Nytrox as “Drop-in” Replacement for Gaseous Oxygen in SmallSat Hybrid Propulsion Systems
Abstract
1. Introduction
2. Background on Green Propellant Options
2.1. Ionic Liquid Propellants
2.2. Hybrid Rockets as a Significantly Safer and More Efficient “Green” Propulsion Alternative
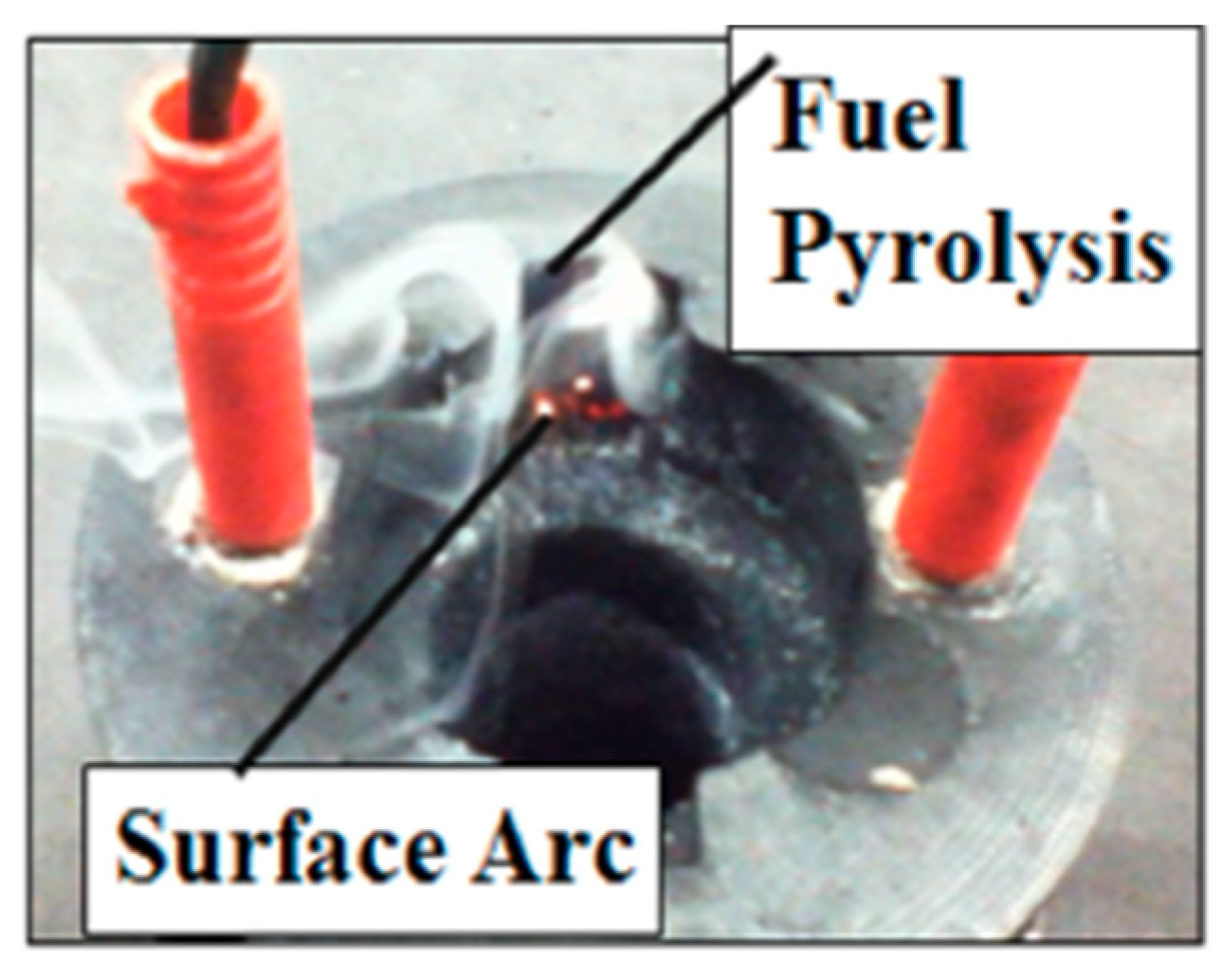
2.3. Hybrid Rocket Low-Power Arc-Ignition System
2.4. Considering the Best Options for “Green” Oxidizers for Hybrid Space Propulsion
2.5. Hazards Associated with Using Nitrous Oxide as a Hybrid Rocket Propellant
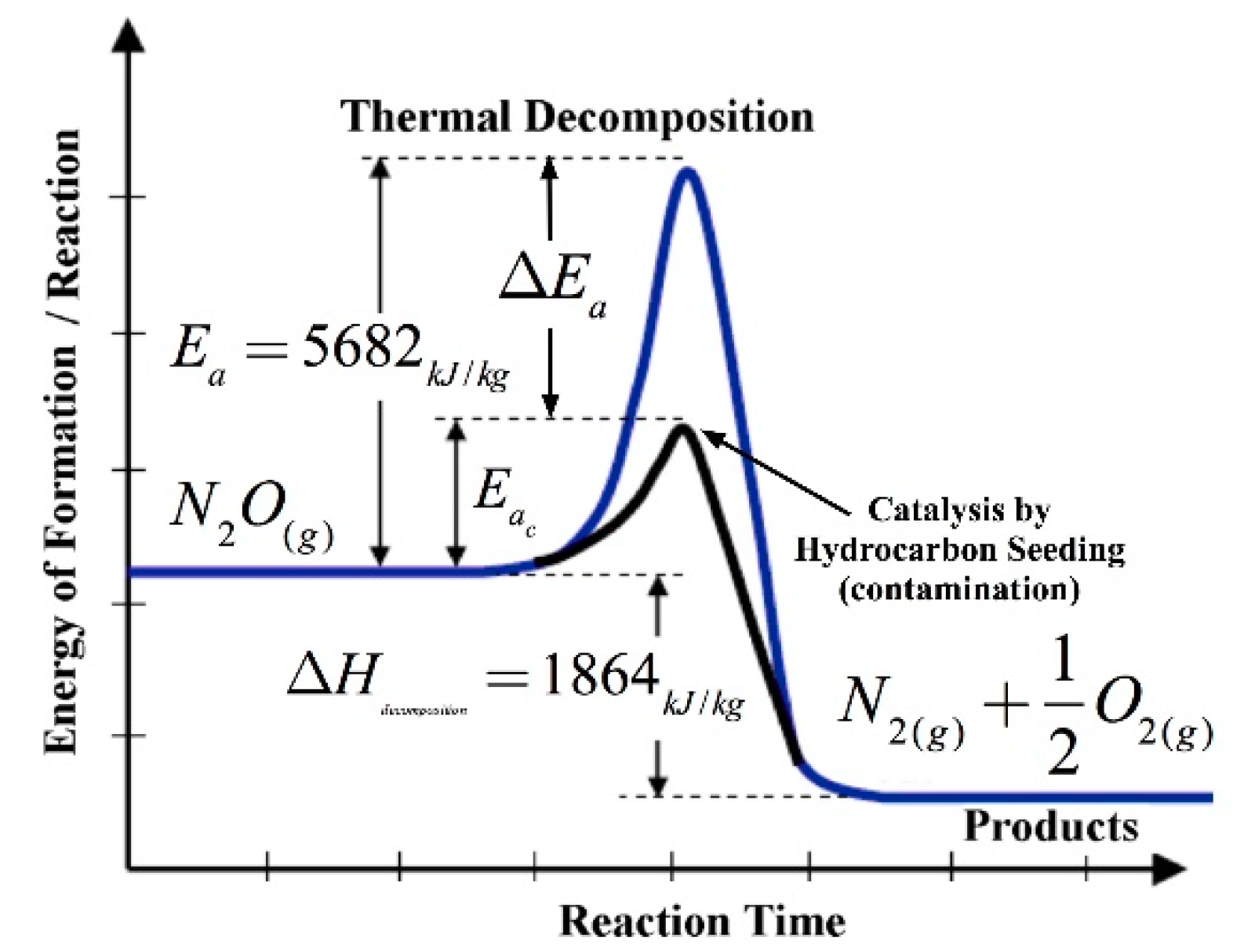
2.6. Mitigation of the N2O Decomposition Hazard
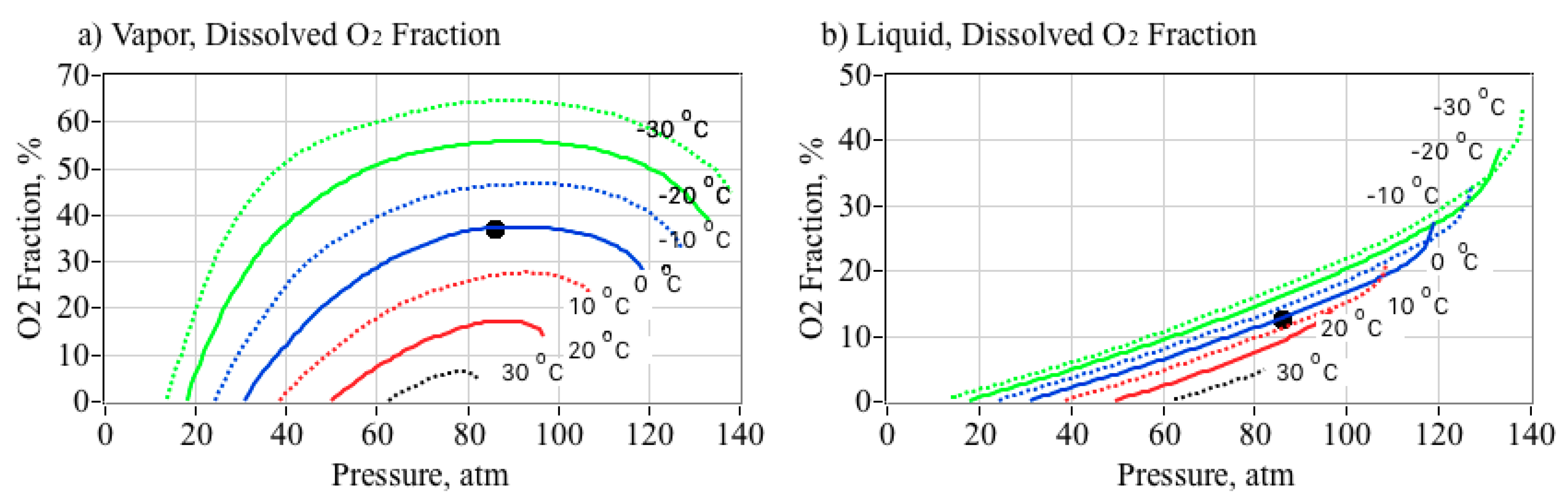
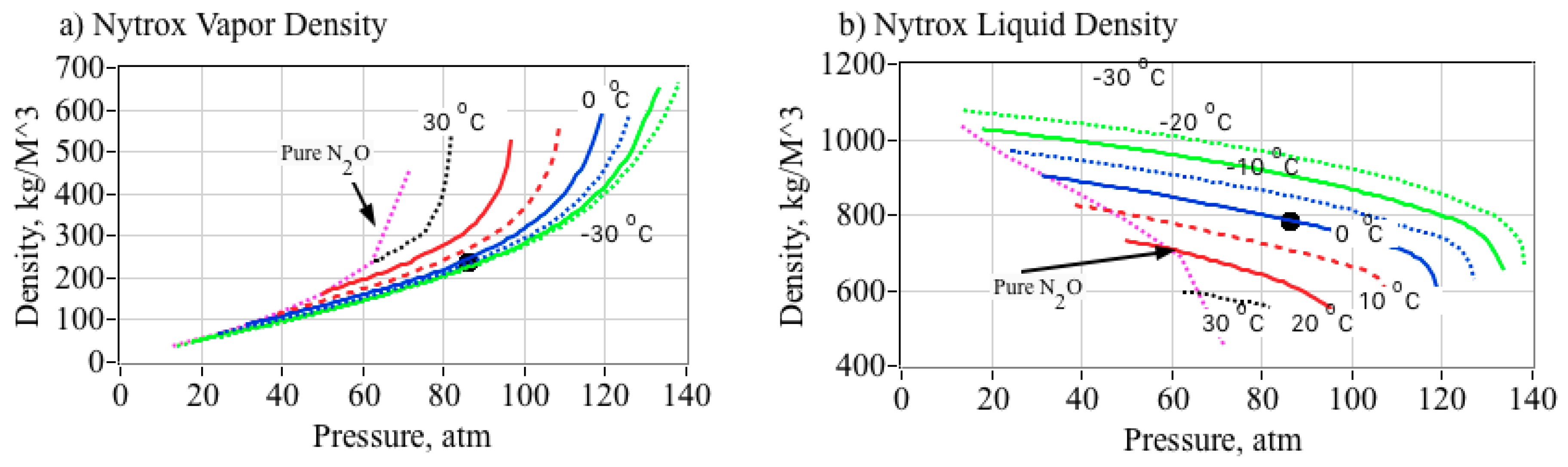
2.7. Effect of O2 in Solution Upon the Nytrox Mixture Density
2.8. Predicted Propulsion Performance of the Nytrox/ABS Hybrid
2.9. Summary of Nytrox Properties and Safety Advantages Compared to Pure Nitrous Oxide
- (1)
- Nytrox is much safer than pure N2O because vapor phase has significant O2 concentration, thereby increasing the minimum ignition energy Ei by three or four orders of magnitude,
- (2)
- The multiple order of magnitude increase in Ei using Nytrox allows for safe self-pressurization with high fluid densities.
- (3)
- Self-pressurization greatly simplifies the system design and eliminates the need for a heavy, separate pressurant system using helium or nitrogen.
- (4)
- Nytrox allows improved Isp performance compared to pure N2O.
- (5)
- From Figure 5b, at the optimal pressure level of 86 atmospheres, the Nytrox liquid density at 0 °C is higher than GOX by a factor approximately 6.4, and allows for a significant improvement in the overall volumetric efficiency of the propellants.
- (6)
- The optimal O/F ratio is significantly reduced when using Nytrox, allowing a larger proportion of the total impulse to be delivered by the high-density fuel component, in this case, ABS.
- (7)
- Maintaining the storage pressure near 80 atmospheres at 0 °C is essential to achieving best volumetric efficiency with Nytrox.
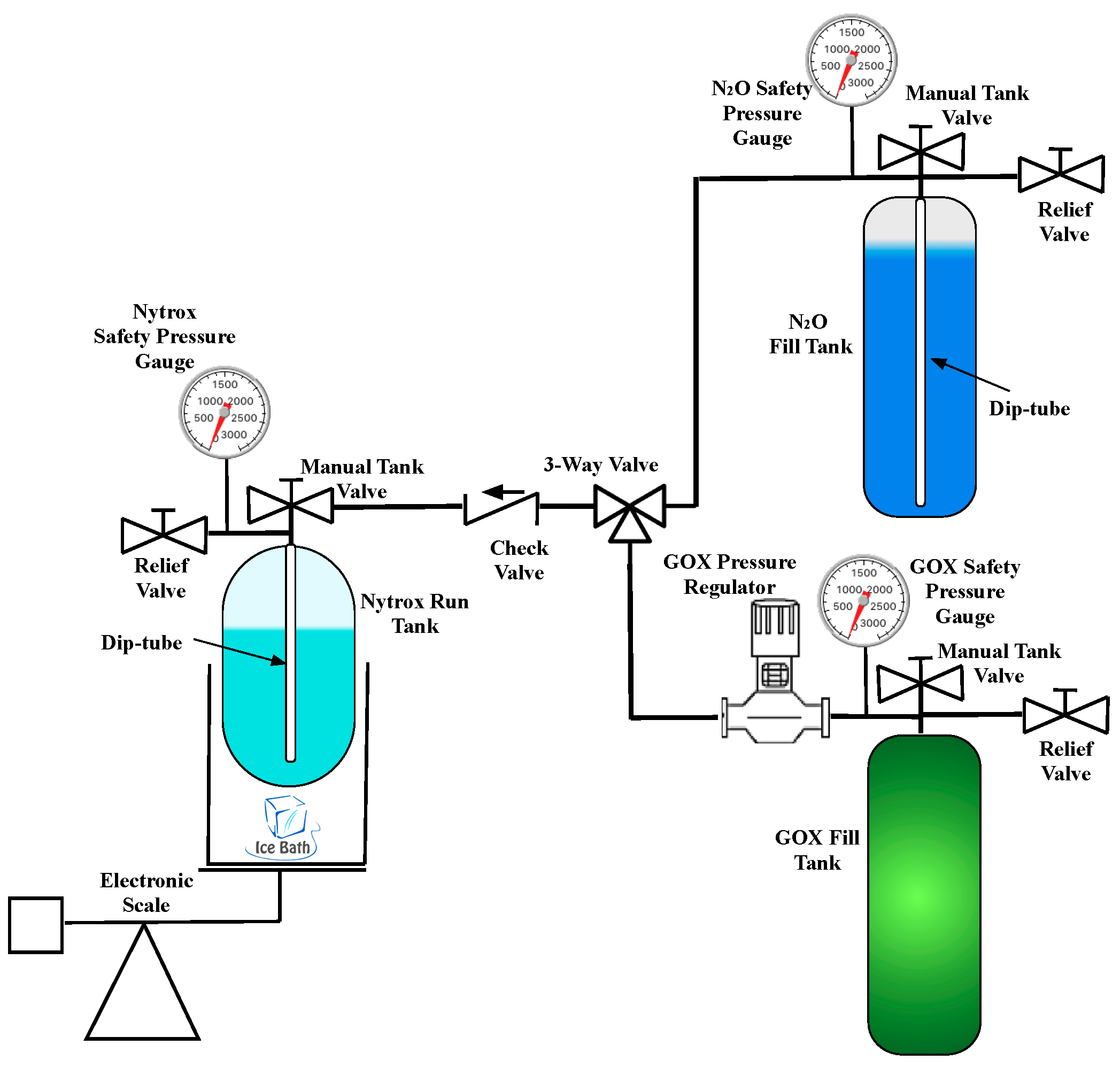
3. Experimental Apparatus, Instrumentation, and Test Procedures
3.1. Nytrox Solution Processing
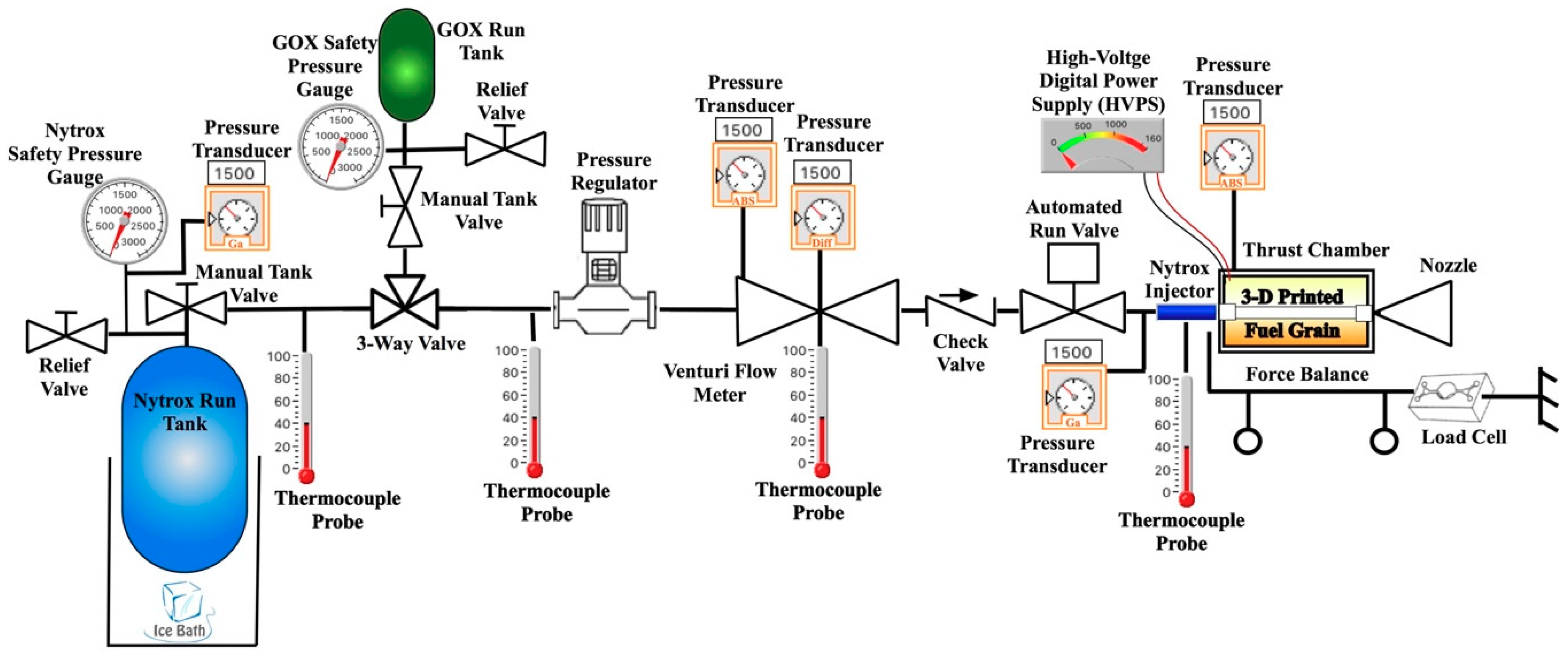
3.2. Hot-Fire Test Apparatus and Instrumentation
3.2.1. Thrust Chamber
3.2.2. Arc-Ignition System
3.2.3. Test Stand Apparatus and Instrumentation
3.3. Hot-Fire Test Procedures
4. Data Analysis Methods
4.1. Calculating the Fuel Mass flow Rate.
4.2. Calculating the Fuel Regression Rate
4.3. Calculating the Motor Performance Parameters
5. Results and Discussion
5.1. Making the Nytrox Mixture
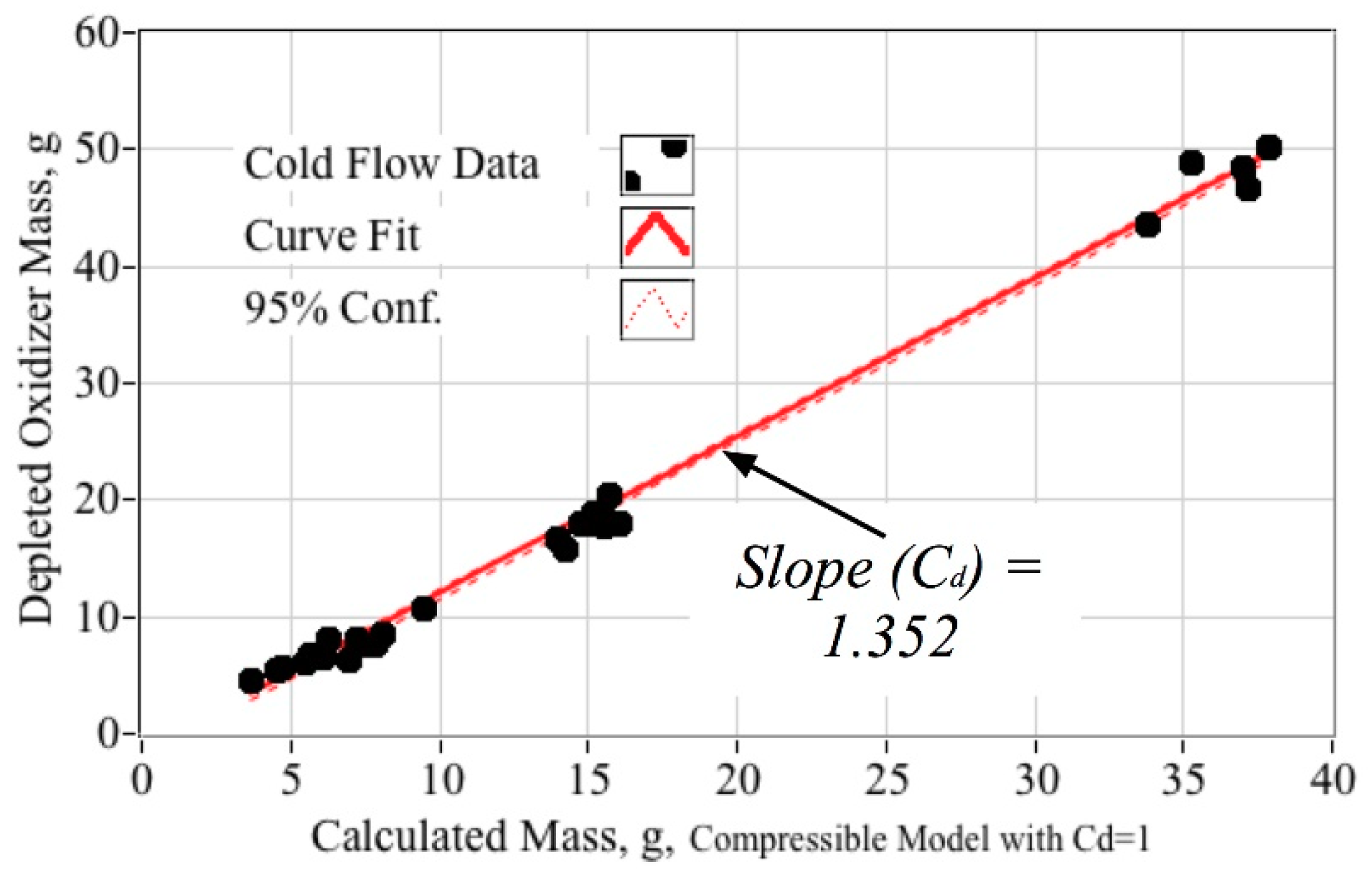
5.2. Oxidizer Venturi Calibration
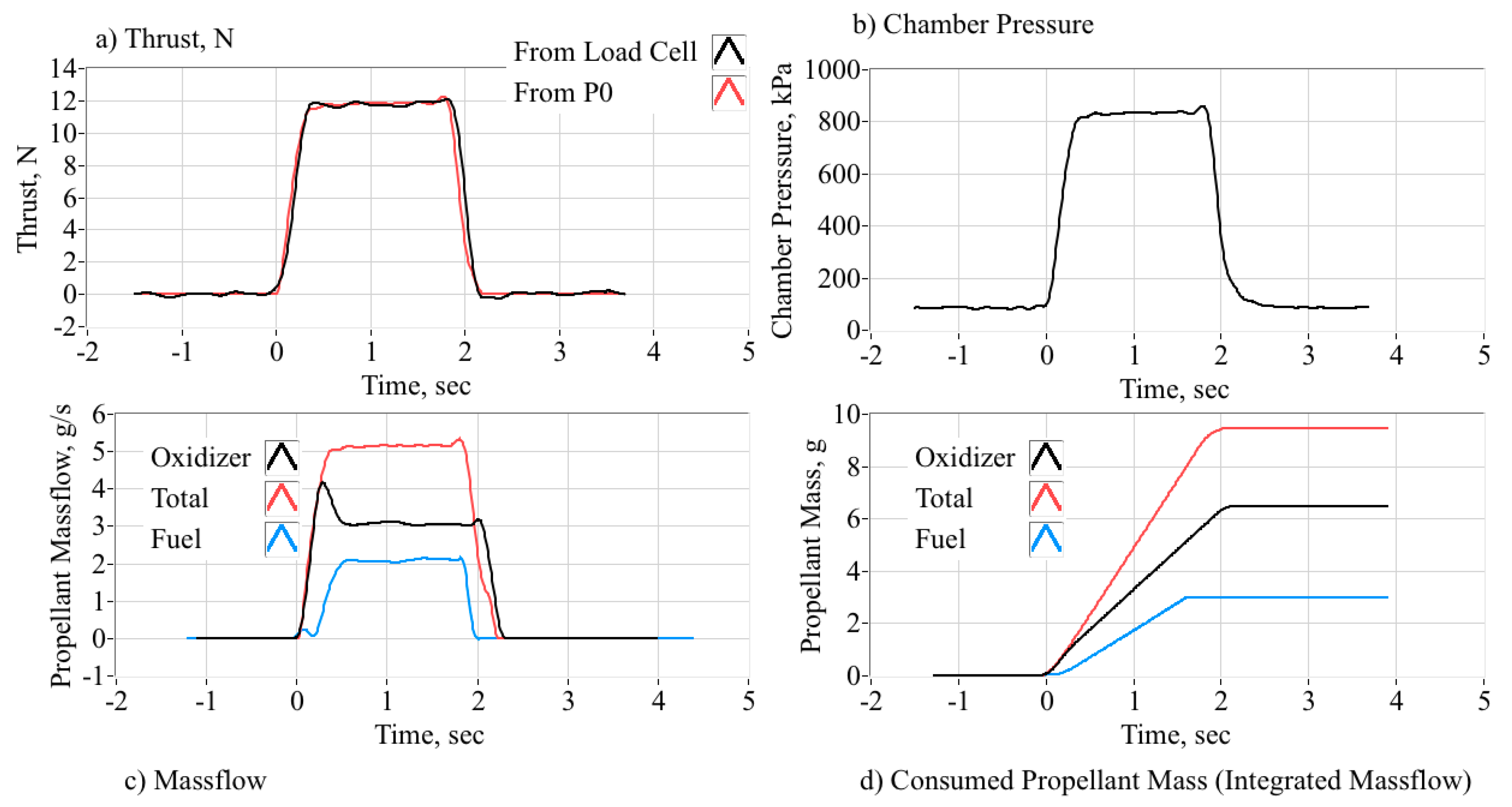
5.3. Baseline O2 Hot-Fire Test Summary
5.4. Nytrox 87 Hot-Fire Test Summary
5.5. GOX/Nytrox Burn Data Comparisons
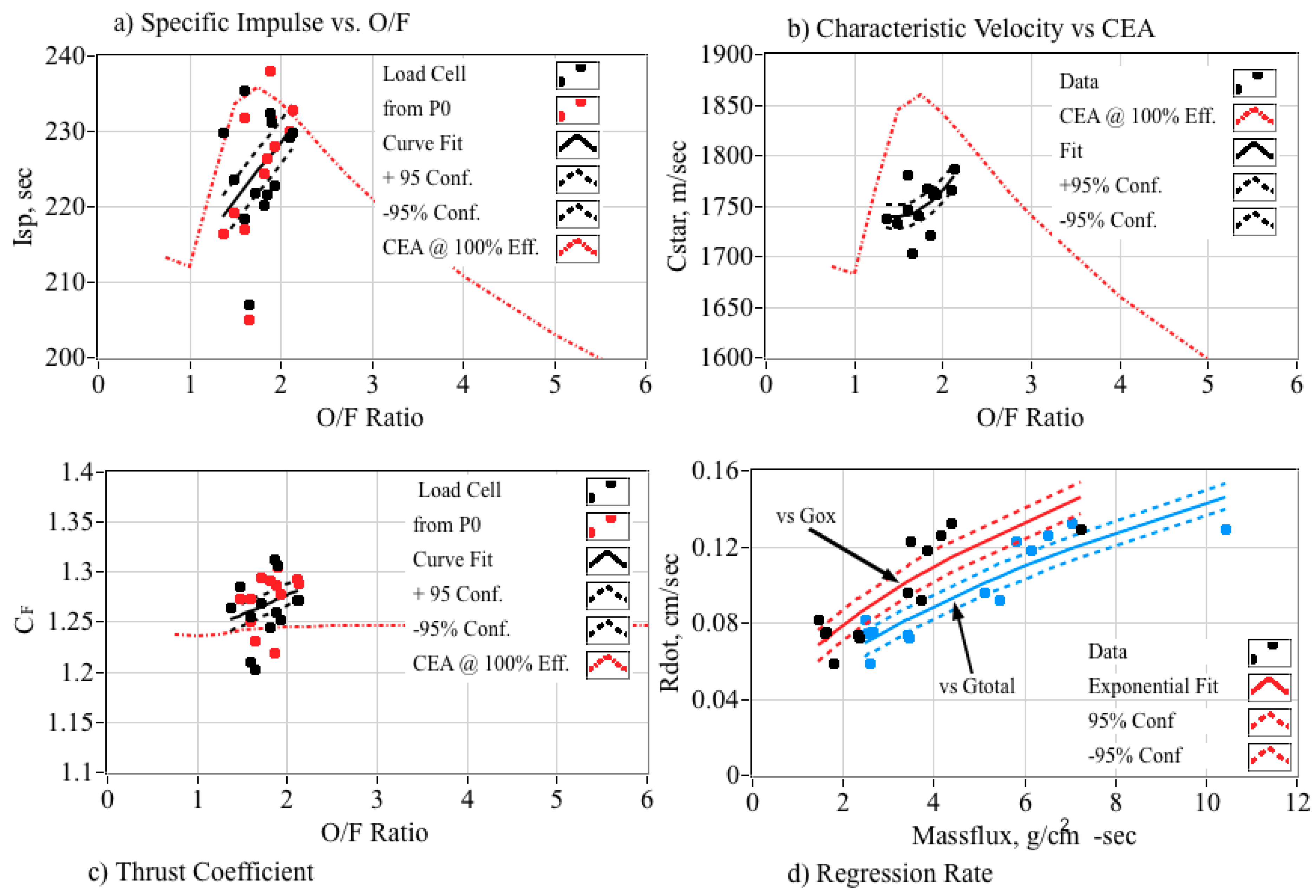
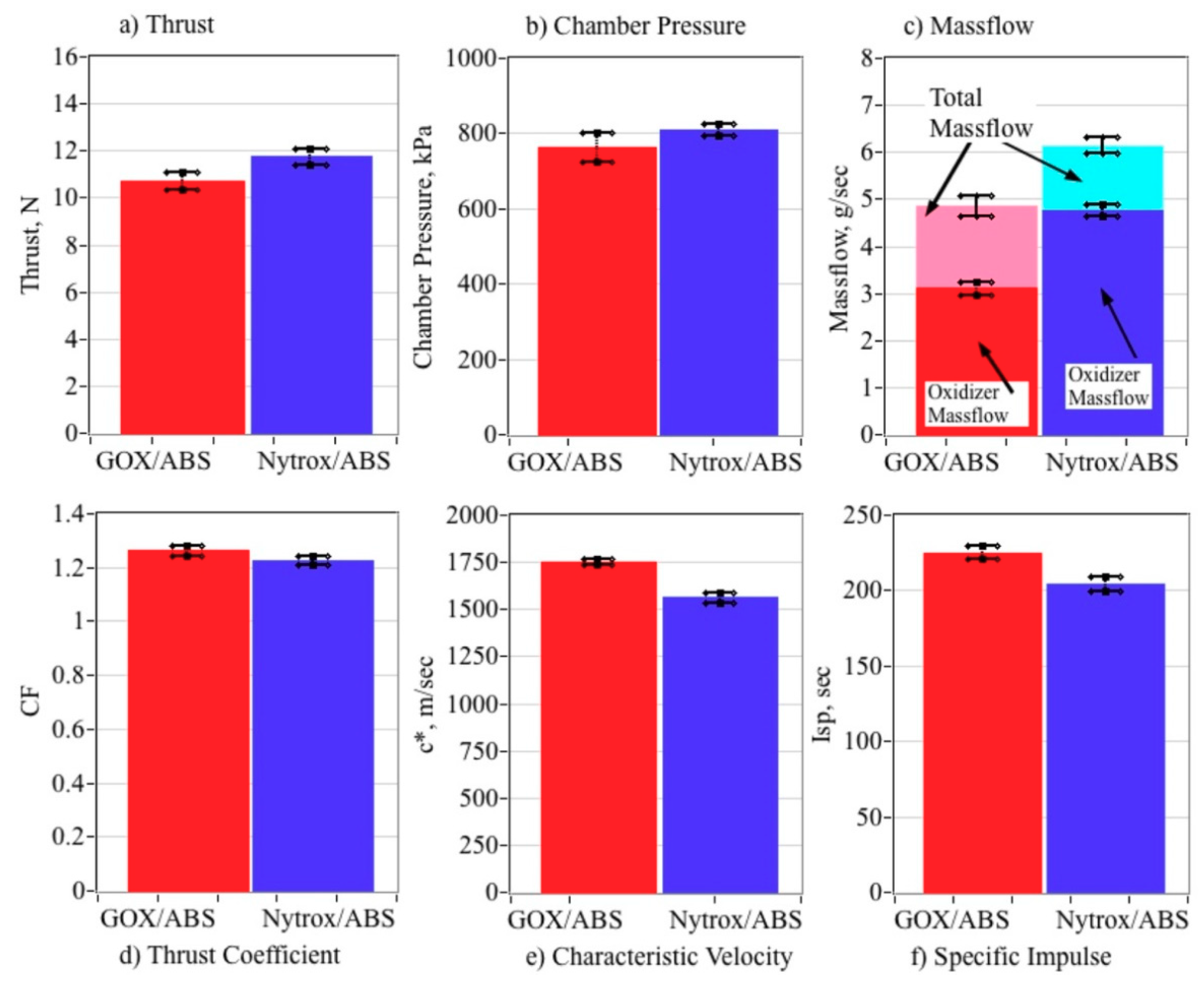
5.5.1. Top-Level Motor Performance Comparisons
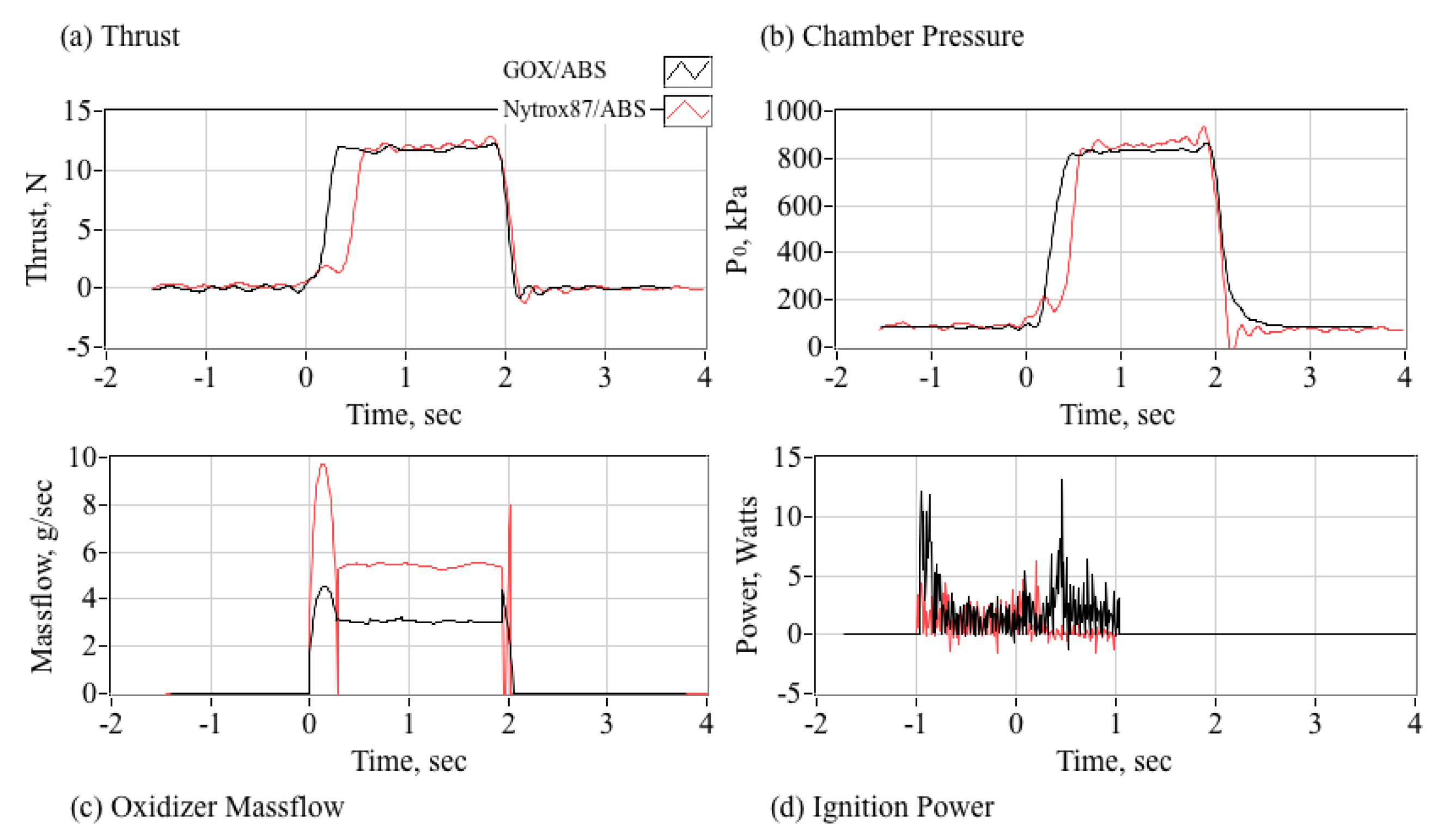
5.5.2. Comparing Combustion Properties
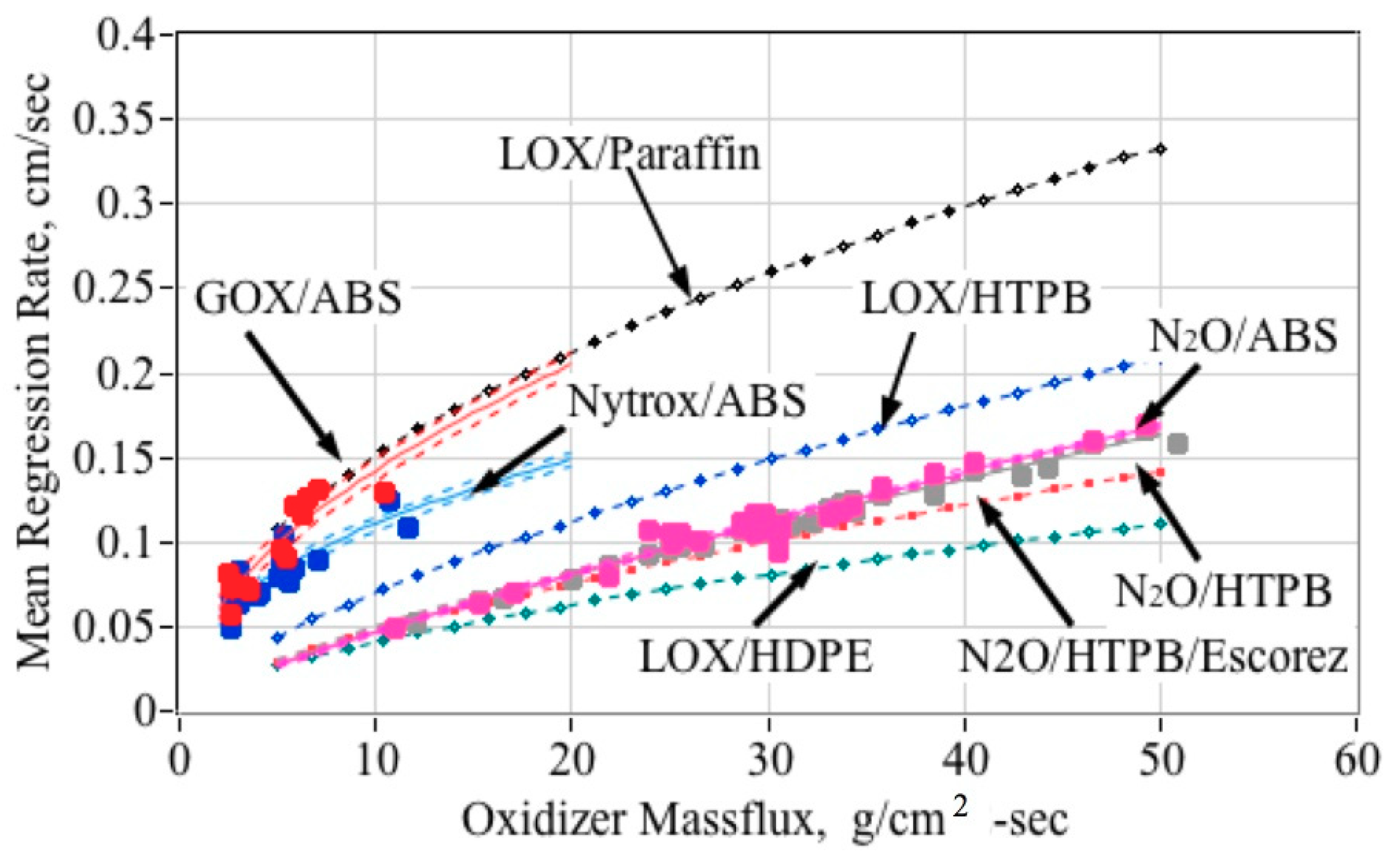
5.5.3. Detailed Fuel Regression Rate Comparisons
5.5.4. Volumetric Efficiency Comparisons
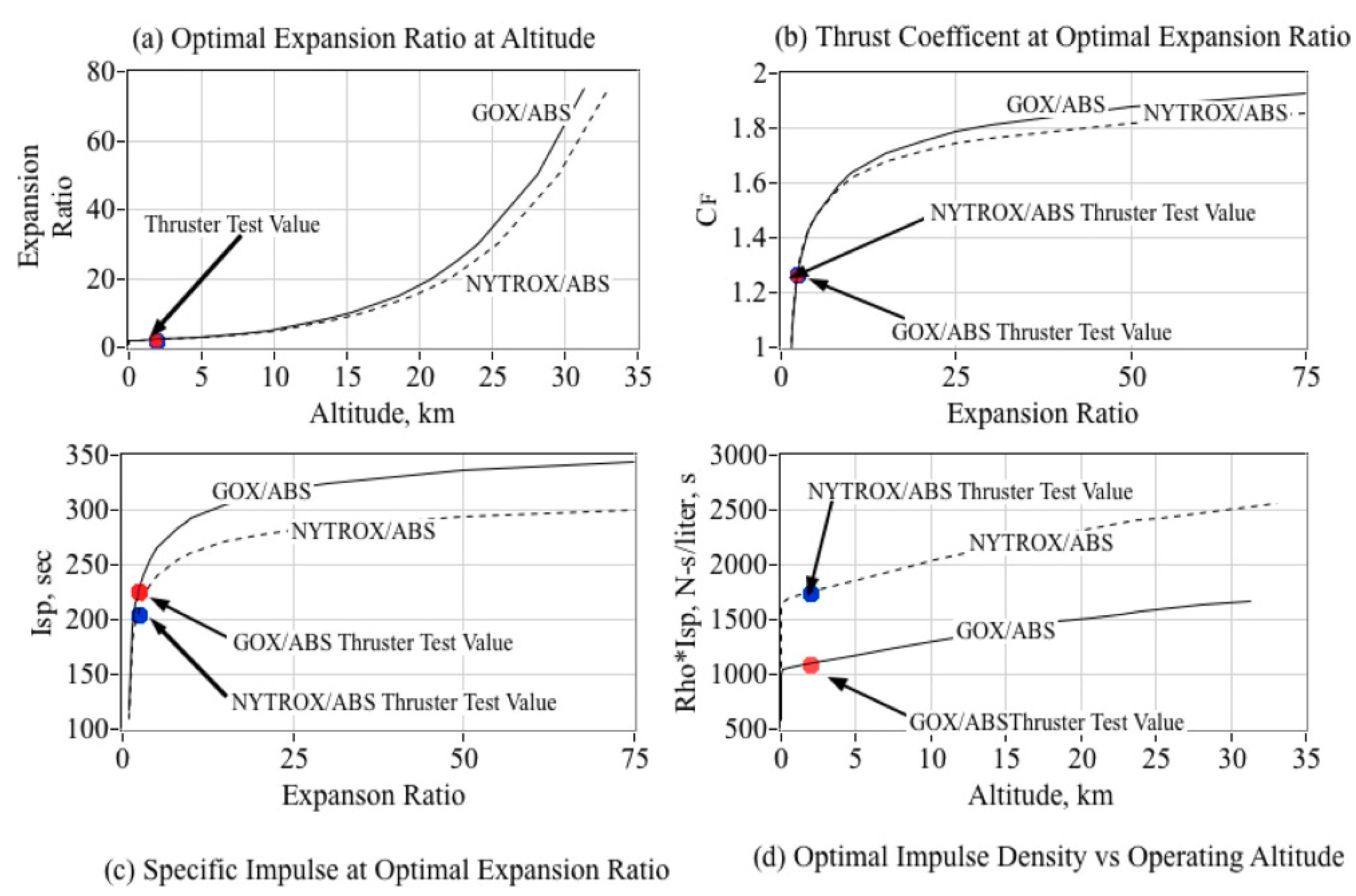
5.5.5. Extrapolating the Specific Impulse to Vacuum Conditions
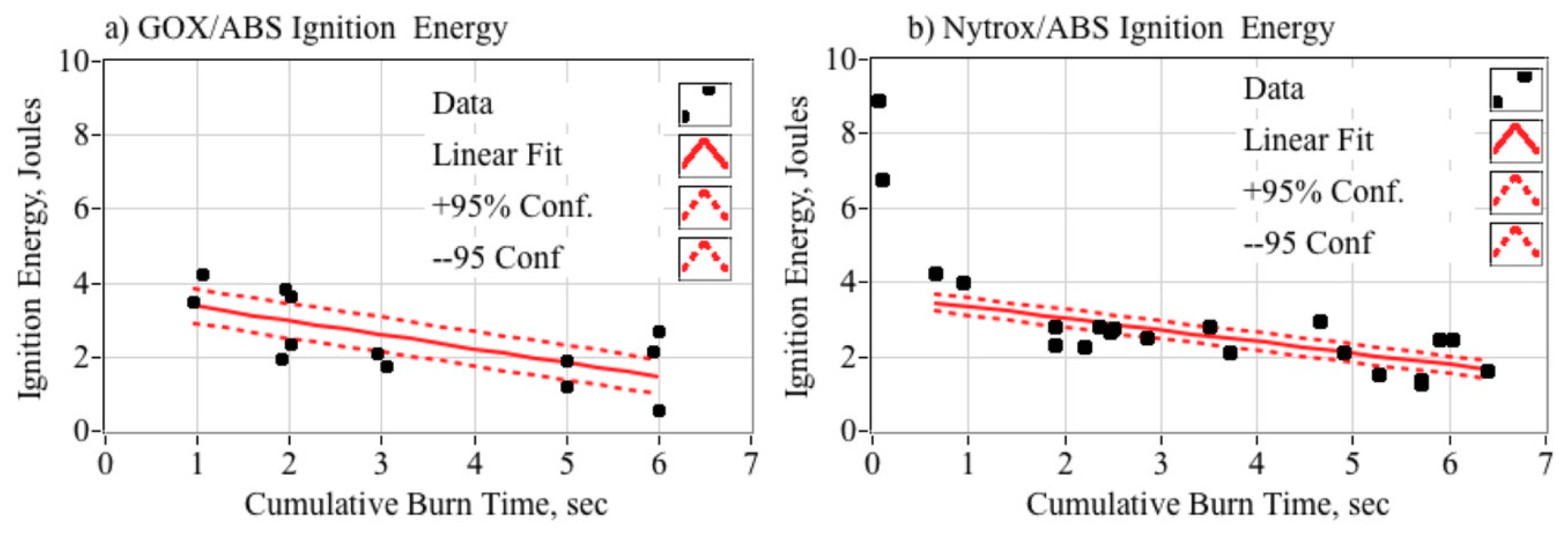
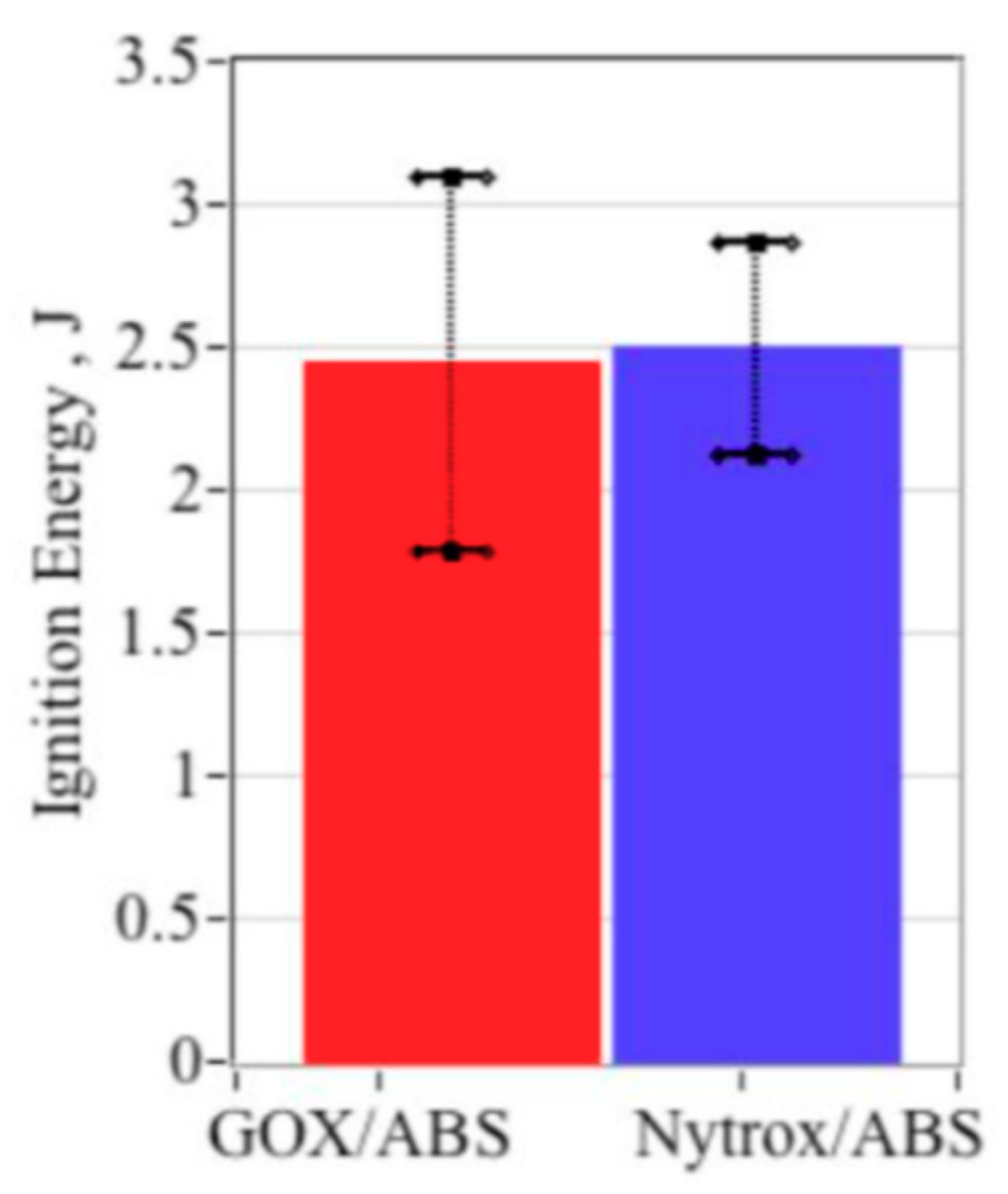
5.5.6. Required Ignition Energy
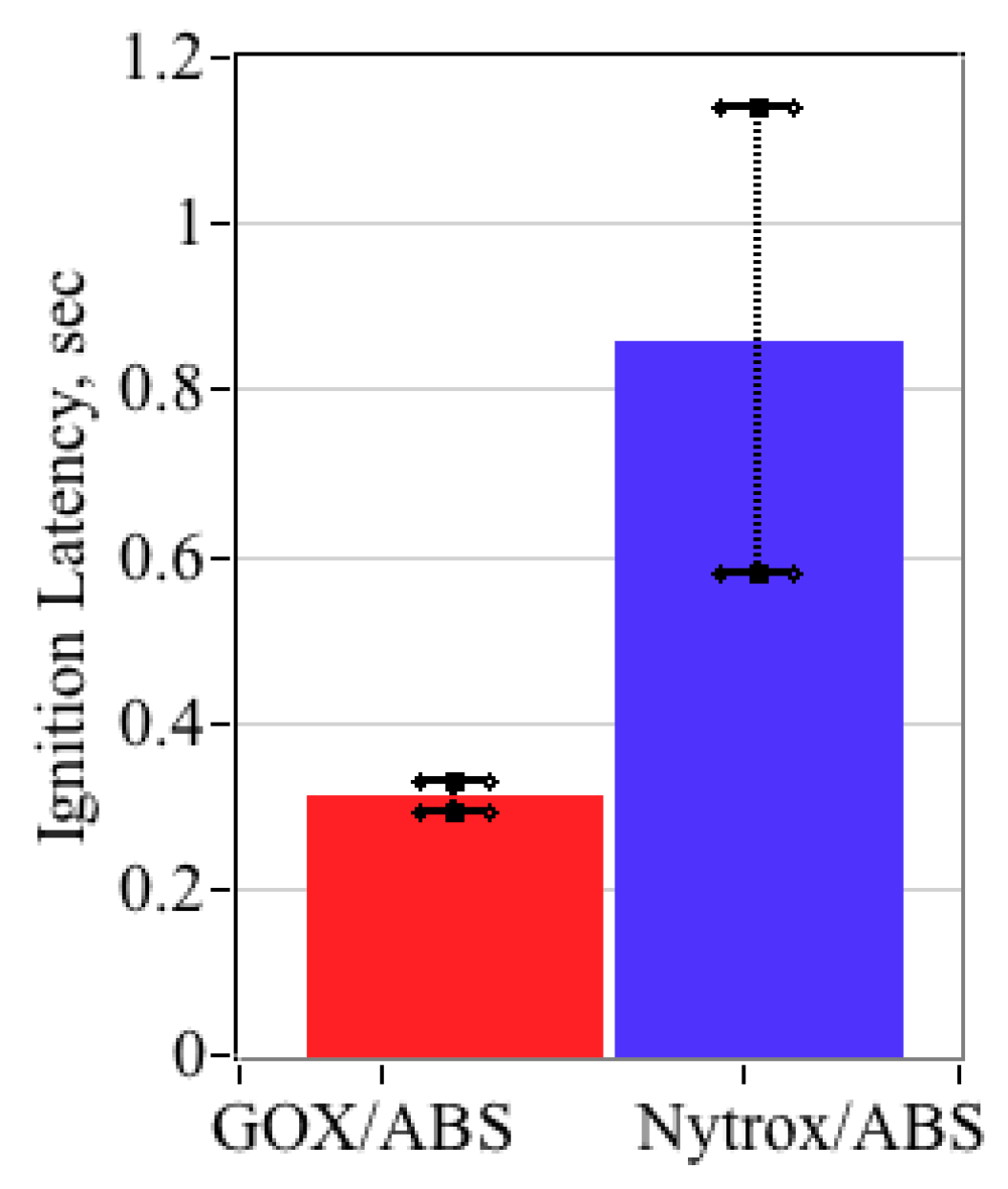
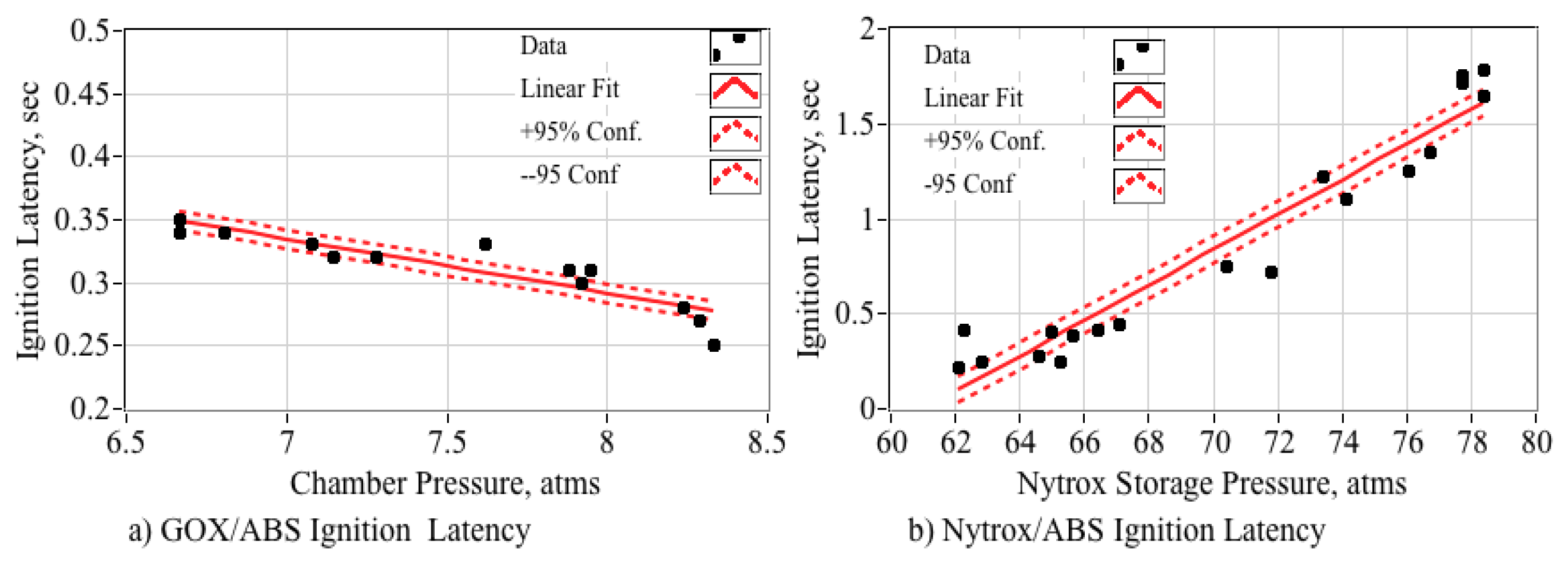
5.5.7. Ignition Latency
5.6. Comparing Nytrox to Existing Space Mono-Propellants
6. Summary and Conclusions
7. Patents
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| Ac | abfuel port cross-sectional area, cm2 |
| Aexit | abnozzle exit area, cm2 |
| A* | abcross sectional area at which local flow chokes, cm2 |
| A1 | abVenturi inlet area, cm2 |
| A2 | abVenturi throat area, cm2 |
| Aexit/A* | abnozzle expansion-ratio |
| Cd | abdischarge coefficient |
| CF | abthrust coefficient |
| abtime averaged thrust coefficient | |
| c* | abcharacteristic velocity of propellants, m/s |
| Ea | abactivation energy for self-sustaining decomposition reaction, kJ/kg |
| Eac | abactivation energy for contaminated solution, kJ/kg |
| Ea | abreduction of energy of activation due to catalysis or hydrocarbon contamination, kJ/kg |
| Ei | abminimum energy required to initiate a deflagration wave in Nytrox mixture, J |
| Eif | abignition energy amplification factor |
| F | abthrust, N |
| Gox | aboxidizer massflux, g/cm2·s |
| Gtot | abtotal massflux, g/cm2·s |
| g0 | abnominal acceleration of gravity, 9.8067 m/s |
| H | abenergy of reaction, kJ/kg |
| Isp | abspecific impulse, s |
| Lport | abfuel grain length, cm |
| Mw | abmolecular weight, g/g-mol |
| Mfuel | abconsumed fuel mass, g |
| Moxl | abconsumed oxidizer mass, g |
| mflue | abfuel mass flow, g/s |
| mox | aboxidizer mass flow, g/s |
| mtotal | abtotal mass flow through the nozzle, g/s |
| O/F | aboxidizer/fuel ratio |
| O/Factual | abactual oxidizer-to-fuel ratio |
| O/Fstoich | abstoichiometric oxidizer-to-fuel ratio |
| O/F | abtime-averaged oxidizer-to-fuel ratio. |
| P1 | abVenturi inlet pressure, psia |
| P2 | abVenturi throat pressure, psia |
| P0 | abchamber pressure or oxidizer flow stagnation pressure, psia |
| pexit | abexit plane static pressure, psia |
| p∞ | abambient pressure, psia |
| Rg | abgas constant, J/kg-K |
| Ru | abuniversal gas constant, 8314.4612 J/kg-mol-K |
| rL | ablongitudinal average of the fuel port radius, cm |
| r0 | abinitial fuel port radius, cm |
| ablongitudinal mean of fuel regression rate, cm/s | |
| abmean regression rate over burn duration, cm/s | |
| abdependent variable for regression rate as fit by power-law curve, cm/s | |
| sg | abspecific gravity with respect to weight of water. |
| T0 | abstagnation temperature, K |
| tburn | abburn time, s |
| t | abgeneric time symbol, s |
| Z | abcompressibility factor |
| μ | abmean value |
| η* | combustion efficiency |
| γ | abof specific heats |
| Θ | abequivalence ratio |
| ρuel | absolid fuel density, g/cm3 |
| ρIsp | abimpulse density, N·s/L standard deviation |
| ABS | abAcrylonitrile Butadiene Styrene |
| ADN | abAmmonium Dinitramide |
| AF-M315E | abHAN Based Ionic Liquid Green Propellant |
| AFWL | abAir Force Weapons Lab |
| ECAPS | abEcological Advanced Propulsion Systems |
| ESTEC | abEuropean Space Agency Space Research and Technology Center |
| FDM | abFused Deposition Modeling |
| GOX | abGaseous Oxygen |
| GPIM | abGreen Propellant Infusion Mission |
| HAN | abHydroxylamine Nitrate |
| HDPE | abHigh Density Polyethylene |
| HPGHP | abHigh-Performance “Green” Hybrid Propulsion |
| HTPB | abHydroxyl-Terminated Polybutadiene |
| HVPS | abHigh Voltage Power Supply |
| IL | abIonic Liquid |
| LMP-103S | abADN-Based Ionic Liquid “Green” Propellant |
| NOS® | abNitrous Oxide Systems (Trademark) |
| P&ID | abPiping and Instrumentation |
| TRL | abTechnology Readiness Level |
| TTL | abTransistor–Transistor Logic |
| USU-PRL | abUtah State University Propulsion Research Laboratory |
References
- Whitmore, S.A.; Bulcher, A.M. Vacuum Test of a Novel Green-Propellant Thruster for Small Spacecraft, AIAA 2017-5044. In Proceedings of the 53rd AIAA/SAE/ASEE Joint Propulsion Conference, AIAA Propulsion and Energy Forum, Atlanta, GA, USA, 10–12 July 2017. [Google Scholar] [CrossRef]
- Bombelli, V. Economic Benefits for the Use of Non-toxic Monopropellants for Spacecraft Applications, AIAA-2003-4783. In Proceedings of the 39th AIAA/ASME/SAE/ASEE Joint Propulsion Conference and Exhibit, Huntsville, AL, USA, 20–23 July 2003. [Google Scholar] [CrossRef]
- Haeseler, D.; Bombelli, V.; Vuillermoz, P.; Lo, R.; Marée, T.; Caramelli, F. Green Propellant Propulsion Concepts for Space Transportation and Technology Development Needs. ESA SP-557. In Proceedings of the 2nd International Conference on Green Propellants for Space Propulsion, Cagliari, Sardinia, Italy, 7–8 June 2004; Available online: https://www.researchgate.net/publication/234265416_Green_Propellant_Propulsion_Concepts_for_Space_Transportation_and_Technology_Development_Needs (accessed on 5 December 2019).
- Goldstein, E. The Greening of Satellite Propulsion. Aerosp. Am. 2012, 26–28. Available online: https://uppsagd.files.wordpress.com/2012/05/aerospace-america-february-2012-the-greening-of-satellite-propulsion-page-26-28.pdf (accessed on 5 December 2019).
- Venkatachalam, S.; Santhosh, G.; Ninan, K.N. An Overview on the Synthetic Routes and Properties of Ammonium Dinitramide (ADN) and other Dinitramide Salts. Propellants Explos. Pyrotech. 2004, 29, 178–187. [Google Scholar] [CrossRef]
- Nagamachi, M.Y.; Oliveira, J.I.; Kawamoto, A.M.; Dutra, R.D. ADN—The new oxidizer around the corner for an environmentally friendly smokeless propellant. J. Aerosp. Technol. Manag. 2009, 1, 153–160. Available online: http://www.redalyc.org/articulo.oa?id=309426551003 (accessed on 5 December 2019).
- Rheingold, A.L.; Cronin, J.T.; Brill, T.B.; Ross, F.K. Structure of Hydroxylammonium Nitrate (HAN) and the Deuterium Homolog. Acta Crystallogr. 1987, 43, 402–404. Available online: https://scripts.iucr.org/cgi-bin/paper?a26870 (accessed on 5 December 2019).
- Hawkins, T.W.; Brand, A.J.; McKay, M.B.; Tinnirello, M. Reduced Toxicity, High Performance Monopropellant at the U.S. Air Force Research Laboratory. AFRL-RZ-ED-TP-2010-219. In Proceedings of the 4th International Association for the Advancement of Space Safety Conference, Huntsville, AL, USA, 19–21 May 2010; Available online: www.dtic.mil/dtic/tr/fulltext/u2/a522113.pdf (accessed on 5 December 2019).
- Anflo, K.; Crowe, B. In-Space Demonstration of an ADN-based Propulsion System, AIAA-2011-5832. In Proceedings of the 47th AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, San Diego, CA, USA, 31 July–03 August 2011. [Google Scholar] [CrossRef]
- Spores, R.A.; Masse, R.; Kimbrel, S. GPIM AF-M315E Propulsion System, AIAA_2013-3849. In Proceedings of the 49th AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, San Jose, CA, USA, 15–17 July 2013; Available online: https://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/20140012587.pdf (accessed on 5 December 2019).
- Sempsrott, D. NASA’s Green Propellant Infusion Mission Deploys. NASA Blog 2019. Available online: https://blogs.nasa.gov/spacex/2019/06/25/nasas-green-propellant-infusion-mission-deploys/ (accessed on 20 October 2019).
- McLean, C.; Marotta, B.; Porter, B. Flight performance of the propulsion subsystem on the green propellant infusion mission. AAS 20-062. In Proceedings of the 30th AIAA/AAS Space Flight Mechanics Meeting, Orlando, FL, USA, 6–10 January 2020; Available online: http://www.univelt.com/AASPapers.html (accessed on 5 December 2019).
- Wilhelm, M.; Negri, M.; Ciezki, H.; Schlechtriem, S. Preliminary tests on thermal ignition of ADN-based liquid monopropellants. Acta Astronaut. 2019, 158, 388–396. [Google Scholar] [CrossRef]
- Anon, AF-M315E Safety Data Sheet. Available online: https://static1.squarespace.com/static/59de9c9c18b27ddf3bac610a/t/5e3af2d0fb8df43f9b2ae377/1580921553250/AF-M315E+SDS.pdf (accessed on 1 December 2019).
- Button, K. Green Propellant. Aerospace America. March 2017. Available online: https://aerospaceamerica.aiaa.org/features/green-propellant/ (accessed on 1 December 2019).
- Persson, M.; Anflo, K.; Dinardi, A. A Family of Thrusters for ADN-Based Monopropellant LMP-103S. AIAA-2012-3815. In Proceedings of the 48th AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Atlanta, GA, USA, 30 July–01 August 2012. [Google Scholar] [CrossRef]
- Brand, A. Reduced Toxicity, High Performance Monopropellant. AFRL-RZ-ED-VG-2011-326. Presentation at the Green Propellant Workshop, Sweden, 12–15 September 2011; p. 23. Available online: https://apps.dtic.mil/dtic/tr/fulltext/u2/a554667.pdf (accessed on 5 December 2019).
- Pokrupa, N.; Anglo, K.; Svensson, O. Spacecraft System Level Design with Regards to Incorporation of a New Green Propulsion System. AIAA-2011-6129. In Proceedings of the 46th AIAA/ASME/SAE/ASEE Joint Propulsion Conference and Exhibit, San Diego, CA, USA, 31 July–3 August 2011. [Google Scholar] [CrossRef]
- Katsumi, T.; Hori, K. Combustion Wave Structure of Hydroxylammonium Nitrate Aqueous Solutions. AIAA 2010-6900. In Proceedings of the 46th AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Nashville, TN, USA, 25–28 July 2010. [Google Scholar] [CrossRef]
- Whitmore, S.A.; Burnside, C.G. Performance Analysis of a High Performance Green Propellant Thruster. NASA Marshall Space Flight Center Faculty Program, NASA TM-2015-218216. December 2015; pp. 125–151. Available online: https://ntrs.nasa.gov/search.jsp?R=20160000456 (accessed on 1 December 2019).
- Lowe, D. The Overselling of Ionic Liquids. Science Magazine, In The Pipeline, 11 June 2013. Available online: http://blogs.sciencemag.org/pipeline/archives/2013/06/11/the_overselling_of_ionic_liquids (accessed on 28 March 2018).
- Anon. Hazard Analysis of Commercial Space Transportation; Vol. 1: Operations, Vol. 2: Hazards, vol. 3: Risk Analysis, PB93-199040, Accession 1988; U.S. Dept. of Transportation: Washington, DC, USA, 1988. Available online: https://rosap.ntl.bts.gov/view/dot/35788 (accessed on 5 December 2019).
- Palermo, E. Fused Deposition Modeling: Most Common 3D Printing Method. LIVESCIENCE. 19 September 2013. Available online: https://www.livescience.com/39810-fused-deposition-modeling.html (accessed on 2 December 2019).
- Whitmore, S.A.; Inkley, N.R.; Merkley, D.P.; Judson, M.I. Development of a Power-Efficient, Restart-Capable Arc Ignitor for Hybrid Rockets. J. Propuls. Power 2015, 31, 1739–1749. [Google Scholar] [CrossRef]
- Whitmore, S.A.; Mathias, S.D.; Harvey, R. High Voltage Breakdown and Arc-Tracking Mechanism of Thermoplastics with Applications to Hybrid Rocket Arc- Ignition. In Proceedings of the 53rd AIAA/SAE/ASEE Joint Propulsion Conference, Atlanta, GA, USA, 10–12 July 2017. AIAA 2017-4601. [Google Scholar] [CrossRef]
- Whitmore, S.A.; Merkley, S.L.; Tonc, L.; Mathias, S.D. Survey of Selected Additively Manufactured Propellants for Arc Ignition of Hybrid Rockets. J. Propuls. Power 2016, 32, 1494–1504. [Google Scholar] [CrossRef]
- Whitmore, S.A. Three-Dimensional Printing of “Green” Fuels for Low-Cost Small Spacecraft Propulsion Systems. J. Spacecr. Rocket. 2017, 54. [Google Scholar] [CrossRef]
- Whitmore, S.A. Additive Manufacturing as an Enabling Technology for “Green” Hybrid Spacecraft Propulsion. In Proceedings of the RAST-1039, Conference on Recent Advances in Space Technology 2015, Istanbul, Turkey, 16–19 June 2015; Available online: https://ieeexplore.ieee.org/document/7208305 (accessed on 5 December 2019).
- Whitmore, S.A.; Merkley, S.L.; Spurrier, Z.S.; Walker, S.D. Development of a Power Efficient, Restartable. Green” Propellant Thruster for Small Spacecraft and Satellites. SSC15-P-34. In Proceedings of the 29th AIAA/USU Conference on Small Satellites, Logan, UT, USA, 8–13 August 2015; Available online: https://digitalcommons.usu.edu/smallsat/2015/all2015/90/ (accessed on 5 December 2019).
- Whitmore, S.A.; Bulcher, A.M. A Green Hybrid Thruster Using Moderately Enriched Compressed Air as the Oxidizer. AIAA 2018-4841. In Proceedings of the 2018 Joint Propulsion Conference, AIAA Propulsion and Energy Forum, Cincinnati, OH, USA, 8–11 July 2018. [Google Scholar] [CrossRef]
- Karabeyoglu, M.A. Nitrous Oxide and Oxygen Mixtures (Nytrox) as Oxidizers for Rocket Propulsion Applications. J. Propuls. Power 2014, 30, 696–706. [Google Scholar] [CrossRef]
- Whitmore, S.A.; Armstrong, I.W.; Heiner, M.C.; Martinez, C.J. High-Performing Hydrogen Peroxide Hybrid Rocket with 3-D Printed and Extruded ABS Fuel. Aeronaut. Aerosp. Open Access J. 2018, 2. [Google Scholar] [CrossRef]
- Whitmore, S.A.; Martinez, C.J.; Merkley, D.P. Catalyst Development for an Arc-Ignited Hydrogen Peroxide/ABS Hybrid Rocket System. Aeronaut. Aerosp. Open Access J. 2018, 2. [Google Scholar] [CrossRef]
- Rommingen, J.E.; Husdal, J. Nammo Hybrid Rocket Propulsion TRL Improvement Program. AIAA 2012-4311. In Proceedings of the 48th AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Atlanta, GA, USA, 30 July–1 August 2012. [Google Scholar] [CrossRef]
- Anthoine, J.; Jean-Yves Lestrade, J.-Y.; Messineo, J.; Casu, S. Performances of a Multi-Pulsed Hybrid Rocket Engine Operating with Highly Concentrated Hydrogen Peroxide. AIAA 2017-4006. In Proceedings of the 53rd AIAA/SAE/ASEE Joint Propulsion Conference, AIAA Propulsion and Energy Forum, July 2017. [Google Scholar] [CrossRef]
- Anon. Safety Standard for Oxygen and Oxygen Systems; NSS 1740.15; NASA Office of Mission Assurance: Washington DC, USA, January 1996. Available online: https://ntrs.nasa.gov/search.jsp?R=19960021046 (accessed on 5 December 2019).
- Rhodes, G.W. Investigation of Decomposition Characteristics of Gaseous and Liquid Nitrous Oxide; Report AD-784 602; Air Force Weapons Laboratory: Kirtland AFB, NM, USA, July 1974; Available online: https://www.freelists.org/archives/arocket/01-2014/pdfEE82jaPU9W.pdf (accessed on 5 December 2019).
- Anon. Occupational Safety and Health Guideline for Nitrous Oxide; US Department of Labor, Occupational Health and Safety Administration. Available online: http://www.osha.gov/SLTC/healthguidelines/nitrousoxide/recognition.html (accessed on 21 September 2018).
- Whitmore, S.A. Additively Manufactured Acrylonitrile-Butadiene-Styrene–Nitrous-Oxide Hybrid Rocket Motor with Electrostatic Igniter. J. Propuls. Power 2015, 31, 1217–1220. [Google Scholar] [CrossRef]
- Zakirov, V.; Sweeting, M.; Goeman, V.; Lawrence, T. Surrey Research on Nitrous Oxide Catalytic Decomposition for Space Applications. SSC00-XI-6. In Proceedings of the 14th Annual AIAA/USU Conference on Small Satellites, August 2000; Available online: https://digitalcommons.usu.edu/cgi/viewcontent.cgi?article=2109&context=smallsat (accessed on 5 December 2019).
- Karabeyoglu, A.; Dyer, J.; Stevens, J.; Cantwell, B. Modeling of N2O Decomposition Events, AIAA 2008-4933. In Proceedings of the 44th AIAA/ASME/SAE/ASEE Joint Propulsion Conference and Exhibit, Hartford, CT, USA, 1–3 July 2008. [Google Scholar] [CrossRef]
- Bracken, A.B.; Broughton, G.B.; Hill, W. Equilibria for Mixtures of Oxygen and Nitrous Oxide and Carbon Dioxide and Their Relevance to the Storage of N2O/O2 Cylinders for Use in Analgesia. J. Phys. D 1970, 3, 1747–1758. [Google Scholar] [CrossRef]
- Peng, D.-Y.; Robinson, D.B. A New Two Constant Equation of State. Ind. Eng. Chem. Fundam. 1976, 15, 59–64. [Google Scholar] [CrossRef]
- Zudkevitch, D.; Joffe, J. Correlation and Prediction of Vapor- Liquid Equilibria with the Redlich-Kwong Equation of State. AIChE J. 1970, 16, 112–119. [Google Scholar] [CrossRef]
- Linstrom, P.; Mallard, W. NIST Chemistry WebBook; NIST Standard Reference Database Number 69, National Inst. of Standards and Technology: Gaithersburg, Maryland, 2018. Available online: http://webbook.nist.gov/ (accessed on 28 June 2011).
- Whitmore, S.A.; Stoddard, R.L. N2O/O2 blends safe and volumetrically efficient oxidizers for small spacecraft hybrid propulsion. Aeronaut. Aerosp. Open Access J. 2019, 3. Available online: https://medcraveonline.com/AAOAJ/AAOAJ-03-00097.pdf (accessed on 5 December 2019). [CrossRef]
- Borisov, A.A.; Troshin, K.Y. Critical Conditions for Nitrous Oxide Ignition. Russ. J. Phys. Chem. 2009. [Google Scholar] [CrossRef]
- Gordon, S.; McBride, B.J. Computer Program for Calculation of Complex Chemical Equilibrium Compositions and Applications. In NASA Technical Report RP-1311; 1994. Available online: https://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/19950013764.pdf (accessed on 5 December 2019).
- Anon. “Pure Gasses”, Airgas, an Air Liquide Company. Pages 39, 40. Available online: http://airgassgcatalog.com/catalog/ (accessed on 2 July 2019).
- Stoddard, R.L. Experimental Investigation of N2O/O2 Mixtures as Volumetrically Efficient Oxidizers for Small Spacecraft Hybrid Propulsion Systems. Master’s Thesis, Utah State University, Logan, UT, USA, December 2019. Available on Digital Commons. Available online: https://digitalcommons.usu.edu/ (accessed on 5 December 2019).
- Anon. Cesaroni Pro38® Hardware. Available online: http://pro38.com/products/pro38/pro38.php (accessed on 5 January 2020).
- Anon. ULTRAVOLT C Series High Voltage CAP-Charging Supplies. Advanced Energy, Inc. Available online: https://www.advancedenergy.com/globalassets/resources-root/data-sheets/ultravolt-c-series-data-sheet.pdf (accessed on 9 December 2019).
- Holley. Nitrous Oxide Systems. Available online: https://www.holley.com/brands/nos/ (accessed on 6 December 2019).
- Anderson, J.D. Modern Compressible Flow, 3rd ed.; The McGraw Hill Companies, Inc.: New York, NY, USA, 2003; Chapter 4; pp. 127–187, ISBN-13: 978-0072424430; Available online: https://libcat.lib.usu.edu/search/i0070016542 (accessed on 5 December 2019).
- Zilliac, G.; Karabeyoglu, M.A. Modeling of Propellant Tank Pressurization. AIAA 2005-3549. In Proceedings of the 41st AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Tucson, AZ, USA, 10–13 July 2005; pp. 1–25. [Google Scholar] [CrossRef]
- Dyer, J.; Doran, E.; Dunn, Z.; Lohner, K. Modeling Feed System Flow Physics for Self-Pressuring Propellants. In Proceedings of the 43rd AIAA/ASME/ SAE/ASEE Joint Propulsion Conference & Exhibit, Cincinnati, OH, USA, 8–11 July 2007. AIAA Paper 2007-5702. [Google Scholar] [CrossRef]
- Whitmore, S.A.; Chandler, S.N. Engineering Model for Self-Pressurizing Saturated-N2O-Propellant Feed Systems. J. Propuls. Power 2010, 26, 706–714. [Google Scholar] [CrossRef]
- Zimmerman, J.E.; Waxman, B.S.; Cantwell, B.; Zilliac, G. Review and Evaluation of Models for Self-Pressurizing Propellant Tank Dynamics. AIAA 2013-4045. In Proceedings of the 49th AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, San Jose, CA, USA, 14–17 July 2013. [Google Scholar] [CrossRef]
- Waxman, B.S.; Zimmerman, J.E.; Cantwell, B.J.; Zilliac, G.G. Mass flow Rate Characterization of Injectors for Use with Self-Pressurizing Oxidizers in Hybrid Rockets. AIAA 2013-3636. In Proceedings of the 49th AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, San Jose, CA, USA, 14–17 July 2013. [Google Scholar] [CrossRef]
- Beckwith, T.G.; Marangoni, R.D.; Lienhard, J.H.V. Mechanical Measurements, 6th ed.; Prentice Hall: Upper Saddle River, NY, USA, 2006; pp. 43–73. [Google Scholar]
- Whitmore, S.A.; Peterson, Z.W.; Eilers, S.D. Comparing Hydroxyl Terminated Polybutadiene and Acrylonitrile Butadiene Styrene as Hybrid Rocket Fuels. J. Propuls. Power 2013, 29, 582–592. [Google Scholar] [CrossRef]
- Whitmore, S.A.; Merkley, S. Radiation Heating Effects on Oxidizer-to-Fuel Ratio of Additively Manufactured Hybrid Rocket Fuels. J. Propuls. Power 2019, 35, 863–878. [Google Scholar] [CrossRef]
- Zilliac, G.; Karabeyoglu, M.A. Hybrid Rocket Fuel Regression Rate Data and Modeling. AIAA 2006-4504. In Proceedings of the 42nd AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Sacramento, CA, USA, 9–12 July 2006. [Google Scholar] [CrossRef]
- Anon. NFPA 704, National Fire Protection Association Hazard Identification System. ACS Chemistry for Life. Available online: https://www.google.com/search?client=firefox-b-1-d&q=NFPA+Hazard+Class (accessed on 10 December 2019).
- Hussain, S.M.; Frazier, J.M. In vitro toxicity assessment of a new series of high energy compounds. Sci. Total Environ. 2001, 247, 151–160. Available online: https://www.ncbi.nlm.nih.gov/pubmed/11453292 (accessed on 25 March 2020). [CrossRef]










| Component | ||||
|---|---|---|---|---|
| Single Port Injector | Diameter: 0.127 cm | |||
| Fuel Grain (ABS) | Diameter: 3.168 cm | Length: 5.1 cm | Initial Weight: 45.0 g 1 | Initial Port Diameter: 0.53 cm |
| Motor Case | Diameter: 3.8 cm | Length: 7.92 cm | Wall Thickness: 1.5 mm | |
| Nozzle (Graphite) | Initial Throat Diameter: 0.345 cm | Exit Diameter: 0.483 cm | Expansion Ratio: 2.07:1, Conical | Exit Angle: 5.0 deg. |
| Batch No. | 1 | 2 | 3 | 4 | 5 | 6 | Mean | Std. Dev |
|---|---|---|---|---|---|---|---|---|
| Final Fill Pressure, kPa | 8704.4 | 8842.3 | 8739.4 | 8760.1 | 8725.6 | 8105.1 | 8745.4 | 269.3 |
| Final Fill Temperature, °C | 0.1 | −0.2 | 0.0 | 0.0 | 0.2 | 0.2 | 0.05 | 0.15 |
| Total Oxidizer Added, kg | 2.867 | 2.921 | 2.916 | 2.864 | 2.865 | 2.854 | 2.887 | 0.029 |
| N2O Added, kg | 2.234 | 2.267 | 2.284 | 2.273 | 2.283 | 2.278 | 2.268 | 0.019 |
| O2 Added to Tank, kg | 0.633 | 0.654 | 0.632 | 0.591 | 0.582 | 0.576 | 0.618 | 0.032 |
| Mean Nytrox Density, g/cm3 | 0.419 | 0.427 | 0.426 | 0.418 | 0.418 | 0.417 | 0.422 | 0.004 |
| Liquid O2 Mass Fraction (%) | 12.63 | 12.99 | 12.72 | 12.78 | 12.69 | 11.13 | 12.76 | 0.68 |
| Vapor O2 Mass Fraction (%) | 37.14 | 37.20 | 37.16 | 37.17 | 37.16 | 36.40 | 37.17 | 0.31 |
| Vapor Fraction in Tank (%) | 37.0 | 36.6 | 35.9 | 37.5 | 37.2 | 32.9 | 36.8 | 01.7 |
| Nytrox Liquid Density, g/cm3 | 0.774 | 0.770 | 0.773 | 0.773 | 0.774 | 0.790 | 0.773 | 0.007 |
| Nytrox Vapor Density, g/cm3 | 0.235 | 0.241 | 0.236 | 0.237 | 0.236 | 0.212 | 0.237 | 0.010 |
| Baseline GOX/ABS Test (13 Total Burns) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Statistic | Thrust, N Load P0 | CF Load P0 | Isp Load P0 | c*, m/s | η* | O/F | P0, kPa | Total Mass Flow, g/s | |||
| μ | 10.70 | 10.90 | 1.262 | 1.273 | 224.9 | 224.8 | 1751.4 | 0.919 | 1.772 | 762.9 | 4.85 |
| σ | 0.645 | 0.617 | 0.031 | 0.026 | 7.52 | 8.83 | 23.84 | 0.089 | 0.228 | 62.82 | 0.349 |
| 95% t-conf. | 0.339 | 0.373 | 0.019 | 0.016 | 4.54 | 5.34 | 14.40 | 0.054 | 0.178 | 37.94 | 0.211 |
| Nytrox/ABS Test (19 Total Burns) | |||||||||||
| μ | 11.75 | 11.70 | 1.227 | 1.222 | 204.4 | 198.4 | 560.84 | 0.927 | 3.464 | 809.7 | 6.14 |
| σ | 0.749 | 0.759 | 0.034 | 0.039 | 10.29 | 11.10 | 57.46 | 0.106 | 0.463 | 32.84 | 0.356 |
| 95% t-conf. | 0.358 | 0.366 | 0.017 | 0.019 | 54.96 | 5.34 | 27.68 | 0.051 | 0.223 | 15.82 | 0.171 |
| Propellant | GOX/ABS | Nytrox87/ABS | N2O/ABS58 | N2O/HTPB58,59 | Paraffin/LOX59 | LOX/HTPB59 | Lox/HTPB-Escorez59 | LOX/HDPE59 |
|---|---|---|---|---|---|---|---|---|
| a coefficient | 0.0428 | 0.0354 | 0.00742 | 0.00795 | 0.0488 | 0.0146 | 0.0099 | 0.0098 |
| n exponent | 0.524 | 0.455 | 0.799 | 0.773 | 0.491 | 0.681 | 0.680 | 0.620 |
| Propellant | Hydrazine | LMP-103S | AF-M315E | Nytrox/ABS Hybrid |
|---|---|---|---|---|
| Flame Temperature | 600–750 °C | 1600 °C | 1900 °C | 3000 °C |
| Vacuum Isp, s | 220–225 | 252 (theory) 235 (delivered) | 266 (theory) 245 (delivered) | 320 (theory) 294 (extrapolated) |
| Specific Gravity | 1.01 | 1.24 | 1.465 | 0.650 (87% N2O) |
| Impulse density, N·s/L | 2270 | 3125 (theory) 2915 (delivered) | 3900 (theory) 3650 (delivered) | 2510 (vacuum, extrapolated) 1750 (ambient, delivered) |
| Preheat Temperature | 315 °C, cold-start capable | 300 °C | 370 °C | N/A none-required |
| Required Ignition Input Energy, Joules | N/A | 12,000 J (10 Watts @ 1200 s) | 27,000 J (15 Watts @ 1800 s | 2.5 J (5 Watts for 500 ms) |
| Propellant Freezing Temperature | 1–2 °C | −7 °C | < 0 °C (forms glass, no freezing point) | −70 °C |
| Cost | $ | $$$ | $$$$ | $ |
| Availability | Readily Available | Restricted Access | Limited Access | Very Widely Available 1 |
| NFPA 704 Hazard Class [64] |  |  |  2 2 |  |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Whitmore, S.A. Nytrox as “Drop-in” Replacement for Gaseous Oxygen in SmallSat Hybrid Propulsion Systems. Aerospace 2020, 7, 43. https://doi.org/10.3390/aerospace7040043
Whitmore SA. Nytrox as “Drop-in” Replacement for Gaseous Oxygen in SmallSat Hybrid Propulsion Systems. Aerospace. 2020; 7(4):43. https://doi.org/10.3390/aerospace7040043
Chicago/Turabian StyleWhitmore, Stephen A. 2020. "Nytrox as “Drop-in” Replacement for Gaseous Oxygen in SmallSat Hybrid Propulsion Systems" Aerospace 7, no. 4: 43. https://doi.org/10.3390/aerospace7040043
APA StyleWhitmore, S. A. (2020). Nytrox as “Drop-in” Replacement for Gaseous Oxygen in SmallSat Hybrid Propulsion Systems. Aerospace, 7(4), 43. https://doi.org/10.3390/aerospace7040043




