Antibiotics and Lipid-Modifying Agents: Potential Drug–Drug Interactions and Their Clinical Implications
Abstract
1. Introduction
2. Literature Research
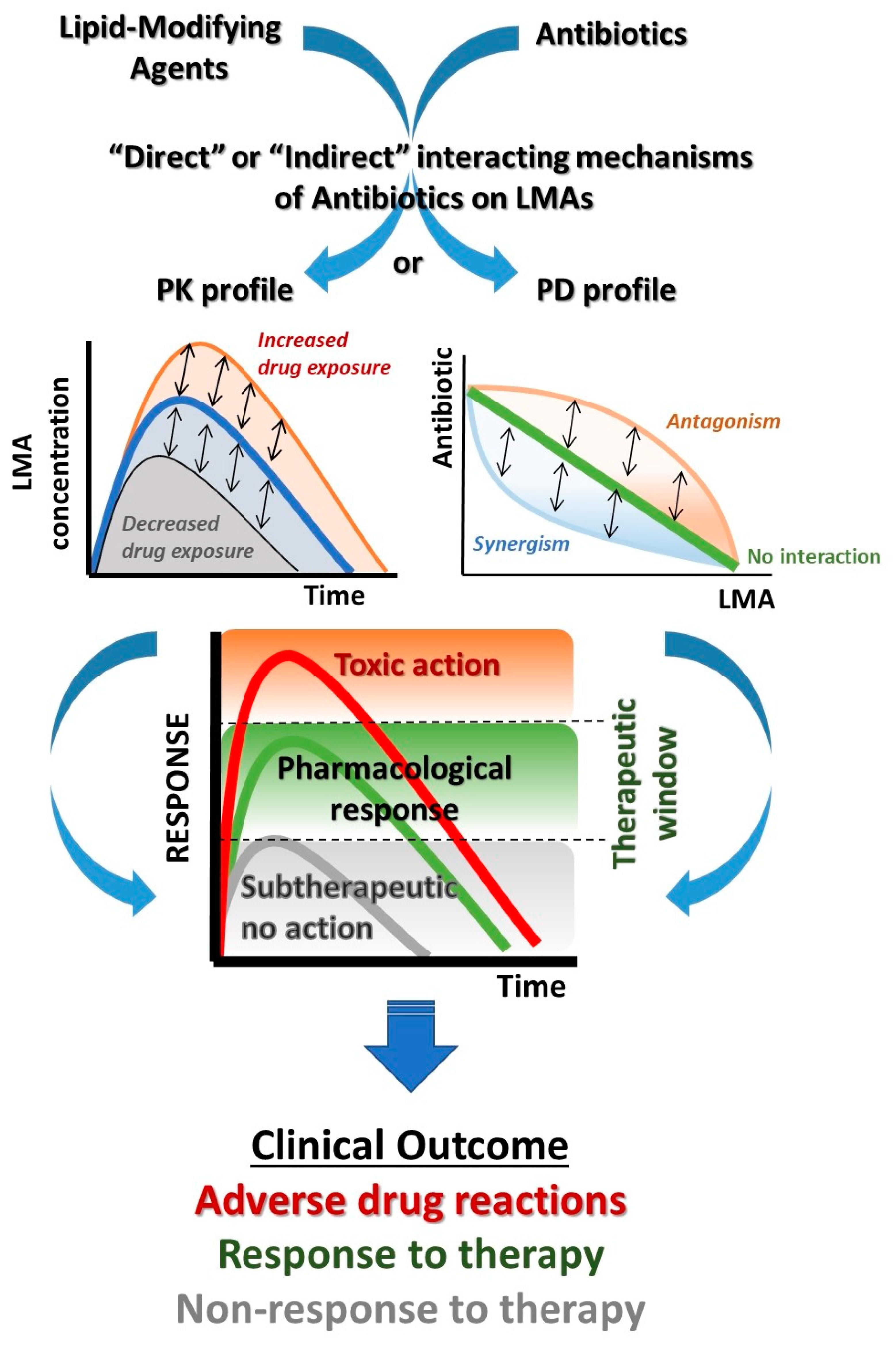
3. Pharmacological Mechanisms of Drug Interactions
4. Antibiotic and LMA Prescription Trends in EU Countries
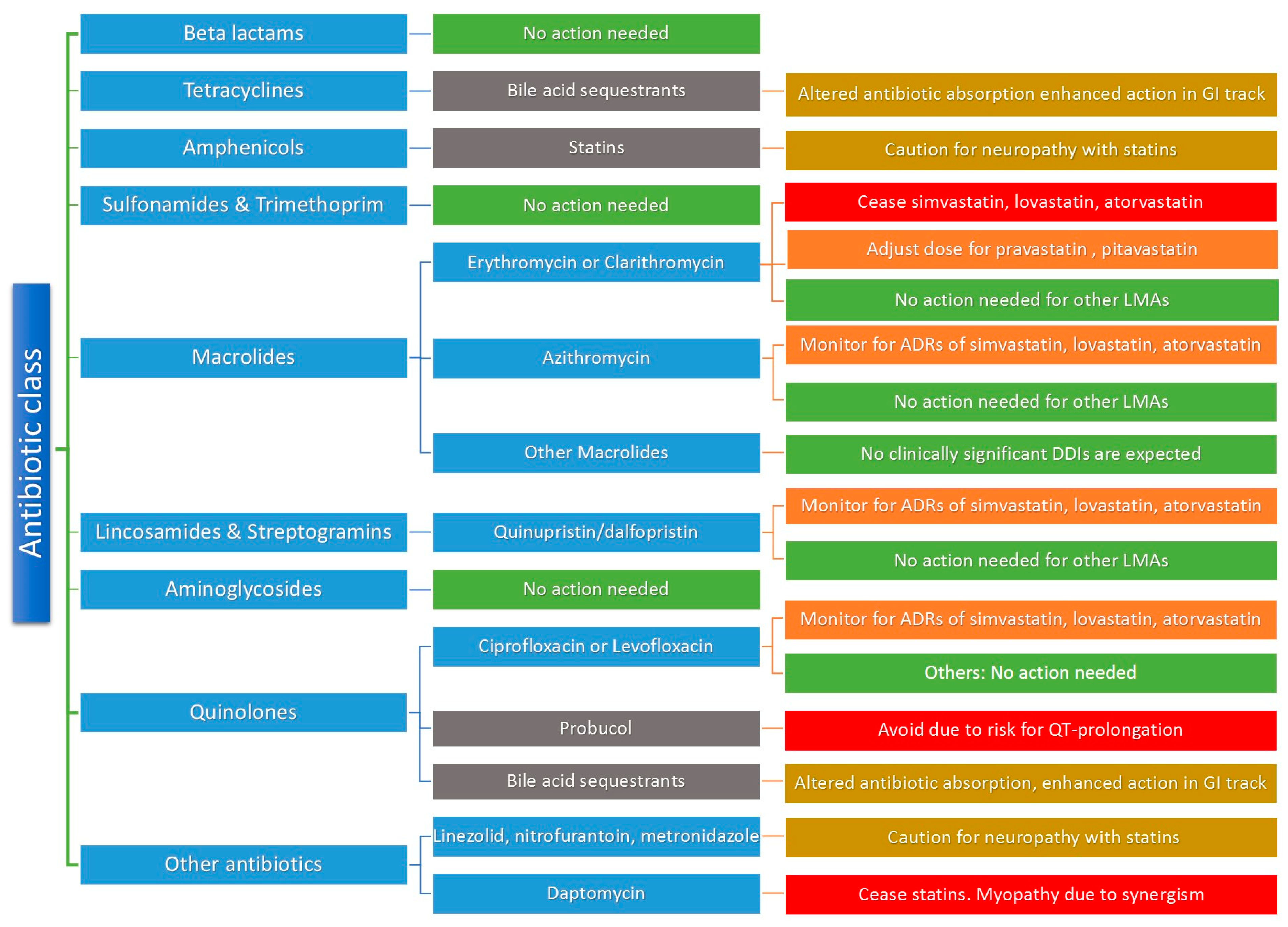
5. Antibiotic and LMA DDIs: Mechanisms and Clinical Significance
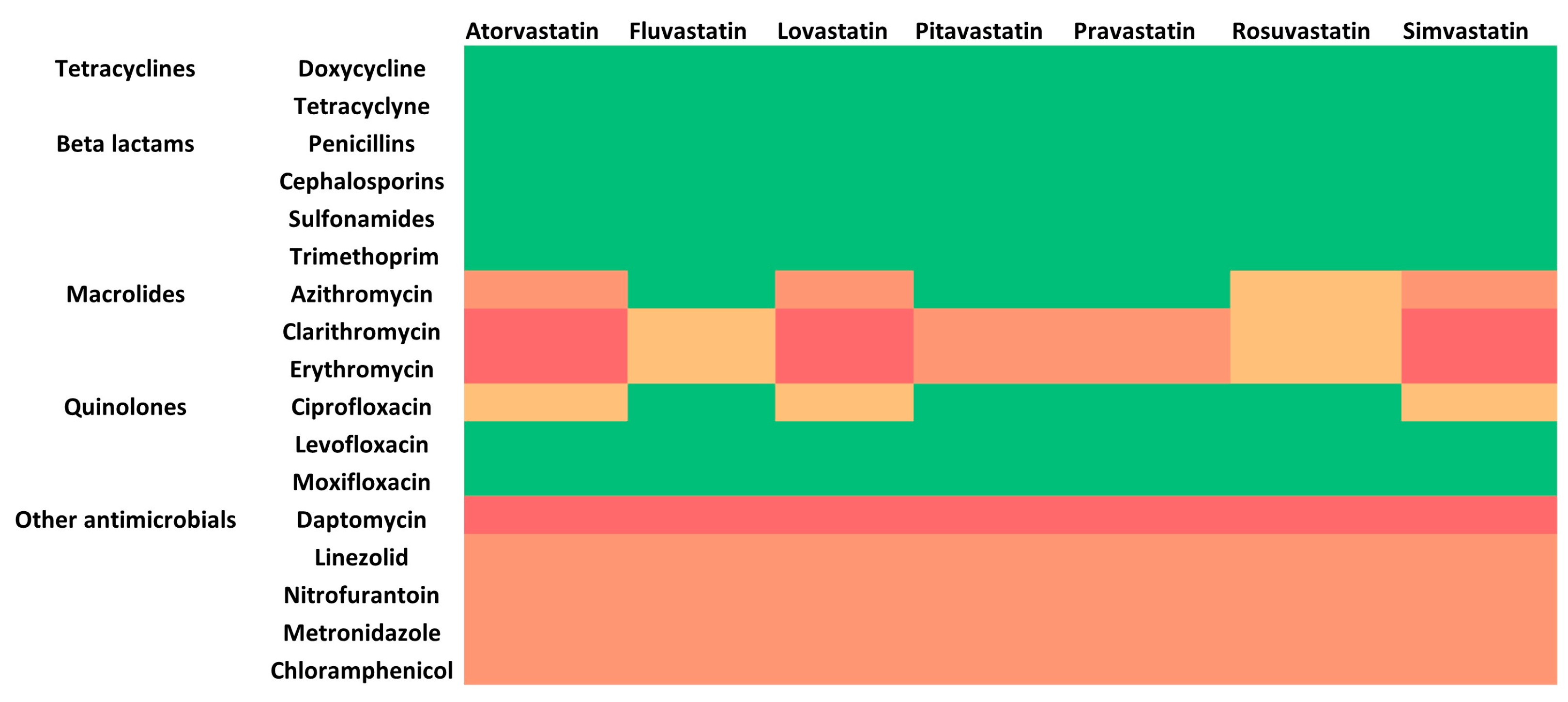
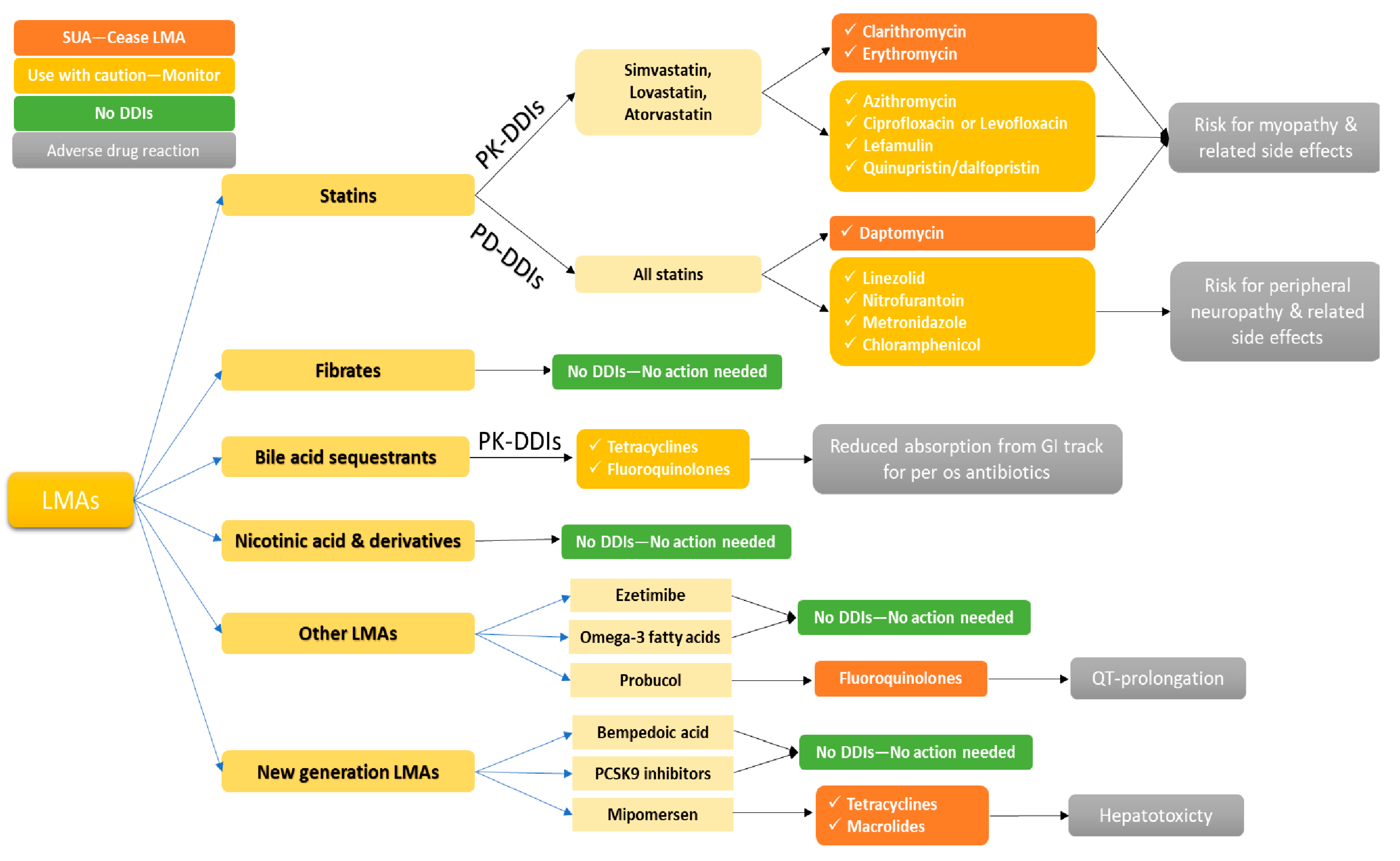
5.1. HMG CoA Reductase Inhibitors and Antibiotics
5.2. Fibrates and Antibiotics
5.3. Bile Acid Sequestrants and Antibiotics
5.4. Other LMAs and Antibiotics
5.5. New Generation LMAs and Potential Interactions with Antibiotics
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grissinger, M. The Five Rights: A Destination Without a Map. Pharm. Ther. 2010, 35, 542. [Google Scholar]
- Marengoni, A.; Onder, G. Guidelines, Polypharmacy, and Drug-Drug Interactions in Patients with Multimorbidity. BMJ 2015, 350, h1059. [Google Scholar] [CrossRef] [PubMed]
- Masnoon, N.; Shakib, S.; Kalisch-Ellett, L.; Caughey, G.E. What Is Polypharmacy? A Systematic Review of Definitions. BMC Geriatr. 2017, 17, 230. [Google Scholar] [CrossRef] [PubMed]
- Scheife, R.T.; Hines, L.E.; Boyce, R.D.; Chung, S.P.; Momper, J.D.; Sommer, C.D.; Abernethy, D.R.; Horn, J.R.; Sklar, S.J.; Wong, S.K.; et al. Consensus Recommendations for Systematic Evaluation of Drug-Drug Interaction Evidence for Clinical Decision Support. Drug Saf. 2015, 38, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Eyler, R.F.; Shvets, K. Clinical Pharmacology of Antibiotics. Clin. J. Am. Soc. Nephrol. 2019, 14, 1080–1090. [Google Scholar] [CrossRef]
- Leekha, S.; Terrell, C.L.; Edson, R.S. General Principles of Antimicrobial Therapy. Mayo Clin. Proc. 2011, 86, 156–167. [Google Scholar] [CrossRef]
- Calhoun, C.; Wermuth, H.R.; Hall, G.A. Antibiotics; StatPearls: Orlando, FL, USA, 2022. [Google Scholar]
- Roberts, S.C.; Zembower, T.R. Global Increases in Antibiotic Consumption: A Concerning Trend for WHO Targets. Lancet Infect. Dis. 2021, 21, 10–11. [Google Scholar] [CrossRef]
- Spernovasilis, N.; Ierodiakonou, D.; Spanias, C.; Mathioudaki, A.; Ioannou, P.; Petrakis, E.C.; Kofteridis, D.P. Doctors’ Perceptions, Attitudes and Practices towards the Management of Multidrug-Resistant Organism Infections after the Implementation of an Antimicrobial Stewardship Programme during the COVID-19 Pandemic. Trop. Med. Infect. Dis. 2021, 6, 20. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control Downloadable Tables: Antimicrobial Consumption—Annual Epidemiological Report for 2021. Available online: https://www.ecdc.europa.eu/en/publications-data/surveillance-antimicrobial-consumption-europe-2021 (accessed on 3 April 2023).
- Allel, K.; Day, L.; Hamilton, A.; Lin, L.; Furuya-Kanamori, L.; Moore, C.E.; Van Boeckel, T.; Laxminarayan, R.; Yakob, L. Global Antimicrobial-Resistance Drivers: An Ecological Country-Level Study at the Human–Animal Interface. Lancet Planet. Health 2023, 7, e291–e303. [Google Scholar] [CrossRef]
- Omar, M.A.; Wilson, J.P. FDA Adverse Event Reports on Statin-Associated Rhabdomyolysis. Ann. Pharmacother. 2002, 36, 288–295. [Google Scholar] [CrossRef]
- Anrys, P.; Petit, A.E.; Thevelin, S.; Sallevelt, B.; Drenth, C.; Soiza, R.L.; Correa-Pérez, A.; Dalleur, O.; De Brauwer, I.; Petrovic, M.; et al. An International Consensus List of Potentially Clinically Significant Drug-Drug Interactions in Older People. J. Am. Med. Dir. Assoc. 2021, 22, 2121–2133.e24. [Google Scholar] [CrossRef]
- Aronson, J.K. Classifying Drug Interactions. Br. J. Clin. Pharmacol. 2004, 58, 343–344. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Straubinger, R.M.; Mager, D.E. Pharmacodynamic Drug-Drug Interactions. Clin. Pharmacol. Ther. 2019, 105, 1395. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Petrie, I.D.; Levy, R.H.; Ragueneau-Majlessi, I. Mechanisms and Clinical Significance of Pharmacokinetic-Based Drug-Drug Interactions with Drugs Approved by the U.S. Food and Drug Administration in 2017. Drug Metab. Dispos. 2019, 47, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Kalliokoski, A.; Niemi, M. Impact of OATP Transporters on Pharmacokinetics. Br. J. Pharmacol. 2009, 158, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Rimac, H.; Dufour, C.; Debeljak, Ž.; Zorc, B.; Bojić, M. Warfarin and Flavonoids Do Not Share the Same Binding Region in Binding to the IIA Subdomain of Human Serum Albumin. Molecules 2017, 22, 1153. [Google Scholar] [CrossRef]
- Eyal, S.; Hsiao, P.; Unadkat, J.D. Drug Interactions at the Blood-Brain Barrier: Fact or Fantasy? Pharmacol. Ther. 2009, 123, 80–104. [Google Scholar] [CrossRef]
- Zanger, U.M.; Schwab, M. Cytochrome P450 Enzymes in Drug Metabolism: Regulation of Gene Expression, Enzyme Activities, and Impact of Genetic Variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar] [CrossRef]
- Jancova, P.; Anzenbacher, P.; Anzenbacherova, E. Phase II Drug Metabolizing Enzymes. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2010, 154, 103–116. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, Y.; Wang, R.; Zhang, J. Gut Microbiota-Mediated Drug-Drug Interaction between Amoxicillin and Aspirin. Sci. Rep. 2019, 9, 16194. [Google Scholar] [CrossRef]
- Yoo, H.H.; Kim, I.S.; Yoo, D.H.; Kim, D.H. Effects of Orally Administered Antibiotics on the Bioavailability of Amlodipine: Gut Microbiota-Mediated Drug Interaction. J. Hypertens. 2016, 34, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Yoo, D.H.; Kim, I.S.; Van Le, T.K.; Jung, I.H.; Yoo, H.H.; Kim, D.H. Gut Microbiota-Mediated Drug Interactions between Lovastatin and Antibiotics. Drug Metab. Dispos. 2014, 42, 1508–1513. [Google Scholar] [CrossRef] [PubMed]
- Spanakis, M.; Sfakianakis, S.; Kallergis, G.; Spanakis, E.G.; Sakkalis, V. PharmActa: Personalized Pharmaceutical Care EHealth Platform for Patients and Pharmacists. J. Biomed. Inform. 2019, 100, 103336. [Google Scholar] [CrossRef]
- Spanakis, M.; Ioannou, P.; Tzalis, S.; Papakosta, V.; Patelarou, E.; Tzanakis, N.; Patelarou, A.; Kofteridis, D.P. Drug-Drug Interactions among Patients Hospitalized with COVID-19 in Greece. J. Clin. Med. 2022, 11, 7172. [Google Scholar] [CrossRef]
- Spanakis, M.; Ioannou, P.; Tzalis, S.; Chouzouri, F.; Patelarou, E.; Kofteridis, D.P.; Antoniou, K.M.; Schiza, S.E.; Patelarou, A.; Tzanakis, N. Evaluation of Drug Interactions in Hospitalized Patients with Respiratory Disorders in Greece. Adv. Respir. Med. 2023, 91, 74–92. [Google Scholar] [CrossRef]
- Spernovasilis, N.; Agouridis, A.P.; Tsioutis, C. Appropriate Antimicrobial Use during the COVID-19 Pandemic: Not Cause for Complacency. Lancet Microbe 2023, 4, e293. [Google Scholar] [CrossRef]
- Blais, J.E.; Wei, Y.; Yap, K.K.; Alwafi, H.; Ma, T.T.; Brauer, R.; Lau, W.C.; Man, K.K.; Siu, C.W.; Tan, K.C.; et al. Trends in Lipid-Modifying Agent Use in 83 Countries. Atherosclerosis 2021, 328, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Stergiou, G.S.; Ntineri, A.; Menti, A.; Kalpourtzi, N.; Vlachopoulos, C.; Liberopoulos, E.N.; Rallidis, L.; Richter, D.; Gavana, M.; Vantarakis, A.; et al. Twenty-First Century Epidemiology of Dyslipidemia in Greece: EMENO National Epidemiological Study. Hell. J. Cardiol. 2023, 69, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Barter, P.J.; Rye, K.A. New Era of Lipid-Lowering Drugs. Pharmacol. Rev. 2016, 68, 458. [Google Scholar] [CrossRef] [PubMed]
- Kitzmiller, J.P.; Mikulik, E.B.; Dauki, A.M.; Murkherjee, C.; Luzum, J.A. Pharmacogenomics of Statins: Understanding Susceptibility to Adverse Effects. Pharmgenomics. Pers. Med. 2016, 9, 97–106. [Google Scholar] [CrossRef]
- Vassy, J.L.; Michael Gaziano, J.; Green, R.C.; Ferguson, R.E.; Advani, S.; Miller, S.J.; Chun, S.; Hage, A.K.; Seo, S.J.; Majahalme, N.; et al. Effect of Pharmacogenetic Testing for Statin Myopathy Risk vs Usual Care on Blood Cholesterol a Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e2027092. [Google Scholar] [CrossRef] [PubMed]
- Estudante, M.; Morais, J.G.; Soveral, G.; Benet, L.Z. Intestinal Drug Transporters: An Overview. Adv. Drug Deliv. Rev. 2013, 65, 1340–1356. [Google Scholar] [CrossRef] [PubMed]
- Link, E.; Parish, S.; Armitage, J.; Bowman, L.; Heath, S.; Matsuda, F.; Gut, I.; Lathrop, M.; Collins, R. SLCO1B1Variants and Statin-Induced Myopathy—A Genomewide Study. N. Engl. J. Med. 2008, 359, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Turongkaravee, S.; Jittikoon, J.; Lukkunaprasit, T.; Sangroongruangsri, S.; Chaikledkaew, U.; Thakkinstian, A. A Systematic Review and Meta-Analysis of Genotype-Based and Individualized Data Analysis of SLCO1B1 Gene and Statin-Induced Myopathy. Pharmacogenomics J. 2021, 21, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Stancu, C.; Sima, A. Statins: Mechanism of Action and Effects. J. Cell. Mol. Med. 2001, 5, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Palleria, C.; Di Paolo, A.; Giofrè, C.; Caglioti, C.; Leuzzi, G.; Siniscalchi, A.; De Sarro, G.; Gallelli, L. Pharmacokinetic Drug-Drug Interaction and Their Implication in Clinical Management. J. Res. Med. Sci. 2013, 18, 600–609. [Google Scholar]
- Price, G.; Patel, D.A. Drug Bioavailability. In xPharm: The Comprehensive Pharmacology Reference; Elsevier: Boston, MA, USA, 2022; pp. 1–2. [Google Scholar] [CrossRef]
- Chong, P.H.; Seeger, J.D.; Franklin, C. Clinically Relevant Differences between the Statins: Implications for Therapeutic Selection. Am. J. Med. 2001, 111, 390–400. [Google Scholar] [CrossRef]
- Di Stasi, S.L.; MacLeod, T.D.; Winters, J.D.; Binder-Macleod, S.A. Effects of Statins on Skeletal Muscle: A Perspective for Physical Therapists. Phys. Ther. 2010, 90, 1530–1542. [Google Scholar] [CrossRef]
- Hylton Gravatt, L.A.; Flurie, R.W.; Lajthia, E.; Dixon, D.L. Clinical Guidance for Managing Statin and Antimicrobial Drug-Drug Interactions. Curr. Atheroscler. Rep. 2017, 19, 46. [Google Scholar] [CrossRef]
- Patel, A.M.; Shariff, S.; Bailey, D.G.; Juurlink, D.N.; Gandhi, S.; Mamdani, M.; Gomes, T.; Fleet, J.; Hwang, Y.J.; Garg, A.X. Statin Toxicity from Macrolide Antibiotic Coprescription. Ann. Intern. Med. 2013, 158, 869–876. [Google Scholar] [CrossRef]
- Hougaard Christensen, M.M.; Bruun Haastrup, M.; Øhlenschlæger, T.; Esbech, P.; Arnspang Pedersen, S.; Bach Dunvald, A.C.; Bjerregaard Stage, T.; Pilsgaard Henriksen, D.; Thestrup Pedersen, A.J. Interaction Potential between Clarithromycin and Individual Statins—A Systematic Review. Basic Clin. Pharmacol. Toxicol. 2020, 126, 307–317. [Google Scholar] [CrossRef]
- Abu Mellal, A.; Hussain, N.; Said, A.S. The Clinical Significance of Statins-Macrolides Interaction: Comprehensive Review of in Vivo Studies, Case Reports, and Population Studies. Ther. Clin. Risk Manag. 2019, 15, 921–936. [Google Scholar] [CrossRef] [PubMed]
- Goldie, F.C.; Brogan, A.; Boyle, J.G. Ciprofloxacin and Statin Interaction: A Cautionary Tale of Rhabdomyolysis. BMJ Case Rep. 2016, 2016, 48. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Yi, Y.; Fan, Y.; Shang, R. Cytochrome P450 Inhibition Potential and Initial Genotoxic Evaluation of 14-O-[(4,6-Diaminopyrimidine-2-Yl)Thioacetyl] Mutilin. Sci. Rep. 2020, 10, 13474. [Google Scholar] [CrossRef]
- José, M.; Elisa, G.-V.; Jordi, V. Macrólidos, Cetólidos y Estreptograminas. Enferm. Infecc. Microbiol. Clin. 2003, 21, 200–208. [Google Scholar] [CrossRef]
- Dare, R.K.; Tewell, C.; Harris, B.; Wright, P.W.; Van Driest, S.L.; Farber-Eger, E.; Nelson, G.E.; Talbot, T.R. Effect of Statin Coadministration on the Risk of Daptomycin-Associated Myopathy. Clin. Infect. Dis. 2018, 67, 1356–1363. [Google Scholar] [CrossRef] [PubMed]
- Golightly, L.K.; Barber, G.R.; Barron, M.A.; Page, R.L. Statins and Daptomycin: Safety Assessment of Concurrent Use and Evaluation of Drug Interaction Liability. Drug Metabol. Drug Interact. 2013, 28, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.R.; Urits, I.; Wolf, J.; Corrigan, D.; Colburn, L.; Peterson, E.; Williamson, A.; Viswanath, O. Drug-Induced Peripheral Neuropathy: A Narrative Review. Curr. Clin. Pharmacol. 2020, 15, 38. [Google Scholar] [CrossRef]
- Green, S.; Holton, A. Drug-Induced Peripheral Neuropathy. Advers. Drug React. Bull. 2016, 300, 1159–1162. [Google Scholar] [CrossRef]
- Vilholm, O.J.; Christensen, A.A.; Zedan, A.H.; Itani, M. Drug-Induced Peripheral Neuropathy. Basic Clin. Pharmacol. Toxicol. 2014, 115, 185–192. [Google Scholar] [CrossRef]
- Wysocki, J.; Belowski, D.; Kalina, M.; Kochanski, L.; Okopien, B.; Kalina, Z. Effects of Micronized Fenofibrate on Insulin Resistance in Patients with Metabolic Syndrome. Int. J. Clin. Pharmacol. Ther. 2004, 42, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.B.; Spence, J.D. Clinical Pharmacokinetics of Fibric Acid Derivatives (Fibrates). Clin. Pharmacokinet. 1998, 34, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Bays, H.E.; Dujovne, C.A. Drug Interactions of Lipid-Altering Drugs. Drug Saf. 1998, 19, 355–371. [Google Scholar] [CrossRef] [PubMed]
- Gallo, D.G.; Bailey, K.R.; Sheffner, A.L. The Interaction between Cholestyramine and Drugs. Proc. Soc. Exp. Biol. Med. 1965, 120, 60–65. [Google Scholar] [CrossRef]
- Patel, P.H.; Can, A.S. Colesevelam; StatPearls: Orlando, FL, USA, 2023. [Google Scholar]
- Morley, V.J.; Kinnear, C.L.; Sim, D.G.; Olson, S.N.; Jackson, L.M.; Hansen, E.; Usher, G.A.; Showalter, S.A.; Pai, M.P.; Woods, R.J.; et al. An Adjunctive Therapy Administered with an Antibiotic Prevents Enrichment of Antibiotic-Resistant Clones of a Colonizing Opportunistic Pathogen. Elife 2020, 9, e58147. [Google Scholar] [CrossRef]
- Phan, B.A.P.; Dayspring, T.D.; Toth, P.P. Ezetimibe Therapy: Mechanism of Action and Clinical Update. Vasc. Health Risk Manag. 2012, 8, 415. [Google Scholar] [CrossRef]
- Kosoglou, T.; Statkevich, P.; Johnson-Levonas, A.O.; Paolini, J.F.; Bergman, A.J.; Alton, K.B. Ezetimibe: A Review of Its Metabolism, Pharmacokinetics and Drug Interactions. Clin. Pharmacokinet. 2005, 44, 467–494. [Google Scholar] [CrossRef]
- Matsuhashi, H.; Onodera, S.; Kawamura, Y.; Hasebe, N.; Kohmura, C.; Yamashita, H.; Tobise, K. Probucol-Induced QT Prolongation and Torsades de Pointes. Jpn. J. Med. 1989, 28, 612–615. [Google Scholar] [CrossRef][Green Version]
- Briasoulis, A.; Agarwal, V.; Pierce, W.J. QT Prolongation and Torsade de Pointes Induced by Fluoroquinolones: Infrequent Side Effects from Commonly Used Medications. Cardiology 2011, 120, 103–110. [Google Scholar] [CrossRef]
- Massaro, M.; Scoditti, E.; Carluccio, M.A.; De Caterina, R. Nutraceuticals and Prevention of Atherosclerosis: Focus on Omega-3 Polyunsaturated Fatty Acids and Mediterranean Diet Polyphenols. Cardiovasc. Ther. 2010, 28, e13–e19. [Google Scholar] [CrossRef] [PubMed]
- Spanakis, M.; Patelarou, E.; Patelarou, A. Drug-Food Interactions with a Focus on Mediterranean Diet. Appl. Sci. 2022, 12, 10207. [Google Scholar] [CrossRef]
- Kim, K.; Ginsberg, H.N.; Choi, S.H. New, Novel Lipid-Lowering Agents for Reducing Cardiovascular Risk: Beyond Statins. Diabetes Metab. J. 2022, 46, 517–532. [Google Scholar] [CrossRef] [PubMed]
- Biogen Inc. Inclisiran (Leqvio) Annex I Summary of Product Characteristics. Published Online 2013. Available online: https://www.ema.europa.eu/en/documents/product-information/leqvio-epar-product-information_en.pdf (accessed on 17 April 2023).
- Committee for Medicinal Products for Human Use (CHMP). Kynamro|European Medicines Agency. 2013. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/kynamro (accessed on 5 April 2023).
- Ballantyne, C.M.; Bays, H.; Catapano, A.L.; Goldberg, A.; Ray, K.K.; Saseen, J.J. Role of Bempedoic Acid in Clinical Practice. Cardiovasc. Drugs Ther. 2021, 35, 853. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, S.R.J. Evidence Based Prescribing: Is the Goal, but Prescribers Still Need Education, Experience, and Common Sense. BMJ 2005, 331, 247. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Guidance for Industry: Clinical Drug Interaction Studies—Cytochrome P450 Enzyme- and Transporter-Mediated Drug Interactions. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/clinical-drug-interaction-studies-cytochrome-p450-enzyme-and-transporter-mediated-drug-interactions (accessed on 23 May 2023).
- European Medicines Agency Investigation of Drug Interactions. Available online: https://www.ema.europa.eu/en/investigation-drug-interactions-scientific-guideline (accessed on 23 May 2023).
- Wiggins, B.S.; Saseen, J.J.; Page, R.L.; Reed, B.N.; Sneed, K.; Kostis, J.B.; Lanfear, D.; Virani, S.; Morris, P.B. Recommendations for Management of Clinically Significant Drug-Drug Interactions With Statins and Select Agents Used in Patients With Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2016, 134, e468–e495. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.; Feely, J. Pharmacokinetic-Pharmacodynamic Drug Interactions with HMG-CoA Reductase Inhibitors. Clin. Pharmacokinet. 2002, 41, 343–370. [Google Scholar] [CrossRef]
- Eljaaly, K.; Alshehri, S.; Bhattacharjee, S.; Al-Tawfiq, J.A.; Patanwala, A.E. Contraindicated Drug-Drug Interactions Associated with Oral Antimicrobial Agents Prescribed in the Ambulatory Care Setting in the United States. Clin. Microbiol. Infect. 2019, 25, 620–622. [Google Scholar] [CrossRef]
- FDA Important Safety Label Changes to Cholesterol-Lowering Statin Drugs. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-important-safety-label-changes-cholesterol-lowering-statin-drugs (accessed on 3 April 2023).
- Gilad, R.; Lampl, Y. Rhabdomyolysis Induced by Simvastatin and Ketoconazole Treatment. Clin. Neuropharmacol. 1999, 22, 295–297. [Google Scholar] [PubMed]
- Gupta, A.K.; Versteeg, S.G.; Shear, N.H. Common Drug-Drug Interactions in Antifungal Treatments for Superficial Fungal Infections. Expert Opin. Drug Metab. Toxicol. 2018, 14, 387–398. [Google Scholar] [CrossRef]
- Kyrklund, C.; Backman, J.T.; Kivistö, K.T.; Neuvonen, M.; Laitila, J.; Neuvonen, P.J. Rifampin Greatly Reduces Plasma Simvastatin and Simvastatin Acid Concentrations. Clin. Pharmacol. Ther. 2000, 68, 592–597. [Google Scholar] [CrossRef]
- Baciewicz, A.M.; Chrisman, C.R.; Finch, C.K.; Self, T.H. Update on Rifampin, Rifabutin, and Rifapentine Drug Interactions. Curr. Med. Res. Opin. 2013, 29, 1–12. [Google Scholar] [CrossRef]
- CDC Antiretrovirals and Rifampin|Recommendations|Managing Drug Interactions in the Treatment of HIV-Related Tuberculosis|Guidelines|TB|CDC. Available online: https://www.cdc.gov/tb/publications/guidelines/tb_hiv_drugs/recommendations02.htm (accessed on 13 April 2023).
- Yip, D.W.; Gerriets, V. Penicillin. In Drug Allergy Test; Elsevier: Boston, MA, USA, 2022; pp. 103–113. [Google Scholar] [CrossRef]
- Kemnic, T.R.; Coleman, M. Trimethoprim Sulfamethoxazole. Mayo Clin. Proc. 2022, 74, 730–734. [Google Scholar] [CrossRef]
- Luft, F.C. Aminoglycoside Interactions with Other Drugs. Nephrotoxic Mech. Drugs Environ. Toxins 1982, 135–150. [Google Scholar] [CrossRef]
- Strandell, J.; Bate, A.; Hägg, S.; Edwards, I.R. Rhabdomyolysis a Result of Azithromycin and Statins: An Unrecognized Interaction. Br. J. Clin. Pharmacol. 2009, 68, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, A.; Oda, S.; Akai, S.; Tsuneyama, K.; Yokoi, T. Establishment of a Drug-Induced Rhabdomyolysis Mouse Model by Co-Administration of Ciprofloxacin and Atorvastatin. Toxicol. Lett. 2018, 291, 184–193. [Google Scholar] [CrossRef]
- Irfan, F.; Karim, S.I. Co-Prescription of Ciprofloxacin and Statins; a Dangerous Combination: Case Report. J. Pak. Med. Assoc. 2020, 70, 1272–1274. [Google Scholar] [CrossRef]
- Hennessy, E.; Adams, C.; Reen, F.J.; O’Gara, F. Is There Potential for Repurposing Statins as Novel Antimicrobials? Antimicrob. Agents Chemother. 2016, 60, 5111–5121. [Google Scholar] [CrossRef]
- Parihar, S.P.; Guler, R.; Brombacher, F. Statins: A Viable Candidate for Host-Directed Therapy against Infectious Diseases. Nat. Rev. Immunol. 2018, 19, 104–117. [Google Scholar] [CrossRef]
- Ko, H.H.T.; Lareu, R.R.; Dix, B.R.; Hughes, J.D. Statins: Antimicrobial Resistance Breakers or Makers? PeerJ 2017, 2017, e3952. [Google Scholar] [CrossRef] [PubMed]
- Masadeh, M.; Mhaidat, N.; Alzoubi, K.; Al-azzam, S.; Alnasser, Z. Antibacterial Activity of Statins: A Comparative Study of Atorvastatin, Simvastatin, and Rosuvastatin. Ann. Clin. Microbiol. Antimicrob. 2012, 11, 13. [Google Scholar] [CrossRef]
- Basyoni, M.M.A.; Fouad, S.A.; Amer, M.F.; Amer, A.F.; Ismail, D.I. Atorvastatin: In-Vivo Synergy with Metronidazole as Anti-Blastocystis Therapy. Korean J. Parasitol. 2018, 56, 105. [Google Scholar] [CrossRef] [PubMed]
- Morales-Alvarez, M.C. Nephrotoxicity of Antimicrobials and Antibiotics. Adv. Chronic Kidney Dis. 2020, 27, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Niedrig, D.; Maechler, S.; Hoppe, L.; Corti, N.; Kovari, H.; Russmann, S. Drug Safety of Macrolide and Quinolone Antibiotics in a Tertiary Care Hospital: Administration of Interacting Co-Medication and QT Prolongation. Eur. J. Clin. Pharmacol. 2016, 72, 859–867. [Google Scholar] [CrossRef] [PubMed]
| LMA 1 | OATP1B1 2 | OATP1B3 2 | P-gp 3 | CYP3A4 4 | CYP2C9 4 |
|---|---|---|---|---|---|
| Atorvastatin | Substrate | Substrate | Substrate | Substrate | - |
| Fluvastatin | Substrate | Substrate | - | - | Substrate |
| Lovastatin | Substrate | Substrate | Substrate | Substrate | Substrate |
| Pitavastatin | Substrate | Substrate | Substrate | Substrate | - |
| Pravastatin | Substrate | Substrate | - | Substrate | Substrate |
| Rosuvastatin | Substrate | Substrate | Substrate | Substrate | Substrate |
| Fenofibrate | Inhibitor | - | Inhibitor | Substrate | Substrate |
| Gemfibrozil | Inhibitor | - | Inhibitor | Substrate | Inhibitor |
| Bezafibrate | Inhibitor | - | - | Substrate | Substrate |
| Ciprofibrate | - | - | - | Substrate | Substrate |
| Ezetimibe | Substrate | Substrate | Substrate | - | - |
| Atorvastatin (10–80 mg/day) | Fluvastatin (40–80 mg/day) | Lovastatin (20–80 mg/day) | Pitavastatin (1–4 mg/day) | Pravastatin (20–80 mg/day) | Rosuvastatin (5–40 mg/day) | Simvastatin (10–80 mg/day) | |
|---|---|---|---|---|---|---|---|
| Azithromycin | Sustain and monitor | - | Sustain and monitor | - | - | - | Sustain and monitor |
| Clarithromycin | Cease | Sustain and monitor | Cease | Adjust to 1 mg/day | Limit to 40 mg/day | Sustain and monitor | Cease |
| Erythromycin | |||||||
| Ciprofloxacin | Sustain and monitor | - | Sustain and monitor | - | - | - | Sustain and monitor |
| Daptomycin | Cease | ||||||
| Linezolid Nitrofurantoin Metronidazole Chloramphenicol | Sustain and monitor | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spanakis, M.; Alon-Ellenbogen, D.; Ioannou, P.; Spernovasilis, N. Antibiotics and Lipid-Modifying Agents: Potential Drug–Drug Interactions and Their Clinical Implications. Pharmacy 2023, 11, 130. https://doi.org/10.3390/pharmacy11040130
Spanakis M, Alon-Ellenbogen D, Ioannou P, Spernovasilis N. Antibiotics and Lipid-Modifying Agents: Potential Drug–Drug Interactions and Their Clinical Implications. Pharmacy. 2023; 11(4):130. https://doi.org/10.3390/pharmacy11040130
Chicago/Turabian StyleSpanakis, Marios, Danny Alon-Ellenbogen, Petros Ioannou, and Nikolaos Spernovasilis. 2023. "Antibiotics and Lipid-Modifying Agents: Potential Drug–Drug Interactions and Their Clinical Implications" Pharmacy 11, no. 4: 130. https://doi.org/10.3390/pharmacy11040130
APA StyleSpanakis, M., Alon-Ellenbogen, D., Ioannou, P., & Spernovasilis, N. (2023). Antibiotics and Lipid-Modifying Agents: Potential Drug–Drug Interactions and Their Clinical Implications. Pharmacy, 11(4), 130. https://doi.org/10.3390/pharmacy11040130









