Abstract
Nitrous oxide (N2O) has attracted increasing attention as an oxidizer for space propulsion systems due to its non-toxic nature and favorable handling characteristics. Its relatively high vapor pressure allows self-pressurization, while its wide storage temperature range makes it attractive for a range of space applications. In parallel with broader efforts to identify alternatives to conventional toxic propellants, numerous studies have investigated liquid propulsion systems based on N2O combined with hydrocarbon fuels, spanning both premixed fuel blends and non-premixed bipropellant configurations. This review summarizes experimental and system-level studies on N2O–hydrocarbon propellant combinations, including ethylene, ethane, ethanol, propane, acetylene, methane, dimethyl ether, and propylene. Results reported by different research groups reveal clear differences among propellant combinations in terms of vapor pressure, thermal stability, chemical reactivity, and ignition delay. These differences have direct implications for injector design, mixing strategies, ignition mechanism, and system safety. By bringing together recent results from the literature, this paper aims to clarify the practical trade-offs associated with fuel selection in N2O-based premixed and bipropellant systems and to provide a useful reference for the design and development of future space propulsion concepts.
1. Introduction
In recent years, the space propulsion community has shown growing interest in non-toxic propellants, largely driven by tighter environmental and occupational safety regulations [1,2]. Regulations such as the European Commission’s Registration, Evaluation, Authorisation and Restriction of Chemicals, REACH, have placed strong constraints on the continued use of highly toxic substances, most notably hydrazine [3,4]. As a result, the practical burden associated with toxic propellants, including specialized handling procedures, safety infrastructure, and long term regulatory compliance, has become increasingly difficult to justify. This has prompted renewed efforts to identify viable alternative propellant options [5,6].
Among the candidate propellants, nitrous oxide, N2O, has attracted sustained attention because it combines benign handling characteristics with propulsion relevant performance [7]. Its use has been actively explored in small propulsion systems, particularly attitude control thrusters for small satellites and CubeSats, where simplicity and operational robustness are critical [8]. A defining feature of nitrous oxide is its high vapor pressure, approximately 50 bar at 293 K, which allows the propellant tank to remain self pressurized [9]. This characteristic removes the need for a separate pressurization system and leads to a simpler and lighter propulsion architecture.
From a performance perspective, nitrous oxide based propulsion systems can achieve relatively high specific impulse values. In bipropellant configurations with hydrocarbon fuels, vacuum specific impulse values on the order of 300 s have been reported (see Table 1), making N2O competitive with conventional low thrust chemical propulsion options [10]. In addition, nitrous oxide can be stored either as a liquid or as a compressed gas over a wide temperature range, approximately from 183 K to 333 K, which enables stable storage at standard sea-level conditions (1 atm, 298 K) without active thermal control [10]. The absence of acute toxicity further reduces operational constraints by eliminating many of the handling, safety, and disposal requirements associated with traditional propellants [11]. Together with its relatively low procurement cost and established industrial supply chain, these factors make nitrous oxide an economically attractive choice for emerging small satellite missions [7,12].

Table 1.
Vacuum specific impulse (), characteristic velocity (c*), reaction temperature (), and toxicity for various propellants: hydrazine, Monomethylhydrazine/Nitrogen Tetroxide (MMH/NTO), Nitrous Oxide (N2O), and Nitrous Oxide/Propane (N2O/C3H8) propellants [13].
Nitrous oxide can be employed in several propulsion configurations. It may be used as a monopropellant through catalytic decomposition, producing thrust via the exothermic breakdown of N2O into nitrogen and oxygen [14,15,16,17,18]. To facilitate this reaction, noble metal catalysts such as Platinum (Pt), Rhodium (Rh), and Iridium (Ir), typically supported on alumina (Al2O3) or zeolites (e.g., ZSM-5), are commonly employed [13,19,20]. These catalysts significantly reduce the decomposition initiation temperature to a range of 350–600 K, compared to the adiabatic decomposition temperature of approximately 1913 K, thereby enabling reliable auto-ignition and sustained monopropellant operation [21,22]. While monopropellant systems offer simplicity, their specific impulse () is inherently limited by the decomposition energy of nitrous oxide alone. Alternatively, it can function as an oxidizer in liquid bipropellant systems when combined with hydrocarbon fuels, as illustrated schematically in Figure 1. In such combustion processes, nitrous oxide serves both as an oxidizing agent that sustains fuel combustion and as a source of additional thermal energy released through its decomposition [23].
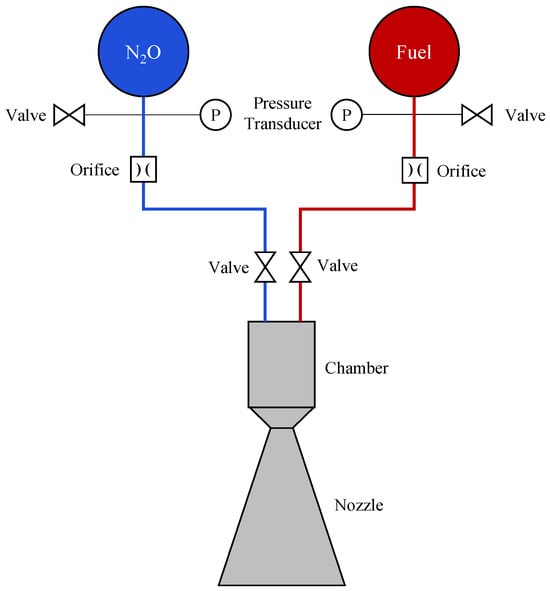
Figure 1.
Schematic of a nitrous oxide and hydrocarbon non-premixed bipropellant propulsion system.
Nitrous oxide has also been employed with hydrocarbon fuels in premixed formulations commonly referred to as Nitrous Oxide Fuel Blends, NOFB [12], as illustrated schematically in Figure 2. This concept offers propulsion performance comparable to that of conventional bipropellant systems while requiring only a single propellant tank, which can provide a substantial mass advantage at the system level [24]. The reduction in tank count and feed system complexity is particularly attractive for small spacecraft with tight volume and mass constraints. However, because the oxidizer and fuel are premixed prior to injection, NOFB systems introduce an inherent risk of flashback. Mitigating this risk requires the implementation of dedicated flashback arrestors and additional safety measures, which can partially offset the simplicity gains and remains an important consideration in system design and operation [25].
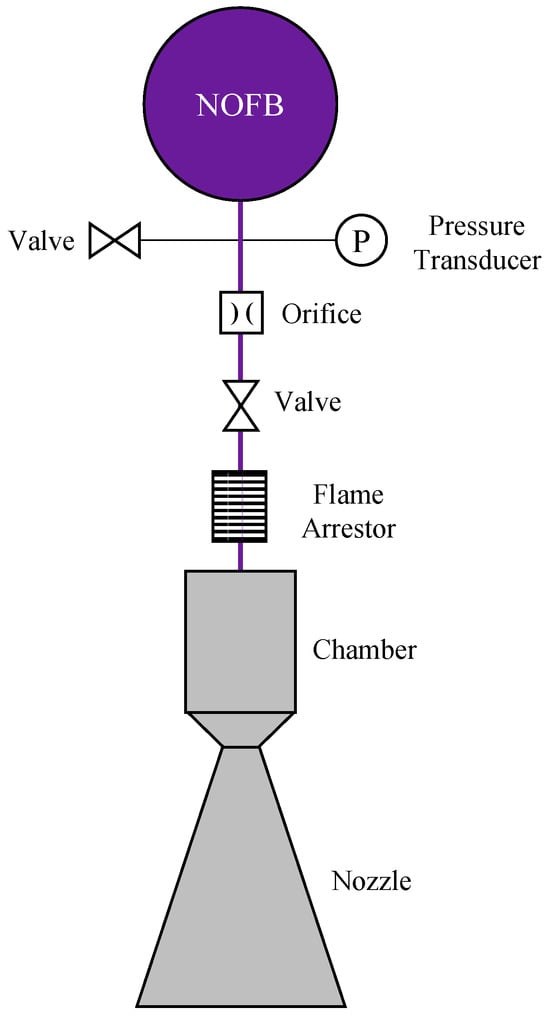
Figure 2.
Schematic of a premixed nitrous oxide fuel blend propulsion system.
A variety of hydrocarbon fuels have been investigated in combination with nitrous oxide, reflecting efforts to balance performance, safety, and system simplicity. Representative fuels include ethane (C2H6), ethylene (C2H4), ethanol (C2H5OH), methane (CH4), and propane (C3H8), each exhibiting distinct physicochemical properties that influence ignition behavior, flame structure, and combustion stability. For instance, Firestar Technologies in the United States classified mixtures of nitrous oxide with ethane, ethylene, or acetylene (C2H2) under the designation Nitrous Oxide Fuel Blends and carried out extensive experimental and system-level studies aimed at small satellite propulsion applications [12,24]. The German Aerospace Center (DLR) investigated premixed propellants composed of nitrous oxide with ethane or ethylene, referred to as Hydrocarbon mixed Nitrous Oxide, HyNOx. In their studies, particular attention was given to combustion safety, especially the mitigation of flashback phenomena. To this end, porous materials were integrated upstream of the injector as passive flashback arrestors [26], and their effectiveness was evaluated through laminar flame experiments and thruster scale testing [25,27]. These efforts highlighted the potential of premixed nitrous oxide hydrocarbon formulations to achieve competitive propulsion performance while maintaining a simplified feed system architecture.
Additional efforts have explored alternative fuel combinations. The European Space Agency conducted combustion tests using premixed nitrous oxide-ethanol propellants, motivated by ethanol’s favorable handling characteristics and widespread availability. However, these experiments revealed significant thermal loading on injector components, and injector damage due to overheating was reported during operation [28,29]. In another notable example, the United States Defense Advanced Research Projects Agency selected a nitrous oxide-acetylene blend for a program targeting low Earth orbit satellite launch applications. Despite promising performance, the program was ultimately terminated following two explosion incidents during ground testing [30]. Together, these studies illustrate both the performance potential and the practical limitations of premixed nitrous oxide-hydrocarbon propellants when applied to thruster systems.
As discussed above, although NOFB systems offer high energy density and clear system-level advantages, the potential for explosive behavior in the vapor phase has been identified as a major limitation. This safety concern is not exclusive to premixed formulations but is also relevant to a broader class of propulsion systems that employ nitrous oxide as an oxidizer, particularly under conditions involving elevated temperatures, pressure transients, or unintended mixing [31]. These findings indicate that combustion behavior and associated safety margins are governed not only by the choice of fuel but also by the overall propellant configuration, mixing strategy, and operating envelope of the propulsion system.
In light of these considerations, this paper provides a comprehensive review of premixed and non-premixed nitrous oxide-based liquid and gaseous propellant systems reported in the literature. The focus is placed on experimentally investigated combustion characteristics, including ignition behavior and combustion stability, across a range of hydrocarbon fuels. In addition, demonstrated thruster concepts and application oriented system implementations are reviewed to highlight how these combustion characteristics translate into practical design choices. By synthesizing results from laboratory-scale experiments and system-level demonstrations, this review aims to clarify the tradeoffs associated with fuel selection and propellant configuration, and to provide a consolidated technical perspective for the development of nitrous oxide based propulsion systems.
2. Nitrous Oxide-Hydrocarbon Propellant Combinations
2.1. Ethylene (C2H4) and Ethane (C2H6)
Ethylene (C2H4) and ethane (C2H6) are among the more frequently studied hydrocarbon fuels in combination with nitrous oxide, and their combustion behavior and flame characteristics have been examined in a range of experimental studies. Owing to their relatively simple molecular structures, these fuels have often been used as reference propellants for investigating fundamental combustion processes as well as system-level feasibility in nitrous-oxide-based propulsion concepts.
Ethylene is a colorless, flammable gas with a high vapor pressure of approximately 41 bar at 273 K [32,33]. This vapor pressure, which is comparable to that of nitrous oxide, facilitates simultaneous injection and rapid mixing of the two components, making ethylene attractive for premixed or near-premixed propulsion configurations [34]. As a result, ethylene has been employed in multiple experimental investigations of nitrous oxide fuel blends to study ignition characteristics, flame stabilization, and flashback behavior.
Ethane is also a colorless and odorless gas, with a vapor pressure of approximately 24.4 bar at 273 K [35]. Although its vapor pressure is lower than that of ethylene, ethane exhibits a higher thermal decomposition temperature and greater thermal stability. These properties reduce the likelihood of premature decomposition and undesired reactions under elevated temperature conditions. Consequently, ethane has often been investigated alongside ethylene as a comparatively more thermally stable fuel option in nitrous oxide fuel blend systems, particularly in studies focusing on combustion stability and flashback mitigation [36].
Several studies have examined the chemical and combustion behavior of nitrous oxide mixed with ethylene and ethane, with particular emphasis on reaction pathways, flame dynamics, and ignition characteristics [23,37,38,39,40,41,42,43]. In a detailed investigation of N2O/C2H4 mixtures, Wang and Zhang [39] measured the laminar burning velocity at an initial temperature of 280 K over an equivalence ratio range of 0.2 to 2.4 and at pressures of 0.5, 1.0, and 2.0 atm. Their results indicated that under near-stoichiometric conditions (), the decomposition of nitrous oxide was primarily governed by the reaction N2O → N2 + O, whereas under fuel-rich conditions (), the reaction N2O + H → N2 + OH became increasingly dominant. It was further reported that variations in pressure had only a limited effect on the laminar burning velocity, while the adiabatic flame temperature increased noticeably with increasing pressure.
Related experimental investigations have also examined the flame characteristics of N2O/C2H4 alongside other nitrous-oxide-based fuel combinations, including N2O/C3H8 and N2O/NH3 [44]. Using a range of combustion channel configurations, high-speed imaging and pressure measurements were employed to analyze flame location, propagation behavior, and quenching diameters. Within this parallel set of experiments, the N2O/C2H4 mixture exhibited more rapid pressure-dependent flame acceleration and a stronger tendency toward detonation development than the N2O/C3H8 and N2O/NH3 mixtures. In addition, the onset temperature for thermal decomposition of the N2O/C2H4 mixture was reported to be approximately 408 K, indicating relatively high thermal sensitivity. This behavior has been attributed to the presence of the carbon–carbon double bond in ethylene, which promotes the formation of reactive intermediates and enhances overall chemical reactivity [32].
Regarding safety and stability, N2O/C2H4 mixtures present significant explosion hazards due to the exothermic decomposition of the oxidizer, which amplifies flame temperature and explosion intensity compared to conventional fuel-air mixtures [45]. In particular, Deflagration-to-Detonation Transition (DDT) occurs most rapidly near stoichiometric conditions (), generating pressure peaks that can compromise flame arresters [46]. Consequently, to mitigate the risk of flashback and detonation, operating at non-stoichiometric compositions and managing initial pressures is recommended [47].
The German Aerospace Center (DLR) has conducted a systematic investigation of premixed N2O/C2H4 combustion, ranging from fundamental flame behavior to thruster demonstrations. Initial studies concentrated on laminar flame speeds, ignition delay times, and flashback characteristics under premixed conditions [48]. To examine flashback mechanisms in greater detail, DLR constructed a dedicated ignition test section equipped with high-speed optical diagnostics. Measurements indicated that flame propagation velocities were approximately 20 m/s immediately after ignition and increased to about 120 m/s during downstream propagation. It was further demonstrated that installing porous bronze and stainless-steel inserts upstream of the combustion zone effectively suppressed flashback events [25]. Alongside these experiments, detailed kinetic analyses of N2O/C2H4 combustion were performed, leading to the formulation of reduced reaction mechanisms suitable for CFD simulations [49,50].
Building on this body of fundamental work, DLR designed and tested a premixed demonstrator combustor operating with N2O/C2H4. Time-resolved wall heat-flux distributions were reconstructed from a combination of experimental measurements and inverse analysis. The resulting heat loads corresponded to combustion temperatures in excess of 3000 K, clearly indicating that passive thermal management would be insufficient and that regenerative cooling is required for sustained operation [51]. Based on this foundation, DLR developed the premixed propellant HyNOx, composed of nitrous oxide blended with ethylene and ethane, as illustrated schematically in Figure 3. Thruster-level tests employed porous flame arrestors upstream of the injector to suppress flashback, together with regenerative cooling to accommodate the elevated combustion temperatures [25,52,53]. At an oxidizer-to-fuel mass ratio of 7, a characteristic velocity (c*) of 1530 m/s was obtained, with c* efficiency ranging between 92% and 96% throughout the test series. However, in the N2O/C2H4 propellant configuration, soot deposition and coking were observed in the cooling channels, indicating severe thermal degradation issues [27,34,54,55,56,57]. As a result, ethylene was later replaced with the more thermally stable ethane. Under otherwise comparable operating conditions, this fuel substitution eliminated injector blockage while still achieving a characteristic velocity of 1578 m/s [36,58]. These observations highlight an inherent trade-off between chemical reactivity and thermal robustness in premixed N2O-based propulsion systems.
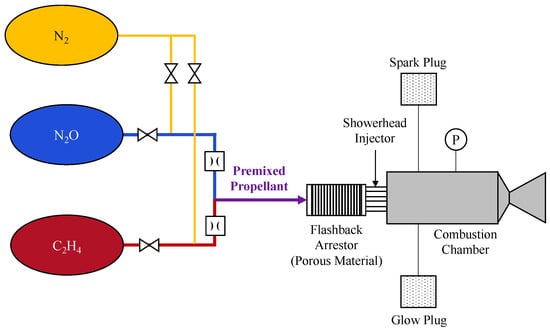
Figure 3.
Schematic of the N2O/C2H4 (HyNOx) propulsion system investigated by the German Aerospace Center (DLR). The schematic was reconstructed by the authors based on the system layout and design details reported in the original literature [57].
In the United States, a complementary application-driven effort was pursued by Firestar Technologies under the NOFBX program, which targeted a throttleable 100 lbf-class (445 N) thruster for Mars ascent-stage propulsion [59]. The premixed propellant consisted of nitrous oxide blended with ethylene, ethane, and acetylene. The thruster design incorporated regenerative cooling, with the propellant routed through cooling channels prior to injection, and employed spark ignition to enable multiple restarts, as shown in Figure 4. Hot-fire tests conducted in 2010 reported a specific impulse of approximately 325 s, demonstrating the practical feasibility and performance potential of nitrous-oxide-based premixed fuel blends [12,24]. In parallel, recent commercial entities have also adopted non-premixed architectures, as exemplified by the reported deployment of N2O/C2H6 bipropellant thrusters by Impulse Space [60].
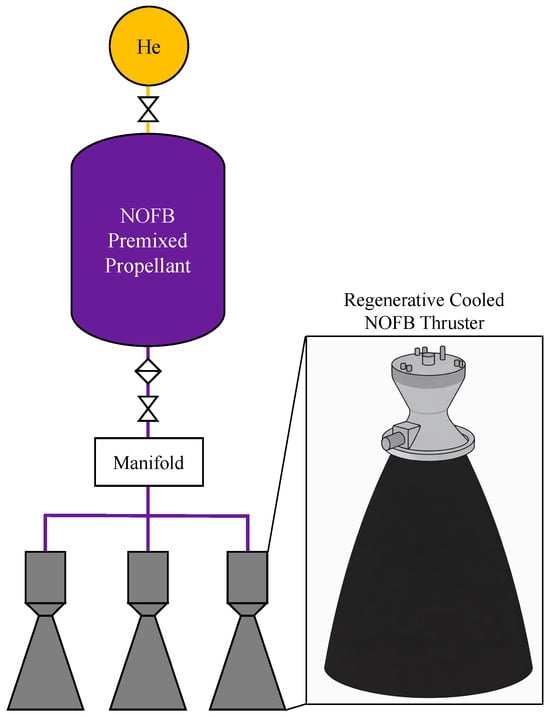
Figure 4.
Illustration of a premixed nitrous oxide/ethylene (N2O/C2H4) thruster concept developed by Firestar Technologies for Nitrous Oxide Fuel Blend (NOFB) applications. This figure was recreated by the authors based on the system layout and design details reported in the original literature [12].
In summary, ethylene and ethane represent the most mature fuel options for nitrous oxide propulsion, widely applicable to both premixed and non-premixed architectures due to their high vapor pressure compatibility. Ethylene is particularly advantageous for premixed configurations due to its high vapor pressure (41 bar), which facilitates favorable mixing with nitrous oxide. However, its high flame speed and thermal sensitivity necessitate stringent flashback arrestor designs and thermal management strategies. Ethane serves as a complementary alternative; while it shares similar self-pressurization capabilities, it exhibits greater thermal stability, offering a practical trade-off that reduces the risk of pre-ignition and injector fouling at the cost of slightly reduced reactivity.
2.2. Ethanol (C2H5OH)
Ethanol (C2H5OH) is a colorless liquid that has long been used in a wide range of civilian and industrial applications, including food additives, preservatives, disinfectants, and fuels. Its non-toxic nature and complete solubility with water make ethanol relatively easy to handle, store, and transport compared with many conventional rocket fuels [61,62]. In propulsion systems, ethanol has historically been employed both as a coolant and as a fuel in combination with high-pressure oxidizers such as liquid oxygen. However, its high reactivity with oxygen and corresponding handling difficulties have motivated interest in alternative oxidizers. In this context, nitrous oxide has been explored as a safer and more manageable oxidizer for use with ethanol in bipropellant propulsion concepts [63].
At standard sea-level conditions, ethanol remains in the liquid phase owing to its low vapor pressure of approximately 0.06 bar at 293 K [64], and it is therefore typically supplied as a liquid fuel. When nitrous oxide is delivered in the gaseous or two-phase state, the resulting N2O/C2H5OH combustion process inherently involves multiphase phenomena. In such configurations, the atomization, evaporation, and mixing of ethanol droplets become key factors governing ignition behavior, flame stabilization, and overall combustion efficiency. Consequently, injector geometry, momentum flux ratio, and propellant injection strategy play a central role in determining system performance.
Motivated by these considerations, several experimental studies have focused on injector-scale and laboratory-scale investigations of N2O/C2H5OH combustion under multiphase conditions. At Korea Aerospace University, for example, a series of experiments employed a tricoaxial injector configuration to systematically examine spray formation and combustion characteristics as a function of the oxidizer-to-fuel momentum flux ratio [65]. The transient ignition flow-field characteristics of such a 50 N-class thruster were visualized using shadowgraph imaging, quantitatively demonstrating the dependence of spray breakup mechanisms on the momentum flux ratio [66].
More extensive system-level developments of ethanol-based nitrous oxide propulsion have been carried out in Japan. Since 2003, the Japan Aerospace Exploration Agency (JAXA) has pursued the development of an N2O/C2H5OH propellant combination, designated NOEL, for use as both the main engine propellant and the attitude control propellant for the third stage of the Epsilon launch vehicle [67,68]. The propulsion system, whose simplified schematic is shown in Figure 5, consisted of two fuel tanks, two oxidizer tanks, and a helium pressurization system, with nitrous oxide stored at pressures of approximately 60 bar. Following initial feasibility studies, JAXA constructed a 2 kN-class breadboard model after 2006 and conducted a series of ground and high-altitude combustion tests. At a mixture ratio of 3.82, the engine achieved a chamber pressure of 1.95 MPa, a vacuum thrust of 1.89 kN, and a characteristic velocity of 1474 m/s, thereby demonstrating the practical viability of the NOEL propulsion concept [61].
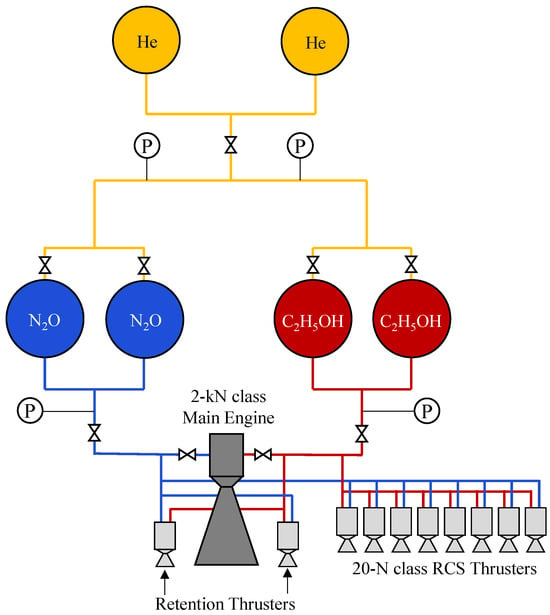
Figure 5.
Simplified schematic of the N2O/C2H5OH (NOEL) propulsion system developed by the Japan Aerospace Exploration Agency for the third stage of the Epsilon launch vehicle. This figure was recreated by the authors based on the system layout and design details reported in the original literature [61].
In addition to these system-level developments, rotating detonation engine (RDE) concept using N2O/C2H5OH propellant has been investigated by research groups in Japan [69,70]. Cylindrical RDEs, schematically illustrated in Figure 6, operating with gaseous or two-phase N2O and liquid ethanol were developed to investigate detonation initiation, wave stability, and combustion characteristics under varying propellant supply conditions and injector momentum angles [69,70]. These investigations highlighted the potential for compact propulsion architectures, demonstrating that the RDE configuration can reduce the combustor size (in terms of characteristic length) by approximately 88% compared to conventional rocket design.
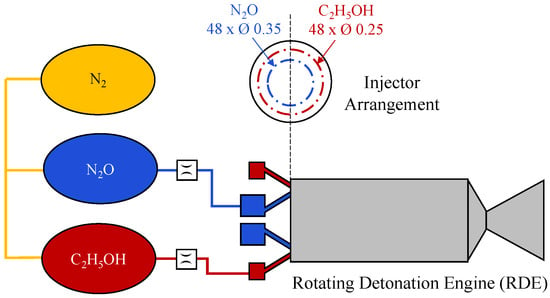
Figure 6.
Schematic of a cylindrical rotating detonation engine featuring a hollow combustion chamber, operating with nitrous oxide and liquid ethanol. This figure was recreated by the authors based on the system layout and design details reported in the original literature [69].
Alternative ignition and combustion-enhancement approaches have also been examined at the thruster scale. For instance, Kakami et al. [71,72] developed a 1 N-class bipropellant thruster using N2O/C2H5OH with plasma arc discharge-assisted combustion. In this configuration (see Figure 7), liquid ethanol and nitrous oxide arc plasma were injected simultaneously into the combustion chamber to promote rapid ethanol evaporation and enhance chemical reactivity. Stable combustion was achieved using a 1 kW arc discharge during ignition, resulting in a thrust of 0.35 N, a specific impulse of 55 s, and a thrust efficiency of 53% [71].
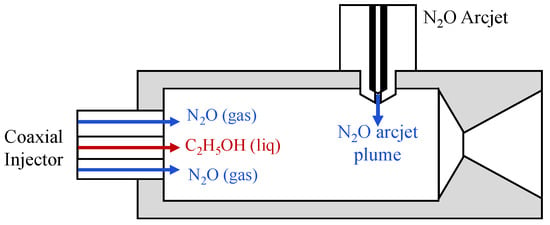
Figure 7.
Schematic of a plasma arc discharge-assisted N2O/C2H5OH bipropellant thruster. This figure was recreated by the authors based on the system layout and design details reported in the original literature [71].
In the United States, larger-scale development efforts have been reported by the New Mexico Institute of Mining and Technology (NMIMT), which developed a 600 N-class bipropellant propulsion system using liquid nitrous oxide and liquid ethanol [73,74]. To reduce the risk of unintended premixing, the fuel and oxidizer were supplied through separate feed lines and supported by a dedicated nitrogen purge line. The nitrous oxide feed line was maintained above its saturation pressure to ensure delivery in the liquid phase. Ignition was provided by an H2/O2 torch igniter, and hot-fire tests demonstrated thrust levels of approximately 577 N with a specific impulse in the range of 250–260 s.
While most N2O/C2H5OH systems rely on non-premixed injection, a notable exception is a program led by the European Space Agency (ESA) in collaboration with TNO and Bradford Engineering in the Netherlands, together with NAMMO Westcott in the United Kingdom. In this effort, a nitrous oxide fuel blend (NOFB) was developed by premixing liquid nitrous oxide and ethanol [29]. Prior to thruster testing, mixture compatibility and chemical stability were assessed by storing both liquids in a 50 bar high-pressure test tube. No decomposition or reaction was observed over approximately 70 h, confirming mixture stability within this pressure range. Uniform mixing was achieved up to an oxidizer-to-fuel ratio of 3.15, whereas phase separation occurred at higher ratios. Based on these findings, NAMMO Westcott developed and tested a 600 N-class thruster with liquid-phase NOFB injection; a schematic of the thruster is shown in Figure 8. The tests demonstrated a combustion efficiency of approximately 97% and a specific impulse of 259 s. However, because the propellant was premixed prior to injection, combustion initiated close to the injector face, resulting in elevated heat fluxes and injector damage despite the absence of flashback. These results underscored the need for further development of flashback arrestor concepts and injector designs suitable for both liquid- and gas-phase NOFB operation [28].
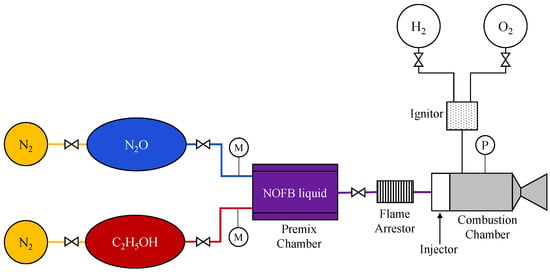
Figure 8.
Schematic of a 600-N-class thruster using a premixed nitrous oxide-ethanol fuel blend (NOFB). This figure was recreated by the authors based on the system layout and design details reported in the original literature [28].
In summary, ethanol is distinguished by its operational safety, non-toxicity, and wide availability, making it a preferred choice for non-premixed bipropellant systems where handling simplicity is prioritized. However, significant differences in phase and vapor pressure between liquid ethanol and gaseous/supercritical nitrous oxide introduce challenges for premixed applications. Successful implementation of premixed N2O/ethanol blends typically requires high-pressure liquid-phase mixing to avoid phase separation, or otherwise relies on atomization-driven combustion in conventional bipropellant architectures.
2.3. Propane (C3H8)
Propane (C3H8) is a saturated hydrocarbon that exists as a gas under standard atmospheric conditions but can be readily liquefied under moderate pressure, which has led to its widespread use as a transportation and storage-friendly fuel. It is a primary constituent of liquefied petroleum gas (LPG) and is typically obtained as a byproduct of natural gas processing or petroleum refining. Owing to its low toxicity, high availability, and established infrastructure, propane has been extensively used as a vehicular and industrial fuel [75,76]. Similar to nitrous oxide, propane can be stored in a self-pressurizing state, with a vapor pressure of approximately 8.6 bar at ambient temperature, making it attractive for compact propulsion system designs that avoid complex pressurization hardware [77].
From a combustion perspective, N2O/C3H8 mixtures have been examined alongside other N2O–hydrocarbon combinations in several experimental and kinetic studies [37,38,78,79,80]. These investigations have generally focused on ignition behavior, flame structure, and detonation characteristics, with propane serving as a representative saturated hydrocarbon fuel. For example, Li et al. [44] studied premixed N2O/C3H8 flames in comparison with N2O/C2H4 and other fuel combinations. The results showed that detonability differs between the two fuels. In particular, ethylene exhibits a shorter deflagration-to-detonation transition (DDT) run-up distance compared to propane, reflecting its higher chemical reactivity. Furthermore, the quenching diameter was reported to range from 0.5 to 0.7 mm for N2O/C2H4, whereas for N2O/C3H8, it lies in the range of 0.7 to 1.2 mm. These findings indicate that propane possesses relatively weaker flame propagation characteristics.
Beyond fundamental combustion studies, propane has also been explored in application-oriented nitrous oxide propulsion concepts, most notably through programs initiated by the Defense Advanced Research Projects Agency (DARPA). Beginning in 2001, DARPA investigated an N2O/C3H8 propellant system, commonly referred to as NOP, as part of a small-scale propulsion development effort associated with the Orbital Express program [77]. A key feature of this concept was the use of catalytic decomposition of nitrous oxide to achieve ignition without an external igniter, as illustrated in Figure 9. In this approach, nitrous oxide is passed through a catalyst bed, where it decomposes exothermically into nitrogen and oxygen. Once sufficiently heated, this oxidizing mixture ignites the injected propane downstream. Complete dissociation of N2O occurs at temperatures on the order of 1915 K, providing a robust thermal source for fuel ignition [77,81]. To elaborate, in collaboration with the University of Alabama in Huntsville (UAH), DARPA conducted detailed studies on the catalytic decomposition of nitrous oxide using iridium-based Shell-405 and cobalt-based ZSM-5 catalysts. Both catalyst formulations were shown to promote sufficient N2O decomposition to enable reliable ignition of hydrocarbon fuels. Using the Shell-405 catalyst, nitrous oxide decomposition was achieved at approximately 478 K, while the introduction of a small amount of hydrocarbon fuel further reduced the effective onset temperature to around 366 K [77,81]. Building on these results, UAH constructed a dedicated NOP propulsion test facility and performed hot-fire tests of a thruster with a characteristic length of m over a wide range of mixture ratios (O/F = 4.89–8.68). The experiments demonstrated thrust levels exceeding 222 N (50 lbf) and specific impulses greater than 160 s. Stable combustion was observed for a nitrous oxide mass flow rate of 0.123 kg/s and mixture ratios between 5 and 6, confirming the viability of the catalytic ignition concept for N2O/C3H8 propulsion systems [13,77,82].
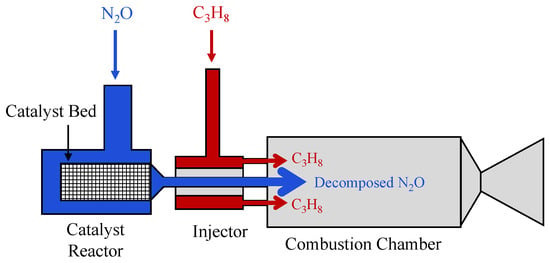
Figure 9.
Schematic of the N2O/C3H8 propulsion concept employing catalytic decomposition of nitrous oxide. This figure was recreated by the authors based on the system layout and design details reported in the original literature [77].
More recently, propane-based nitrous oxide propulsion has also been adopted in small-satellite applications. Hyperion Technologies in the Netherlands developed a compact bipropellant propulsion module employing an N2O/C3H8 combination for attitude control of 6U CubeSats [83]. The system occupies a 2U volume and features extensive use of additive manufacturing for structural and fluidic components. To reduce overall system volume, the propane tank was integrated concentrically within the nitrous oxide tank. A short-duration hot-fire test of approximately 2 s, conducted at 283 K and atmospheric pressure, demonstrated a thrust of 0.75 N, a specific impulse of 290 s, and a combustion efficiency of 94%. Similarly, Valiant Space (Australia) utilized this propellant combination for a 20 N-class thruster, which was slated for orbital deployment aboard a SpaceX Falcon 9 mission [84].
In summary, propane offers a favorable balance of high energy density and logistical convenience, with self-pressurization capabilities suitable for compact systems. However, its vapor pressure is considerably lower than that of nitrous oxide, which limits its utility in passive premixed blow-down systems compared to ethylene. Consequently, propane has been most effectively utilized in systems employing catalytic decomposition of nitrous oxide to trigger auto-ignition, or in standard spark-ignited bipropellant thrusters where simple storage is more critical than maximizing flame speed.
2.4. Other Fuels: Acetylene (C2H2), Methane (CH4), DME (CH3OCH3), and Propylene (C3H6)
Beyond the hydrocarbon fuels discussed above, several other candidates—including acetylene (C2H2), methane (CH4), dimethyl ether (DME, CH3OCH3), and propylene (C3H6)—have also been explored in combination with nitrous oxide, though with comparatively limited scope. While these fuels have not yet reached the same level of technological maturity or system-level demonstration as ethylene-, ethane-, ethanol-, or propane-based systems, prior experimental and analytical studies indicate that they exhibit distinctive combustion characteristics and handling properties when paired with N2O. This subsection therefore reviews representative investigations that illustrate the potential of these alternative fuels in nitrous-oxide-based propulsion applications.
First, acetylene (C2H2) is a colorless gas that is generally regarded as non-toxic, although it exhibits anesthetic effects at high concentrations. Owing to its high formation energy, acetylene is thermodynamically unstable and chemically reactive. Its unsaturated molecular structure, characterized by a carbon–carbon triple bond, enables rapid reactions with a wide range of elements and compounds. In particular, acetylene-oxygen mixtures exhibit an exceptionally wide explosive range, which necessitates strict separation from oxidizers during storage and handling [85]. During combustion, acetylene decomposes rapidly to form highly reactive radical species, which strongly accelerate chain-branching reactions. As a result, acetylene flames exhibit substantially higher burning velocities than those of saturated hydrocarbons. Consistent with this behavior, experimental studies have shown that the flame velocity of N2O/C2H2 mixtures is nearly twice that observed for corresponding mixtures using methane (CH4) or propane (C3H8) as the fuel [79]. While this high reactivity is attractive from a performance standpoint, it also raises significant challenges with respect to combustion control and system safety [86].
A prominent application-oriented effort involving N2O/C2H2 was pursued under DARPA’s Airborne Launch Assist Space Access (ALASA) program initiated in 2011. The program aimed to develop a low-cost launch system capable of delivering a 45 kg payload to orbit at a target cost below 100 USD per pound [87]. The propulsion concept relied on a premixed nitrous oxide-acetylene fuel blend (NOFB), intended to function effectively as a monopropellant and to enable air-launch operations from an F-15E aircraft. However, the extreme chemical sensitivity of the propellant proved difficult to manage. Two explosions occurred during ground testing, highlighting the inherent instability of the premixed N2O/C2H2 system. These safety issues ultimately led DARPA to terminate the ALASA launch vehicle development program in late 2015 [30].
Second, methane (CH4) is a colorless and odorless gas at standard sea-level conditions and constitutes the primary component of natural gas and biogas. Owing to its non-toxic nature, low cost, and high availability, methane has recently attracted significant attention as a cryogenic liquid propellant for large-scale rocket engines [88]. However, with a normal boiling point of 111.65 K and a critical temperature of 190.56 K [89], methane must be stored under cryogenic conditions to remain in the liquid phase. As a result, methane exists exclusively as a gas at room temperature, which makes premixing or co-storage with nitrous oxide particularly challenging.
From a combustion standpoint, the characteristics of N2O/CH4 mixtures have been examined in a number of experimental and kinetic studies alongside other N2O–hydrocarbon combinations [37,78,90]. These studies consistently indicate that methane exhibits relatively low chemical reactivity when combined with nitrous oxide. In particular, ignition delay times for CH4-containing mixtures have been reported to be up to five times longer than those for fuels such as ethylene (C2H4) and ethane (C2H6) under comparable conditions [91]. Similarly, the laminar burning velocities of N2O/CH4 mixtures are significantly lower than those of mixtures employing more reactive fuels such as acetylene (C2H2) or propane (C3H8) [79]. Moreover, increasing the oxidizer concentration in N2O/CH4 flames has been shown to weaken flame stability and promote extinction [92].
Consistent with these combustion characteristics, applications of N2O/CH4 propellants at the thruster or system level have remained limited. Villanueva et al. [93] investigated plasma-assisted combustion of premixed CH4/N2O/Ar flames using a nanosecond pulsed dielectric barrier discharge, focusing on ignition enhancement and NOx emissions. While plasma actuation increased reactive radical concentrations and improved fuel conversion, NOx emissions were found to increase under fuel-lean conditions, highlighting potential trade-offs associated with plasma-assisted operation. Beyond these fundamental combustion studies, thruster-level demonstrations of N2O/CH4 propellants remain scarce. Recently, however, a preliminary study reported the design and hot-fire testing of a small-scale premixed N2O/CH4 thruster [94]. In that work, methane and nitrous oxide were premixed upstream of the injector and ignited using a low-current arc plasma igniter integrated with the combustion chamber. Short-duration hot-firing tests demonstrated stable ignition and sustained combustion, providing an initial indication of the feasibility of plasma-ignited premixed N2O/CH4 propulsion.
Third, dimethyl ether (DME) is an oxygenated organic compound with the chemical formula CH3OCH3. Although it shares the same molecular formula (C2H6O) as ethanol, DME has a distinct molecular structure and belongs to the ether family rather than alcohols. DME is essentially non-carcinogenic and exhibits very low toxicity, and it is typically produced via the catalytic dehydration of methanol, during which water is removed to form the ether [95]. Owing to its high cetane number, DME possesses excellent ignition quality and clean, soot-free combustion characteristics, which has led to significant interest in its use as a next-generation fuel for compression-ignition engines [96].
Laminar burning velocities of N2O/DME mixtures have been measured to characterize the fundamental combustion behavior of this propellant combination [96]. Building on these results, a bipropellant propulsion system employing N2O/DME was initially investigated using a non-premixed configuration. However, repeated spark ignition caused intermittent ignition and extinction, preventing sustained stable combustion [97]. To improve characteristic velocity performance and combustion stability, a premixed propulsion concept was subsequently adopted, as illustrated in Figure 10. In this configuration, both nitrous oxide and DME were stored in the liquid phase and supplied to the combustion chamber after vaporization. A coil-type mixer was installed upstream of the injector to promote homogenization of the gaseous propellants through secondary flows generated by tube curvature. Hot-fire tests of the premixed thruster achieved a maximum characteristic velocity efficiency of approximately 84.5%. The performance shortfall relative to ideal values was attributed to a combination of factors, including injector flow velocity, flame stabilization limits, and mixing effectiveness within the premixed flow path [98].
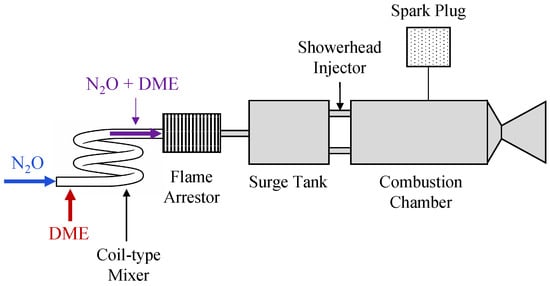
Figure 10.
Schematic of the premixed N2O/Dimethyl Ether (DME) propulsion system employing a coil-type mixer. This figure was recreated by the authors based on the system layout and design details reported in the original literature [98].
Next, propylene (C3H6), also known as propene, is an unsaturated hydrocarbon that exhibits higher chemical reactivity than propane due to the presence of a carbon–carbon double bond. In terms of physical properties, it possesses a vapor pressure of approximately 11.5 bar at 293 K, which is sufficient to enable self-pressurization [99]. The properties of propylene offer distinct advantages of better ignition and flame stabilization compared to propane, while retaining relatively benign handling characteristics compared to more unstable fuels such as acetylene or ethylene [76]. These features have motivated the exploration of N2O/C3H6 propellant combinations for small-scale propulsion applications.
Based on publicly available information, Dawn Aerospace has developed N2O/C3H6 bipropellant thrusters in the 1 N and 20 N thrust classes, referred to as B1 and B20 [100]. These thrusters are reported to have been employed in CubeSat propulsion systems and space transportation vehicles. In the absence of detailed peer-reviewed documentation, these developments nonetheless suggest that propylene is a viable fuel option for compact nitrous-oxide-based propulsion architectures.
In addition to these commercial developments, laboratory-scale investigations have also been carried out within the authors’ research group to further examine N2O/C3H6 combustion behavior. A 15 N-class bipropellant thruster operating on nitrous oxide and propylene was designed and tested under ground conditions (see Figure 11). Both pulse-mode and continuous firing tests were successfully demonstrated, with firing durations of up to 5 s. The measured characteristic exhaust velocities ranged from 1470 to 1500 m/s. These results confirm the practical feasibility of propylene as a hydrocarbon fuel for nitrous-oxide-based bipropellant thrusters and support its potential as a viable alternative to more extensively studied fuel options.
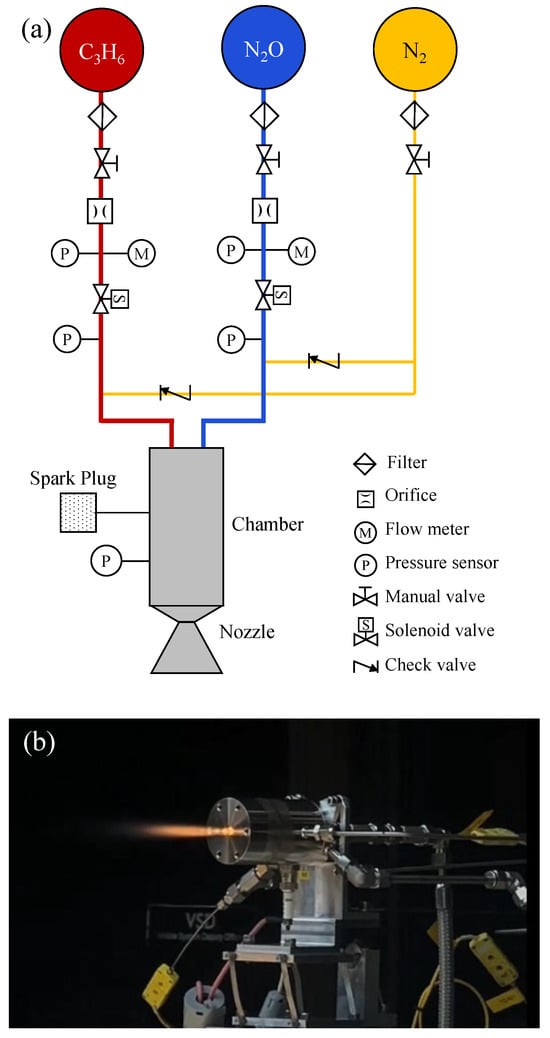
Figure 11.
(a) Schematic of a 15-N-class N2O/C3H6 bipropellant thruster developed for (b) laboratory-scale combustion tests.
Lastly, ammonia (NH3) has recently been explored as a carbon-free fuel in combination with nitrous oxide. Despite its low reactivity, preliminary thruster-level results using plasma-assisted ignition indicate the potential feasibility of N2O/NH3 storable bipropellant propulsion [101].
It should also be noted that nitrous oxide has been widely studied as an oxidizer in hybrid rocket engines in combination with various solid fuels and injector concepts [102,103,104,105]. Because hybrid propulsion involves combustion physics and system architectures that differ fundamentally from those of liquid bipropellant thrusters, a detailed discussion is beyond the scope of the present review, which focuses on liquid-phase propulsion systems. Readers are instead guided to Montanaro et al. [106] for comprehensive discussions of N2O-based hybrid rocket propulsion.
In summary, alternative hydrocarbons introduced in this section exhibit distinct trade-offs between performance and operational feasibility. Acetylene delivers the highest burning velocities but poses severe safety risks, as evidenced by detonation incidents in premixed configurations. Methane, while abundant, is limited in small-scale N2O thrusters due to its low reactivity and the complexity of cryogenic storage. Propylene emerges as a promising intermediate candidate, offering higher reactivity than propane while maintaining safer handling characteristics than acetylene. Meanwhile, oxygenated fuels like DME offer good ignition qualities but face challenges related to mixing and combustion stability in premixed modes.
3. Conclusions
This review has surveyed the combustion characteristics and propulsion-system developments of nitrous oxide (N2O) combined with a range of hydrocarbon fuels, encompassing both premixed fuel blends (NOFBs) and bipropellant configurations. Owing to its self-pressurizing capability, non-toxicity, and favorable storage properties, N2O has emerged as a promising oxidizer for small- and medium-scale spacecraft propulsion, motivating extensive experimental and system-level investigations.
Across the fuels reviewed, including ethylene, ethane, ethanol, propane, acetylene, methane, dimethyl ether, and propylene, distinct trends can be identified. Highly reactive unsaturated fuels such as ethylene and acetylene enable rapid ignition and high flame speeds, which are advantageous for premixed operation but impose stringent requirements on flashback control and thermal management. More thermally stable fuels, including ethane and propane, offer improved robustness and safety margins, lending themselves to bipropellant or catalytically assisted ignition concepts. Oxygenated fuels such as ethanol and DME provide favorable handling and ignition characteristics, though their lower vapor pressures and multiphase behavior introduce additional injector and mixing challenges. Methane, while attractive in large cryogenic propulsion systems, exhibits limited applicability in N2O-based thrusters due to its low reactivity and storage incompatibility. Propylene represents an intermediate option, balancing reactivity and storability, with emerging evidence of practical feasibility at the thruster level.
Overall, the reviewed studies demonstrate that fuel selection plays a decisive role in determining feasible propulsion architectures, ignition strategies, and thermal-management requirements for N2O-based systems. No single hydrocarbon fuel is universally optimal, and the choice must be guided by mission objectives, thrust class, operational mode, and system-level constraints. The comparative overview provided in this review, together with the summarized development status of N2O–hydrocarbon thrusters, offers a consolidated technical perspective to assist in the propellant selection and design of future nitrous-oxide-based propulsion systems.
Author Contributions
Conceptualization, all authors; methodology, E.J. and M.L.; validation, E.J.; formal analysis, all authors; investigation, all authors; resources, M.L.; writing, all authors; funding acquisition, E.S.J. and M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (RS-2024-00449141).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mayer, A.; Wieling, W. Green propulsion research at TNO the Netherlands. Trans. Aerosp. Res. 2018, 4, 5–32. [Google Scholar] [CrossRef]
- Nosseir, A.E.S.; Cervone, A.; Pasini, A. Review of state-of-the-art green monopropellants: For propulsion systems analysts and designers. Aerospace 2021, 8, 20. [Google Scholar] [CrossRef]
- Biedenkopf, K. EU chemicals regulation: Extending its experimentalist REACH. In Extending Experimentalist Governance; Oxford University Press: Oxford, UK, 2015; pp. 107–136. [Google Scholar]
- Keller, W.C. Toxicity assessment of hydrazine fuels. Aviat. Space Environ. Med. 1988, 59, A100–A106. [Google Scholar] [PubMed]
- Sackheim, R.L.; Masse, R.K. Green propulsion advancement: Challenging the maturity of monopropellant hydrazine. J. Propuls. Power 2014, 30, 265–276. [Google Scholar] [CrossRef]
- Aggarwal, R.; Patel, I.; Sharma, P. Green propellant: A study. Int. J. Latest Trends Eng. Technol. 2015, 6, 83–87. [Google Scholar]
- Zakirov, V.; Sweeting, M.; Lawrence, T.; Sellers, J. Nitrous oxide as a rocket propellant. Acta Astronaut. 2001, 48, 353–362. [Google Scholar] [CrossRef]
- Palacz, T. Nitrous oxide application for low-thrust and low-cost liquid rocket engine. In Proceedings of the 7th European Conference for Aero-Space Sciences, Milano, Italy, 3–6 July 2017; pp. 3–6. [Google Scholar]
- Zakirov, V.; Sweeting, M.; Goeman, V.; Lawrence, T. Surrey research on nitrous oxide catalytic decomposition for space applications. In Proceedings of the 14th AIAA/USU Conference on Small Satellites, Logan, UT, USA, 21–24 August 2000. [Google Scholar]
- Taylor, R. Safety and performance advantages of nitrous oxide fuel blends (NOFBX) propellants for manned and unmanned spaceflight applications. A Safer Space Safer World 2012, 699, 67. [Google Scholar]
- Pregger, T.; Schiller, G.; Cebulla, F.; Dietrich, R.U.; Maier, S.; Thess, A.; Lischke, A.; Monnerie, N.; Sattler, C.; Clercq, P.L.; et al. Future fuels—Analyses of the future prospects of renewable synthetic fuels. Energies 2019, 13, 138. [Google Scholar] [CrossRef]
- Mungas, G.; Vozoff, M.; Rishikof, B. NOFBX: A new non-toxic, Green propulsion technology with high performance and low cost. In Proceedings of the 63 International Astronautical Congress, Naples, Italy, 1–5 October 2012; pp. 1–5. [Google Scholar]
- Schulte, G.; Werling, L.; Gernoth, A.; Hagen, D. Development and Testing of a Nitrous Oxide–Propane Rocket Engine. In Proceedings of the 39th AIAA/ASME/SAE/ASEE Joint Propulsion Conference and Exhibit, Astrium Space Infrastructure, Huntsville, AL, USA, 20–23 July 2003. [Google Scholar]
- Haag, G.; Sweeting, M.; Richardson, G. Low cost propulsion development for small satellites at the surrey space centre. In Proceedings of the 13th Small Satellite Conference, Logan, UT, USA, 23–26 August 1999; pp. 1–10. [Google Scholar]
- Zakirov, V.; Sweeting, M.; Lawrence, T. An Update on Surrey Nitrous Oxide Catalytic Decomposition Research; SSC01-XI-2. In Proceedings of the 15th Annual Conference on Small Satellites, Logan, UT, USA, 13–14 August 2001. [Google Scholar]
- Tarantini, V.; Risi, B.; Spina, R.; Orr, N.G.; Zee, R.E. Development of a nitrous oxide-based monopropellant thruster for small spacecraft. In Proceedings of the Small Satellites Systems and Services (4S) Symposium, Valletta, Malta, 30 May–3 June 2016; Volume 5. [Google Scholar]
- Kim, M.; Kim, T. N2O Decomposition on Ru/Si-doped Alumina Catalyst for Monopropellant Thruster. J. Propuls. Energy 2025, 5, 89–98. [Google Scholar] [CrossRef]
- Cai, G.; Sun, W.; Fang, J.; Li, M.; Cong, Y.; Yang, Z. Design and performance characterization of a sub-Newton N2O monopropellant thruster. Aerosp. Sci. Technol. 2012, 23, 439–451. [Google Scholar] [CrossRef]
- Liu, Z.; He, F.; Ma, L.; Peng, S. Recent advances in catalytic decomposition of N2O on noble metal and metal oxide catalysts. Catal. Surv. Asia 2016, 20, 121–132. [Google Scholar] [CrossRef]
- Di Martino, G.D.; Gallo, G.; Mungiguerra, S.; Festa, G.; Savino, R. Design and testing of a monopropellant thruster based on N2O decomposition in Pd/Al2O3 pellets catalytic bed. Acta Astronaut. 2021, 180, 460–469. [Google Scholar] [CrossRef]
- Gaidei, T.P.; Kokorin, A.I.; Pillet, N.; Srukova, M.E.; Khaustova, E.S.; Shmurak, G.G.; Yaroshenko, N.T. The catalytic activity of metallic and deposited oxide catalysts in the decomposition of nitrous oxide. Russ. J. Phys. Chem. A 2007, 81, 895–900. [Google Scholar] [CrossRef]
- Hendley, C.T., IV; Connell, T.L., Jr.; Wilson, D.; Young, G. Catalytic decomposition of nitrous oxide for use in hybrid rocket motors. J. Propuls. Power 2021, 37, 474–478. [Google Scholar] [CrossRef]
- Razus, D. Nitrous oxide: Oxidizer and promoter of hydrogen and hydrocarbon combustion. Ind. Eng. Chem. Res. 2022, 61, 11329–11346. [Google Scholar] [CrossRef]
- Mungas, G.; Fisher, D.; Vozoff, J.; Villa, M. NOFBXTM Single Stage to Orbit Mars Ascent Vehicle. In Proceedings of the 2012 IEEE Aerospace Conference, Big Sky, MT, USA, 3–10 March 2012; Institute of Electrical and Electronics Engineers: New York, NY, USA, 2012; pp. 1–11. [Google Scholar]
- Werling, L.; Lauck, F.; Freudenmann, D.; Röcke, N.; Ciezki, H.; Schlechtriem, S. Experimental investigation of the flame propagation and flashback behavior of a green propellant consisting of N2O and C2H4. J. Energy Power Eng. 2017, 11, 735–752. [Google Scholar] [CrossRef][Green Version]
- Thomas, G.; Oakley, G.; Bambrey, R. Fundamental studies of explosion arrester mitigation mechanisms. Process Saf. Environ. Prot. 2020, 137, 15–33. [Google Scholar] [CrossRef]
- Werling, L.; Hörger, T.; Ciezki, H.; Schlechtriem, S. Experimental and Theoretical Analysis of the Combustion Efficiency and the Heat Loads on a N2O/C2H4 Green Propellant Combustion Chamber. In Proceedings of the 8th European Conference for Aeronautics and Space Sciences, Madrid, Spain, 1–4 July 2019. [Google Scholar]
- Waugh, I.; Moore, E.; Macfarlane, J.; Watts, A.; Mayer, A.E.H.J. Testing of a novel nitrous-oxide and ethanol fuel blend. In Proceedings of the Space Propulsion Conference, Seville, Spain, 14–18 May 2018; pp. 14–18. [Google Scholar]
- Mayer, A.E.H.J.; Wieling, W.; Watts, A.; Poucet, M.; Waugh, I.; Macfarlane, J.; Valencia-Bel, F. European Fuel Blend development for in-space propulsion. In Proceedings of the Space Propulsion Conference, Seville, Spain, 14–18 May 2018; Volume 14. [Google Scholar]
- Walker, M.; Launchbury, J.; Tompkins, S.; Pallota, B.; Hepburn, M.; Sanchez, J. Innovation at DARPA; Technical Report; Defense Advanced Research Projects Agency (DARPA): Arlington, VA, USA, 2016. [Google Scholar]
- Merrill, C. Nitrous Oxide Explosive Hazards; Technical Paper and Briefing Charts AFRL-RZ-ED-TP-2008-184; Air Force Research Laboratory (AFMC), AFRL/RZSP, Edwards Air Force Base: Edwards, CA, USA, 2008. [Google Scholar]
- Sundaram, K.M.; Shreehan, M.M.; Olszewski, E.F. Ethylene. Kirk-Othmer Encycl. Chem. Technol. 2000, 10, 593–632. [Google Scholar]
- Douslin, D.R.; Harrison, R.H. Pressure, volume, temperature relations of ethylene. J. Chem. Thermodyn. 1976, 8, 301–330. [Google Scholar] [CrossRef]
- Werling, L.; Perakis, N.; Müller, S.; Hauck, A.; Ciezki, H.; Schlechtriem, S. Hot firing of a N2O/C2H4 premixed green propellant: First combustion tests and results. In Proceedings of the Space Propulsion Conference, Rome, Italy, 1–6 May 2016. [Google Scholar]
- Friend, D.G.; Ingham, H.; Fly, J.F. Thermophysical properties of ethane. J. Phys. Chem. Ref. Data 1991, 20, 275–347. [Google Scholar] [CrossRef]
- Werling, L.K.; Hörger, T.; Manassis, K.; Grimmeisen, D.; Wilhelm, M.; Erdmann, C.; Ciezki, H.K.; Schlechtriem, S.; Richter, S.; Torsten, M.; et al. Nitrous oxide fuels blends: Research on premixed monopropellants at the german aerospace center (DLR) since 2014. In Proceedings of the American Institute of Aeronautics and Astronautics Propulsion and Energy Forum, Online, 24–26 August 2020; p. 3807. [Google Scholar]
- Hageman, M.D.; Hopper, D.R.; Knadler, M. Detonation limits and velocity deficits of CH4, C2H4, C2H6 and C3H8 with N2O in a small diameter tube. In Proceedings of the American Institute of Aeronautics and Astronautics Propulsion and Energy 2019 Forum, Indianapolis, IN, USA, 19–22 August 2019; p. 3952. [Google Scholar]
- Shimamori, H.; Fessenden, R.W. Mechanism of thermal electron attachment in N2O and N2O–hydrocarbon mixtures in the gas phase. J. Chem. Phys. 1978, 68, 2757–2766. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, H. Laminar burning velocities of C2H4/N2O flames: Experimental study and its chemical kinetics mechanism. Combust. Flame 2019, 202, 362–375. [Google Scholar] [CrossRef]
- Li, Y.H.; Hsu, C.H.; Lin, P.H.; Chen, C.H. Thermal effect and oxygen-enriched effect of N2O decomposition on soot formation in ethylene diffusion flames. Fuel 2022, 329, 125430. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, R.; Xu, S.; Gong, X. Theoretical Study on the Gas-Phase Oxidation Mechanism of Ethylene by Nitrous Oxide. Propellants Explos. Pyrotech. 2022, 47, e202200082. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, H.Y.; Feng, J.C.; Zheng, D. Experimental investigation of auto-ignition of ethylene-nitrous oxide propellants in rapid compression machine. Fuel 2021, 288, 119688. [Google Scholar] [CrossRef]
- Yang, M.; Yang, Y.; Liao, C.; Tang, C.; Zhou, C.W.; Huang, Z. The auto-ignition boundary of ethylene/nitrous oxide as a promising monopropellant. Combust. Flame 2020, 221, 64–73. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, R.; Li, Z.; Xu, S.; Pan, F.; Xie, L. Studies on the flame propagation characteristic and thermal hazard of the premixed N2O/fuel mixtures. Def. Technol. 2020, 16, 564–570. [Google Scholar] [CrossRef]
- Wang, L.Q.; Ma, H.H.; Shen, Z.W.; Pan, J. A comparative study of the explosion behaviors of H2 and C2H4 with air, N2O and O2. Fire Saf. J. 2021, 119, 103260. [Google Scholar] [CrossRef]
- Bangalore Venkatesh, P.; Meyer, S.E.; Bane, S.P.; Grubelich, M.C. Deflagration-to-detonation transition in nitrous oxide/oxygen-fuel mixtures for propulsion. J. Propuls. Power 2019, 35, 944–952. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, H.; Wang, C. Detonation velocity behavior and scaling analysis for ethylene-nitrous oxide mixture. Appl. Therm. Eng. 2017, 127, 671–678. [Google Scholar] [CrossRef]
- Naumann, C.; Kick, T.; Methling, T.; Braun-Unkhoff, M.; Riedel, U. Ethene/dinitrogen oxide-a green propellant to substitute hydrazine: Investigation on its ignition delay time and laminar flame speed. In Proceedings of the 26th International Colloquium on the Dynamics of Explosions and Reactive Systems, Boston, MA, USA, 30 July–4 August 2017. [Google Scholar]
- Janzer, C.; Richter, S.; Naumann, C.; Methling, T. “Green propellants” as a hydrazine substitute: Experimental investigations of ethane/ethene-nitrous oxide mixtures and validation of detailed reaction mechanism. Counc. Eur. Aerosp. Soc. Space J. 2022, 14, 151–159. [Google Scholar] [CrossRef]
- Naumann, C.; Kick, T.; Methling, T.; Braun-Unkhoff, M.; Riedel, U. Ethene/nitrous oxide mixtures as green propellant to substitute hydrazine: Reaction mechanism validation. Int. J. Energetic Mater. Chem. Propuls. 2020, 19, 65–71. [Google Scholar] [CrossRef]
- Perakis, N.; Werling, L.; Ciezki, H.; Schlechtriem, S. Numerical Calculation of Heat Flux Profiles in a N2O/C2H4 Premixed Green Propellant Combustor using an Inverse Heat Conduction Method. In Proceedings of the Space Propulsion Conference, Rome, Italy, 2–6 May 2016; Volume 1. [Google Scholar]
- Werling, L.; Jooß, Y.; Wenzel, M.; Ciezki, H.K.; Schlechtriem, S. A premixed green propellant consisting of N2O and C2H4: Experimental analysis of quenching diameters to design flashback arresters. Int. J. Energetic Mater. Chem. Propuls. 2018, 17, 241–262. [Google Scholar] [CrossRef]
- Werling, L.; Müller, S.; Hauk, A.; Ciezki, H.K.; Schlechtriem, S. Pressure drop measurement of porous materials: Flashback arrestors for a N2O/C2H4 premixed green propellant. In Proceedings of the 52nd AIAA/SAE/ASEE Joint Propulsion Conference, Salt Lake City, UT, USA, 25–27 July 2016; p. 5094. [Google Scholar]
- Werling, L.; Gernoth, A.; Schlechtriem, S. Investigation of the Combustion and Ignition Process of a Nitrous Oxide/Ethene Fuel Blend. In Proceedings of the Space Propulsion Conference, Cologne, Germany, 19–22 May 2014. [Google Scholar]
- Perakis, N.; Hochheimer, B.; Werling, L.; Gernoth, A.; Schlechtriem, S. Development of an Experimental Demonstrator Unit Using Nitrous Oxide/Ethylene Premixed Bipropellant for Satellite Applications. In Proceedings of the Space Propulsion Conference 2014, German Aerospace Center (DLR), Lampoldshausen, Cologne, Germany, 19–22 May 2014. [Google Scholar]
- Werling, L.; Perakis, N.; Hochheimer, B.; Ciezki, H.; Schlechtriem, S. Experimental investigations based on a demonstrator unit to analyze the combustion process of a nitrous oxide/ethene premixed green bipropellant. In Proceedings of the 5th Council of European Aerospace Societies Air & Space Conference, Delft, The Netherlands, 7–11 September 2015; Volume 7. [Google Scholar]
- Werling, L.; Bätz, P. Parameters influencing the characteristic exhaust velocity of a nitrous oxide/ethene green propellant. J. Propuls. Power 2022, 38, 254–266. [Google Scholar] [CrossRef]
- Negri, M.; Werling, L.; Lauck, F.; Goos, E.; Wischek, J.; Besel, Y.; Valencia-Bel, F. High Performance Propellant Development-Overview of Development Activities Regarding Premixed, Green N2O/C2H6 Monopropellants; Paper ID 93. In Proceedings of the 8th Space Propulsion Conference Estoril, Portugal, Estoril, Portugal, 9–13 May 2022. [Google Scholar]
- Mungas, G.; Vozoff, M.; Rishikof, B.; Strack, D.; London, A.; Fryer, J.; Buchanan, L. NOFBX® Monopropulsion Overview. In Proceedings of the 14th Annual FAA Commercial Space Transportation Conference, Washington, DC, USA, 9–10 February 2011; Volume 626, pp. 755–8819. [Google Scholar]
- SpaceNews. Vast selects Impulse Space for Haven-1 Space Station Propulsion. 2023. Available online: https://spacenews.com/vast-selects-impulse-space-for-haven-1-space-station-propulsion/ (accessed on 14 January 2026).
- Tokudome, S.; Yagishita, T.; Goto, K.; Suzuki, N.; Yamamoto, T.; Daimoh, Y. An Experimental Study of an N2O/Ethanol Propulsion System with 2 kN Thrust Class BBM. Trans. Jpn. Soc. Aeronaut. Space Sci. Aerosp. Technol. Jpn. 2021, 19, 186–192. [Google Scholar]
- Mota, F.A.d.S.; Hinckel, J.N.; Rocco, E.M.; Schlingloff, H. Modeling and analysis of a LOX/Ethanol liquid rocket engine. J. Aerosp. Technol. Manag. 2018, 10, e3018. [Google Scholar] [CrossRef]
- Grayson, G.; Watts, D. Nitrous oxide and ethanol propulsion concepts for a crew space vehicle. In Proceedings of the 43rd AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Cincinnati, OH, USA, 8–11 July 2007; p. 5462. [Google Scholar]
- Dillon, H.E.; Penoncello, S.G. A fundamental equation for calculation of the thermodynamic properties of ethanol. Int. J. Thermophys. 2004, 25, 321–335. [Google Scholar] [CrossRef]
- Lee, I.; Son, M.; Koo, J. Atomization and combustion characteristics of ethanol/nitrous oxide at various momentum flux ratios. Energy Fuels 2014, 28, 2770–2779. [Google Scholar] [CrossRef]
- Kim, D.; Park, J.; Yu, M.; Lee, K.; Koo, J. Visualization of Transient Ignition Flow-field in a 50 N Scale N2O/C2H5OH Thruster. J. Korean Soc. Propuls. Eng. 2014, 18, 11–18. [Google Scholar] [CrossRef]
- Tokudome, S.; Yagishita, T.; Habu, H.; Shimada, T.; Daimou, Y. Experimental study of an N2O/ethanol propulsion system. In Proceedings of the 43rd AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Cincinnati, OH, USA, 8–11 July 2007; p. 5464. [Google Scholar]
- Tokudome, S.; Goto, K.; Yagishita, T.; Suzuki, N.; Yamamoto, T. An experimental study of a nitrous oxide/ethanol (NOEL) propulsion system. In Proceedings of the American Institute of Aeronautics and Astronautics Propulsion and Energy 2019 Forum, Indianapolis, IN, USA, 19–22 August 2019; p. 4429. [Google Scholar]
- Sato, T.; Nakata, K.; Ishihara, K.; Itouyama, N.; Matsuoka, K.; Kasahara, J.; Kawasaki, A.; Nakata, D.; Eguchi, H.; Uchiumi, M.; et al. Combustion structure of a cylindrical rotating detonation engine with liquid ethanol and nitrous oxide. Combust. Flame 2024, 264, 113443. [Google Scholar] [CrossRef]
- Ishihara, K.; Sato, T.; Kimura, T.; Nakajima, K.; Nakata, K.; Itouyama, N.; Kawasaki, A.; Matsuoka, K.; Matsuyama, K.; Kasahara, J.; et al. Nitrous Oxide/Ethanol Cylindrical Rotating Detonation Engine for Sounding Rocket Space Flight. J. Spacecr. Rocket. 2025, 62, 44–54. [Google Scholar] [CrossRef]
- Kakami, A.; Ishibashi, T.; Ideta, K.; Tachibana, T. One-Newton class thruster using arc discharge assisted combustion of nitrous oxide/ethanol bipropellant. Vacuum 2014, 110, 172–176. [Google Scholar] [CrossRef]
- Kakami, A.; Egawa, T.; Yamamoto, N.; Tachibana, T. Plasma-assisted combustion of N2O/Ethanol propellant for Space Propulsion. In Proceedings of the 46th AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Nashville, TN, USA, 25–28 July 2010; p. 6806. [Google Scholar]
- Youngblood, S.H. Design and Testing of a Liquid Nitrous Oxide and Ethanol Fueled Rocket Engine. ProQuest Number: 1606125. Master’s Thesis, New Mexico Institute of Mining and Technology, Socorro, NM, USA, 2015. [Google Scholar]
- Phillip, J.; Youngblood, S.; Grubelich, M.; Saul, W.V.; Hargather, M.J. Development and testing of a nitrous-oxide/ethanol bi-propellant rocket engine. In Proceedings of the 52nd AIAA/SAE/ASEE Joint Propulsion Conference, Salt Lake City, UT, USA, 25–27 July 2016; p. 5092. [Google Scholar]
- Lemmon, E.W.; McLinden, M.O.; Wagner, W. Thermodynamic properties of propane. III. A reference equation of state for temperatures from the melting line to 650 K and pressures up to 1000 MPa. J. Chem. Eng. Data 2009, 54, 3141–3180. [Google Scholar] [CrossRef]
- Chao, J.; Wilhoit, R.C.; Zwolinski, B.J. Ideal gas thermodynamic properties of ethane and propane. J. Phys. Chem. Ref. Data 1973, 2, 427–438. [Google Scholar] [CrossRef]
- Herdy, R. Nitrous oxide/hydrocarbon fuel advanced chemical propulsion: DARPA contract overview. In Proceedings of the 17th Annual Thermal and Fluids Analysis Workshop (TFAWS 2006), College Park, ML, USA, 7–11 August 2006. [Google Scholar]
- Powell, O.A.; Papas, P.; Dreyer, C.B. Hydrogen-and C1–C3 Hydrocarbon-Nitrous Oxide Kinetics in Freely Propagating and Burner-Stabilized Flames, Shock Tubes, and Flow Reactors. Combust. Sci. Technol. 2010, 182, 252–283. [Google Scholar] [CrossRef]
- Powell, O.A.; Papas, P.; Dreyer, C. Laminar burning velocities for hydrogen-, methane-, acetylene-, and propane-nitrous oxide flames. Combust. Sci. Technol. 2009, 181, 917–936. [Google Scholar] [CrossRef]
- Dubkov, K.A.; Parfenov, M.V.; Kharitonov, A.S. Gas-phase oxidation of a propane–propylene mixture by nitrous oxide. Ind. Eng. Chem. Res. 2020, 59, 14157–14162. [Google Scholar] [CrossRef]
- Balasubramanyam, M.; Moser, M.; Sharp, D. Catalytic ignition of nitrous oxide with propane/propylene mixtures for rocket motors. In Proceedings of the 41st AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Tucson, AZ, USA, 10–13 July 2005; p. 3919. [Google Scholar]
- Tiliakos, N.; Tyll, J.; Herdy, R.; Sharp, D.; Moser, M.; Smith, N. Development and testing of a nitrous oxide/propane rocket engine. In Proceedings of the 37th Joint Propulsion Conference and Exhibit, Salt Lake City, UT, USA, 8–11 July 2001; p. 3258. [Google Scholar]
- Powell, S.; Knop, T.; Engelen, S. Experimental Evaluation of a Green Bi-Propellant Thruster for Small Satellite Applications; SSC16-X-8. In Proceedings of the 30th Annual Small Satellite Conference on Small Satellites, Hyperion Technologies B.V., Delft, The Netherlands, 6–11 August 2016. [Google Scholar]
- ABC News. Queensland Space Startup Valiant Space Eyes Rocket Launch as SpaceX Looms Large. Available online: https://www.abc.net.au/news/2022-11-17/qld-space-rockets-valiant-space-start-up-company-spacex/101660100 (accessed on 14 January 2026).
- Pässler, P.; Hefner, W.; Buckl, K.; Meinass, H.; Meiswinkel, A.; Wernicke, H.J.; Ebersberg, G.; Müller, R.; Bässler, J.; Behringer, H.; et al. Acetylene. In Ullmann’s Encyclopedia of Industrial Chemistry; John Wiley Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Aldous, K.; Bailey, B.; Rankin, J. Burning velocity of the premixed nitrous-oxide/acetylene flame and its influence on burner design. Anal. Chem. 1972, 44, 191–194. [Google Scholar] [CrossRef]
- Spravka, J.J.; Jorris, T.R. Current hypersonic and space vehicle flight test instrumentation challenges. In Proceedings of the American Institute of Aeronautics and Astronautics Flight Testing Conference, Dallas, TX, USA, 22–26 June 2015; p. 3224. [Google Scholar]
- Neill, T.; Judd, D.; Veith, E.; Rousar, D. Practical uses of liquid methane in rocket engine applications. Acta Astronaut. 2009, 65, 696–705. [Google Scholar] [CrossRef]
- Friend, D.G.; Ely, J.F.; Ingham, H. Thermophysical properties of methane. J. Phys. Chem. Ref. Data 1989, 18, 583–638. [Google Scholar] [CrossRef]
- Powell, O.; Miller, J.; Dreyer, C.; Papas, P. Characterization of hydrocarbon/nitrous oxide propellant combinations. In Proceedings of the 46th AIAA Aerospace Sciences Meeting and Exhibit, Reno, NV, USA, 7–10 January 2008; p. 999. [Google Scholar]
- Mével, R.; Shepherd, J. Ignition delay-time behind reflected shock waves of small hydrocarbons–nitrous oxide (–oxygen) mixtures. Shock Waves 2015, 25, 217–229. [Google Scholar] [CrossRef]
- Newman-Lehman, T.; Grana, R.; Seshadri, K.; Williams, F. The structure and extinction of nonpremixed methane/nitrous oxide and ethane/nitrous oxide flames. Proc. Combust. Inst. 2013, 34, 2147–2153. [Google Scholar] [CrossRef]
- Villanueva, B.N.; Lin, P.H.; Li, Y.H.; Honra, J.P. Effect of plasma assisted combustion on emissions of a premixed nitrous oxide fuel blend. Results Eng. 2025, 25, 104251. [Google Scholar] [CrossRef]
- Lee, T.Y.; Kim, T. Design and Performance Evaluation of CH4/N2O Premixed Propellant Thruster. SSRN 2025, SSRN:5237487. Available online: https://ssrn.com/abstract=5237487 (accessed on 10 December 2025).
- Semelsberger, T.A.; Borup, R.L.; Greene, H.L. Dimethyl ether (DME) as an alternative fuel. J. Power Sources 2006, 156, 497–511. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Tachibana, T. Burning velocities of dimethyl ether (DME)–nitrous oxide (N2O) mixtures. Fuel 2018, 217, 160–165. [Google Scholar] [CrossRef]
- Asakura, T.; Hayashi, S.; Yano, Y.; Kakami, A. Influence of Injector for Performance of N2O/DME Bipropellant Thruster. Trans. Jpn. Soc. Aeronaut. Space Sci. Aerosp. Technol. Jpn. 2018, 16, 177–180. [Google Scholar] [CrossRef]
- Kakami, A.; Kuranaga, A.; Yano, Y. Premixing-type liquefied gas bipropellant thruster using nitrous oxide/dimethyl ether. Aerosp. Sci. Technol. 2019, 94, 105351. [Google Scholar] [CrossRef]
- Chao, J.; Zwolinski, B.J. Ideal gas thermodynamic properties of ethylene and propylene. J. Phys. Chem. Ref. Data 1975, 4, 251–262. [Google Scholar] [CrossRef]
- SatNews. Dawn Aerospace’s Smallsat Green Propellant Thruster Proves Itself on-orbit with D-Orbit’s ION Space Tug. 2021. Available online: https://news.satnews.com/2021/05/04/dawn-aerospaces-smallsat-green-propellant-thruster-proves-itself-on-orbit-with-d-orbits-ion-space-tug/ (accessed on 14 December 2025).
- Lee, J.; Kim, S.; Jo, H.; Lee, A.; Kang, H. Ammonia-based storable bipropellant (N2O/NH3) thruster utilizing dual-mode plasma ignition for enhanced propellant flexibility. Fuel 2026, 406, 136893. [Google Scholar] [CrossRef]
- Okuda, R.; Komizu, K.; Tsuji, A.; Miwa, T.; Fukada, M.; Yokobori, S.; Soeda, K.; Kamps, L.; Nagata, H. Fuel regression characteristics of axial-injection end-burning hybrid rocket using nitrous oxide. J. Propuls. Power 2022, 38, 759–770. [Google Scholar] [CrossRef]
- Gallo, G.; Kamps, L.; Hirai, S.; Carmicino, C.; Harunori, N. Prediction of the fuel regression-rate in a HDPE single port hybrid rocket fed by liquid nitrous oxide. Combust. Flame 2024, 259, 113160. [Google Scholar] [CrossRef]
- Lee, S.; Ugolini, V.M.P.; Jung, E.; Kwon, S. Demonstration of Polyethylene Nitrous Oxide Catalytic Decomposition Hybrid Thruster with Dual-Catalyst Bed Preheated by Hydrogen Peroxide. Aerospace 2025, 12, 158. [Google Scholar] [CrossRef]
- Whitmore, S.A. Additively manufactured acrylonitrile-butadiene-styrene–nitrous-oxide hybrid rocket motor with electrostatic igniter. J. Propuls. Power 2015, 31, 1217–1220. [Google Scholar] [CrossRef]
- Montanaro, A.; Piazzullo, D.; Tortorici, D.; Ingenito, A.; Allocca, L. Green rocket propulsion: Overview of nitrous oxide applications with emphasis on hybrid systems. Fuel 2026, 406, 137036. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.