Need for Expansion of Pharmacy Education Globally for the Growing Field of Nanomedicine
Abstract
1. Introduction
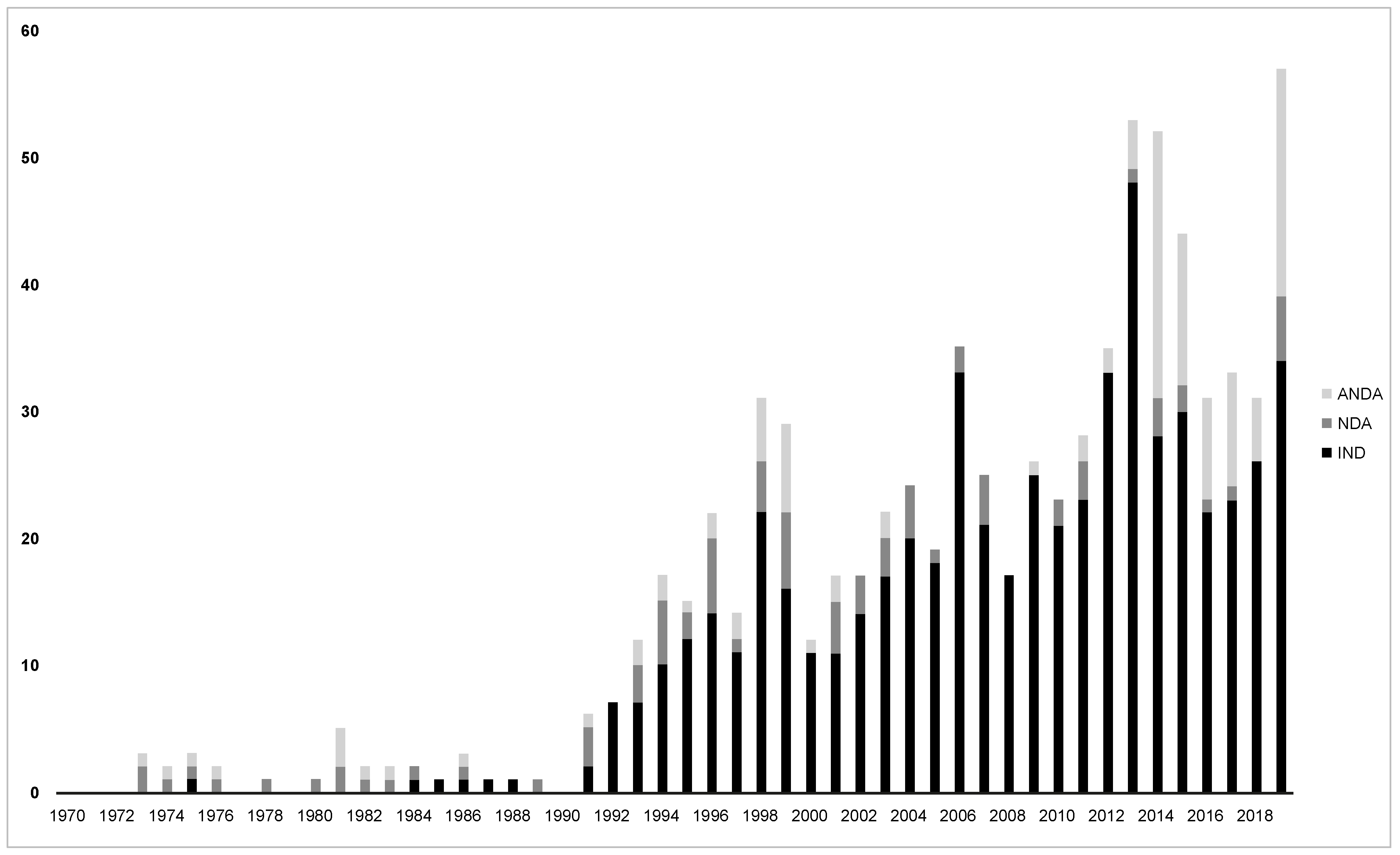
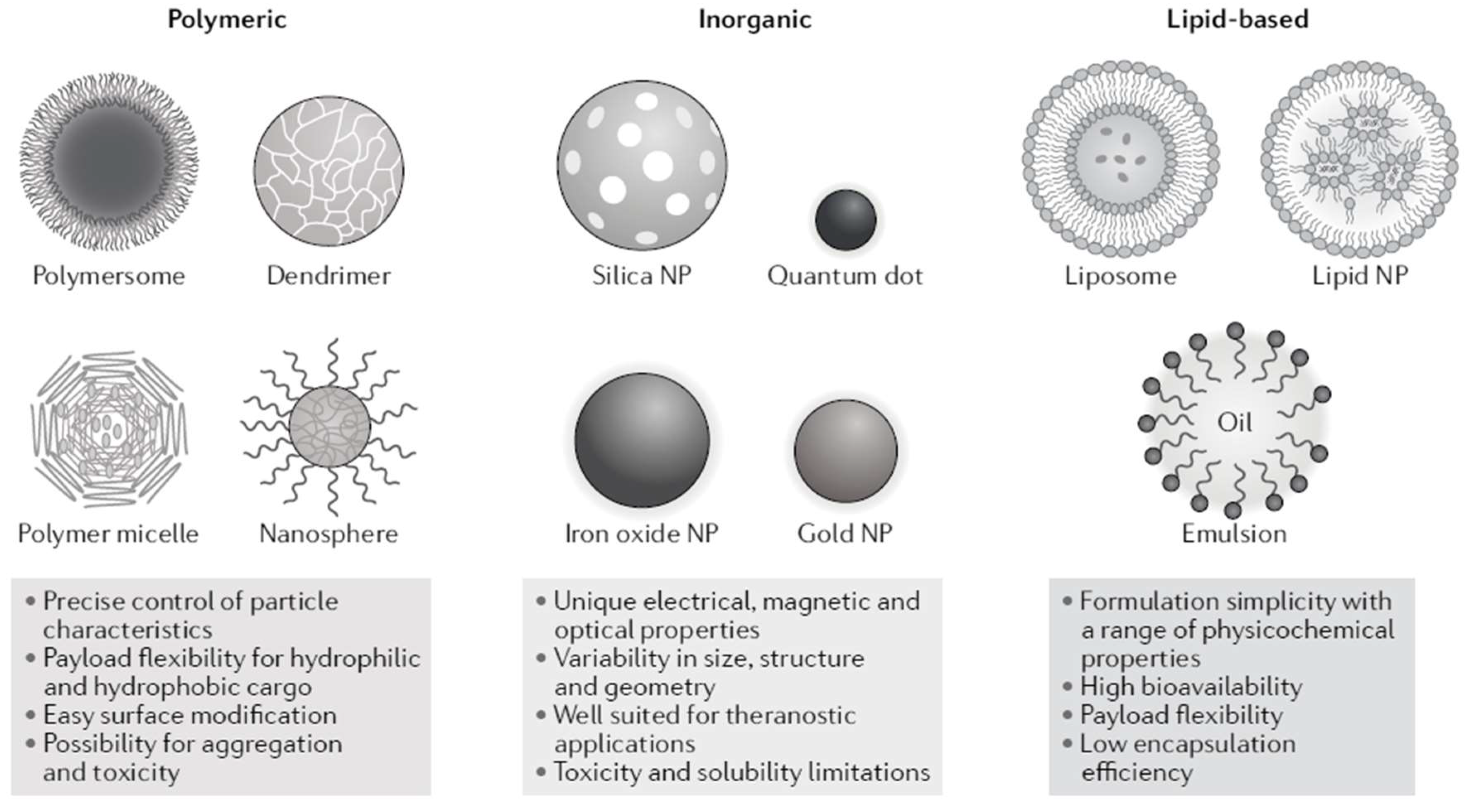
2. The Nanomedicine Science Domain
3. Challenges in Regulatory Evaluation of Nanomedicines
4. Nanomedicine Coverage in Pharmacy Education
5. Opportunities to Incorporate Nanomedicine Educational Content: A Global Snapshot
5.1. Studies in Pharmacy and Pharmaceutical Sciences in Switzerland
Nanomedicine Education at the University of Geneva’s School of Pharmacy
5.2. Studies in Pharmacy and the Pharmaceutical Sciences in Singapore
Nanomedicine Education at NUS
- To acquire the knowledge and critically examine the innovative approaches taken by pharmaceutical industries and scientists to develop efficient drug delivery systems and/or diagnostic devices.
- To present a selected topic and share own knowledge on nanomedicine through small group discussions.
- To be able to summarize key information and data from the literature in a concise, relevant, and conducive manner.
- (1)
- Availability of a small class (usually less than 50 students), which enables the easier organization of students and teaching material; and
- (2)
- The intention is to have all students participate in discussions: in fact, even students that are sitting outside the semi-circles might be asked for their opinion at any point in time. This is perceived as particularly beneficial when we want to discuss controversial or difficult topics.
5.3. Studies in Pharmacy and the Pharmaceutical Sciences in the United Kingdom
5.4. Studies in Pharmacy and the Pharmaceutical Sciences in the United States
Midwestern University, Glendale, Arizona
5.5. Studies in Pharmacy and the Pharmaceutical Sciences in China
6. Summary on the Importance of Nanomedicine Education for Future Pharmacists
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- US_FDA. Nanotechnology—Over a Decade of Progress and Innovation. 2020. Available online: https://www.fda.gov/media/140395/download (accessed on 21 February 2021).
- Ventola, C.L. Progress in nanomedicine: Approved and Investigational nanodrugs. Pharm. Ther. 2017, 42, 742–755. [Google Scholar]
- Duncan, R.; Gaspar, R. Nanomedicine(s) under the microscope. Mol. Pharm. 2011, 8, 2101–2141. [Google Scholar] [CrossRef] [PubMed]
- European_Medicines_Agency. Reflection Paper on Surface Coatings: General Issues for Consideration Regarding Parenteral Administration of Coated Nanomedicine Products. 2013. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-surface-coatings-general-issues-consideration-regarding-parenteral-administration_en.pdf (accessed on 21 February 2021).
- US_FDA. Drug Products, Including Biological Products, that Contain Nanomaterials Guidance for Industry. 2017. Available online: https://www.fda.gov/files/drugs/published/Drug-Products--Including-Biological-Products--that-Contain-Nanomaterials---Guidance-for-Industry.pdf (accessed on 21 February 2021).
- D’Mello, S.R.; Cruz, C.N.; Chen, M.L.; Kapoor, M.; Lee, S.L.; Tyner, K.M. The evolving landscape of drug products containing nanomaterials in the United States. Nat. Nanotechnol. 2017, 12, 523–529. [Google Scholar] [CrossRef]
- Marques, M.R.C.; Choo, Q.; Ashtikar, M.; Rocha, T.C.; Bremer-Hoffmann, S.; Wacker, M.G. Nanomedicines-Tiny particles and big challenges. Adv. Drug Deliv. Rev. 2019, 151–152, 23–43. [Google Scholar] [CrossRef]
- Wacker, M.G. Nanotherapeutics—Product development along the “nanomaterial” discussion. J. Pharm. Sci. 2014, 103, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Wacker, M.G.; Proykova, A.; Santos, G.M.L. Dealing with nanosafety around the globe-Regulation vs. innovation. Int. J. Pharm. 2016, 509, 95–106. [Google Scholar] [CrossRef]
- Crommelin, D.J.A.; van Hoogevest, P.; Storm, G. The role of liposomes in clinical nanomedicine development. What now? Now what? J. Control. Release 2020, 318, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Barenholz, Y. Doxil(R)—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Nanomedicine and the COVID-19 vaccines. Nat. Nanotechnol. 2020, 15, 963. Available online: https://www.nature.com/articles/s41565-020-00820-0 (accessed on 21 February 2021). [CrossRef]
- Junghanns, J.U.; Muller, R.H. Nanocrystal technology, drug delivery and clinical applications. Int. J. Nanomed. 2008, 3, 295–309. [Google Scholar]
- Geisser, P.; Burckhardt, S. The pharmacokinetics and pharmacodynamics of iron preparations. Pharmaceutics 2011, 3, 12–33. [Google Scholar] [CrossRef]
- Marden, E.; Ntai, I.; Bass, S.; Fluhmann, B. Correction to: Reflections on FDA draft guidance for products containing nanomaterials: Is the abbreviated new drug application (ANDA) a suitable pathway for nanomedicines? AAPS J. 2018, 20, 104. [Google Scholar] [CrossRef]
- Wittayanukorn, S.; Rosenberg, M.; Schick, A.; Hu, M.; Wang, Z.; Babiskin, A.; Lionberger, R.; Zhao, L. Factors that have an impact on abbreviated new drug application (ANDA) submissions. Ther. Innov. Regul. Sci. 2020, 54, 1372–1381. [Google Scholar] [CrossRef]
- Halamoda Kenzaoui, B.; Box, H.; Van Elk, M.; Gaitan, S.; Geertsma, R.; Gainza Lafuente, E.; Owen, A.; Del Pozo, A.; Roesslein, M.; Bremer, S. Anticipation of Regulatory Needs for Nanotechnology-Enabled Health Products; Publications Office of the European Union: Luxembourg, 2019. [Google Scholar]
- de Vlieger, J.S.; Crommelin, D.J.; Tyner, K.; Drummond, D.C.; Jiang, W.; McNeil, S.E.; Neervannan, S.; Crist, R.M.; Shah, V.P. Report of the AAPS guidance forum on the FDA draft guidance for industry: “Drug products, including biological products, that contain nanomaterials”. AAPS J. 2019, 21, 56. [Google Scholar] [CrossRef] [PubMed]
- Nikravesh, N.; Borchard, G.; Hofmann, H.; Philipp, E.; Fluhmann, B.; Wick, P. Factors influencing safety and efficacy of intravenous iron-carbohydrate nanomedicines: From production to clinical practice. Nanomedicine 2020, 26, 102178. [Google Scholar] [CrossRef] [PubMed]
- Nothnagel, L.; Wacker, M.G. How to measure release from nanosized carriers? Eur. J. Pharm. Sci. 2018, 120, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, S.; Braner, S.; Modh, H.; Tan, A.X.X.; Mast, M.P.; Chichakly, K.; Albrecht, V.; Wacker, M.G. A physiologically-based nanocarrier biopharmaceutics model to reverse-engineer the in vivo drug release. Eur. J. Pharm. Biopharm. 2020, 153, 257–272. [Google Scholar] [CrossRef] [PubMed]
- Pai, A.B. Evaluating plasma pharmacokinetics of intravenous iron formulations: Judging books by their covers? Clin. Pharm. 2015, 54, 323–324. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alphandery, E. Iron oxide nanoparticles for therapeutic applications. Drug Discov. Today 2020, 25, 141–149. [Google Scholar] [CrossRef]
- Muhlebach, S. Regulatory challenges of nanomedicines and their follow-on versions: A generic or similar approach? Adv. Drug Deliv. Rev. 2018, 131, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Klein, K.; Stolk, P.; De Bruin, M.L.; Leufkens, H.G.M.; Crommelin, D.J.A.; De Vlieger, J.S.B. The EU regulatory landscape of non-biological complex drugs (NBCDs) follow-on products: Observations and recommendations. Eur. J. Pharm. Sci. 2019, 133, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Weissig, V.; Elbayoumi, T.; Fluhmann, B.; Barton, A. The growing field of nanomedicine and its relevance to pharmacy curricula. Am. J. Pharm. Educ. 2021, 85, 8331. [Google Scholar] [CrossRef]
- Hertig, J.B.; Shah, V.P.; Flühmann, B.; Mühlebach, S.; Stemer, G.; Surugue, J.; Moss, R.; Di Francesco, T. Tackling the challenges of nanomedicines: Are we ready? Am. J. Health Syst. Pharm. 2021, 78, 1047–1056. [Google Scholar] [CrossRef] [PubMed]
- Weissig, V.; Guzman-Villanueva, D. Nanopharmaceuticals (part 2): Products in the pipeline. Int. J. Nanomed. 2015, 10, 1245–1257. [Google Scholar] [CrossRef]
- das Neves, J. Nanotechnology inclusion in pharmaceutical sciences education in portugal. Am. J. Pharm. Educ. 2018, 82, 6403. [Google Scholar] [CrossRef]
- Catalogue Des Objectifs De Formation En Pharmacie 2016. 2016. Available online: https://www.bag.admin.ch/dam/bag/fr/dokumente/berufe-gesundheitswesen/medizinalberufe/eidg-pruefungen-universitaerer-medizinalberufe/pharmazie/lernzielkatalog-pharmazie1.pdf.download.pdf/lernzielkatalog-pharmazie-version-2.pdf (accessed on 21 February 2021).
- QS_Rankings. World University Rankings. 2021. Available online: https://www.topuniversities.com/universities/national-university-singapore-nus (accessed on 21 February 2021).
- General_Pharmaceutical_Council. Standards for the Initial Education and Training of Pharmacists; General_Pharmaceutical_Council: London, UK, 2021. [Google Scholar]
- Quality Assurance Agency for Higher Education. The Frameworks for Higher Education Qualifications of UK Degree-Awarding Bodies; Quality Assurance Agency for Higher Education: Gloucester, UK, 2014. [Google Scholar]
- Lewis, G. Another 65 Pharmacy-Led Sites Join COVID-19 Vaccination Programme. Available online: https://www.chemistanddruggist.co.uk/CD006956/Another-65-pharmacy-led-sites-join-COVID-19-vaccination-programme (accessed on 21 February 2021).
- Greene, J.M.; Fuller, K.A.; Persky, A.M. Practical tips for integrating clinical relevance into foundational science courses. Am. J. Pharm. Educ. 2018, 82, 6603. [Google Scholar] [CrossRef]
- Brown, K.P.; Raccor, B.S.; Hilgers, R.H.; Breivogel, C.S. Interdisciplinary pharmaceutical sciences activity within a pharmacy practice skills course. Curr. Pharm. Teach. Learn. 2019, 11, 270–276. [Google Scholar] [CrossRef]
- AACP. American Association of Colleges of Pharmacy Strategic Plan. Available online: https://www.aacp.org/sites/default/files/2022-01/aacp-strategic-plan--2021-2024.pdf (accessed on 21 February 2021).
- Al-Nemrawi, N.K.; AbuAlSamen, M.M.; Alzoubi, K.H. Awareness about nanotechnology and its applications in drug industry among pharmacy students. Curr. Pharm. Teach. Learn. 2020, 12, 274–280. [Google Scholar] [CrossRef]
- Maerten-Rivera, J.L.; Chen, A.M.; Augustine, J.; d’Assalenaux, R.; Lee, K.C.; Lindsey, C.C.; Malcom, D.R.; Mauro, L.S.; Pavuluri, N.; Rudolph, M.J.; et al. Co-curriculum implementation and assessment in accredited doctor of pharmacy programs. Am. J. Pharm. Educ. 2020, 84, 7569. [Google Scholar] [CrossRef]
- Shcherbakova, N. Pharmaceutical industry in a global context elective course: Implementation and preliminary outcomes. Int. J. Pharm. Pract. 2018, 26, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, V.; Goldman, D.; Wang, C.; Keller, M.; Chen, S. Looking ahead to 2030: Survey of evolving needs in pharmacy education. Pharmacy 2021, 9, 59. [Google Scholar] [CrossRef]
- Baines, D.; Norgaard, L.S.; Babar, Z.U.; Rossing, C. The fourth industrial revolution: Will it change pharmacy practice? Res. Social Adm. Pharm. 2020, 16, 1279–1281. [Google Scholar] [CrossRef] [PubMed]
- New, J.; Garner, S.; Ragucci, K.; Spencer, A. An advanced clinical track within a doctor of pharmacy program. Am. J. Pharm. Educ. 2012, 76, 43. [Google Scholar] [CrossRef] [PubMed]
- Piascik, P. CAPE outcomes 2013: Building on two decades of advances to guide the future of pharmacy education. Am. J. Pharm. Educ. 2013, 77, 160. [Google Scholar] [CrossRef]
| Adequacy of Characterization of the Material Structure and Its Function |
|---|
| Complexity of the material structure |
| Understanding the mechanism by which the physicochemical properties of the material impact its biological effects (e.g., effect of particle size on pharmacokinetic parameters) |
| Understanding the in vivo release mechanism based on the material physicochemical properties |
| Predictability of in vivo release based on established in vitro release methods |
| Physical and chemical stability |
| Maturity of the nanotechnology (including manufacturing and analytical methods) |
| Potential impact of manufacturing changes, including in-process controls and the robustness of the control strategy on critical quality attributes * of the drug product |
| Physical state of the material upon administration |
| Route of administration |
| Dissolution, bioavailability, distribution, biodegradation, accumulation, and their predictability based on physicochemical parameters and animal studies |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barton, A.E.; Borchard, G.; Wacker, M.G.; Pastorin, G.; Saleem, I.Y.; Chaudary, S.; Elbayoumi, T.; Zhao, Z.; Flühmann, B. Need for Expansion of Pharmacy Education Globally for the Growing Field of Nanomedicine. Pharmacy 2022, 10, 17. https://doi.org/10.3390/pharmacy10010017
Barton AE, Borchard G, Wacker MG, Pastorin G, Saleem IY, Chaudary S, Elbayoumi T, Zhao Z, Flühmann B. Need for Expansion of Pharmacy Education Globally for the Growing Field of Nanomedicine. Pharmacy. 2022; 10(1):17. https://doi.org/10.3390/pharmacy10010017
Chicago/Turabian StyleBarton, Amy E., Gerrit Borchard, Matthias G. Wacker, Giorgia Pastorin, Imran Y. Saleem, Shaqil Chaudary, Tamer Elbayoumi, Zhigang Zhao, and Beat Flühmann. 2022. "Need for Expansion of Pharmacy Education Globally for the Growing Field of Nanomedicine" Pharmacy 10, no. 1: 17. https://doi.org/10.3390/pharmacy10010017
APA StyleBarton, A. E., Borchard, G., Wacker, M. G., Pastorin, G., Saleem, I. Y., Chaudary, S., Elbayoumi, T., Zhao, Z., & Flühmann, B. (2022). Need for Expansion of Pharmacy Education Globally for the Growing Field of Nanomedicine. Pharmacy, 10(1), 17. https://doi.org/10.3390/pharmacy10010017









