Results of a multicentre, randomised, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum 2006, 54, 2793–2806. [Google Scholar]
- Chen, X.Q.; Peng, J.W.; Lin, G.N.; Li, M.; Xia, Z.J. The effect of prophylactic lamivudine on hepatitis B virus reactivation in HBsAg-positive patients with diffuse large B-cell lymphoma undergoing prolonged rituximab therapy. Med. Oncol. 2012, 29, 1237–1241. [Google Scholar] [CrossRef]
- Cairo, M.S.; Coiffier, B.; Reiter, A.; Younes, A. TLS Expert Panel. Recommendations for the evaluation of risk and prophylaxis of tumour lysis syndrome (TLS) in adults and children with malignant diseases: An expert TLS panel consensus. Br. J. Haematol. 2010, 149, 578–586. [Google Scholar] [CrossRef]
- Darmon, M.; Ciroldi, M.; Thiery, G.; Schlemmer, B.; Azoulay, E. Clinical review: Specific aspects of acute renal failure in cancer patients. Crit Care 2006, 10, 211. [Google Scholar] [CrossRef]
- Verma, S.K. Updated cardiac concerns with rituximab use: A growing challenge. Indian Heart J. 2016, 68, 246–248. [Google Scholar] [CrossRef] [PubMed]
- Patil, V.B.; Lunge, S.B.; Doshi, B.R. Managing a cardiac side effect of rituximab. Indian J. Drugs Dermatol. 2020, 9, 6372–6374. [Google Scholar]
- Emery, P.; Fleischmann, R.; Filipowicz-Sosnowska, A.; Schechtman, J.; Szczepanski, L.; Kavanaugh, A.; Racewicz, A.J.; van Vollenhoven, R.F.; Li, N.F.; Agarwal, S.; et al. The efficacy and safety of rituximab in patients with active rheumatoid arthritis despite methotrexate treatment: Results of a phase IIB randomised, double-blind, placebo-controlled, dose-ranging trial. Arthritis Rheum 2006, 54, 1390–1400. [Google Scholar] [CrossRef]
- Gellrich, S.; Muche, J.M.; Wilks, A.; Jasch, K.C.; Voit, C.; Fischer, T.; Audring, H.; Sterry, W. Systemic eight-cycle anti-CD20 monoclonal antibody (rituximab) therapy in primary cutaneous B-cell lymphomas--an application observation. Br. J. Dermatol. 2005, 153, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Scheinfeld, N. A review of rituximab in cutaneous medicine. Dermatol. Online J. 2006, 12, 3. [Google Scholar] [CrossRef]
- Urru, S.A.M.; Spila Alegiani, S.; Guella, A.; Traversa, G.; Campomori, A. Safety of switching between rituximab biosimilars in onco-hematology. Sci. Rep. 2021, 11, 5956. [Google Scholar] [CrossRef]
- Jurczak, W.; Długosz Danecka, M.; Buske, C. Rituximab biosimilars for lymphoma in Europe. Expert Opin. Biol. Ther. 2019, 19, 1045–1056. [Google Scholar] [CrossRef] [PubMed]
- Cheesman, S. Introduction of Biosimilar Rituximab: A Hospital Perspective. Hemasphere 2020, 5, 515. [Google Scholar] [CrossRef]
- Song, N.K.; Musa, H.; Soriano, M.; Batger, M.; Hawkins, B.; Ramzan, I.; Hibbs, D.E.; Ong, J.A. Safety and efficacy comparisons of rituximab biosimilars to the reference product in patients with cancer: A systematic meta-analysis review. J. Pharm. Pract. Res. 2022, 52, 332–356. [Google Scholar] [CrossRef]
- Lee, S.; Lee, H.; Kim, E. Comparative Efficacy and Safety of Biosimilar Rituximab and Originator Rituximab in Rheumatoid Arthritis and Non-Hodgkin’s Lymphoma: A Systematic Review and Meta-analysis. BioDrugs 2019, 33, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Ayer, T.; Nastoupil, L.J.; Rose, A.C.; Flowers, C.R. Comparing the Cost-Effectiveness of Rituximab Maintenance and Radioimmunotherapy Consolidation versus Observation Following First-Line Therapy in Patients with Follicular Lymphoma. Value Health 2015, 18, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.S.; de Andrade, J.P.; Freitas, D.A.; Gonçalves, É.S.D.; Borges, D.L.; Carvalho, L.M.A.; Noronha, K.V.M.S.; Andrade, M.V. Cost-Effectiveness Analysis of Rituximab for Chronic Lymphocytic Leukemia Using a Semi-Markovian Model Approach in R. Value Health Reg. Issues 2023, 36 (Suppl. C), 10–17. [Google Scholar] [CrossRef]
- Rognoni, C.; Bertolani, A.; Jommi, C. Budget Impact Analysis of Rituximab Biosimilar in Italy from the Hospital and Payer Perspectives. Glob. Reg. Health Technol. Assess. 2018, 5, 2284240318784289. [Google Scholar] [CrossRef]
- Gulácsi, L.; Brodszky, V.; Baji, P.; Rencz, F.; Péntek, M. The Rituximab Biosimilar CT-P10 in Rheumatology and Cancer: A Budget Impact Analysis in 28 European Countries. Adv. Ther. 2017, 34, 1128–1144. [Google Scholar] [CrossRef]
- Tsimberidou, A.M. Ofatumumab in the treatment of chronic lymphocytic leukaemia. Drugs Today 2010, 46, 451–461. [Google Scholar] [CrossRef]
- Laurenti, L.; Innocenti, I.; Autore, F.; Sica, S.; Efremov, D.G. New developments in the management of chronic lymphocytic leukaemia: Role of ofatumumab. OncoTargets Ther. 2016, 9, 421–429. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Raedler, L.A. Arzerra (ofatumumab) receives FDA approval for patients with previously untreated chronic lymphocytic leukaemia for use in combination with chlorambucil. Oncol. Pract. Manag. 2014, 4, 6. Available online: https://oncpracticemanagement.com/issues/2014/october-2014-vol-4-no-6/arzerra-ofatumumab-receives-fda-approval-for-patients-with-previously-untreated-chronic-lymphocytic-leukemia-for-use-in-combination-with-chlorambucil (accessed on 5 October 2025).
- Hatswell, A.J.; Thompson, G.J.; Maroudas, P.A.; Sofrygin, O.; Delea, T.E. Estimating outcomes and cost effectiveness using a single-arm clinical trial: Ofatumumab for double-refractory chronic lymphocytic leukemia. Cost Eff. Resour. Alloc. 2017, 15, 8. [Google Scholar] [CrossRef]
- Soini, E.; Martikainen, J.; Nousiainen, T. Cost-effectiveness of first-line chronic lymphocytic leukemia treatments when full-dose fludarabine is unsuitable. Clin. Ther. 2016, 38, 889–904.e14. [Google Scholar] [CrossRef][Green Version]
- Chen, Z.; Cheng, Y.; DeRemer, D.; Diaby, V. Cost-effectiveness and drug wastage of immunotherapeutic agents for hematologic malignancies: A systematic review. Expert Rev. Pharmacoecon. Outcomes Res. 2021, 21, 923–941. [Google Scholar] [CrossRef]
- Lakhotia, R.; Melani, G.; Pittaluga, S.; Gordon, M.J.; Phelan, J.D.; Muppidi, J.R.; Tadese, A.; Evans, S.; Jaffe, E.S.; Staudt, L.M.; et al. The anti-CD47 antibody magrolimab with obinutuzumab and venetoclax in relapsed or refractory indolent B-cell lymphomas. Br. J. Haematol. 2025. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, J. Obinutuzumab in chronic lymphocytic leukaemia. Future Oncol. 2015, 11, 2503–2513. [Google Scholar] [CrossRef] [PubMed]
- Teeling, J.L.; Mackus, W.J.; Wiegman, L.J.; van den Brakel, J.H.; Beers, S.A.; French, R.R.; van Meerten, T.; Ebeling, S.; Vink, T.; Slootstra, J.W.; et al. The biological activity of human CD20 monoclonal antibodies is linked to unique epitopes on CD20. J. Immunol. 2006, 177, 362–372. [Google Scholar] [CrossRef]
- Herter, S.; Herting, F.; Mundigl, O.; Waldhauer, I.; Weinzierl, T.; Fauti, T.; Muth, G.; Ziegler-Landesberger, D.; Van Puijenbroek, E.; Lang, S.; et al. Preclinical activity of the type II CD20 antibody GA101 (obinutuzumab) compared with rituximab and ofatumumab in vitro and in xenograft models. Mol. Cancer Ther. 2013, 12, 2031–2042. [Google Scholar] [CrossRef]
- Al-Sawaf, O.; Fischer, K.; Engelke, A.; Pflug, N.; Hallek, M.; Goede, V. Obinutuzumab in chronic lymphocytic leukaemia: Design, development and place in therapy. Drug Des. Devel. Ther. 2017, 11, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.S.; Clemmons, A.B. Obinutuzumab: A novel anti-CD20 monoclonal antibody for chronic lymphocytic leukaemia. J. Adv. Pract. Oncol. 2015, 6, 370–374. [Google Scholar]
- Bourrier, N.; Landego, I.; Bucher, O.; Squires, M.; Streu, E.; Hibbert, I.; Whiteside, T.; Gibson, S.B.; Geirnaert, M.; Johnston, J.B.; et al. Real world risk of infusion reactions and efficacy of front-line obinutuzumab plus chlorambucil compared with other front-line treatments for chronic lymphocytic leukaemia. BMC Cancer 2022, 22, 148. [Google Scholar] [CrossRef]
- Pullarkat, P.; Lei, M.; Redd, R.; Rana, R.; Lou, U.; Sorial, M.; Rowen, B.; Kim, E.B.; Medrano, A.; Haydu, J.E.; et al. Short-duration infusion of obinutuzumab with venetoclax in chronic lymphocytic leukaemia: A prospective observational study. Blood Neoplasia 2025, 2, 100075. [Google Scholar] [CrossRef]
- Wang, C.; Dong, Y.; Men, P.; Zhang, R.; Xiao, Y.; Bu, Y.; Qin, Y.; Zhang, X.; Dou, Q.; Yang, Y.; et al. Efficacy, safety and cost-effectiveness of obinutuzumab in patients with follicular lymphoma: A rapid review. Front. Pharmacol. 2025, 15, 1426772. [Google Scholar] [CrossRef]
- Spencer, S.J.; Guzauskas, G.F.; FeLizzi, F.; Launonen, A.; Dawson, K.; Veenstra, D.L.; Masaquel, A. Cost-effectiveness of obinutuzumab versus rituximab biosimilars for previously untreated follicular lymphoma. J. Manag. Care Spec. Pharm. 2021, 27, 615–624. [Google Scholar] [CrossRef]
- Do, N.; Thielen, F.W. Cost-effectiveness of venetoclax plus obinutuzumab versus chlorambucil plus obinutuzumab for the first-line treatment of adult patients with chronic lymphocytic leukemia: An extended societal view. Value Health 2023, 26, 477–486. [Google Scholar] [CrossRef]
- Salisbury, J.R.; Rapson, N.T.; Codd, J.D.; Rogers, M.V.; Nethersell, A.B. Immunohistochemical analysis of CDw52 antigen expression in non-Hodgkin’s lymphomas. J. Clin. Pathol. 1994, 47, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Rowan, W.; Tite, J.; Topley, P.; Brett, S.J. Cross-linking of the CAMPATH-1 antigen (CD52) mediates growth inhibition in human B- and T-cell lymphoma cell lines, and subsequent emergence of CD52-deficient cells. Immunology 1998, 95, 427–436. [Google Scholar] [CrossRef]
- Christian, B.A.; Lin, T.S. Antibody therapy for chronic lymphocytic leukaemia. Semin. Hematol. 2008, 45, 95–103. [Google Scholar] [CrossRef][Green Version]
- Keating, M.J.; Flinn, I.; Jain, V.; Binet, J.L.; Hillmen, P.; Byrd, J.; Albitar, M.; Brettman, L.; Santabarbara, P.; Wacker, B.; et al. Therapeutic role of alemtuzumab (Campath-1H) in patients who have failed fludarabine: Results of a large international study. Blood 2002, 99, 3554–3561. [Google Scholar] [CrossRef] [PubMed]
- Stilgenbauer, S.; Winkler, D.; Kr öber, A.; Kienle, D.; Hallek, M.; Hensel, M.; Lengfelder, E.; Trümper, L.; Dreger, P.; Jäger, U.; et al. Subcutaneous Campath-1H (alemtuzumab) in fludarabine-refractory CLL: Interim analysis of the CLL2H study of the German CLL Study Group (GCLLSG). Blood 2004, 104, 478. [Google Scholar] [CrossRef]
- Eden, R.E.; Coviello, J.M. Chronic Myelogenous Leukaemia; StatPearls Publishing: Treasure Isalnd, FL, USA, 2025.
- Amadori, S.; Stasi, R. Monoclonal antibodies and immunoconjugates in acute myeloid leukaemia. Best Pract. Res. Clin. Haematol. 2006, 19, 715–736. [Google Scholar] [CrossRef]
- Kondo, M. Lymphoid and myeloid lineage commitment in multipotent hematopoietic progenitors. Immunol. Rev. 2010, 238, 37–46. [Google Scholar] [CrossRef]
- Weir, A.B. Hazard Identification and Risk Assessment for Biologics Targeting the Immune System. Immunotoxicology 2008, 5, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Al-Taie, A.; Sheta, N. Clinically approved monoclonal antibodies-based immunotherapy: Association with glycemic control and impact role of clinical pharmacist for cancer patient care. Clin. Ther. 2024, 46, e29–e44. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.V., Jr. Compounding with biotechnology products, Part 2: Product-specific considerations. Int. J. Pharm. Compd. 2022, 26, 446–466. [Google Scholar] [PubMed]
- Suárez-Casillas, P.; Lora-Escobar, S.J.; Montecatine-Alonso, E.; Li, T.; Acosta-García, H. Stability of thermolabile drugs at room temperature: A review. Farm. Hosp. 2025, 49, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Stabilis. Blinatumomab—Stability in Solutions. Stabilis Database. 2025. Available online: https://stabilis.org/Monographie.php?IdMolecule=960&codeLangue=EN-en (accessed on 2 October 2025).
- Stabilis. Rituximab—Stability in solutions. Stabilis Database. 2025. Available online: https://stabilis.org/Monographie.php?IdMolecule=280 (accessed on 5 October 2025).
- Stabilis. Obinutuzumab—Stability in Solutions. Stabilis Database. 2025. Available online: https://stabilis.org/Monographie.php?IdMolecule=977 (accessed on 5 October 2025).
- Stabilis. Alemtuzumab—Stability in Solutions. Stabilis Database. 2025. Available online: https://stabilis.org/Monographie.php?IdMolecule=558 (accessed on 25 September 2025).

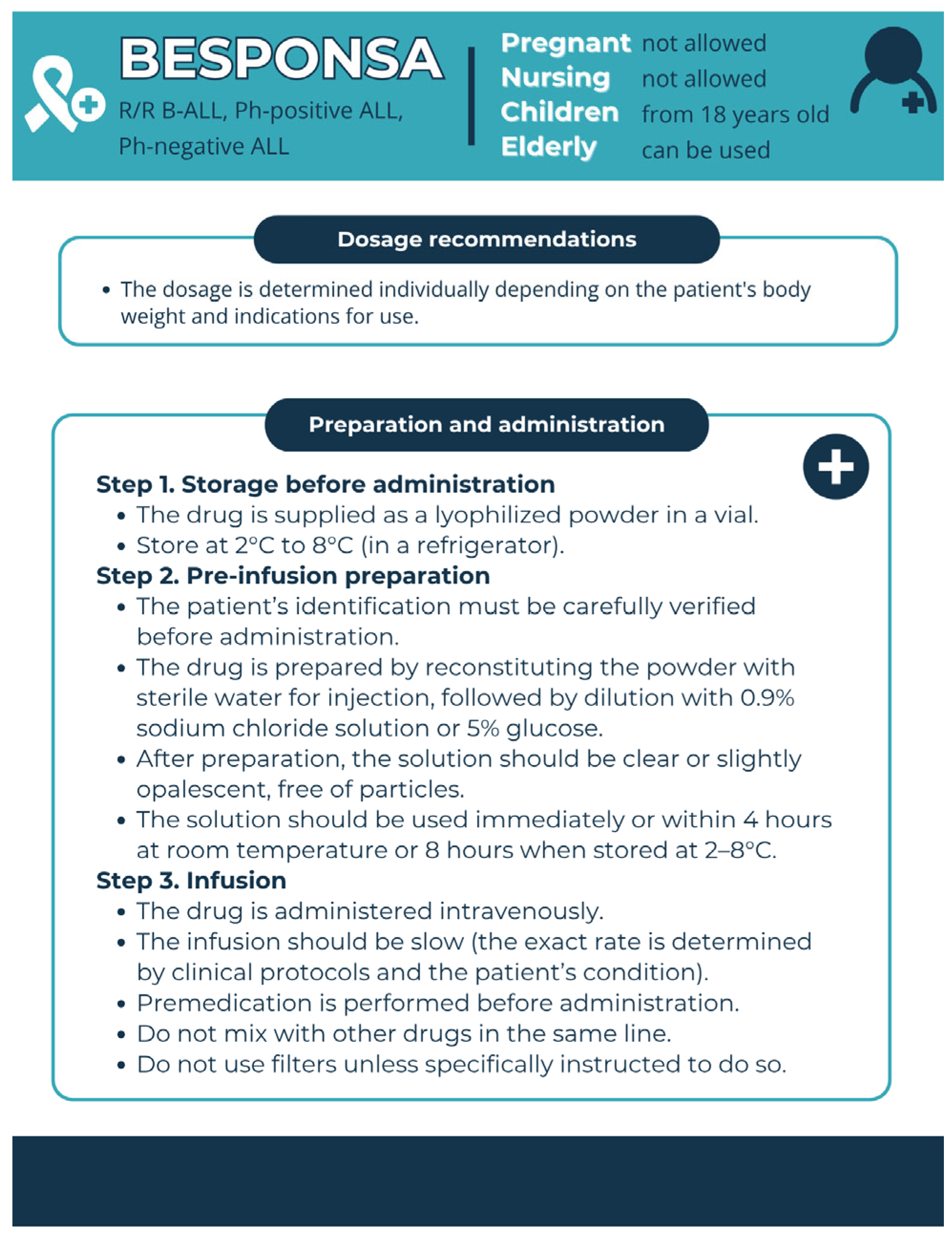
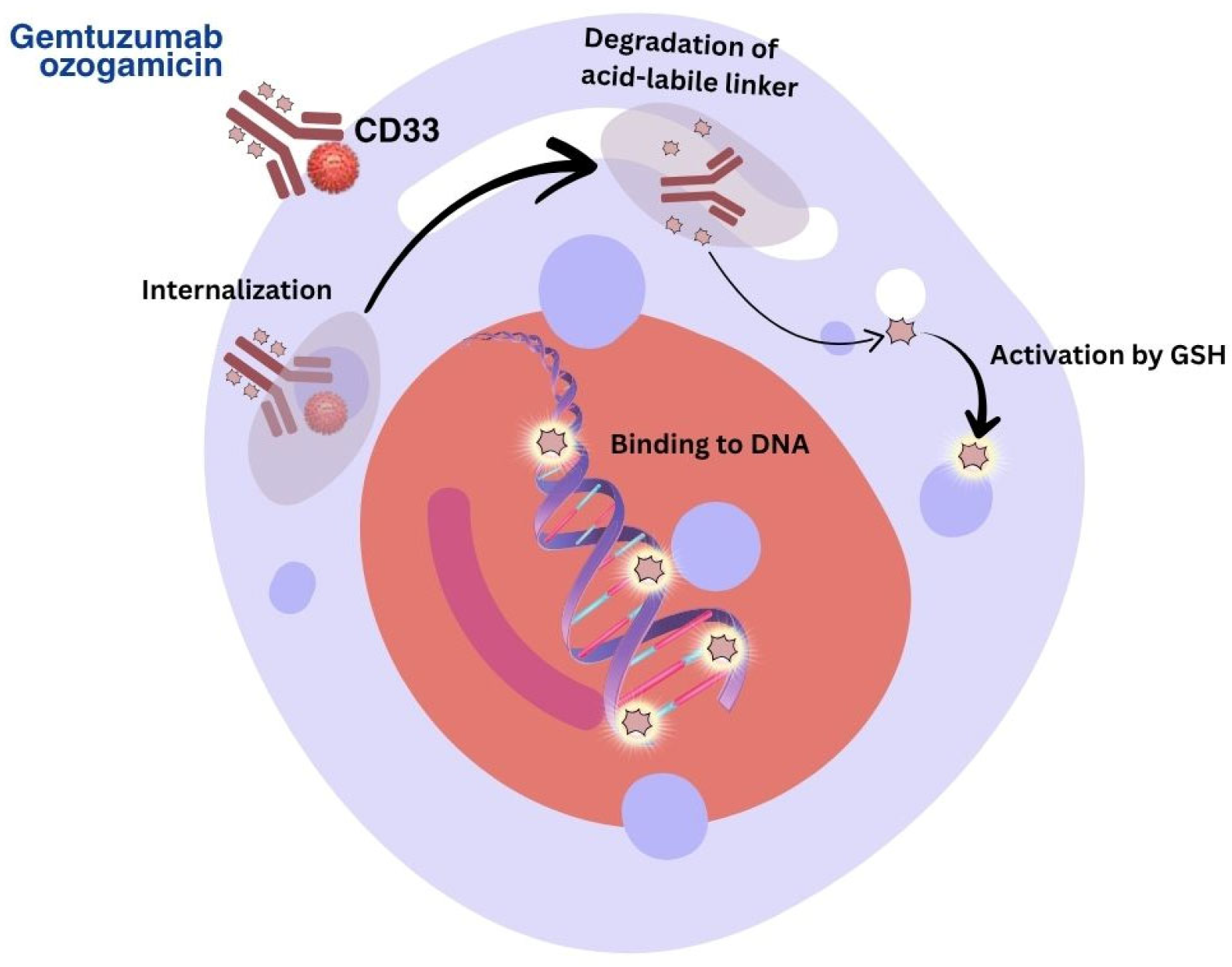
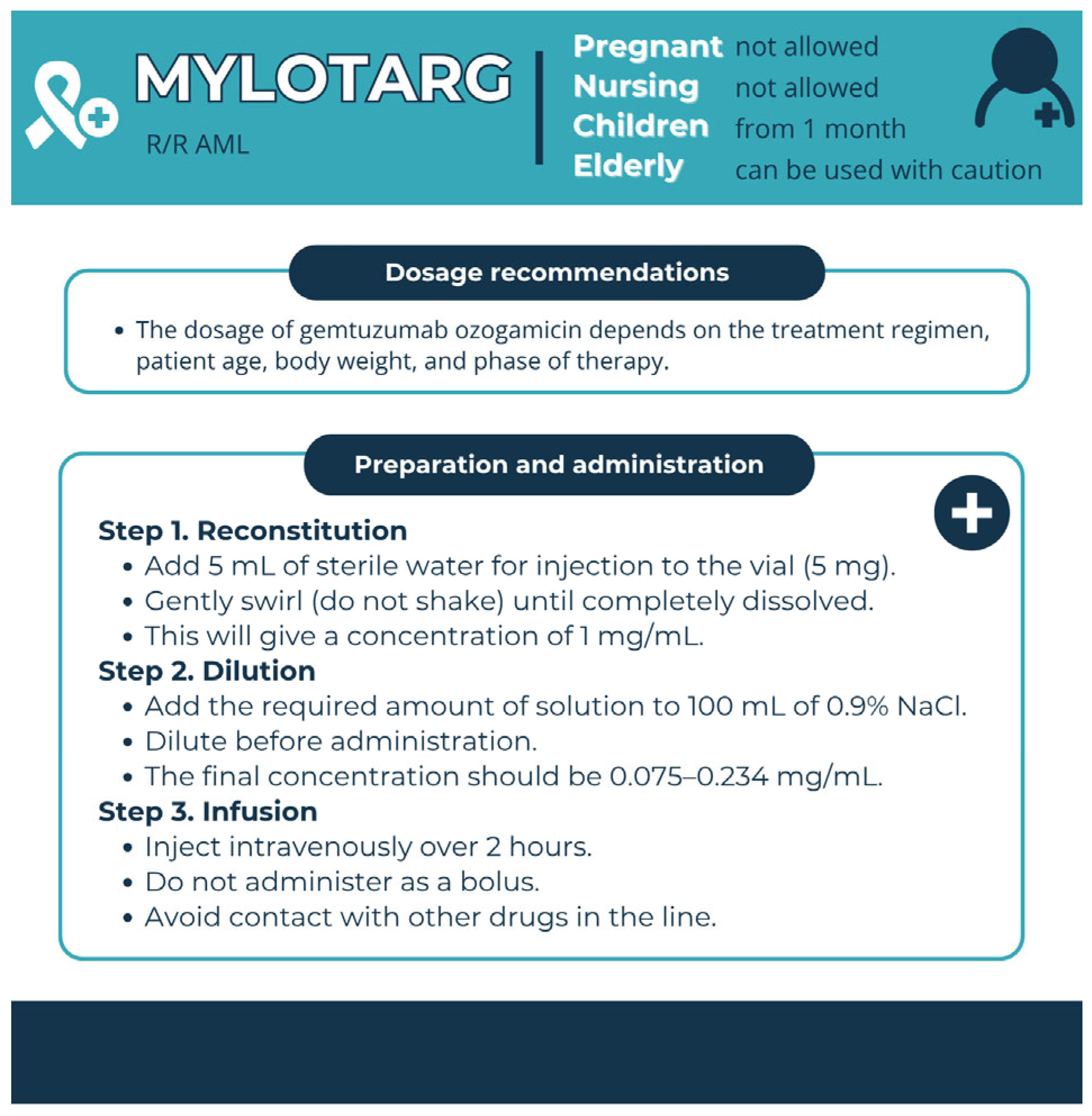
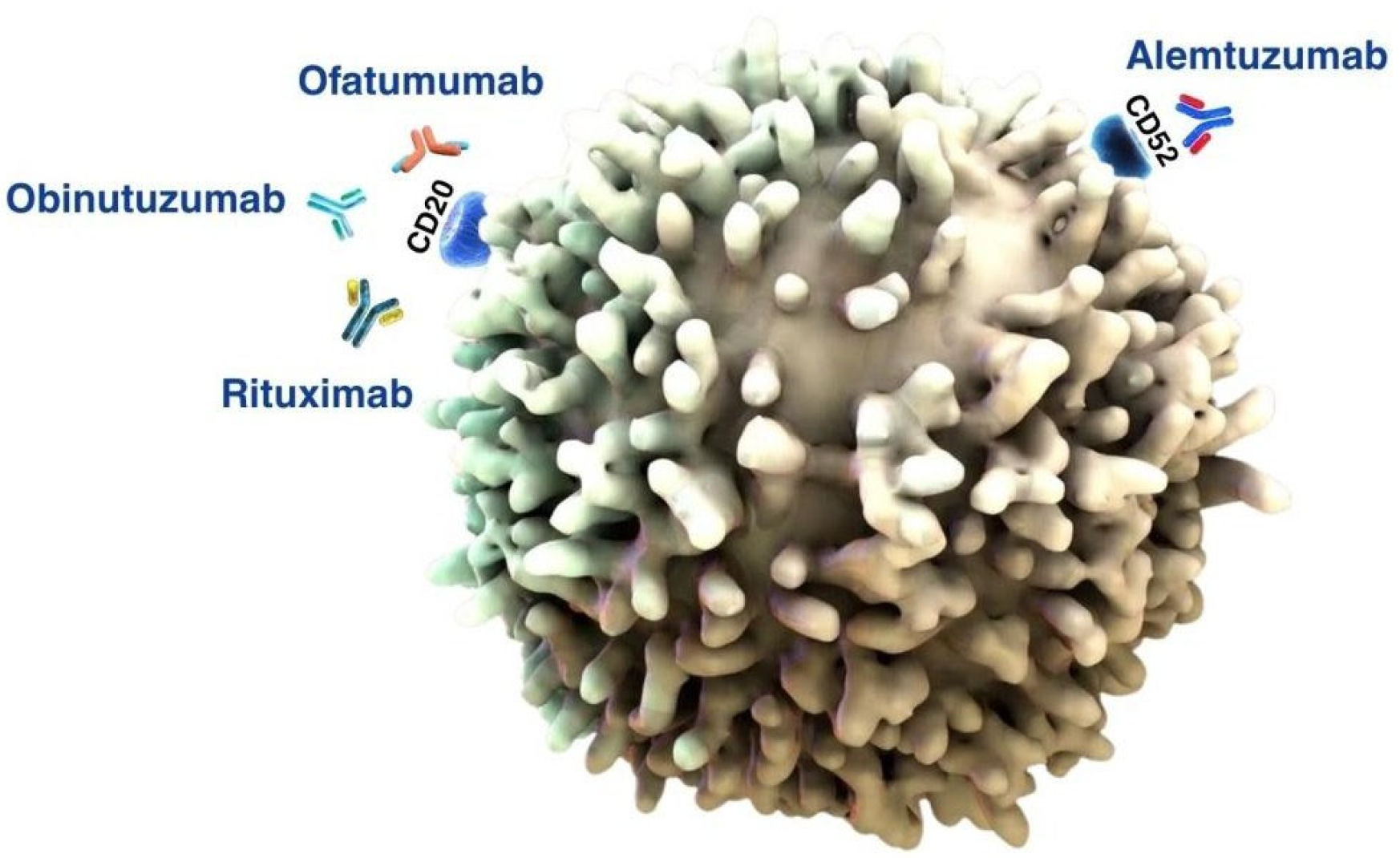
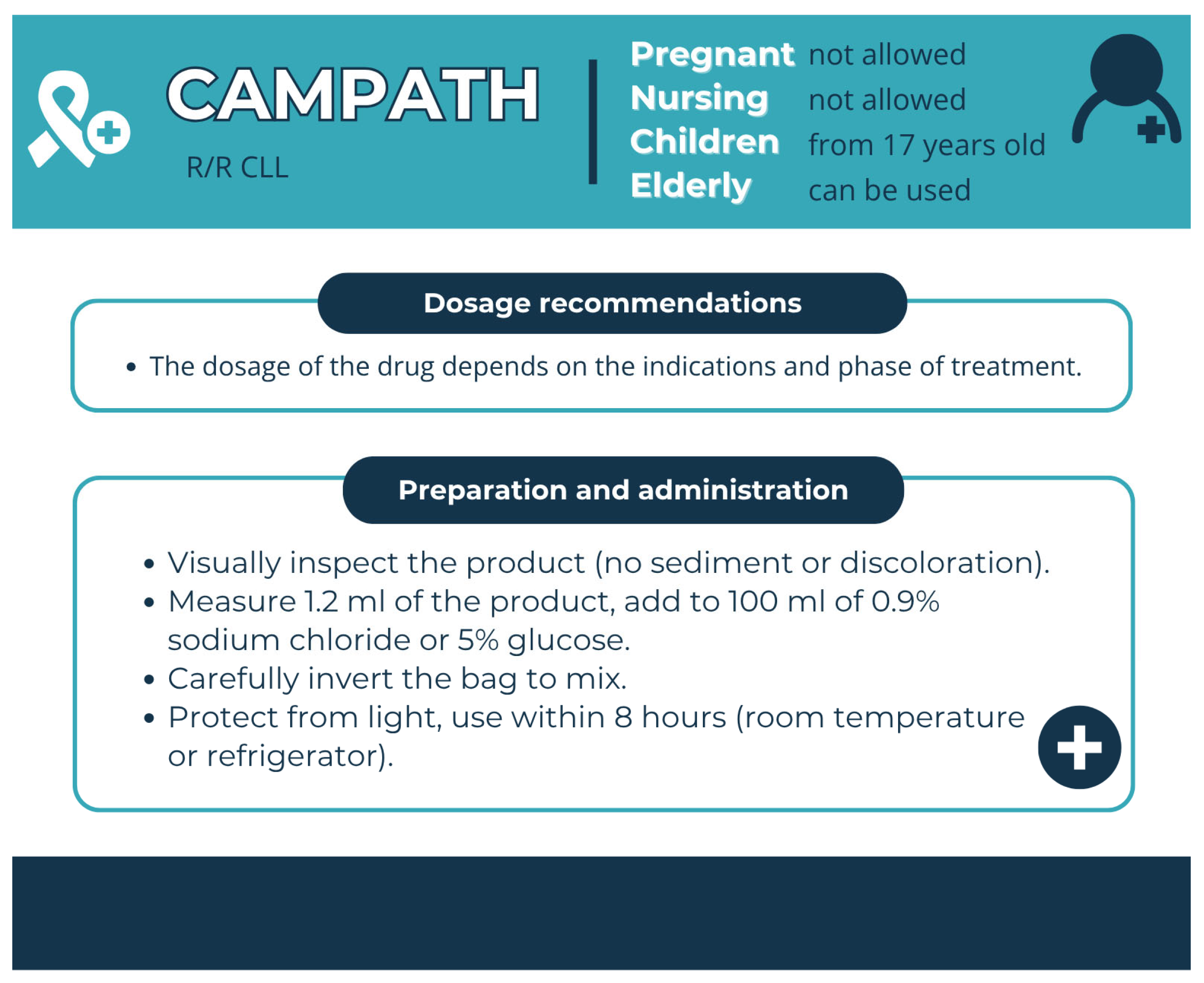
| Medicinal Product | Drug Group | Risks Associated with Use | Pharmaceutical Care | Premedication |
|---|---|---|---|---|
| Blincyto (Blinatumomab) | BsAb | Neurotoxicity | Dose adjustment based on patient’s history | Premedication with prednisone: Adults: 100 mg 1 h before the first dose of each cycle. Children: 5 mg only before the first dose in the first cycle and after a break of more than 3 h in the first cycle. Premedication with dexamethasone: Adults: 20 mg 1 h before the first dose of each cycle, dose increase and breaks longer than 3 h. Children: 5 mg only before the first dose in the first cycle, before dose increase and after breaks longer than 3 h in the first cycle. |
| CRS | Choice of symptomatic therapy | |||
| Besponsa (InO) | Conjugated mAb | Hepatotoxicity | Reduce the number of doses, warn to avoid the use of alkylators | Before administration of the drug, premedication with corticosteroids, antipyretics, and antihistamines is recommended. For patients with circulating lymphoblasts, cytoreduction with a combination of hydroxyurea, steroids, and/or vincristine is recommended before the first administration of the drug, until the peripheral blast level reaches no more than 10,000/mm3. |
| Infusion-related reactions | Control of premedication, symptomatic treatment | |||
| TLS | Selection of prophylactic agents | |||
| Cardiotoxicity | Suspension of the use of drugs that prolong QT | |||
| Mylotarg (GO) | Conjugated mAb | VOD | Fractionated dosing, control of drug interaction with azoles | One hour prior to administration, adult patients should be premedicated with 650 mg of acetaminophen orally and 50 mg of diphenhydramine orally or intravenously, and 30 min before infusion—methylprednisolone 1 mg/kg or an equivalent dose of another corticosteroid. Children should be premedicated with acetaminophen 15 mg/kg (maximum 650 mg), diphenhydramine 1 mg/kg (maximum 50 mg), and methylprednisolone 1 mg/kg orally or intravenously; Additional doses of acetaminophen and diphenhydramine may be administered every 4 h after the initial dose of the previous treatment. |
| Infusion-related reactions | Premedication and symptomatic treatment | |||
| TLS | Preventive measures | |||
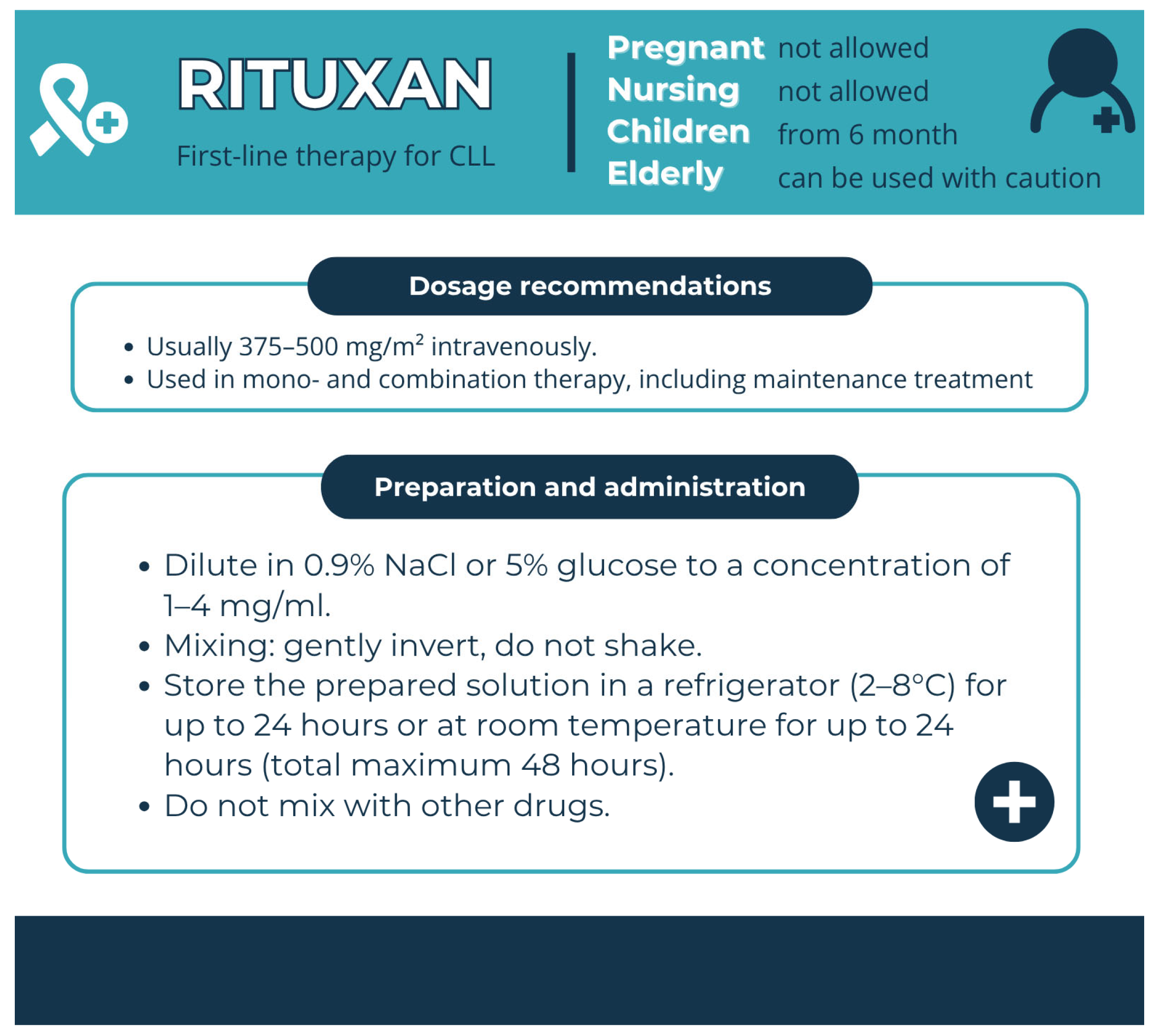
| Rituxan (Rituximab) | Chimeric mAb | Infusion-related reactions, cardiotoxicity | Premedication and preventive measures, control of interaction with drugs affecting the cardiovascular system | Premedication with acetaminophen and an antihistamine should be administered prior to each infusion of the drug. For adult patients receiving RITUXAN at an infusion rate of 90 min, the glucocorticoid component of the chemotherapy regimen should be administered prior to infusion. For paediatric patients with mature B-cell NHL/B-ALL, premedication with acetaminophen and H1-antihistamine (diphenhydramine or equivalent) should be administered 30–60 min before the start of each intravenous infusion of the drug. In patients with CLL during treatment and for 12 months after treatment, if necessary. |
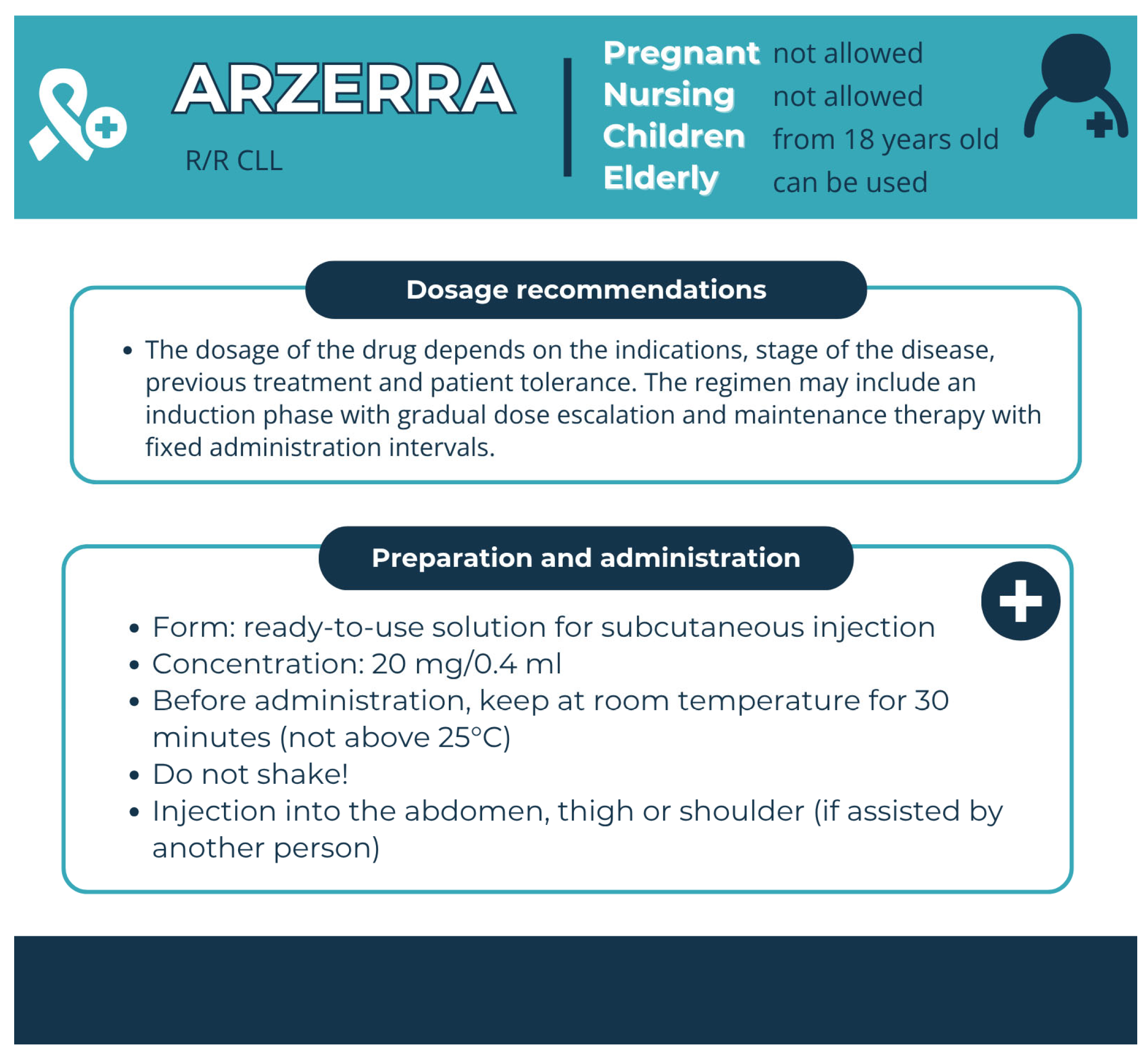
| Arzerra (Ofatumumab) | Human mAb | Patients should receive the following premedication 30 min to 2 h prior to each infusion. Previously untreated CLL: Oral acetaminophen 1000 mg (or equivalent). Oral or intravenous antihistamine (diphenhydramine 50 mg or cetirizine 10 mg or equivalent). Intravenous corticosteroid (prednisolone 50 mg or equivalent). | ||
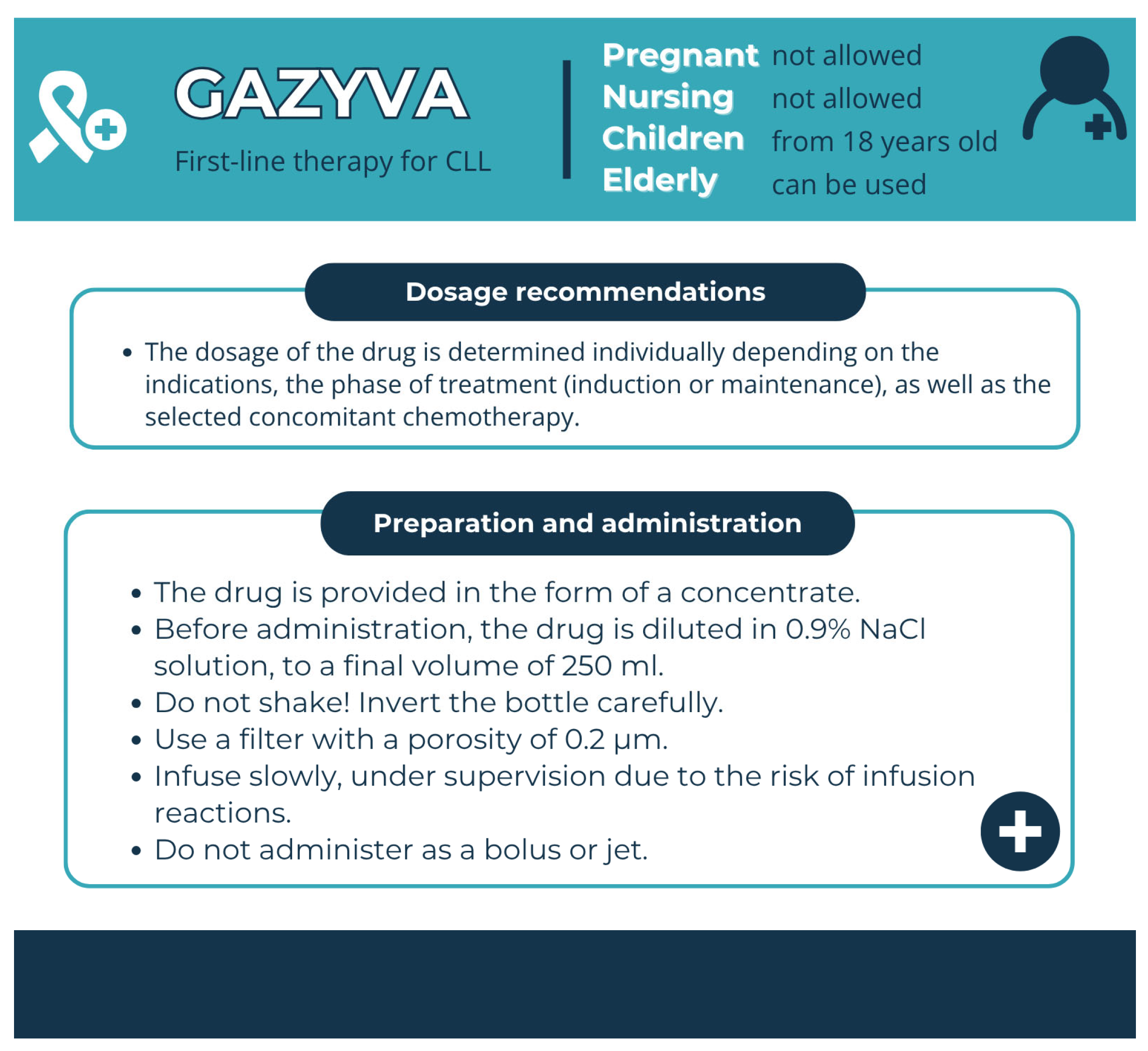
| Gazyva (Obinutuzumab) | Humanised mAb | Infusion reactions associated with the first dose | Premedication | Cycle 1 CLL (days 1 and 2): For all patients: Intravenous glucocorticoid: 20 mg dexamethasone or 80 mg methylprednisolone to be completed at least 1 h prior to infusion. Paracetamol (acetaminophen): 650–1000 mg administered at least 30 min prior to infusion Antihistamine (e.g., 50 mg diphenhydramine) administered at least 30 min prior to infusion. All subsequent cycles For all patients: Acetaminophen 650–1000 mg 30 min before infusion. |
| Thrombocytopenia | Control of discontinuation of medications that may provoke the risk of bleeding | |||
| Hypertensive crisis | Discontinuation of antihypertensive agents | |||
| Campath (Alemtuzumab) | Humanised mAb | Infusion toxicity | Enhanced dosing regimen, premedication | Premedication with diphenhydramine (50 mg) and acetaminophen (500–1000 mg) should be administered 30 min prior to the first infusion and each dose escalation. Administer trimethoprim / sulfamethoxazole twice daily three times a week (or equivalent) as prophylaxis for Pneumocystis pneumonia. Administer famciclovir 250 mg BID or equivalent as prophylaxis for herpes. |
| Name of the Drug | Dosage Form | Container | Excipients | Shelf Life | Storage | |||
|---|---|---|---|---|---|---|---|---|
| Refrigerator | Freezing | Light-Protection | Lit. Data | |||||
| Blincyto (Blinatumomab) | Powder for concentrate and solution for infusion, 38.5 micrograms | Type I glass vial with elastomeric rubber stopper and aluminium seal with flip off cap | Citric acid monohydrate (E330); Trehalose dihydrate; Lysine hydrochloride; Polysorbate 80 (E433); Sodium hydroxide (for pH-adjustment) | Unopened vial, 5 years | 2–8 °C | prohibited | Store in the original carton | |
| 10 mL solution (stabiliser) | Type I glass vial with elastomeric rubber stopper and aluminium seal with flip off cap | Citric acid monohydrate (E330); Lysine hydrochloride; Polysorbate 80 (E433); Sodium hydroxide (for pH adjustment); Water for injections | ||||||
| Besponsa (InO) | Liophil powder, 1 g | Type I amber glass vial with chlorobutyl rubber stopper and crimp seal with flip off cap | Sucrose Polysorbate 80; Sodium chloride; Tromethamine | Unopened vial, 5 years | 2–8 °C | prohibited | Store in the original carton | 1 year at ≤25 °C [174] |
| Mylotarg (GO) | 4.5 mg as a lyophilized cake or powder in a single-dose vial for reconstitution and dilution | Amber Type 1 glass vial, with butyl rubber stopper and crimp seal with flip-off cap containing 5 mg GO | Dextran 40; Sucrose; Sodium chloride; Sodium dihydrogen phosphate monohydrate; Disodium hydrogen phosphate anhydrous | Unopened vial, 5 years | 2–8 °C | prohibited | Store in the original carton | |
| Mabthera (Rituxan Rituximab) | Mabthera 500 mg concentrate in solution for infusion | Clear Type I glass vials with butyl rubber stopper | Sodium citrate (E331); Polysorbate 80 (E433); Sodium chloride; Sodium hydroxide (for pH adjustment) (E524); Hydrochloric acid (for pH adjustment) (E507); Water for injections | Unopened vial, 3 years | 2–8 °C | prohibited | Store in the original carton | 24 h at ≤25 °C [174] |
| Arzerra (Ofatumumab) | Arzerra 100 mg concentrate in solution for infusion. Arzerra 1000 mg concentrate in solution for infusion | Clear Type I glass vial with a bromobutyl rubber stopper and aluminium over-seal, containing 5 mL of concentrate in solution for infusion | Arginine; Sodium acetate (E262); Sodium chloride; Polysorbate 80 (E433); Edetate disodium (E386); Hydrochloric acid (E507) (for pH-adjustment); Water for injections | Unopened vial, 3 years | 2–8 °C | prohibited | Store in the original carton | |
| Gazyvaro (Obiuntuzumab) | 1000 mg concentrate in solution for infusion [174] | Amber Type 1 glass vial, with butyl rubber stopper | Histidine Histidine hydrochloride monohydrate Trehalose dihydrate Poloxamer 188 Water for injections | Unopened vial, 3 years | 2–8 °C | prohibited | Store in the original carton | 24 h at ≤25 °C [174] |
| Campath (Alemtuzumab) | Single-use transparent glass vials containing 30 mg of alemtuzumab in 1 mL of solution | A sterile, clear, colourless, isotonic solution (pH 6.8–7.4) in a single-dose vial for intravenous administration | 30 mg of alemtuzumab, 8.0 mg of sodium chloride, 1.44 mg of disodium phosphate, 0.2 mg of potassium chloride, 0.2 mg of monobasic potassium phosphate, 0.1 mg of polysorbate 80, and 0.0187 mg of disodium edetate dihydrate | Unopened vial, 3 years | 2–8 °C | prohibited | Store in the original carton | 1 month at 30 ± 2 °C and 3 months at 25 ± 2 °C [174] |
| Name of the Drug | Container Type | Concentration | Temperature Regime | Storage Time |
|---|---|---|---|---|
| Blincyto (Blinatumomab) | vials | 12.5 μg/ml | 2–8 °C | 24 h |
| 23–27 °C | 4 h | |||
| polyolefin and ethylene vinyl acetate infusion bags | 0.26 μg/mL | 2–8 °C | 10 days | |
| 23–27 °C | 96 h | |||
| Besponsa (InO) | Use immediately after preparation. | |||
| Mylotarg (GO) | Use immediately after preparation. | |||
| Mabthera (Rituxan Rituximab) | glass vials | 10 mg/mL | 23–32 °C | 21 days |
| partially used vials | 2–4 °C | 28 days | ||
| 25 °C | 15 days | |||
| dilution of the solution in 0.9% NaCl in polyethylene bags. | 1 mg/mL | 2–8 °C | 31 days | |
| 23–27 °C | 30 days | |||
| 28–32 °C | 14 days | |||
| polypropylene syringes | 2–8 °C | 31 days | ||
| polyolefin bags | 2–4 °C | 28 days | ||
| 25 °C | 15 days | |||
| polypropylene syringes | 120 mg/mL | 2–8 °C | 28 days | |
| 30 °C | 24 h | |||
| 1–4 mg/mL in 0.9% NaCl | 2–8 °C | 30 days | ||
| <30 °C | 24 h | |||
| 1–4 mg/mL in 5% glucose | 2–8 °C | 24 h | ||
| 25 °C | 12 h | |||
| For biosimilars | polyolefin bags | 1 mg/mL | 2–8 °C | up to 180 days |
| Arzerra (Ofatumumab) | in solutions prepared on the basis of a 0.9% sodium chloride solution | 0.3 mg/mL and 2 mg/mL | 25 °C | 48 h |
| Gazyvaro (Obiuntuzumab) | PVC and polyolefin bags | 0.4–20 mg/mL | 2–8 °C | 24 h |
| <30 °C | 48 h | |||
| Campath (Alemtuzumab) | in solutions prepared from 0.9% sodium chloride solution or 5% glucose solution | 0.1 mg/mL | 2–8 °C | 8 h |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryzhuk, A.; Kovalenko, S.M.; Georgiyants, M.; Vysotska, K.; Georgiyants, V. Monoclonal Antibodies as a Breakthrough in Personalised Leukaemia Therapy: What Pharmacists and Doctors Should Know. Pharmacy 2025, 13, 169. https://doi.org/10.3390/pharmacy13060169
Ryzhuk A, Kovalenko SM, Georgiyants M, Vysotska K, Georgiyants V. Monoclonal Antibodies as a Breakthrough in Personalised Leukaemia Therapy: What Pharmacists and Doctors Should Know. Pharmacy. 2025; 13(6):169. https://doi.org/10.3390/pharmacy13060169
Chicago/Turabian StyleRyzhuk, Anastasiia, Sergiy M. Kovalenko, Marine Georgiyants, Kateryna Vysotska, and Victoriya Georgiyants. 2025. "Monoclonal Antibodies as a Breakthrough in Personalised Leukaemia Therapy: What Pharmacists and Doctors Should Know" Pharmacy 13, no. 6: 169. https://doi.org/10.3390/pharmacy13060169
APA StyleRyzhuk, A., Kovalenko, S. M., Georgiyants, M., Vysotska, K., & Georgiyants, V. (2025). Monoclonal Antibodies as a Breakthrough in Personalised Leukaemia Therapy: What Pharmacists and Doctors Should Know. Pharmacy, 13(6), 169. https://doi.org/10.3390/pharmacy13060169







