Surrogate Models and Related Combustion Reaction Mechanisms for a Coal-Derived Alternative Jet Fuel and Its Blends with a Traditional RP-3
Abstract
1. Introduction
2. Experimental Methods
2.1. GC × GC Analysis
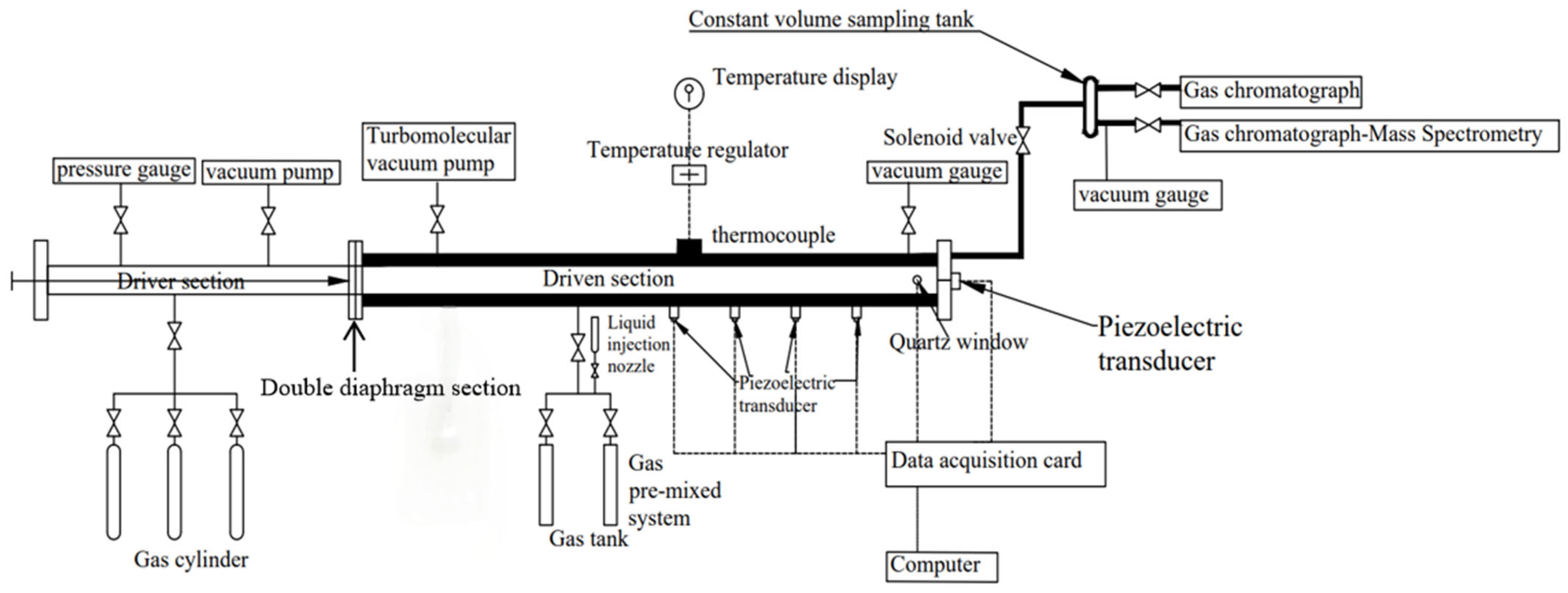
2.2. Shock-Tube Experiment
2.3. Kinetic Modeling Approach
3. Results and Discussion
3.1. Physicochemical Properties
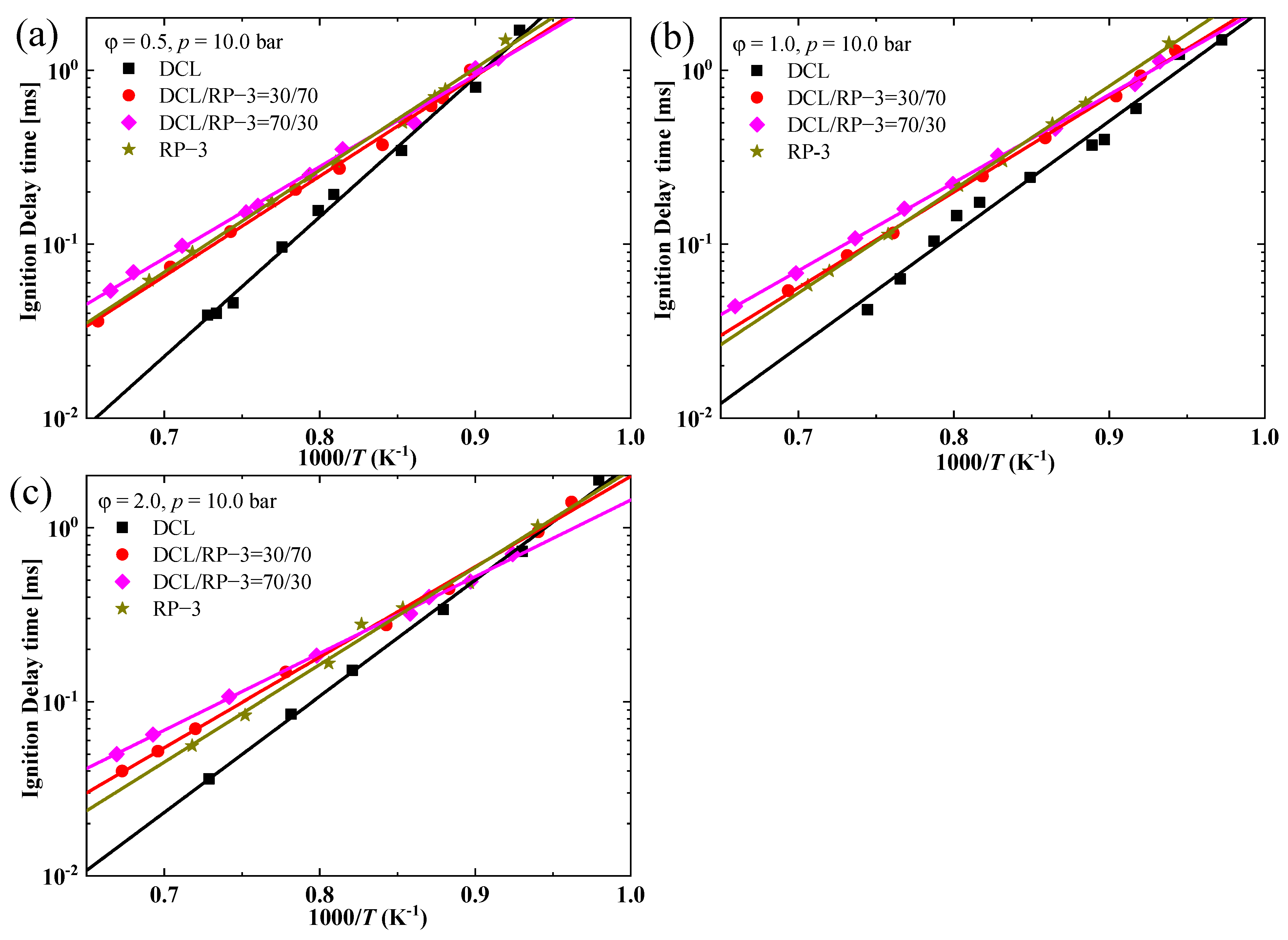
3.2. Ignition Properties of the Blends
3.3. Surrogate Models and Combustion Reaction Mechanism
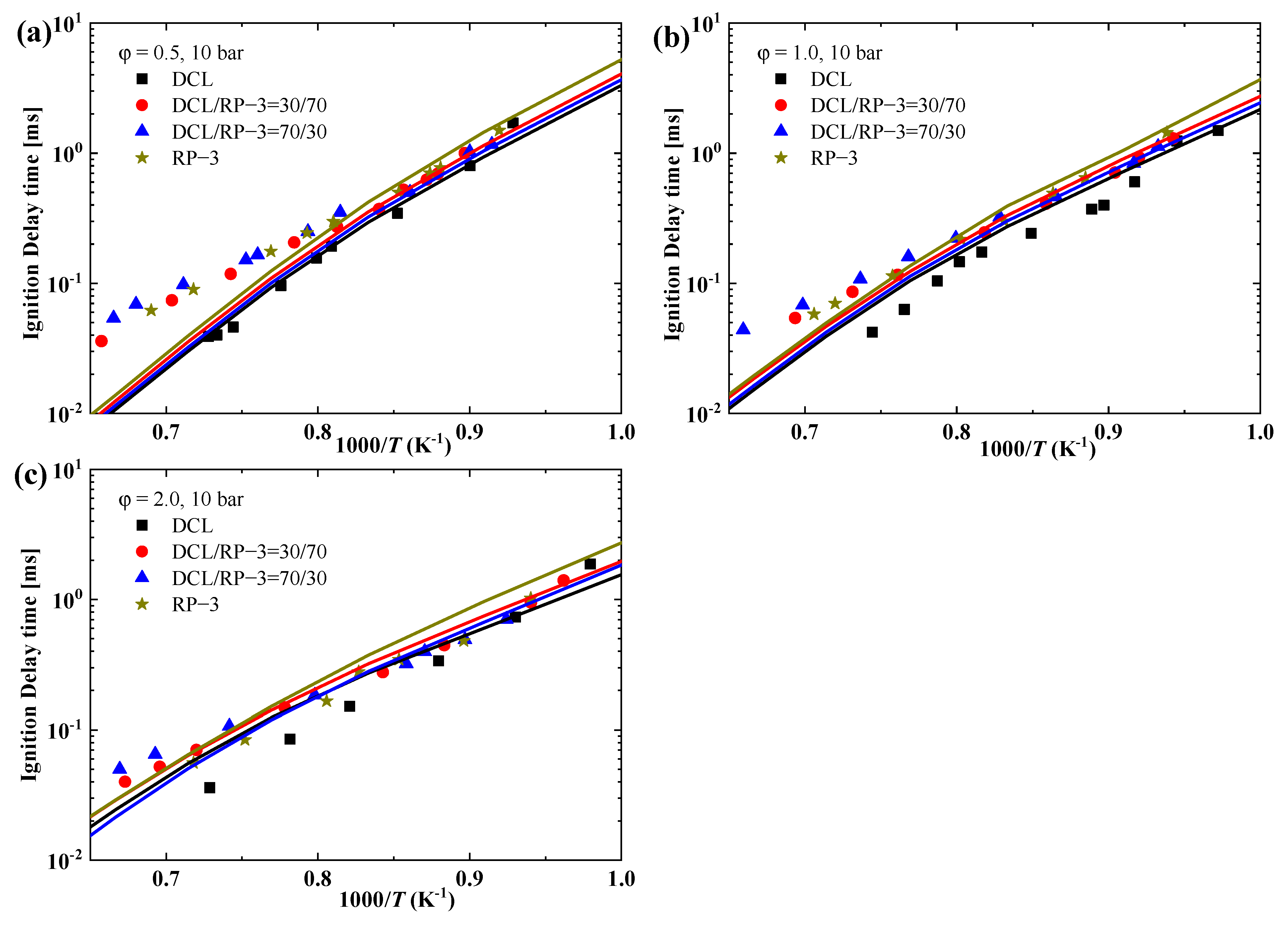
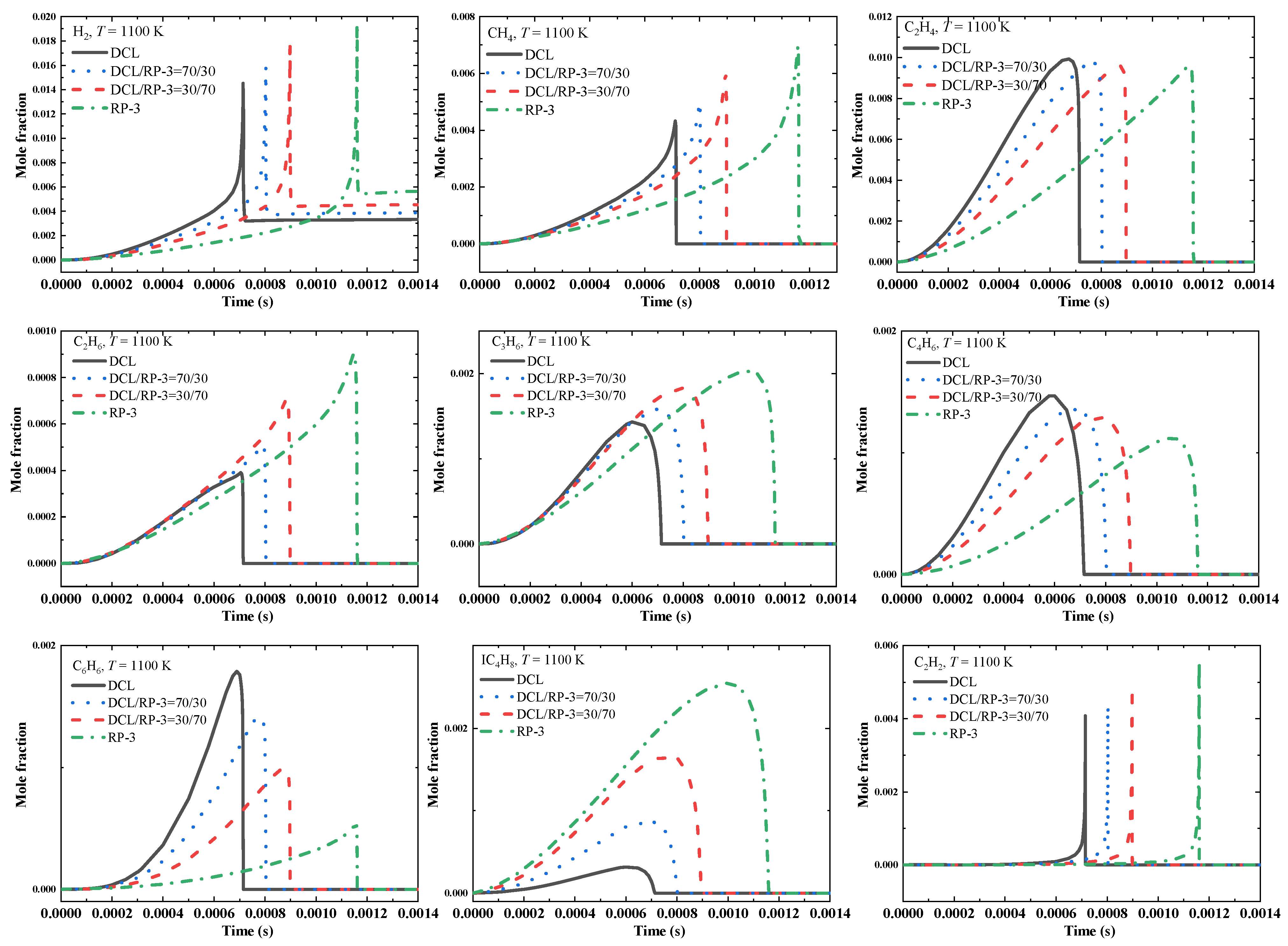
3.4. Kinetic Modeling Results
4. Conclusions
- Advanced physicochemical analysis and chemical compositions were carried out for the DCL jet fuel and its blends with RP-3. Generally, the major physicochemical properties of the DCL jet fuel satisfied most of the airworthiness certification standards, and it is cleaner, with a lower freezing point (−66.0 °C compared with that of −52.5 °C of RP-3);
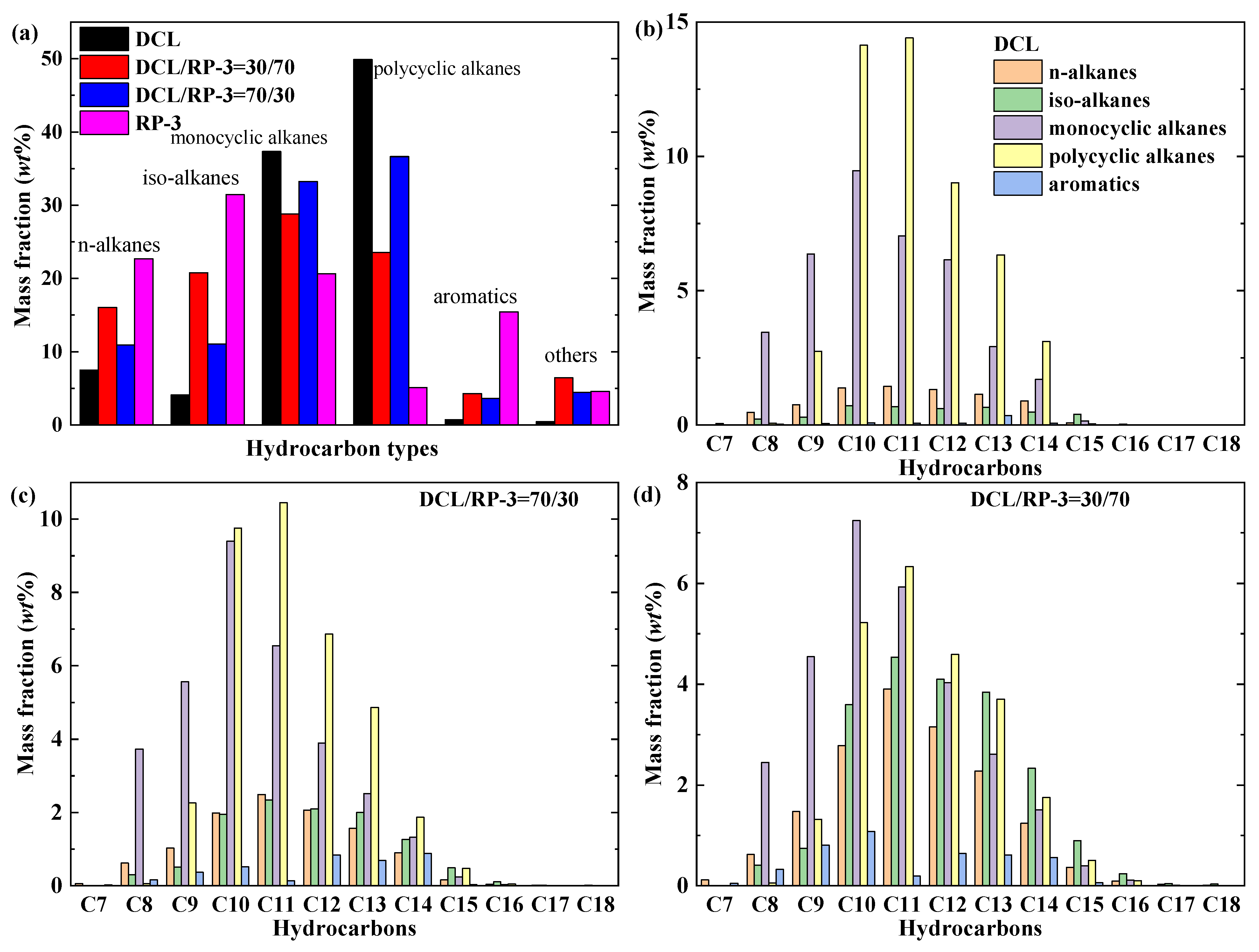
- Advanced GC × GC analysis revealed that the DCL jet fuel is mainly composed of cyclic alkanes (87.21% mass fraction) with a small number of n/iso-alkanes (11.59%), and the carbon numbers are primarily distributed within the C9–C14 range;
- The IDTs of the blended fuels of DCL and RP-3 with different volume ratios do not exhibit a large difference, and they tend to be close to the IDTs of RP-3 within the studied combustion conditions, i.e., equivalence ratios of 0.5, 1.0, and 2.0 and temperature ranges 1030–1530 K, with pressure at approximately 10 bar;
- ROP analysis indicated that major intermediates, including 1,3-butadiene and benzene, can be largely formed due to the dehydrogenation and ring-opening reactions of cyclic alkanes during the ignition process of DCL, while the quantities of other intermediates, including hydrogen, methane, propene, acetylene, iso-butene, ethylene, and ethane, increase as the volume ratios of RP-3 increase;
- Sensitivity analysis results highlighted that the ignition properties of the studied jet fuels are significantly affected by the chemical compositions and molecular structures of the jet fuels. Future studies on more accurate chemical composition analysis and other combustion properties, including laminar flame speeds and soot, should be conducted for surrogate model and mechanism optimization, which is critical for airworthiness certification and large-scale commercial applications of the DCL jet fuels.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| DCL | Direct coal liquefaction |
| GC × GC | Two-dimensional gas chromatography |
| HPST | High-pressure shock tube |
| IDT | Ignition delay time |
References
- Pires, A.P.P.; Han, Y.L.; Kramlich, J.; Garcia-Perez, M. Chemical Composition and Fuel Properties of Alternative Jet Fuels. Bioresources 2018, 13, 2632–2657. [Google Scholar] [CrossRef]
- Zhang, C.; Hui, X.; Lin, Y.Z.; Sung, C.J. Recent development in studies of alternative jet fuel combustion: Progress, challenges, and opportunities. Renew. Sustain. Energy Rev. 2016, 54, 120–138. [Google Scholar] [CrossRef]
- Mochida, I.; Okuma, O.; Yoon, S.H. Chemicals from Direct Coal Liquefaction. Chem. Rev. 2014, 114, 1637–1672. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Rolfe, A.; Rezvani, S.; Herrador, J.M.H.; Franco, F.; Pinto, F.; Snape, C.; Hewitt, N. Converting brown coal to synthetic liquid fuels through direct coal liquefaction technology:Techno-economicevaluation. Int. J. Energy Res. 2020, 44, 11827–11839. [Google Scholar] [CrossRef]
- Khodakov, A.Y.; Chu, W.; Fongarland, P. Advances in the development of novel cobalt Fischer-Tropsch catalysts for synthesis of long-chain hydrocarbons and clean fuels. Chem. Rev. 2007, 107, 1692–1744. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; He, Y.; Tan, L.; Zhang, P.; Peng, X.; Oruganti, A.; Yang, G.; Abe, H.; Wang, Y.; Tsubaki, N. Integrated tuneable synthesis of liquid fuels via Fischer–Tropsch technology. Nat. Catal. 2018, 1, 787–793. [Google Scholar] [CrossRef]
- Sankaran, V. Research and development needs in combustion modeling. Appl. Energy Combust. Sci. 2025, 21, 100307. [Google Scholar] [CrossRef]
- Naik, C.V.; Puduppakkam, K.V.; Modak, A.; Meeks, E.; Wang, Y.L.; Feng, Q.Y.; Tsotsis, T.T. Detailed chemical kinetic mechanism for surrogates of alternative jet fuels. Combust. Flame 2011, 158, 434–445. [Google Scholar] [CrossRef]
- Dagaut, P.; Karsenty, F.; Dayma, G.; Dievart, P.; Hadj-Ali, K.; Mze-Ahmed, A.; Braun-Unkhoff, M.; Herzler, J.; Kathrotia, T.; Kick, T.; et al. Experimental and detailed kinetic model for the oxidation of a Gas to Liquid (GtL) jet fuel. Combust. Flame 2014, 161, 835–847. [Google Scholar] [CrossRef]
- Dagaut, P.; Dievart, P. Combustion of synthetic jet fuels: Naphthenic cut and blend with a gas-to-liquid (GtL) jet fuel. Proc. Combust. Inst. 2017, 36, 433–440. [Google Scholar] [CrossRef]
- Valco, D.; Gentz, G.; Allen, C.; Colket, M.; Edwards, T.; Gowdagiri, S.; Oehlschlaeger, M.A.; Toulson, E.; Lee, T. Autoignition behavior of synthetic alternative jet fuels: An examination of chemical composition effects on ignition delays at low to intermediate temperatures. Proc. Combust. Inst. 2015, 35, 2983–2991. [Google Scholar] [CrossRef]
- Melder, J.; Zinsmeister, J.; Grein, T.; Jürgens, S.; Köhler, M.; Oßwald, P. Comprehensive Two-Dimensional Gas Chromatography: A Universal Method for Composition-Based Prediction of Emission Characteristics of Complex Fuels. Energy Fuels 2023, 37, 4580–4595. [Google Scholar] [CrossRef]
- Heyne, J.; Bell, D.; Feldhausen, J.; Yang, Z.; Boehm, R. Towards fuel composition and properties from Two-dimensional gas chromatography with flame ionization and vacuum ultraviolet spectroscopy. Fuel 2022, 312, 122709. [Google Scholar] [CrossRef]
- Wu, Z.; Mao, Y.; Raza, M.; Zhu, J.; Feng, Y.; Wang, S.; Qian, Y.; Yu, L.; Lu, X. Surrogate fuels for RP-3 kerosene formulated by emulating molecular structures, functional groups, physical and chemical properties. Combust. Flame 2019, 208, 388–401. [Google Scholar] [CrossRef]
- Dooley, S.; Won, S.H.; Chaos, M.; Heyne, J.; Ju, Y.; Dryer, F.L.; Kumar, K.; Sung, C.-J.; Wang, H.; Oehlschlaeger, M.A.; et al. A jet fuel surrogate formulated by real fuel properties. Combust. Flame 2010, 157, 2333–2339. [Google Scholar] [CrossRef]
- Kim, D.; Martz, J.; Abdul-Nour, A.; Yu, X.; Jansons, M.; Violi, A. A six-component surrogate for emulating the physical and chemical characteristics of conventional and alternative jet fuels and their blends. Combust. Flame 2017, 179, 86–94. [Google Scholar] [CrossRef]
- Kathrotia, T.; Oßwald, P.; Naumann, C.; Richter, S.; Köhler, M. Combustion kinetics of alternative jet fuels, Part-II: Reaction model for fuel surrogate. Fuel 2021, 302, 120736. [Google Scholar] [CrossRef]
- Kathrotia, T.; Oßwald, P.; Zinsmeister, J.; Methling, T.; Köhler, M. Combustion kinetics of alternative jet fuels, Part-III: Fuel modeling and surrogate strategy. Fuel 2021, 302, 120737. [Google Scholar] [CrossRef]
- Yang, Z.-Y.; Zeng, P.; Wang, B.-Y.; Jia, W.; Xia, Z.-X.; Liang, J.; Wang, Q.-D. Ignition characteristics of an alternative kerosene from direct coal liquefaction and its blends with conventional RP-3 jet fuel. Fuel 2021, 291, 120258. [Google Scholar] [CrossRef]
- Richter, S.; Kukkadapu, G.; Westbrook, C.K.; Braun-Unkhoff, M.; Naumann, C.; Köhler, M.; Riedel, U. A combined experimental and modeling study of combustion properties of an isoparaffinic alcohol-to-jet fuel. Combust. Flame 2022, 240, 111994. [Google Scholar] [CrossRef]
- Abi Nurazaq, W.; Wang, W.-C.; Lin, J.-K. The properties of sustainable aviation fuel II: Laminar flame speed. Energy 2024, 294, 130946. [Google Scholar] [CrossRef]
- Guzman, J.; Brezinsky, K. Experimental and modeling study of the oxidation of F-24 jet fuel, and its mixture with an iso-paraffinic synthetic jet fuel, ATJ. Combust. Flame 2021, 224, 108–125. [Google Scholar] [CrossRef]
- Yang, Z.; Kosir, S.; Stachler, R.; Shafer, L.; Anderson, C.; Heyne, J.S. A GC × GC Tier α combustor operability prescreening method for sustainable aviation fuel candidates. Fuel 2021, 292, 120345. [Google Scholar] [CrossRef]
- Wang, Q.-D.; Wang, B.-Y.; Yao, Q.; Liang, J.; Zeng, P.; Liu, J.-G.; Xia, Z.-X. An experimental and kinetic modeling study on the ignition property of an alternative gas to liquid jet fuel. Combust. Flame 2025, 271, 113805. [Google Scholar] [CrossRef]
- Wang, Q.-D.; Zeng, P.; Yao, Q.; Liang, J.; Wang, B.-Y.; Huang, F.; Liu, J.-G.; Xia, Z.-X. An experimental and kinetic modeling study on the ignition kinetics of a sustainable aviation fuel and its blends with a traditional RP-3 jet fuel. Fuel 2025, 380, 133191. [Google Scholar] [CrossRef]
- Wang, B.-Y.; Zeng, P.; He, R.; Li, F.; Yang, Z.-Y.; Xia, Z.-X.; Liang, J.; Wang, Q.-D. Single-Pulse Shock Tube Experimental and Kinetic Modeling Study on Pyrolysis of a Direct Coal Liquefaction-Derived Jet Fuel and Its Blends with the Traditional RP-3 Jet Fuel. ACS Omega 2021, 6, 18442–18450. [Google Scholar] [CrossRef]
- Fang, X.Y.; Huang, X.Y.; Chen, W.K.; Qiao, X.Q.; Ju, D.H. Development of a skeletal surrogate mechanism for emulating combustion characteristics of diesel from direct coal liquefaction. Combust. Flame 2020, 218, 84–97. [Google Scholar] [CrossRef]
- Wang, F.; Rijal, D. Sustainable Aviation Fuels for Clean Skies: Exploring the Potential and Perspectives of Strained Hydrocarbons. Energy Fuels 2024, 38, 4904–4920. [Google Scholar] [CrossRef]
- Panigrahy, S.; Liang, J.; Ghosh, M.K.; Wang, Q.-D.; Zuo, Z.; Nagaraja, S.; Mohamed, A.A.E.-S.; Kim, G.; Vasu, S.S.; Curran, H.J. An experimental and detailed kinetic modeling study of the pyrolysis and oxidation of allene and propyne over a wide range of conditions. Combust. Flame 2021, 233, 111578. [Google Scholar] [CrossRef]
- Liang, J.; Zhao, C.; Zhao, Z.; Wang, X.; Jia, M.-X.; Wang, Q.-D.; Zhang, Y.; Zhao, F. An experimental and kinetic modeling study on the high-temperature ignition and pyrolysis characteristics of cyclohexylamine. Combust. Flame 2023, 252, 112769. [Google Scholar] [CrossRef]
- Wang, Q.-D.; Sun, Y.; Zhao, Z.; Zhang, Y.; Zhao, F.; Li, Y.; Liang, J. Ignition kinetics of nitrocyclohexane behind reflected shock waves in inert and air environments. Combust. Flame 2023, 255, 112865. [Google Scholar] [CrossRef]
- Morley, C. Gaseq: A Chemical Equilibrium Program for Windows, 0.79. 2005. Available online: http://www.gaseq.co.uk/ (accessed on 17 September 2010).
- Petersen, E.L.; Rickard, M.J.A.; Crofton, M.W.; Abbey, E.D.; Traum, M.J.; Kalitan, D.M. A facility for gas- and condensed-phase measurements behind shock waves. Meas. Sci. Technol. 2005, 16, 1716–1729. [Google Scholar] [CrossRef]
- Guzman, J.; Kukkadapu, G.; Brezinsky, K.; Westbrook, C. Experimental and modeling study of the pyrolysis and oxidation of an iso-paraffinic alcohol-to-jet fuel. Combust. Flame 2019, 201, 57–64. [Google Scholar] [CrossRef]
- Baigmohammadi, M.; Patel, V.; Nagaraja, S.; Ramalingam, A.; Martinez, S.; Panigrahy, S.; Mohamed, A.A.E.-S.; Somers, K.P.; Burke, U.; Heufer, K.A.; et al. Comprehensive Experimental and Simulation Study of the Ignition Delay Time Characteristics of Binary Blended Methane, Ethane, and Ethylene over a Wide Range of Temperature, Pressure, Equivalence Ratio, and Dilution. Energy Fuels 2020, 34, 8808–8823. [Google Scholar] [CrossRef]
- Goodwin, D.G.; Speth, R.L.; Moffat, H.K.; Weber, B.W. Cantera: An Object-oriented Software Toolkit for Chemical Kinetics, Thermodynamics, and Transport Processes; Version 2.4.0; Cantera: Tulsa, OK, USA, 2018. [Google Scholar]
- Davidson, D.F.; Hanson, R.K. Recent advances in shock tube/laser diagnostic methods for improved chemical kinetics measurements. Shock Waves 2009, 19, 271–283. [Google Scholar] [CrossRef]
- Sung, C.J.; Curran, H.J. Using rapid compression machines for chemical kinetics studies. Prog. Energy Combust. Sci. 2014, 44, 1–18. [Google Scholar] [CrossRef]
- Wang, Q.-D.; Fang, Y.-M.; Wang, F.; Li, X.-Y. Systematic analysis and reduction of combustion mechanisms for ignition of multi-component kerosene surrogate. Proc. Combust. Inst. 2013, 34, 187–195. [Google Scholar] [CrossRef]
- Curran, H.J. Developing detailed chemical kinetic mechanisms for fuel combustion. Proc. Combust. Inst. 2019, 37, 57–81. [Google Scholar] [CrossRef]
- Bugler, J.; Marks, B.; Mathieu, O.; Archuleta, R.; Camou, A.; Gregoire, C.; Heufer, K.A.; Petersen, E.L.; Curran, H.J. An ignition delay time and chemical kinetic modeling study of the pentane isomers. Combust. Flame 2016, 163, 138–156. [Google Scholar] [CrossRef]
- Darcy, D.; Tobin, C.J.; Yasunaga, K.; Simmie, J.M.; Wurmel, J.; Metcalfe, W.K.; Niass, T.; Ahmed, S.S.; Westbrook, C.K.; Curran, H.J. A high pressure shock tube study of n-propylbenzene oxidation and its comparison with n-butylbenzene. Combust. Flame 2012, 159, 2219–2232. [Google Scholar] [CrossRef]
- El-Sabor Mohamed, A.A.; Sahu, A.B.; Panigrahy, S.; Baigmohammadi, M.; Bourque, G.; Curran, H. The effect of the addition of nitrogen oxides on the oxidation of propane: An experimental and modeling study. Combust. Flame 2022, 245, 112306. [Google Scholar] [CrossRef]
- Wang, Q.-D.; Panigrahy, S.; Yang, S.; Martinez, S.; Liang, J.; Curran, H.J. Development of Multipurpose Skeletal Core Combustion Chemical Kinetic Mechanisms. Energy Fuels 2021, 35, 6921–6927. [Google Scholar] [CrossRef]
- Dong, S.; Wagnon, S.W.; Pratali Maffei, L.; Kukkadapu, G.; Nobili, A.; Mao, Q.; Pelucchi, M.; Cai, L.; Zhang, K.; Raju, M.; et al. A new detailed kinetic model for surrogate fuels: C3MechV3.3. Appl. Energy Combust. Sci. 2022, 9, 100043. [Google Scholar] [CrossRef]
- Zeng, M.R.; Li, Y.Y.; Yuan, W.H.; Li, T.Y.; Wang, Y.Z.; Zhou, Z.Y.; Zhang, L.D.; Qi, F. Experimental and kinetic modeling study of laminar premixed decalin flames. Proc. Combust. Inst. 2017, 36, 1193–1202. [Google Scholar] [CrossRef]
- Fang, R.; Kukkadapu, G.; Wang, M.; Wagnon, S.W.; Zhang, K.; Mehl, M.; Westbrook, C.K.; Pitz, W.J.; Sung, C.-J. Fuel molecular structure effect on autoignition of highly branched iso-alkanes at low-to-intermediate temperatures: Iso-octane versus iso-dodecane. Combust. Flame 2020, 214, 152–166. [Google Scholar] [CrossRef]
- Wang, H.; Dames, E.; Sirjean, B.; Sheen, D.A.; Tangko, R.; Violi, A.; Lai, J.Y.W.; Egolfopoulos, F.N.; Davidson, D.F.; Hanson, R.K.; et al. JetSurF II: A High Temperature Chemical Kinetic Model of n-Alkane (up to n-Dodecane), Cyclohexane, and Methyl-, Ethyl-, n-Propyl and n-Butyl-Cyclohexane Oxidation at High Temperatures. Available online: https://web.stanford.edu/group/haiwanglab/JetSurF/JetSurF2.0/ (accessed on 17 September 2010).
- Liu, Y.-X.; Richter, S.; Naumann, C.; Braun-Unkhoff, M.; Tian, Z.-Y. Combustion study of a surrogate jet fuel. Combust. Flame 2019, 202, 252–261. [Google Scholar] [CrossRef]
- Wang, H.; Xu, R.; Wang, K.; Bowman, C.T.; Hanson, R.K.; Davidson, D.F.; Brezinsky, K.; Egolfopoulos, F.N. A physics-based approach to modeling real-fuel combustion chemistry—I. Evidence from experiments, and thermodynamic, chemical kinetic and statistical considerations. Combust. Flame 2018, 193, 502–519. [Google Scholar] [CrossRef]
- Yu, L.; Wu, Z.Y.; Qiu, Y.; Qian, Y.; Mao, Y.B.; Lu, X.C. Ignition delay times of decalin over low-to-intermediate temperature ranges: Rapid compression machine measurement and modeling study. Combust. Flame 2018, 196, 160–173. [Google Scholar] [CrossRef]
- Kukkadapu, G.; Whitesides, R.; Wang, M.; Wagnon, S.W.; Mehl, M.; Westbrook, C.K.; McCormick, R.; Sung, C.-J.; Pitz, W.J. Development of a diesel surrogate for improved autoignition prediction: Methodology and detailed chemical kinetic modeling. Appl. Energy Combust. Sci. 2023, 16, 100216. [Google Scholar] [CrossRef]




| Fuel (DCL/RP-3 Blends) | φ | xFuel (mol%) | xO2 (mol%) | xN2 (mol%) | Avg. P5 (bar) | T5 Range (K) |
|---|---|---|---|---|---|---|
| 30/70 blends in volume | 0.5 | 0.64 | 20.87 | 78.50 | 10.02 | 1110–1530 |
| 1.0 | 1.27 | 20.73 | 78.00 | 9.94 | 1060–1450 | |
| 2.0 | 2.50 | 20.48 | 77.03 | 10.23 | 1030–1490 | |
| 70/30 blends in volume | 0.5 | 0.64 | 20.87 | 78.50 | 10.03 | 1090–1510 |
| 1.0 | 1.27 | 20.73 | 78.00 | 10.16 | 1070–1520 | |
| 2.0 | 2.50 | 20.48 | 77.03 | 10.13 | 1080–1500 |
| Property | DCL | DCL/RP-3 | RP-3 | Standard | |
|---|---|---|---|---|---|
| 30/70 | 70/30 | ||||
| Acidity (mg KOH/g) | 0.002 | — | — | 0.002 | ASTM D3242, ≤0.015 |
| aromatics (% vol) | 0.9 | — | — | 16.8 | ASTM D1319, ≤25 |
| sulfur (mass %) | <0.0001 | — | — | 0.066 | ASTM D5453, ≤0.3 |
| sulfur, mercaptan (mass %) | <0.0003 | — | — | 0.0012 | ASTM D3227, ≤0.003 |
| flash point (°C) | 45.5 | — | — | 47.5 | ASTM D1655, ≥38 |
| density 15 °C (kg/m3) | 827.4 | — | — | 799.7 | ASTM D4052, 775–840 |
| freezing point (°C) | −66.0 | — | — | −52.5 | ASTM D5972, ≤−47 |
| viscosity, −20 °C (mm2/s) | 4.528 | 4.395 | 4.242 | 4.230 | ASTM D445, ≤8.0 |
| Net heat of combustion (MJ/kg) | 42.992 | 43.257 | 43.362 | 43.296 | ASTM D3338, ≥42.8 |
| smoke point (mm) | 25.2 | 26.1 | 27.6 | 26.6 | ASTM D1332, ≥25.0 |
| naphthalene (vol %) | 0.0091 | 0.72 | 0.34 | 0.81 | ASTM D1840, ≤3.0 |
| Surrogate Component | DCL | DCL/RP-3 = 30/70 | DCL/RP-3 = 70/30 | RP-3 |
|---|---|---|---|---|
| n-Undecane | 6.85 | 16.11 | 10.47 | 22.60 |
| Iso-dodecane | 3.46 | 19.11 | 9.72 | 28.76 |
| n-Butyl cyclohexane | 38.08 | 32.21 | 35.46 | 22.90 |
| Decalin | 51.61 | 26.70 | 39.65 | 5.28 |
| n-Propyl benzene | 0.00 | 5.87 | 4.70 | 20.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.-D.; Du, L.; Wang, B.-Y.; Yao, Q.; Liang, J.; Zeng, P.; Xia, Z.-X. Surrogate Models and Related Combustion Reaction Mechanisms for a Coal-Derived Alternative Jet Fuel and Its Blends with a Traditional RP-3. Aerospace 2025, 12, 505. https://doi.org/10.3390/aerospace12060505
Wang Q-D, Du L, Wang B-Y, Yao Q, Liang J, Zeng P, Xia Z-X. Surrogate Models and Related Combustion Reaction Mechanisms for a Coal-Derived Alternative Jet Fuel and Its Blends with a Traditional RP-3. Aerospace. 2025; 12(6):505. https://doi.org/10.3390/aerospace12060505
Chicago/Turabian StyleWang, Quan-De, Lan Du, Bi-Yao Wang, Qian Yao, Jinhu Liang, Ping Zeng, and Zu-Xi Xia. 2025. "Surrogate Models and Related Combustion Reaction Mechanisms for a Coal-Derived Alternative Jet Fuel and Its Blends with a Traditional RP-3" Aerospace 12, no. 6: 505. https://doi.org/10.3390/aerospace12060505
APA StyleWang, Q.-D., Du, L., Wang, B.-Y., Yao, Q., Liang, J., Zeng, P., & Xia, Z.-X. (2025). Surrogate Models and Related Combustion Reaction Mechanisms for a Coal-Derived Alternative Jet Fuel and Its Blends with a Traditional RP-3. Aerospace, 12(6), 505. https://doi.org/10.3390/aerospace12060505






