Colon Cancer Pharmacogenetics: A Narrative Review
Abstract
:1. Introduction
2. Colon Cancer Overview
2.1. Signs and Symptoms
2.2. Risk Factors
2.3. Pharmacological Therapy
2.3.1. Chemotherapy
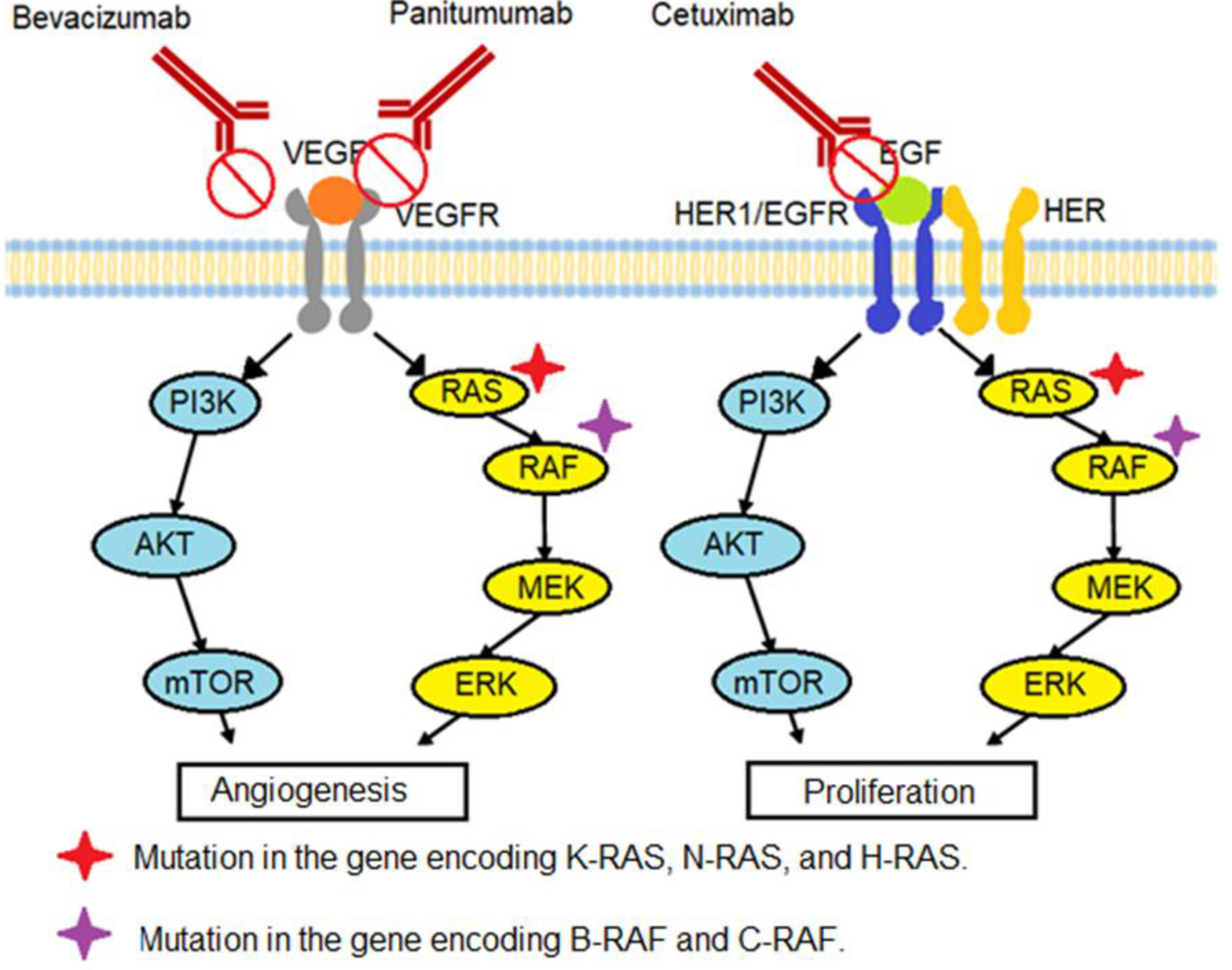
2.3.2. Biological Drugs and Immunotherapy
Angiogenesis
Sustained Proliferative Signaling
Evasion of Immune System
2.3.3. Therapy Safety
3. Pharmacogenetics Related to Colon Cancer
3.1. K-RAS/N-RAS/H-RAS Gene Mutation
3.2. B-RAF/C-RAF Gene Mutation
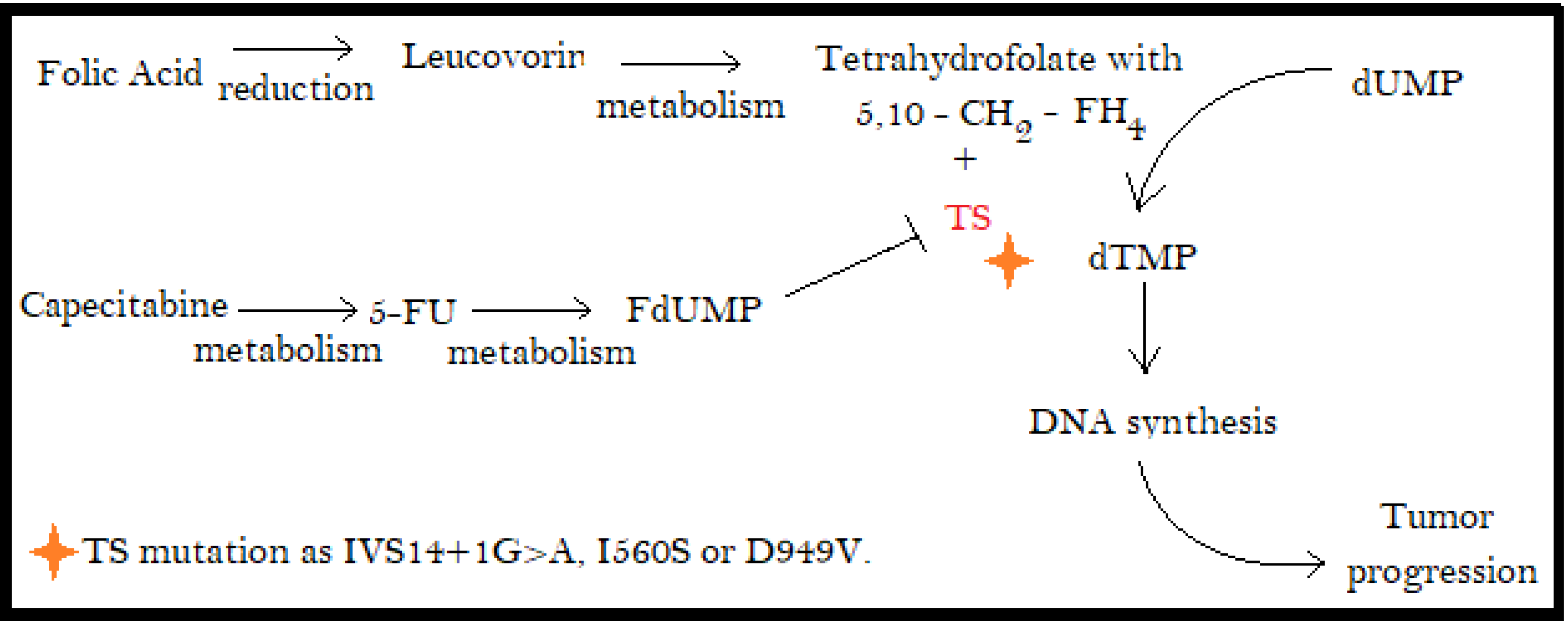
3.3. DPDY Genotype
3.4. Thymidylate Synthase (TS)
3.5. Methylenetetrahydrofolate Reductase (MTHFR) Gene
3.6. ATP Binding Cassette Transporter B (ABCB1 and ABCB2)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bassotti, G.; Battaglia, E. Physiology of the Colon. In Colon, Rectum and Anus: Anatomic, Physiologic and Diagnostic Bases for Disease Management; Ratto, C., Parello, A., Donisi, L., Litta, F., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Gema, L. Positorio institucional de la Universidad de Cantabria [Internet]. Cantabria, Spain. Monografía Sobre el Intestino Grueso: Enfermedades Inflamatorias Intestinales. [Updated 2014; Cited 2020 November 03]. Available online: https://repositorio.unican.es/xmlui/bitstream/handle/10902/5116/LopezMoraG.pdf?sec (accessed on 29 May 2022).
- Greenwood-Van Meerveld, B.; Johnson, A.; Grundy, D. Gastrointestinal Physiology and Function. Gastrointest. Pharmacol. 2017, 239, 1–16. [Google Scholar]
- Bleier, J.I.S.; Wilkins, K.B. Colonic Physiology. In The ASCRS Manual of Colon and Rectal Surgery; Steele, S.R., Hull, T.L., Hyman, N., Maykel, J.A., Read, T.E., Whitlow, C.B., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 29–36. [Google Scholar]
- Ali Koc, M.; Utku Celik, S.; Akyol, C. Colon Cancer. In Current Trends in Cancer Management; Streba, L., Ionut Gheonea, D., Schenker, M., Eds.; IntechOpen: London, UK, 2019. [Google Scholar]
- The Global Cancer Observatory. Colon; The Global Cancer Observatory: Lyon, France, 2019; pp. 1–2. [Google Scholar]
- The Global Cancer Observatory. Costa Rica; The Global Cancer Observatory: Lyon, France, 2019; pp. 1–2. [Google Scholar]
- Angell, H.; Bruni, D.; Barrett, J.; Herbst, R.; Galon, J. The Immunoscore: Colon Cancer and Beyond. Clin. Cancer Res. 2020, 26, 332–339. [Google Scholar] [CrossRef] [Green Version]
- Dineen, S.; Rodríguez-Bigas, M. Colorectal Cancer: Molecular Biology and Inherited Cancer Syndromes. In Textbook of Complex General Surgical Oncology; McGraw Hill, Education: Shanghai, China, 2018. [Google Scholar]
- American Cancer Society. About Colorectal Cancer; American Cancer Society: Atlanta, GA, USA, 2020. [Google Scholar]
- Hoehn, R.S.; Smith, J.J. Adjuvant Chemotherapy for Colon Cancer. Dis. Colon Rectum. 2019, 62, 274–278. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Colorectal Cancer Early Detection, Diagnosis, and Staging; American Cancer Society: Atlanta, Georgia, 2020. [Google Scholar]
- Sáez-López, P.; Filipovich Vegas, E.; Martinez Peromingo, J.; Jimenez Mola, S. Cáncer colorrectal en el anciano. Tratamiento quirúrgico, quimioterápico y aportación desde la geriatría. Rev. Esp. Geriatría Gerontol. 2017, 52, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Alfaro-Alfaro, Á.E.; Alpízar-Cambronero, V.; Duarte-Rodríguez, A.I.; Feng-Feng, J.; Rosales-Leiva, C.; Mora-Román, J.J. C-ficocianinas: Modulación del sistema inmune y su posible aplicación como terapia contra el cáncer. Rev. Tecnol. En Marcha. 2020, 33, 125–139. [Google Scholar] [CrossRef]
- Rubin, D.C.; Shaker, A.; Levin, M.S. Chronic intestinal inflammation: Inflammatory bowel disease and colitis-associated colon cancer. Front. Immunol. 2012, 3, 107. [Google Scholar] [CrossRef] [Green Version]
- Ullman, T.A.; Itzkowitz, S.H. Intestinal Inflammation and Cancer. Gastroenterology 2011, 140, 1807–1816. [Google Scholar] [CrossRef]
- Danese, S.; Mantovani, A. Inflammatory bowel disease and intestinal cancer: A paradigm of the Yin–Yang interplay between inflammation and cancer. Oncogene 2010, 29, 3313–3323. [Google Scholar] [CrossRef] [Green Version]
- Al-Harbi, S.A.; Al-Judaibi, A.A. Relationship between Escherichia coli and colon cancer. GSC Biol. Pharm. Sci. 2020, 12, 188–193. [Google Scholar] [CrossRef]
- Cordero-García, E.; Serrano, B. Microbiota, Epigenética y respuesta a medicamentos en cáncer de colon. Rev. Médica De La Univ. Costa Rica 2020, 14, 81–92. [Google Scholar] [CrossRef]
- Sears, C.L.; Garrett, W.S. Microbes, Microbiota, and Colon Cancer. Cell Host Microbe 2014, 15, 317–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobhani, I.; Amiot, A.; Le Baleur, Y.; Levy, M.; Auriault, M.-L.; Van Nhieu, J.T.; Delchier, J.C. Microbial dysbiosis and colon carcinogenesis: Could colon cancer be considered a bacteria-related disease? Ther. Adv. Gastroenterol. 2013, 6, 215–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irrazábal, T.; Belcheva, A.; Girardin, S.E.; Martin, A.; Philpott, D.J. The Multifaceted Role of the Intestinal Microbiota in Colon Cancer. Mol. Cell 2014, 54, 309–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.S.; Menter, D.G.; Kopetz, S. Right Versus Left Colon Cancer Biology: Integrating the Consensus Molecular Subtypes. J. Natl. Compr. Canc. Netw. 2017, 15, 411–419. [Google Scholar] [CrossRef] [Green Version]
- Salem, M.E.; Weinberg, B.A.; Xiu, J.; El-Deiry, W.S.; Hwang, J.J.; Gatalica, Z.; Philip, P.A.; Shields, A.F.; Lenz, H.-J.; Marshall, J.L. Comparative molecular analyses of left-sided colon, right-sided colon, and rectal cancers. Oncotarget 2017, 8, 86356–86368. [Google Scholar] [CrossRef] [Green Version]
- National Comprehensive Cancer Network. Clinical Practice Guideline in Oncology (NCCN Guideline) Colon Cancer (European Edition); National Comprehensive Cancer Network: Plymouth Meeting, PA, USA, 2016. [Google Scholar]
- National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Colon Cancer Version 4; National Comprehensive Cancer Network: Plymouth Meeting, PA, USA, 2020. [Google Scholar]
- Martinez-Balibrea, E.; Martínez-Cardús, A.; Ginés, A.; de Porras, V.R.; Moutinho, C.; Layos, L.; Manzano, J.L.; Bugés, C.; Bystrup, S.; Esteller, M.; et al. Tumor-Related Molecular Mechanisms of Oxaliplatin Resistance. Mol. Cancer Ther. 2015, 14, 1767–1776. [Google Scholar] [CrossRef] [Green Version]
- Hussain, S. On a New Proposed Mechanism of 5-Fluorouracil-Mediated Cytotoxicity. Trends Cancer 2020, 6, 365–368. [Google Scholar] [CrossRef]
- Schneiders, F.L.; van den Berg, H.P.; Peters, G.J.; Verheul, H.M.W.; van der Vliet, H.J. Severe toxicity of capecitabine following uncomplicated treatment with 5-fluorouracil/leucovorin. Med. Oncol. 2011, 28, 1136–1139. [Google Scholar] [CrossRef]
- Fujita, K. Irinotecan, a key chemotherapeutic drug for metastatic colorectal cancer. World J. Gastroenterol. 2015, 21, 12234. [Google Scholar] [CrossRef]
- Zhao, Y.; Adjei, A.A. Targeting Angiogenesis in Cancer Therapy: Moving Beyond Vascular Endothelial Growth Factor. Oncologist 2015, 20, 660–673. [Google Scholar] [CrossRef] [Green Version]
- Papadopoulos, N.; Martin, J.; Ruan, Q.; Rafique, A.; Rosconi, M.P.; Shi, E.; Pyles, E.A.; Yancopoulos, G.D.; Stahl, N.; Wiegand, S.J. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis 2012, 15, 171–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, Q.; Bi, L.; Ren, Y.; Song, S.; Wang, Q.; Wang, Y. Advances in studies of tyrosine kinase inhibitors and their acquired resistance. Mol. Cancer 2018, 17, 36. [Google Scholar] [CrossRef] [PubMed]
- Marcinkowska, E.; Gocek, E. Tyrosine Kinase Signaling Pathways in Normal and Cancer Cells. In Resistance to Tyrosine Kinase Inhibitors; Focosi, D., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–25. [Google Scholar]
- Calle, X.; Jiménez-Gallegos, D.; Muñoz-Córdova, F.; Sánchez, P.; Lavandero, S. Mecanismo sensor y de adaptación a los niveles de oxígeno y su implicancia en las enfermedades cardiovasculares: A propósito del Premio Nobel de Fisiología-Medicina 2019. Rev. Chil. Cardiol. 2019, 38, 225–235. [Google Scholar] [CrossRef] [Green Version]
- Hurwitz, H.; Fehrenbacher, L.; Novotny, W.; Cartwright, T.; Hainsworth, J.; Heim, W.; Berlin, J.; Baron, A.; Griffing, S.; Holmgren, E.; et al. Bevacizumab plus Irinotecan, Fluorouracil, and Leucovorin for Metastatic Colorectal Cancer. N. Engl. J. Med. 2004, 350, 2335–2342. [Google Scholar] [CrossRef] [Green Version]
- Colucci, G.; Gebbia, V.; Paoletti, G.; Giuliani, F.; Caruso, M.; Gebbia, N.; Cartenì, G.; Agostara, B.; Pezzella, G.; Manzione, L.; et al. Phase III Randomized Trial of FOLFIRI Versus FOLFOX4 in the Treatment of Advanced Colorectal Cancer: A Multicenter Study of the Gruppo Oncologico Dell’Italia Meridionale. J. Clin. Oncol. 2005, 23, 4866–4875. [Google Scholar] [CrossRef] [Green Version]
- Shin, D.; Lee, Y.J.; Choi, J.; Lee, D.; Park, M.; Petkova, M. A phase I, randomized, single-dose pharmacokinetic study comparing sb8 (bevacizumab biosimilar) with reference bevacizumab in healthy volunteers. Cancer Chemother Pharmacol. 2020, 86, 567–575. [Google Scholar] [CrossRef]
- Ngo, D.T.M.; Williams, T.; Horder, S.; Kritharides, L.; Vardy, J.; Mandaliya, H.; Nordman, I.I.C.; Lynam, J.; Bonaventura, T.; Sverdlov, A.L. Factors Associated with Adverse Cardiovascular Events in Cancer Patients Treated with Bevacizumab. J. Clin. Med. 2020, 9, 2664. [Google Scholar] [CrossRef]
- Qu, C.Y. Value of bevacizumab in treatment of colorectal cancer: A meta-analysis. World J. Gastroenterol. 2015, 21, 5072. [Google Scholar] [CrossRef]
- Mésange, P.; Poindessous, V.; Sabbah, M.; Escargueil, A.E.; de Gramont, A.; Larsen, A.K. Intrinsic bevacizumab resistance is associated with prolonged activation of autocrine VEGF signaling and hypoxia tolerance in colorectal cancer cells and can be overcome by nintedanib, a small molecule angiokinase inhibitor. Oncotarget 2014, 5, 4709–4721. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.H.; Wang, Y.Z.; Tu, J.; Liu, C.W.; Yuan, Y.J.; Lin, R.; He, W.L.; Cai, S.R.; He, Y.L.; Ye, J.N. Anti-EGFR therapy in metastatic colorectal cancer: Mechanisms and potential regimens of drug resistance. Gastroenterol. Rep. 2020, 8, 179–191. [Google Scholar] [CrossRef]
- Petrelli, F.; Borgonovo, K.; Barni, S. The predictive role of skin rash with cetuximab and panitumumab in colorectal cancer patients: A systematic review and meta-analysis of published trials. Target Oncol. 2013, 8, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Ghartimagar, D.; Thapa, S. Oncogenes-the basics. J. Biomed. Sci. 2018, 3, 35–37. [Google Scholar] [CrossRef] [Green Version]
- Krasinskas, A.M. EGFR Signaling in Colorectal Carcinoma. Pathol Res. Int. 2011, 2011, 932932. [Google Scholar] [CrossRef]
- Markman, B.; Javier Ramos, F.; Capdevila, J.; Tabernero, J. EGFR and KRAS in Colorectal Cancer. In Advances in Clinical Chemistry; Elsevier: Amsterdam, The Netherlands, 2010; pp. 71–119. [Google Scholar] [CrossRef]
- Fornasier, G.; Francescon, S.; Baldo, P. An Update of Efficacy and Safety of Cetuximab in Metastatic Colorectal Cancer: A Narrative Review. Adv. Ther. 2018, 35, 1497–1509. [Google Scholar] [CrossRef] [PubMed]
- Deeks, E.D. Pembrolizumab: A Review in Advanced Melanoma. Drugs 2016, 76, 375–386. [Google Scholar] [CrossRef]
- McDermott, D.F.; Atkins, M.B. PD-1 as a potential target in cancer therapy. Cancer Med. 2013, 2, 662–673. [Google Scholar] [CrossRef]
- Sznol, M.; Chen, L. Antagonist Antibodies to PD-1 and B7-H1 (PD-L1) in the Treatment of Advanced Human Cancer. Clin. Cancer Res. 2013, 19, 1021–1034. [Google Scholar] [CrossRef] [Green Version]
- Rozali, E.N.; Hato, S.V.; Robinson, B.W.; Lake, R.A.; Lesterhuis, W.J. Programmed Death Ligand 2 in Cancer-Induced Immune Suppression. Clin. Dev. Immunol. 2012, 2012, 656340. [Google Scholar] [CrossRef]
- Huang, P.W.; Chang, J.W.C. Immune checkpoint inhibitors win the 2018 Nobel Prize. Biomed. J. 2019, 42, 299–306. [Google Scholar] [CrossRef]
- Lau, D.; Cunningham, D.; Gillbanks, A.; Crux, R.; Powell, R.; Kalaitzaki, E.; Annels, N.E.; Sclafani, F.; Gerlinger, M.; Chau, I.; et al. POLEM: Avelumab plus fluoropyrimidine-based chemotherapy as adjuvant treatment for stage III dMMR or POLE exonuclease domain mutant colon cancer—A phase III randomized study. J. Clin. Oncol. 2019, 37 (Suppl. 15), TPS3615. [Google Scholar] [CrossRef]
- Torres-González, S.; Morales-Sánchez, M.A.; Gómez-Molinar, V.M. Alopecia por medicamentos. Rev. Cent Derm. Pascua. 2016, 25, 5–10. [Google Scholar]
- Bartolomé-Alonso, A.; Pardal-Refoyo, J.L. Revisión sobre prevención y tratamiento de la mucositis oral en cáncer de cabeza y cuello. Rev. ORL 2019, 10, 269. [Google Scholar] [CrossRef] [Green Version]
- De Jesús Sobrevilla Calvo, P.; Sobrevilla Moreno, N.; Ochoa Carrillo, F.J. Neutropenia inducida por quimioterapia: El punto de vista del oncólogo. Gac Mex Oncol. 2016, 15, 344–349. [Google Scholar] [CrossRef] [Green Version]
- American Cancer Society. Chemotherapy for Colorectal Cancer [Internet]. American Cancer Society. 2020. Available online: https://www.cancer.org/cancer/colon-rectal-cancer/treating/chemotherapy.html (accessed on 10 October 2020).
- Miranda Poma, J.; Ostios García, L. Protocolo diagnóstico y terapéutico de las náuseas y vómitos en el paciente oncológico. Med.-Programa Médica Contin Acreditado 2017, 12, 2070–2075. [Google Scholar] [CrossRef]
- Hou, J.; Guo, C.; Li, G.; Lv, G. Chemotherapy Induced Diarrhea: A Case Report. J. Cancer Prev. Curr. Res. 2018, 9, 1. [Google Scholar] [CrossRef] [Green Version]
- Squeff, M.; Otal, M.; Boncimpain, C.; Moreno, C.; Mercau, S.; Gorosito, M.; Fernández Bussy, R.A. Eritrodisestesia o síndrome mano pie presentación de dos casos y revisión de la literatura. Arch. Argent Dermatol. 2016, 66, 169–172. [Google Scholar]
- Salas, J.; Pérez, J. Cardiotoxicidad de los quimioterapéuticos diferentes a antraciclinas de la lista oficial de medicamentos de la Caja Costarricense del Seguro Social. Rev. Costarric. Cardiol. 2019, 21, 7–13. [Google Scholar] [CrossRef]
- Gebremedhn, E.G.; Shortland, P.J.; Mahns, D.A. The incidence of acute oxaliplatin-induced neuropathy and its impact on treatment in the first cycle: A systematic review. BMC Cancer 2018, 18, 410. [Google Scholar] [CrossRef] [Green Version]
- Sociedad Española de Oncología Médica. [Internet] Spain. Blasco, A.; Caballero, C. Toxicidad de los Tratamientos Oncológicos. Sociedad Española de Oncología Médica, [updated 2019; cited 2020 Nov 06]. Available online: https://seom.org/guia-actualizada-de-tratamientos/toxicidad-de-los-tratamientos-oncologicos (accessed on 29 May 2022).
- Parel, M.; Ranchon, F.; Nosbaum, A.; You, B.; Vantard, N.; Schwiertz, V.; Gourc, C.; Gauthier, N.; Guedat, M.-G.; He, S.; et al. Hypersensitivity to oxaliplatin: Clinical features and risk factors. BMC Pharm. Toxicol. 2014, 15, 1. [Google Scholar] [CrossRef] [Green Version]
- Boyer, A.-S.; Walter, D.; Sørensen, C.S. DNA replication and cancer: From dysfunctional replication origin activities to therapeutic opportunities. Semin. Cancer Biol. 2016, 37–38, 16–25. [Google Scholar] [CrossRef]
- Quiñones, L.; Roco, Á.; Cayún, J.P.; Escalante, P.; Miranda, C.; Varela, N.; Meneses, F.; Gallegos, B.; Zaruma-Torres, F.; Lares-Asseff, I. Farmacogenómica como herramienta fundamental para la medicina personalizada: Aplicaciones en la práctica clínica. Rev. Med. Chile 2017, 145, 483–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cremolini, C.; Loupakis, F.; Antoniotti, C.; Lupi, C.; Sensi, E.; Lonardi, S.; Mezi, S.; Tomasello, G.; Ronzoni, M.; Zaniboni, A.; et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: Updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 2015, 16, 1306–1315. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Köhne, C.-H.; Hitre, E.; Zaluski, J.; Chien, C.-R.C.; Makhson, A.; D’Haens, G.; Pintér, T.; Lim, R.; Bodoky, G.; et al. Cetuximab and Chemotherapy as Initial Treatment for Metastatic Colorectal Cancer. N. Engl. J. Med. 2009, 360, 1408–1417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douillard, J.-Y.; Siena, S.; Cassidy, J.; Tabernero, J.; Burkes, R.; Barugel, M.; Humblet, Y.; Bodoky, G.; Cunningham, D.; Jassem, J.; et al. Randomized, Phase III Trial of Panitumumab with Infusional Fluorouracil, Leucovorin, and Oxaliplatin (FOLFOX4) Versus FOLFOX4 Alone as First-Line Treatment in Patients with Previously Untreated Metastatic Colorectal Cancer: The PRIME Study. J. Clin. Oncol. 2010, 28, 4697–4705. [Google Scholar] [CrossRef]
- Li, Z.N.; Zhao, L.; Yu, L.F.; Wei, M.J. BRAF and KRAS mutations in metastatic colorectal cancer: Future perspectives for personalized therapy. Gastroenterol. Rep. 2020, 8, 192–205. [Google Scholar] [CrossRef]
- Tie, J.; Gibbs, P.; Lipton, L.; Christie, M.; Jorissen, R.; Burgess, A.W.; Croxford, M.; Jones, I.; Langland, R.; Kosmider, S.; et al. Optimizing targeted therapeutic development: Analysis of a colorectal cancer patient population with the BRAFV600E mutation. Int. J. Cancer 2011, 128, 2075–2084. [Google Scholar] [CrossRef]
- Corcoran, R.B.; Atreya, C.E.; Falchook, G.S.; Kwak, E.L.; Ryan, D.P.; Bendell, J.C.; Hamid, O.; Messersmith, W.A.; Daud, A.; Kurzrock, R.; et al. Combined BRAF and MEK Inhibition with Dabrafenib and Trametinib in BRAF. J. Clin. Oncol. 2015, 33, 4023–4031. [Google Scholar] [CrossRef] [Green Version]
- Morris, V.; Overman, M.J.; Jiang, Z.-Q.; Garrett, C.; Agarwal, S.; Eng, C.; Kee, B.; Fogelman, D.; Dasari, A.; Wolff, R.; et al. Progression-Free Survival Remains Poor Over Sequential Lines of Systemic Therapy in Patients with BRAF-Mutated Colorectal Cancer. Clin. Colorectal. Cancer 2014, 13, 164–171. [Google Scholar] [CrossRef] [Green Version]
- Yokota, T.; Ura, T.; Shibata, N.; Takahari, D.; Shitara, K.; Nomura, M.; Kondo, C.; Mizota, A.; Utsunomiya, S.; Muro, K.; et al. BRAF mutation is a powerful prognostic factor in advanced and recurrent colorectal cancer. Br. J. Cancer 2011, 104, 856–862. [Google Scholar] [CrossRef] [Green Version]
- Zlobec, I.; Bihl, M.P.; Schwarb, H.; Terracciano, L.; Lugli, A. Clinicopathological and protein characterization of BRAF - and K-RAS -mutated colorectal cancer and implications for prognosis. Int. J. Cancer. 2010, 127, 367–380. [Google Scholar] [CrossRef]
- Laurent-Puig, P.; Cayre, A.; Manceau, G.; Buc, E.; Bachet, J.-B.; Lecomte, T.; Rougier, P.; Lievre, A.; Landi, B.; Boige, V.; et al. Analysis of PTEN, BRAF, and EGFR Status in Determining Benefit from Cetuximab Therapy in Wild-Type KRAS. J. Clin. Oncol. 2009, 27, 5924–5930. [Google Scholar] [CrossRef] [PubMed]
- Lunenburg, C.A.; Henricks, L.M.; Guchelaar, H.-J.; Swen, J.J.; Deenen, M.J.; Schellens, J.H.; Gelderblom, H. Prospective DPYD genotyping to reduce the risk of fluoropyrimidine-induced severe toxicity: Ready for prime time. Eur. J. Cancer. 2016, 54, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Esquivel, A.; Chinchilla, R.; Valle, M. C677T and A1298C MTHFR gene polymorphisms and response to fluoropyrimidine-based chemotherapy in Mestizo patients with metastatic colorectal cancer. Anticancer. Res. 2020, 40, 4263–4270. [Google Scholar] [CrossRef] [PubMed]
- Ab Mutalib, N.; Md Yusof, N.; Abdul, S.; Jamal, R. Pharmacogenomics DNA Biomarkers in Colorectal Cancer: Current update. Front. Pharmacol. 2017, 8, 736. [Google Scholar] [CrossRef] [Green Version]
- Bandrés, E. Pharmacogenomics in colorectal cancer: The first step for individualized-therapy. World J. Gastroenterol. 2007, 13, 5888. [Google Scholar] [CrossRef]
- Funke, S.; Brenner, H.; Chang-Claude, J. Pharmacogenetics in colorectal cancer: A systematic review. Pharmacogenomics 2008, 9, 1079–1099. [Google Scholar] [CrossRef]
- Strimpakos, A.; Syrigos, K.; Saif, M. Pharmacogenetics and biomarkers in colorectal cancer. Pharm. J. 2009, 9, 147–160. [Google Scholar] [CrossRef] [Green Version]
- López-Cortés, A.; Paz-y-Miño, C.; Guerrero, S.; Jaramillo-Koupermann, G.; León Cáceres, Á.; Intriago-Baldeón, D.; García-Cárdenas, J.M.; Guevara-Ramírez, P.; Armendáriz-Castillo, I.; Leone, P.E.; et al. Pharmacogenomics, biomarker network, and allele frequencies in colorectal cancer. Pharm. J. 2019, 20, 136–158. [Google Scholar] [CrossRef] [Green Version]
- Olivera, G.; Sendra, L.; Herrero, M.; Puig, C.; Aliño, S. Colorectal cancer: Pharmacogenetics support for the correct drug prescription. Pharmacogenomics 2019, 20, 741–763. [Google Scholar] [CrossRef]
- Xie, P.; Mo, J.-L.; Liu, J.-H.; Li, X.; Tan, L.-M.; Zhang, W.; Zhou, H.-H.; Liu, Z.-Q. Pharmacogenomics of 5-fluorouracil in colorectal cancer: Review and update. Cell Oncol. 2020, 43, 989–1001. [Google Scholar] [CrossRef]
- Marsh, S.; Yu, J.; Hoskins, J. Colorectal cancer pharmacogenomics. Curr. Colorectal Cancer Rep. 2006, 2, 217–224. [Google Scholar] [CrossRef]
| Drug Combination | Treatment/Dose | Administration Day | Treatment Cycle |
|---|---|---|---|
| FOLFOX | Oxaliplatin 85 mg/m2 intravenous (IV) | 1 | 14 days |
| Leucovorin 400 mg/m2 IV | 1 | 14 days | |
| 5-FU 400 mg/m2 as IV bolus | 1 | 14 days | |
| 5-FU 1200 mg/m2/day as continuous IV infusion | 2, 3 | 14 days | |
| CapeOX | Oxaliplatin 130 mg/m2 for 2 h | 1 | 21 days |
| Capecitabine 1000 mg/m2 twice a day orally | 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14 | 21 days | |
| FOLFIRI | Irinotecan 180 mg/m2 during 30–90 min | 1 | 14 days |
| Leucovorin 400 mg/m2 IV at the same time as irinotecan | 1 | 14 days | |
| 5-FU 400 mg/m2 as IV bolus | 1 | 14 days | |
| 5-FU 1200 mg/m2/day as continuous IV infusion | 1, 2 | 14 days |
| Drug Combination | Treatment/Dose | Administration Day | Treatment Cycle |
|---|---|---|---|
| FOLFOX + Bevacizumab | FOLFOX | ||
| Bevacizumab 5 mg/kg IV | 1 | 14 days | |
| FOLFOX + Panitumumab * | FOLFOX | ||
| Panitumumab 6 mg/kg IV for 60 min | 1 | 14 days | |
| FOLFOX + Cetuximab * | FOLFOX | ||
| Cetuximab 500 mg/m2 IV for 2 h | 1 | 14 days | |
| CapeOX + Bevacizumab | CapeOX Bevacizumab 7.5 mg/kg IV | 1 | 21 days |
| FOLFIRI + Bevacizumab | FOLFIRI Bevacizumab 5 mg/kg IV | 1 | 14 days |
| FOLFIRI+ Cetuximab * | FOLFIRI Cetuximab 500 mg/m2 IV for 2 h | 1 | 14 days |
| FOLFIRI + Panitumumab * | FOLFIRI Panitumumab 6 mg/kg IV for 60 min | 1 | 14 days |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfaro Alfaro, Á.E.; Murillo Castillo, B.; Cordero García, E.; Tascón, J.; Morales, A.I. Colon Cancer Pharmacogenetics: A Narrative Review. Pharmacy 2022, 10, 95. https://doi.org/10.3390/pharmacy10040095
Alfaro Alfaro ÁE, Murillo Castillo B, Cordero García E, Tascón J, Morales AI. Colon Cancer Pharmacogenetics: A Narrative Review. Pharmacy. 2022; 10(4):95. https://doi.org/10.3390/pharmacy10040095
Chicago/Turabian StyleAlfaro Alfaro, Álvaro Esteban, Brayan Murillo Castillo, Eugenia Cordero García, Javier Tascón, and Ana I. Morales. 2022. "Colon Cancer Pharmacogenetics: A Narrative Review" Pharmacy 10, no. 4: 95. https://doi.org/10.3390/pharmacy10040095
APA StyleAlfaro Alfaro, Á. E., Murillo Castillo, B., Cordero García, E., Tascón, J., & Morales, A. I. (2022). Colon Cancer Pharmacogenetics: A Narrative Review. Pharmacy, 10(4), 95. https://doi.org/10.3390/pharmacy10040095







