Abstract
The global burden of the COVID-19 pandemic has not only disrupted healthcare delivery but has also compromised patients’ access to healthcare on account of the scarcity of medications and trained healthcare professionals. COVID-19 has been particularly challenging for patient subpopulations constituting immunocompromised individuals, geriatric patients, and those afflicted by chronic ailments. Reports indicate that diminished kidney function in chronic kidney disease (CKD) renders patients highly susceptible to complications during COVID-19 treatment. Pharmacists, being medication experts, have a significant role in making treatment decisions during COVID-19 infection. This article describes pharmacists’ interventions for monitoring and managing COVID-19 in patients with CKD. Given the massive increase in off-label use of medications to treat COVID-19, pharmacists can contribute substantially towards dosing decisions, reporting adverse medication events, and managing drug–drug interactions in COVID-19 patients suffering from CKD. In addition to traditional methods of delivering their services, the pharmacist should also adopt innovative tele-health systems to optimize patient care and ensure that patients receive safe and effective therapy during the pandemic.
1. Introduction
The devastating SARS-CoV-2 infection continues to persist in different parts of the world, expanding the scope of further investigations for both treatment and prevention. By July 2022, the pandemic had infected more than 500 million people and caused 6.4 million deaths [1]. Most importantly, patients with co-morbidities have been more vulnerable to the virus than the healthy population [2]. Chronic kidney disease (CKD) afflicts 10–15% of the global population, who are then at greater risk of contracting COVID-19 [3]. Frequent hospital visits by patients with CKD and end-stage renal disease expose them to the newer variants of the COVID-19 virus. Moreover, studies from China and New York have shown that 30% of COVID-19 patients have had varying degrees of kidney damage. Although the angiotensin-converting enzyme (ACE)-2 receptor (distributed abundantly in the lungs) is the dominant port of entry into human cells, SARS-CoV-2 also penetrates into extrapulmonary organs, causing further damage to the kidneys [4]. The exact mechanism behind renal manifestations in COVID-19 patients is still unclear. Multiple complex pathways could be involved, including direct viral entry into the kidneys and the disruption of the homeostasis maintained by the renin-angiotensin system [5,6]. Nevertheless, severe COVID-19 cases are accompanied by a cytokine storm, leading to systemic inflammation and hypercoagulability, resulting in multiple organ complications that progress to kidney injury [7]. Such patients present high proteinuria, hematuria, elevated serum creatinine, and blood urea, possibly augmented by a diminished oxygen supply [8]. Moreover, mortality from pneumonia is 14–16% higher among CKD patients infected with SARS CoV-19 than in the general population [9]. Providing specialized intensive care for vulnerable patients requires a multidisciplinary approach, involving pharmacists who can suitably assist physicians by selecting the effective therapeutic regimen and individualizing and evaluating recommended therapies, thereby ensuring the optimal utilization of resources [10].
Beyond supplying and dispensing medications, community pharmacists also participate actively in addressing patients’ medication-related issues and promoting the rational use of medications, especially in vulnerable sub-populations, e.g., CKD patients. Community pharmacists are embracing newer roles within tele-pharmacy services, the home delivery of medications, the delivery of timely and reliable drug information services, promoting adherence, and supplying infection preventive items [11].
This article discusses the specialized treatment options for COVID-19 infection in CKD patients, especially those strategies that require dose modification, preventive strategies, enhancing the pharmacists’ role in patient care, and hosting telehealth facilities for assuaging the incidence of COVID-19 infection in CKD patients.
Role of Pharmacists toward CKD Patients during a Pandemic
COVID-19 infections in CKD patients require multiple interventions by pharmacists, who can play a major role in optimizing medication safety. During the pandemic, pharmacists’ contributions have demonstrated benefits toward improving patients’ health outcomes for chronic conditions, including CKD [12]. Comprehensive assessment of a patient’s medication therapy, offering home delivery and tele-pharmacy, providing appropriate medication information and counseling, identifying and preventing potential medication-related problems, and promoting medication adherence are some of these. Moreover, each patient with pre-dialyzing or dialyzing CKD may require a comprehensive, highly individualized, and variable drug regimen. It is the duty of pharmacists to review each patient’s renal disease burden and suggest medication adjustments accordingly. Pharmacists can contribute significantly to patient care, and the current scenario necessitates the recruitment and training of more community pharmacists to serve CKD patients [13]. Moreover, community pharmacists can promote medication adherence, which is a principal duty in pharmacy practice. Menon and Sander have reported instances of low adherence in low–middle-income countries [14]. Community pharmacists can initiate early interventions with timely reminders, improving medication adherence and proper medication administration, and therefore potentially redefining pharmacists’ roles in chronic illness management [15].
Low–middle-income countries have experienced substantial disruption to medication supplies on account of shortages, factory closures, and lockdowns. In this context, trained pharmacists can substitute out-of-stock drugs with the most appropriate agents available, after verifying this with physicians. This ensures the continuity of therapy for the patient and raises the value of the pharmacy profession [16]. In the long run, promoting competencies in the services of pharmacists would prepare the world for overcoming future healthcare crises.
Pharmacists make significant contributions to administrative and practice-based areas of COVID-19 pharmaceutical care (Figure 1). Li et al. proposed a model to optimize the care of CKD patients by creating a physician–pharmacist collaborative agreement that allows nephrologists to refer patients to a pharmacist to optimize their medication management. The practice transitioned into a tele-health model during the COVID-19 pandemic by using the patient portals of electronic health records for the remote blood monitoring of CKD patients. This also resulted in positive outcomes that benefited highly vulnerable patient populations [17]. Schütze et al. reported that medication optimization improved for CKD patients after incorporating a clinical pharmacist in the nephrology clinic in ambulatory care settings [18]. Aghili et al. highlighted the value clinical pharmacists presented by minimizing drug–drug interactions for CKD patients. This clinical pharmacist–prescriber collaboration helps minimize drug interactions and improve the positive therapeutic outcomes in kidney patients [19]. Sub-optimal medication adherence is a significant threat and a great challenge in CKD patients. Islahudin et al. created an innovative prediction model (to check for adherence in CKD) that helps improve medication adherence and therapeutic outcomes for CKD patients [20]. Another study by Liu et al. highlighted the proficiency of the clinical pharmacist in identifying drug-related problems (DRPs) in CKD patients and ensuring their safe and effective use of medications [21].
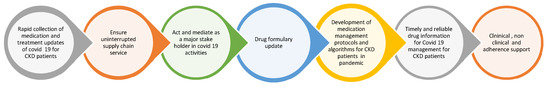
Figure 1.
Administrative and dedicated practice-based roles of pharmacists toward CKD patients.
2. Treating COVID-19 in CKD Patients: A Pharmacist’s Perspective
The treatment of COVID-19 patients with underlying CKD needs special consideration. Renal dysfunction can alter the drug’s elimination, thereby causing sub-therapeutic or supra-therapeutic drug concentrations that require close monitoring and/or dose adjustments. While COVID-19 patients are frequently prescribed antivirals, monoclonal antibodies, steroids, and anticoagulants [9,22], determining the renal function of CKD patients is critical for therapeutic success. Consequently, treatment of COVID-19 in patients with CKD requires individualized therapy and a more focused approach from the pharmacist. Figure 2 describes the major clinical roles of pharmacists in treating COVID-19 infection in CKD patients.
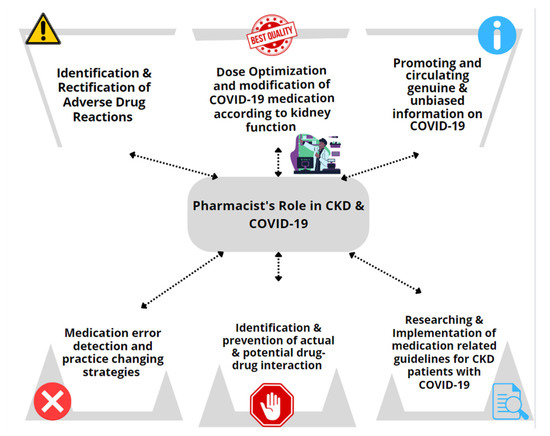
Figure 2.
Pharmacists’ clinical role in managing patients with COVID-19 and CKD.
The sudden surge in the approval of antivirals for treating COVID-19 has created a knowledge gap around dosing protocols for special populations. Favipiravir is currently recommended at a dose of 1.6 g, twice daily, on the first day, followed by 600 mg, twice daily, for 7 to 14 days. Favipiravir is among the most effective treatment options for COVID-19 infection in end-stage kidney disease (ESKD) and does not require dose adjustments in patients with mild to moderate kidney impairment. However, favipiravir should be avoided when the estimated glomerular filtration rate (eGFR) is <30 mL/min [23]. On account of a dearth of safety information, favipiravir requires close monitoring for the possible persistent rise in serum creatinine (in the absence of other nephrotoxic drugs) and vigorous hemodynamic evaluation in case of acute hypotension, hypertensive emergency, sepsis, and dehydration. If the patient encounters any side effects, then urgent intervention is required via considering dosage adjustments, minimizing the duration of the therapy, or withholding treatment if necessary. Favipiravir attains similar blood concentrations in hemodialysis patients and those with normal kidney function, suggesting its potential use in the CKD population [24]. The Institute for Safe Medication Practice (ISMP) has categorized favipiravir within a list of drugs that have a high risk of causing patient harm if used inappropriately [25]. A pharmacist can play a crucial role in selecting, dosing, and monitoring favipiravir treatment in COVID-19 patients with CKD.
A report from India suggested that remdesivir was well tolerated in patients with kidney damage. Remdesivir does not elevate serum creatinine, even in dialysis patients [26]. Moreover, remdesivir can potentially reduce COVID-19 recovery time, supported by evidence from a clinical trial [27]. In this context, some guidelines recommend that dexamethasone should be co-administrated along with remdesivir. If steroids are contraindicated, baricitnib can be combined with remdesivir. The duration of therapy is restricted to 10 days for severe COVID-19 in a patient, and each hospitalized patient can be treated initially with 200 mg, once, followed by 100 mg, daily, for 5 days. However, in non-hospitalized patients, treatment should be limited to 2 to 3 days [28]. The recommended dose of baricitinib (in patients with CrCl ≥ 60 mL/min) is 4 mg, once daily, orally, as part of a combination regimen for 14 days or until discharge, whichever comes first. The dose should be 2 mg, given orally, once daily, if CrCl is between 30 to <60 mL/min, and is not recommended in patients with CrCl < 15 mL/min or on dialysis because 75% of baricitinib is excreted renaly [29]. Although remdesivir has offered significant benefits to patients with CKD, there are a few concerns. Sulfobutylether beta-cyclodextrin, an excipient in the formulation of remdesvir, can accumulate in patients with impaired kidney function; although, there are no reports of clinically significant adverse effects. The above reason is possibly the motive behind manufacturers not recommending remdesivir when CrCl ≤ 30 mL/min. Remdesivir can be given to selected patients whenever the benefits outweigh the risks, because significant toxicity is unlikely under 5–10 days of treatment. An observational study in ESKD reported an elevation of remdesivir’s metabolite GS-441524 after 5 days of therapy, where approximately 50% was removed through hemodialysis [26,30]. In such situations, pharmacists can contribute to patient care by streamlining dosing schedules and ensuring appropriate therapy and closely monitoring drug–drug interactions, particularly with other nephrotoxic medications.
The Food and Drug Administration (FDA), in December 2021, granted emergency use authorization for molnupiravir for mild–moderate COVID-19 outpatients with a high risk of progression to severe illness. Molnupiravir should be initiated as soon as possible after COVID-19 diagnosis, ideally within 5 days of the onset of symptoms. The recommended dose of molnupiravir is 800 mg, every 12 h, for 5 days. A missed dose can be taken within 10 h from the scheduled time (but not later), with dosing resumed at the next scheduled administration time. Molnupiravir is safe in CKD and does not necessitate dose adjustments [31]. Likewise, nirmatrelvir and ritonavir were approved, yet dose adjustments for their combination are essential when CrCl is <60 mL/min, and contraindicated when CrCl is <15 mL/min [23]. The combination therapy should be initiated at the earliest possible time following a COVID-19 diagnosis, ideally within 5 days of the onset of symptoms. The recommended dose for nirmatrelvir is 300 mg, and for ritonavir is 100 mg, every 12 h for 5 days, when CrCl ≥ 60 mL/min. At a CrCl rate of 30 to <60 mL/min, the dose should be halved (nirmatrelvir to 150 mg and ritonavir to 100 mg) and is not recommended when CrCl < 30 mL/min or in dialysis patients. A missed dose may be taken only if within 8 h from the scheduled time but not after >8 h [32]. Some guidelines propose 300 mg of nirmatrelvir and 100 mg of ritonavir on day 1 and then 150 mg of nirmatrelvir and 100 mg of ritonavir, once daily, for 4 more days when CrCl < 30 mL/min and under dialysis [33]. Pharmacists have a significant responsibility as educators and counselors during the current pandemic.
Certain antiviral drugs with good renal safety profiles should not be considered for COVID-19 because of their limited therapeutic benefit. For example, despite the combination of lopinavir and ritonavir having uncompromised clearance for renal impairment and plasma concentrations unaffected by dialysis, these two antivirals did not improve 28-day mortality or the length of hospital stay, and all reported outcomes similar to standard care (without antiviral) [34]. The National Institute of Health (NIH) COVID-19 treatment guidelines do not recommend lopinavir/ritonavir and other similar agents such as darunavir/cobicistat, azithromycin/chloroquine, and hydroxychloroquine for COVID-19 infection [35].
CKD patients with COVID-19 infections, presenting with mild symptoms for 7 days or less, may be recruited for monoclonal antibody therapy. Sotrovimab, or casirivimab and imdevimab combination, bypass renal elimination and therefore need no dose adjustments. Similarly, tocilizumab, which increases the survival rate of COVID-19 victims, does not require any dose adjustment in mild to moderate renal impairment. However, there are studies in cases of severe renal impairment, creating challenges when administering tocilizumab in ESKD patients [22]. Meanwhile, there are alternatives to tocilizumab, i.e., sarilumab [36] and balmanivimab/etesevimab, reserved for COVID-19 post-exposure prophylaxis and mild to moderate COVID-19 infections, which do not require dose adjustment in renal impairment [37]. Table 1 elaborates on the standard and optimized doses of the most common medications being prescribed for COVID-19.

Table 1.
Standard and adjusted doses based on renal impairment for widely utilized medicines against COVID-19.
Corticosteroids are widely prescribed for inflammatory conditions and show a good response. Likewise, steroids suppress the intense phase of inflammation in COVID-19 and the cytokine storm, thereby preventing multi-organ damage. The Randomized Evaluation of COVID-19 Therapy (RECOVERY) trial reported corticosteroid’s potential to reduce mortality in patients requiring oxygenation or ventilation. However, corticosteroids are not considered for mild to moderate infections because of the risk of secondary infections and hyperglycemia. In CKD patients, corticosteroids do not require dosage adjustment even in renal replacement therapy, but patients should be routinely monitored for untoward events [38].
Immunosuppressants, after renal transplantations, can possibly exacerbate COVID-19 complications. A study in pediatric kidney transplanted patients suggested that dose modification or discontinuation of immunosuppressants in COVID-19 positive patients require case-by-case considerations after assessing the risk–benefit ratio [39]. Moreover, although not recommended in CKD patients, NSAIDs may be required during COVID-19 to suppress hyperthermia if the benefit outweighs the risk.
Further, prophylaxis of venous thromboembolism is essential in COVID-19 patients, irrespective of its risk. However, therapeutic modifications may be needed in CKD patients. For those with CrCL > 30 mL/min, anticoagulants, such as unfractionated heparin, enoxaparin, or fondaparinux, may be considered. Besides, a dose reduction label for enoxaparin, and a ‘not recommended’ label for fondaparinux are assigned to those with a CrCl < 30 mL/min [40]. The dose for enoxaparin in patients with CrCl ≥ 30 mL/min is 40 mg SC, once daily, which should be reduced to 30 mg SC, once daily in patients with CrCl < 30 mL/min. It is recommended that the use of enoxaparin be avoided if possible, in patients with dialysis. Moreover, the prophylactic anticoagulant therapy should be discontinued upon discharge or after recovery from COVID-19, when venous thromboembolism risks are resolved [41]. Direct oral anticoagulants are not recommended by chest 2020 guidelines for hospitalized patients due to concerns of an increased risk of bleeding from drug–drug interactions [42]. However, experts consider extended prophylaxis for high-risk patients after discharge, with a direct oral anticoagulant if the CrCl is >30 mL/min, but contraindicated in severe renal failure. The need to select the appropriate anticoagulant and calculate the CrCl and adjust the dose accordingly has widened the scope of pharmacists’ involvement in the treatment of COVID-19 patients with CKD. Communicating and discussing these matters with the physician is of supreme importance, although such practices remain sub-optimal among pharmacists in general.
CKD patients are generally hypertensive and will be on ACE inhibitors or angiotensin receptor blockers (ARBs). No evidence suggests that ACE inhibitors or ARBs exacerbate COVID-19 infections. Unless contra-indicated or substituted with an alternative by the physician, patients are recommended to continue using them [43].
2.1. Identification of Adverse Drug Reactions and Drug-Related Errors
COVID-19 has precipitated a healthcare emergency, promoting a number of “off-label prescriptions”. A retrospective observational study at a tertiary care hospital in Malaysia found 246 adverse events in 1080 samples. The highest number of adverse events was reported for the gastrointestinal system (43.5%), followed by the hepatobiliary (36.2%) and cardiac systems (16.3%). The number of adverse events reported was statistically significant and higher in patients with co-morbid conditions, particularly CKD (50%), cardiovascular diseases (39.7%), and those on atazanavir (52.7%), chloroquine (36.8%, and lopinavir/ritonavir (34.6%). While the investigators relied on a trigger tool for adverse event reporting, the pharmacist contributed remarkably to this reporting [44]. This study has reinforced the vital role of pharmacists in pharmacovigilance that promotes medication safety. Similarly, the highest adverse drug event reported by the pharmacist (81.8%) was described in a cross-sectional study in 71 hospitals, particularly for COVID-19 patients in Brazil [45].
Detection, reporting, and formulating a solution for adverse drug reactions and drug-related problems are among the prime responsibilities of a pharmacist. Each event should be justified with evidence from authentic sources. New and vital information generated from such intensive reporting of adverse events can decrease similar occurrences in the future by increasing vigilance, hence augmenting the standard of patient care.
2.2. Counteracting Drug-Drug Interactions in COVID-19 Patients with CKD
The rise in the global prevalence of CKD mandates the careful monitoring of potential drug–drug interactions. Impaired kidney function alters drug concentrations in COVID-19 patients, potentially reducing their efficacy or enhancing their toxicity. Concomitant drug–drug interactions and higher doses are acceptable only if the benefits outweigh the risks [46]. For instance, tramadol and remdesivir could precipitate a critical drug–drug interaction, and both medications need dose adjustments in CKD. The combination has the potential for an acute pain crisis, secondary to the drug–drug interaction [47]. Similar events can happen during poly-pharmacy, which is common in patients with CKD diagnosed with COVID-19. This would increase the chances for more drug–drug interactions. A study by Schütze et al. reported improved outcomes in medication optimization in CKD patients after incorporating a clinical pharmacist role in a nephrology clinic in ambulatory care settings [48]. With sound knowledge of both pharmacodynamics and pharmacokinetics, pharmacists can suggest suitable modifications to the frequency, dosage, or drugs to minimize drug–drug interactions and subsequent adverse effects.
2.3. Tele-Pharmacy Based Pharmaceutical Care Services for CKD Patients
The COVID-19 pandemic has greatly impacted CKD/ESKD patients, especially those in vulnerable groups, by limiting regular access to health care [49]. Many strategies have emerged for ensuring best practices for safe healthcare delivery. For example, CKD patients who require regular pharmaceutical care during the COVID-19 pandemic can use tele-pharmacy technology to minimize face-to-face interaction [50]. The impact of telepharmacy-based pharmaceutical care services on chronic diseases has been widely researched [51,52]. Johnstone et al. documented 667 clinical pharmacy interventions via tele-pharmacy among CKD patients in Queensland, Australia [48]. Amkreutz et al. reported on the implementation of tele-pharmaceutical expert consultation (in addition to tele-intensive care unit services) and mention 26 recommendations for dosage adjustments in renal failure patients, 11 of which were implemented [53].
Keeys, while highlighting the nighttime pharmaceutical care services in a community hospital in the USA, documented interventions such as dosage adjustment and adverse drug reaction management among patients with renal insufficiency. Another study evaluating medication errors in rural critical access hospitals in the North Dakota Telepharmacy Project (NDTP) documented 182 renal-dosing-related interventions [10]. During the COVID-19 pandemic in Jordan, Hammour et al. reported the delivery of 557 (10.1%) nephrology-related prescriptions from the hospital pharmacy department using an internet-based drug delivery platform model. Similarly, a Spanish tele-healthcare-based study reported erythropoiesis-stimulating agents as the most frequently requested medication via the tele-healthcare services, where 285 CKD and cancer patients requested this medication [54].
Clinical pharmacists employed tele-monitoring services to adjust the drug dosages in a tertiary care hospital in Thailand for COVID-19 patients with severe kidney injury undergoing continuous renal replacement therapy [55]. Another Thailand-based study documented 37 (15.04%) DRPs among 93 (18.64%) CKD patients receiving home drug delivery during the COVID-19 pandemic [56]. A cluster-randomized study, which included CKD patients, documented a statistically significant (p < 0.001) decrease in systolic and diastolic blood pressure when patients were provided with intervention via tele-monitoring services.
The above publications underline the inevitable need for tele-pharmacy-based pharmaceutical care services for vulnerable patients. Published research demonstrates that the delivery of a comprehensive range of pharmaceutical care to renal failure patients can be continued only with tele-pharmacy services included. Innovative technology offers boundless opportunity to improve access to pharmaceutical care for patients with CKD or ESKD.
2.4. Pharmacists Managed Home Delivery Service for CKD Patients
Patients with co-morbidities are more likely to contract the COVID-19 virus and have worse outcomes. In particular, CKD patients with additional underlying medical conditions are at a significantly higher risk. Based on their risk assessment, it is therefore important to minimize their visits to healthcare facilities to avoid the transmission of the virus. In this situation, the home delivery of medication by pharmacists brings with it commendable advantages. In compliance with the international COVID-19 recommendations for pharmacists and the pharmacy workforce, as well as country-specific COVID-19 guidelines, many pharmacies around the world provide daily home deliveries of drugs, essential supplies, and dialysis consumables [57,58].
2.5. Prevention and Management Strategies for Pandemic Related Risks
Chang et al. reported frequent infections in non-dialyzing CKD patients, which increased when eGFR decreases [59]. Similarly, the prevalence of infection can increase with hospital visits and direct patient contact during dialysis. Proper preventative strategies and precautions should be available for CKD patients who avail services in dialysis centers. Streamlined guidelines and recommendations for hand hygiene, isolation precautions, and the timely identification of COVID-19-positive patients are some examples of good practice. Isolation and precautions are essential in dialysis units because multiple patients visit the same dialysis area frequently. There should be a system that aids the early detection of COVID-19 cases that would protect other CKD patients at risk of infection.
3. Conclusions
Patients with CKD are at higher risk of developing complications and may face more severe symptoms upon contracting COVID-19 infection. Antivirals, monoclonal antibodies, steroids, anticoagulants, and CKD medications are a part of the standard treatment regimen as per the COVID-19 treatment guidelines and updates issued by health authorities across the globe. While more studies are still underway to measure the impact of COVID-19 on CKD patients, pharmacists can play a major role in identifying, preventing, and managingDRPs, performing dose adjustments based on renal function, and counseling and motivating patients about the importance of medication adherence and lifestyle modifications for preventing complications in CKD. Pharmacists can also act as medication experts through tele-medicine by providing drug information, especially in places where the pandemic has severely restricted COVID-19 patients from hospital access. Pharmacists have really been at the front and center from the very beginning of the situation. Looking ahead, several things are going to change as a result of what pharmacists have done during the crisis, especially the scope for expanding practice and the increased demand for the types of services that pharmacists provide.
Author Contributions
Conceptualization, M.S.K., S.A.T., S.A.P. and S.A. (Suhaj Abdulsalim); methodology, S.A.P., M.S.K., S.A.P. and H.M.; formal analysis, M.S.K., S.A.T., S.A.P., S.A. (Savera Arain), S.A. (Suhaj Abdulsalim), S.B.S., A.K.K. and M.K.U.; data curation, S.A.T., M.S.K., A.K.K., M.M.A.A., H.M.; writing—original draft preparation, S.A.T., S.A. (Savera Arain), M.S.K., M.K.U., A.K.K., S.A.P., S.B.S. and H.M.; writing—review and editing, S.A.T., H.M., S.A. (Suhaj Abdulsalim), M.S.K., M.K.U., S.A.P., H.M., M.M.A.A. and A.K.K.; supervision, M.K.U., S.A. (Suhaj Abdulsalim), S.B.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/table (accessed on 12 July 2022).
- Petrilli, C.M.; Jones, S.A.; Yang, J.; Rajagopalan, H.; O’Donnell, L.; Chernyak, Y.; Tobin, K.A.; Cerfolio, R.J.; Francois, F.; Horwitz, L.I. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: Prospective cohort study. BMJ 2020, 369, m1966. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Luo, R.; Wang, K.; Zhang, M.; Wang, Z.; Dong, L.; Li, J.; Yao, Y.; Ge, S.; Xu, G. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020, 97, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Chen, K.; Zou, J.; Han, P.; Hao, J.; Han, Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front. Med. 2020, 14, 185–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Rojas, M.A.; Vega-Vega, O.; Bobadilla, N.A. Is the kidney a target of SARS-CoV-2? Am. J. Physiol. Renal. Physiol. 2020, 318, F1454–F1462. [Google Scholar] [CrossRef]
- Del Valle, D.M.; Kim-Schulze, S.; Huang, H.-H.; Beckmann, N.D.; Nirenberg, S.; Wang, B.; Lavin, Y.; Swartz, T.H.; Madduri, D.; Stock, A.; et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020, 26, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Jose, R.J.; Manuel, A. COVID-19 cytokine storm: The interplay between inflammation and coagulation. Lancet Respir. Med. 2020, 8, e46–e47. [Google Scholar] [CrossRef]
- Naicker, S.; Yang, C.-W.; Hwang, S.-J.; Liu, B.-C.; Chen, J.-H.; Jha, V. The Novel Coronavirus 2019 epidemic and kidneys. Kidney Int. 2020, 97, 824–828. [Google Scholar] [CrossRef] [PubMed]
- Henry, B.M.; Lippi, G. Chronic kidney disease is associated with severe coronavirus disease 2019 (COVID-19) infection. Int. Urol. Nephrol. 2020, 52, 1193–1194. [Google Scholar] [CrossRef] [Green Version]
- Scott, D.M.; Friesner, D.L.; Rathke, A.M.; Doherty-Johnsen, S. Medication error reporting in rural critical access hospitals in the North Dakota Telepharmacy Project. Am. J. Health Syst. Pharm. 2014, 71, 58–67. [Google Scholar] [CrossRef]
- Okoro, R.N. COVID-19 pandemic: The role of community pharmacists in chronic kidney disease management supportive care. Res. Soc. Adm. Pharm. 2021, 17, 1925–1928. [Google Scholar] [CrossRef]
- Wang, D.; Liu, Y.; Zeng, F.; Shi, C.; Cheng, F.; Han, Y.; Zhang, Y. Evaluation of the role and usefulness of clinical pharmacists at the Fangcang Hospital during COVID-19 outbreak. Int. J. Clin. Pract. 2021, 75, e14271. [Google Scholar] [CrossRef]
- Zekan, L.; Mestrovic, A.; Perisin, A.S.; Bukic, J.; Leskur, D.; Rusic, D.; Modun, D. Improving community pharmacists’ clinical knowledge to detect and resolve drug-related problems in Croatia: A before/after survey study investigating the efficacy of an educational intervention. BMJ Open 2020, 10, e034674. [Google Scholar] [CrossRef]
- Menon, S.; Sander, J.W. Effects of the COVID-19 pandemic on medication adherence: In the case of antiseizure medications, A scoping review. Seizure 2021, 93, 81–87. [Google Scholar] [CrossRef]
- Saha, S.K.; Adhikary, A.; Jha, A. Enhancement in medication adherence amidst COVID-19 using active reminders. Eur. Phys. J. Spec. Top. 2022, 1–8. [Google Scholar] [CrossRef]
- Kretchy, I.A.; Asiedu-Danso, M.; Kretchy, J.-P. Medication management and adherence during the COVID-19 pandemic: Perspectives and experiences from low-and middle-income countries. Res. Soc. Adm. Pharm. 2021, 17, 2023–2026. [Google Scholar] [CrossRef]
- Li, H.; Radhakrishnan, J. A pharmacist-physician collaborative care model in chronic kidney disease. J. Clin. Hypertens. 2021, 23, 2026–2029. [Google Scholar] [CrossRef]
- Schütze, A.; Hohmann, C.; Haubitz, M.; Radziwill, R.; Benöhr, P. Medicines optimization for patients with chronic kidney disease in the outpatient setting: The role of the clinical pharmacist. Int. J. Pharm. Pract. 2021, 29, 587–597. [Google Scholar] [CrossRef]
- Aghili, M.; Kasturirangan, M.N. Management of Drug–Drug Interactions among Critically Ill Patients with Chronic Kidney Disease: Impact of Clinical Pharmacist’s Interventions. Indian J. Crit. Care Med. 2021, 25, 1226–1231. [Google Scholar] [CrossRef]
- Islahudin, F.; Lee, F.Y.; Kadir, T.N.I.T.A.; Abdullah, M.Z.; Makmor-Bakry, M. Continuous medication monitoring: A clinical model to predict adherence to medications among chronic kidney disease patients. Res. Soc. Adm. Pharm. 2021, 17, 1831–1840. [Google Scholar] [CrossRef]
- Liu, X.-X.; Wang, H.-X.; Hu, Y.-Y.; Zhu, X.-T.; Tan, X.; Yang, Y.; Hang, Y.-F.; Zhu, J.-G. Drug-related problems identified by clinical pharmacists in nephrology department of a tertiary hospital in China—A single center study. Ann. Palliat. Med. 2021, 10, 8701–8708. [Google Scholar] [CrossRef]
- Jonny; Violetta, L.; Kartasasmita, A.S.; Roesli, R.M.A.; Rita, C. Pharmacological Treatment Options for Coronavirus Disease-19 in Renal Patients. Int. J. Nephrol. 2021, 2021, e4078713. [Google Scholar] [CrossRef]
- Marra, F.; Smolders, E.J.; El-Sherif, O.; Boyle, A.; Davidson, K.; Sommerville, A.J.; Marzolini, C.; Siccardi, M.; Burger, D.; Gibbons, S.; et al. Recommendations for Dosing of Repurposed COVID-19 Medications in Patients with Renal and Hepatic Impairment. Drugs R D 2021, 21, 9–27. [Google Scholar] [CrossRef]
- Koshi, E.; Saito, S.; Okazaki, M.; Toyama, Y.; Ishimoto, T.; Kosugi, T.; Hiraiwa, H.; Jingushi, N.; Yamamoto, T.; Ozaki, M.; et al. Efficacy of favipiravir for an end stage renal disease patient on maintenance hemodialysis infected with novel coronavirus disease 2019. CEN Case Rep. 2020, 10, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Gök, S.; Bahçecioğlu, Ö.F.; Durmuş, M.; Gün, Z.Ü.; Ersoy, Y.; Aytemur, Z.A.; Ulutaş, Ö. The safety profile of favipiravir in COVID-19 patients with severe renal impairment. Int. J. Clin. Pract. 2021, 75, e14938. [Google Scholar] [CrossRef] [PubMed]
- Thakare, S.; Gandhi, C.; Modi, T.; Bose, S.; Deb, S.; Saxena, N.; Katyal, A.; Patil, A.; Patil, S.; Pajai, A.; et al. Safety of Remdesivir in Patients With Acute Kidney Injury or CKD. Kidney Int. Rep. 2020, 6, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the Treatment of COVID-19—Final report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef] [PubMed]
- Goldman, J.D.; Lye, D.C.B.; Hui, D.S.; Marks, K.M.; Bruno, R.; Montejano, R.; Spinner, C.D.; Galli, M.; Ahn, M.-Y.; Nahass, R.G.; et al. Remdesivir for 5 or 10 Days in Patients with Severe Covid-19. N. Engl. J. Med. 2020, 383, 1827–1837. [Google Scholar] [CrossRef]
- Kalil, A.C.; Patterson, T.F.; Mehta, A.K.; Tomashek, K.M.; Wolfe, C.R.; Ghazaryan, V.; Marconi, V.C.; Ruiz-Palacios, G.M.; Hsieh, L.; Kline, S.; et al. Baricitinib plus Remdesivir for Hospitalized Adults with COVID-19. N. Engl. J. Med. 2021, 384, 795–807. [Google Scholar] [CrossRef]
- Lê, M.P.; Le Hingrat, Q.; Jaquet, P.; Wicky, P.-H.; Bunel, V.; Massias, L.; Visseaux, B.; Messika, J.; Descamps, D.; Mal, H.; et al. Removal of Remdesivir’s Metabolite GS-441524 by Hemodialysis in a Double Lung Transplant Recipient with COVID-19. Antimicrob. Agents Chemother. 2020, 64, e01521-20. [Google Scholar] [CrossRef]
- Hiremath, S.; McGuinty, M.; Argyropoulos, C.; Brimble, K.S.; Brown, P.A.; Chagla, Z.; Cooper, R.; Hoar, S.; Juurlink, D.; Treleaven, D.; et al. Prescribing Nirmatrelvir/Ritonavir for COVID-19 in Advanced CKD. Clin. J. Am. Soc. Nephrol. 2022, 17, 1–4. [Google Scholar] [CrossRef]
- Jayk Bernal, A.; Gomes da Silva, M.M.; Musungaie, D.B.; Kovalchuk, E.; Gonzalez, A.; Delos Reyes, V.; Martín-Quirós, A.; Caraco, Y.; Williams-Diaz, A.; Brown, M.L.; et al. Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients. N. Engl. J. Med. 2022, 386, 509–520. [Google Scholar] [CrossRef]
- Ltd, B.P.G. Two New Oral Antivirals for COVID-19: ▼Molnupiravir and ▼Nirmatrelvir Plus Ritonavir. DTB 2022, 60, 73–77. [Google Scholar]
- Lopinavir and Ritonavir: Drug Information–UpToDate. Available online: https://www.uptodate.com/contents/lopinavir-and-ritonavir-drug-information (accessed on 7 June 2022).
- Information on COVID-19 Treatment, Prevention and Research. COVID-19 Treatment Guidelines. Available online: https://www.covid19treatmentguidelines.nih.gov/ (accessed on 7 June 2022).
- Lescure, F.-X.; Honda, H.; Fowler, R.A.; Lazar, J.S.; Shi, G.; Wung, P.; Patel, N.; Hagino, O.; Bazzalo, I.J.; Casas, M.M.; et al. Sarilumab in patients admitted to hospital with severe or critical COVID-19: A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir. Med. 2021, 9, 522–532. [Google Scholar] [CrossRef]
- Commissioner O of the Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibody for Treatment of COVID-19. FDA 2020. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibody-treatment-covid-19 (accessed on 7 June 2022).
- The RECOVERY Collaborative Group. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef]
- Saeed, B. Impact of COVID-19 Pandemic on Management of Pediatric Kidney Transplant Recipients. Exp. Clin. Transplant. 2021, 19, 894–898. [Google Scholar] [CrossRef]
- Shafiee, M.A.; Hosseini, S.F.; Mortazavi, M.; Emami, A.; Zadeh, M.M.; Moradi, S.; Shaker, P. Anticoagulation therapy in COVID-19 patients with chronic kidney disease. J. Res. Med Sci. 2021, 26, 63. [Google Scholar] [CrossRef]
- Moores, L.K.; Tritschler, T.; Brosnahan, S.; Carrier, M.; Collen, J.F.; Doerschug, K.; Holley, A.B.; Jimenez, D.; Le Gal, G.; Rali, P.; et al. Prevention, Diagnosis, and Treatment of VTE in Patients With Coronavirus Disease 2019: CHEST Guideline and Expert Panel Report. Chest 2020, 158, 1143–1163. [Google Scholar] [CrossRef]
- Pawar, A.; Gagne, J.J.; Gopalakrishnan, C.; Iyer, G.; Tesfaye, H.; Brill, G.; Chin, K.; Bykov, K. Association of Type of Oral Anticoagulant Dispensed With Adverse Clinical Outcomes in Patients Extending Anticoagulation Therapy Beyond 90 Days After Hospitalization for Venous Thromboembolism. JAMA 2022, 327, 1051–1060. [Google Scholar] [CrossRef]
- Li, J.; Li, S.-X.; Zhao, L.-F.; Kong, D.-L.; Guo, Z.-Y. Management recommendations for patients with chronic kidney disease during the novel coronavirus disease 2019 (COVID-19) epidemic. Chronic Dis. Transl. Med. 2020, 6, 119–123. [Google Scholar] [CrossRef]
- Lee, J.Y.; Ang, A.S.Y.; Ali, N.M.; Ang, L.M.; Omar, A. Incidence of adverse reaction of drugs used in COVID-19 management: A retrospective, observational study. J. Pharm. Policy Pract. 2021, 14, 84. [Google Scholar] [CrossRef]
- Melo, J.R.R.; Duarte, E.C.; de Moraes, M.V.; Fleck, K.; Silva, A.S.D.N.E.; Arrais, P.S.D. Adverse drug reactions in patients with COVID-19 in Brazil: Analysis of spontaneous notifications of the Brazilian pharmacovigilance system. Cad. Saude Publica 2021, 37, e00245820. [Google Scholar] [CrossRef]
- Marquito, A.B.; Fernandes, N.M.; Colugnati, F.A.; Paula, R.B. Identifying potential drug interactions in chronic kidney disease patients. Brazilian J. Nephrol. 2014, 36, 26–34. [Google Scholar] [CrossRef]
- Teoli, D.; Thompson, V.; Wright, J.; Ho, I.; Vlaminck, B.; Miller, G.; Feely, M. Acute Pain Crisis Caused by Tramadol Remdesivir Drug–Drug Interaction. J. Palliat. Med. 2021, 24, 1582–1584. [Google Scholar] [CrossRef]
- Johnstone, L. Telepharmacy and chronic kidney disease—A Making Tracks investment strategy. In Proceedings of the 14th National Rural Health Conference, Canberra, Australia, 26–29 March 2017; pp. 26–29. [Google Scholar]
- Divyaveer, S.; Jha, V. COVID-19 and care for patients with chronic kidney disease: Challenges and lessons. FASEB BioAdv. 2021, 3, 569–576. [Google Scholar] [CrossRef]
- Kilova, K.; Mihaylova, A.; Peikova, L. Opportunities of information communication technologies for providing pharmaceutical care in the COVID-19 pandemic. Pharmacia 2021, 68, 9–14. [Google Scholar] [CrossRef]
- Omboni, S.; Tenti, M. Telepharmacy for the management of cardiovascular patients in the community. Trends Cardiovasc. Med. 2019, 29, 109–117. [Google Scholar] [CrossRef]
- Margolis, K.L.; Asche, S.E.; Bergdall, A.R.; Dehmer, S.P.; Groen, S.E.; Kadrmas, H.M.; Kerby, T.J.; Klotzle, K.J.; Maciosek, M.; Michels, R.D.; et al. Effect of Home Blood Pressure Telemonitoring and Pharmacist Management on Blood Pressure Control: A cluster randomized clinical trial. JAMA 2013, 310, 46–56. [Google Scholar] [CrossRef] [Green Version]
- Amkreutz, J.; Lenssen, R.; Marx, G.; Deisz, R.; Eisert, A. Medication safety in a German telemedicine centre: Implementation of a telepharmaceutical expert consultation in addition to existing tele-intensive care unit services. J. Telemed. Telecare 2020, 26, 105–112. [Google Scholar] [CrossRef]
- Bejarano, A.P.; Santos, P.V.; Robustillo-Cortés, M.D.L.A.; Gómez, E.S.; Rubio, M.D.S. Implementation of a novel home delivery service during pandemic. Eur. J. Hosp. Pharm. 2021, 28, e120–e123. [Google Scholar] [CrossRef]
- Surapat, B.; Sungkanuparph, S.; Kirdlarp, S.; Lekpittaya, N.; Chunnguleum, K. Role of clinical pharmacists in telemonitoring for patients with Coronavirus Disease 2019 (COVID-19). J. Clin. Pharm. Ther. 2021, 46, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Chaomuang, N.; Dede, A.J.O.; Saokaew, S.; Umnuaypornlert, A. Effects of home drug delivery on drug-related problems: Preliminary evidence for improved patient outcomes during the COVID-19 pandemic in Thailand. J. Am. Pharm. Assoc. 2022, 62, 1206–1213.e3. [Google Scholar] [CrossRef] [PubMed]
- Mash, R.J.; Schouw, D.; Daviaud, E.; Besada, D.; Roman, D. Evaluating the implementation of home delivery of medication by community health workers during the COVID-19 pandemic in Cape Town, South Africa: A convergent mixed methods study. BMC Health Serv. Res. 2022, 22, 98. [Google Scholar] [CrossRef] [PubMed]
- AlAbbasi, H.K.; Thorakkattil, S.A.; Mohiuddin, S.I.; Nemr, H.S.; Jabbour, R.; Al-Ghamdi, F. Implementation and effectiveness of drive-through medication pick-up and home delivery services. A patient safety initiative during COVID-19 pandemic. J. Patient Saf. Risk Manag. 2021, 26, 179–186. [Google Scholar] [CrossRef]
- Chang, C.-H.; Fan, P.-C.; Kuo, G.; Lin, Y.-S.; Tsai, T.-Y.; Chang, S.-W.; Tian, Y.-C.; Lee, C.-C. Infection in Advanced Chronic Kidney Disease and Subsequent Adverse Outcomes after Dialysis Initiation: A Nationwide Cohort Study. Sci. Rep. 2020, 10, 2938. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).