Silicone-based thermal protection materials generally comprise a silicone matrix reinforced with functional fillers and ablation-resistant fibers. Given the relatively low intrinsic strength of silicone rubber, incorporating fillers is essential for enhancing its mechanical properties [
31]. Furthermore, adding fibers can facilitate the formation of a network structure within the material, which strengthens the char layer and improves its adhesion to the underlying matrix. This study systematically investigates how different types of fillers and fibers influence the ablation resistance of silicone rubber composites.
The fabricated composites were characterized using field emission scanning electron microscopy (FESEM). Their thermal stability was evaluated via thermogravimetric analysis (TGA) conducted in air, with the temperature ramped from room temperature to 1000 °C at a heating rate of 5 K/min—simulating the atmospheric environment inside an SFRJ combustion chamber. Although the TGA heating rate (5 K/min) is much lower than the rapid heating rates (>1000 K/s) encountered in actual SFRJ operation, this controlled approach provides valuable comparative data on the relative thermal stability and decomposition mechanisms of different composite formulations under oxidative conditions.
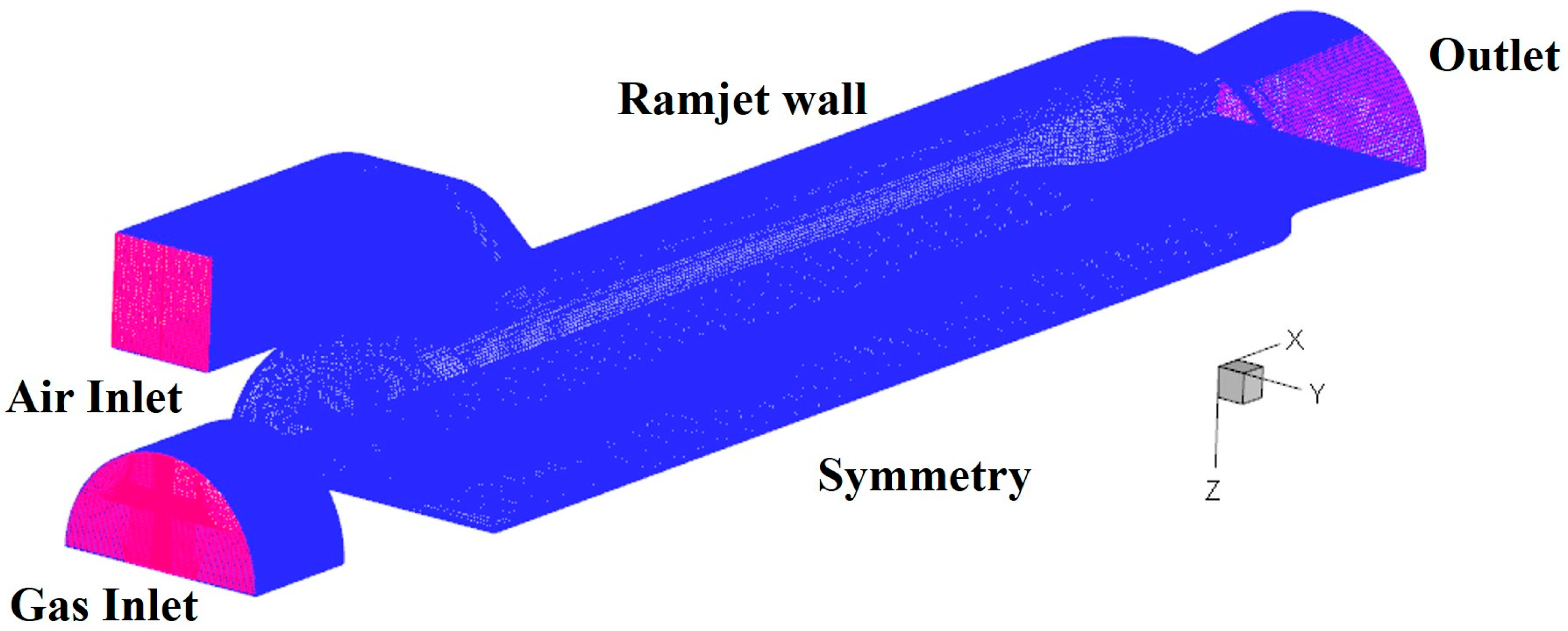
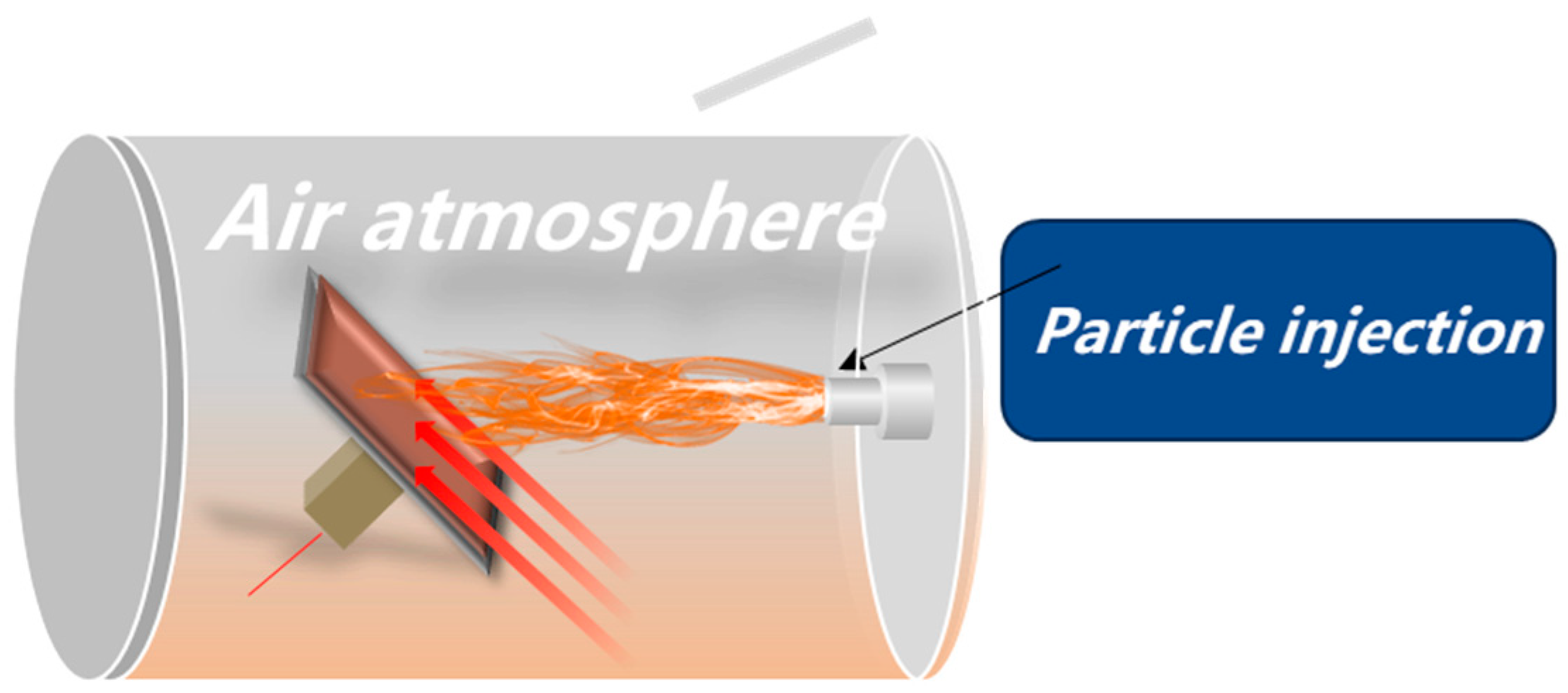
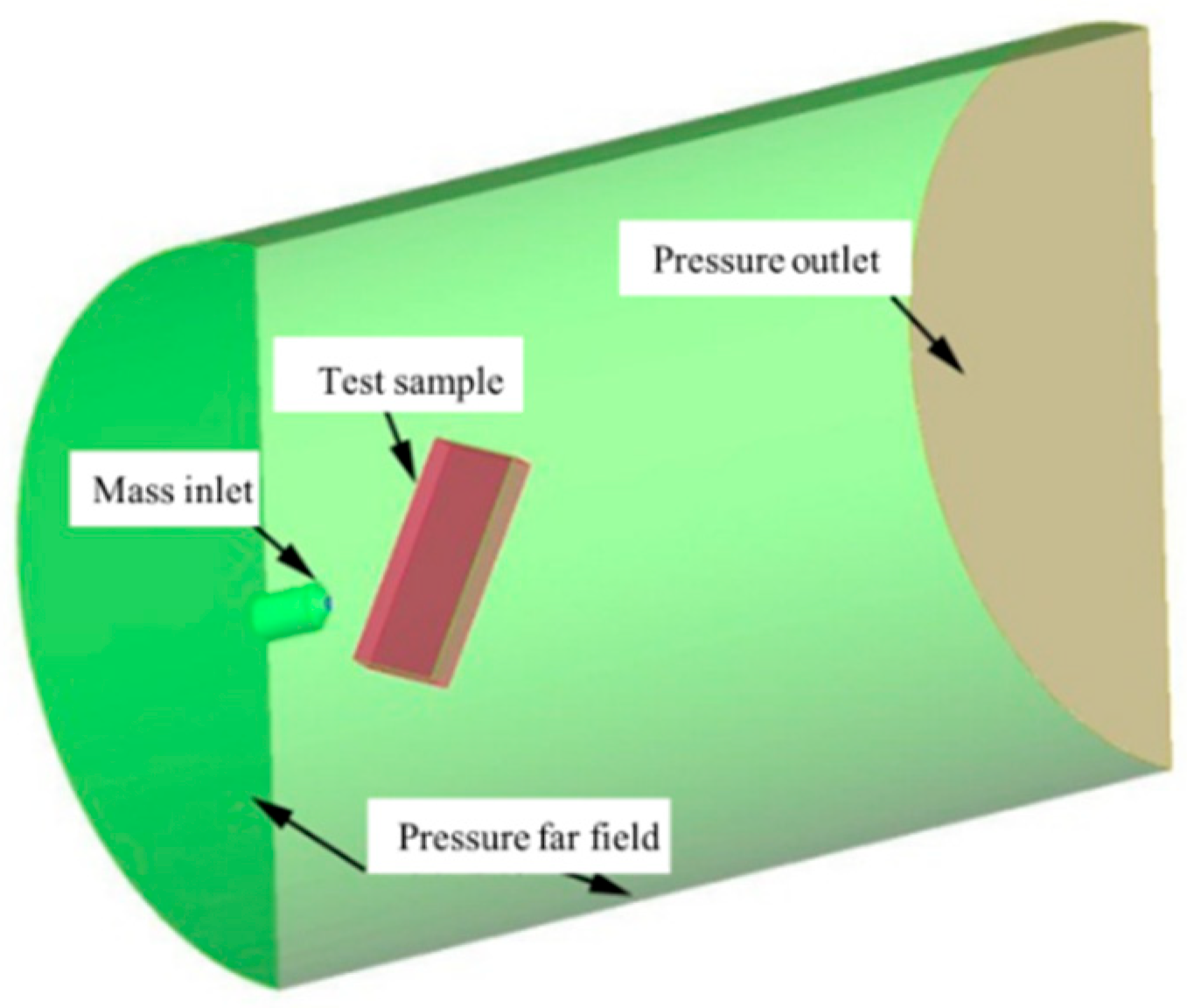
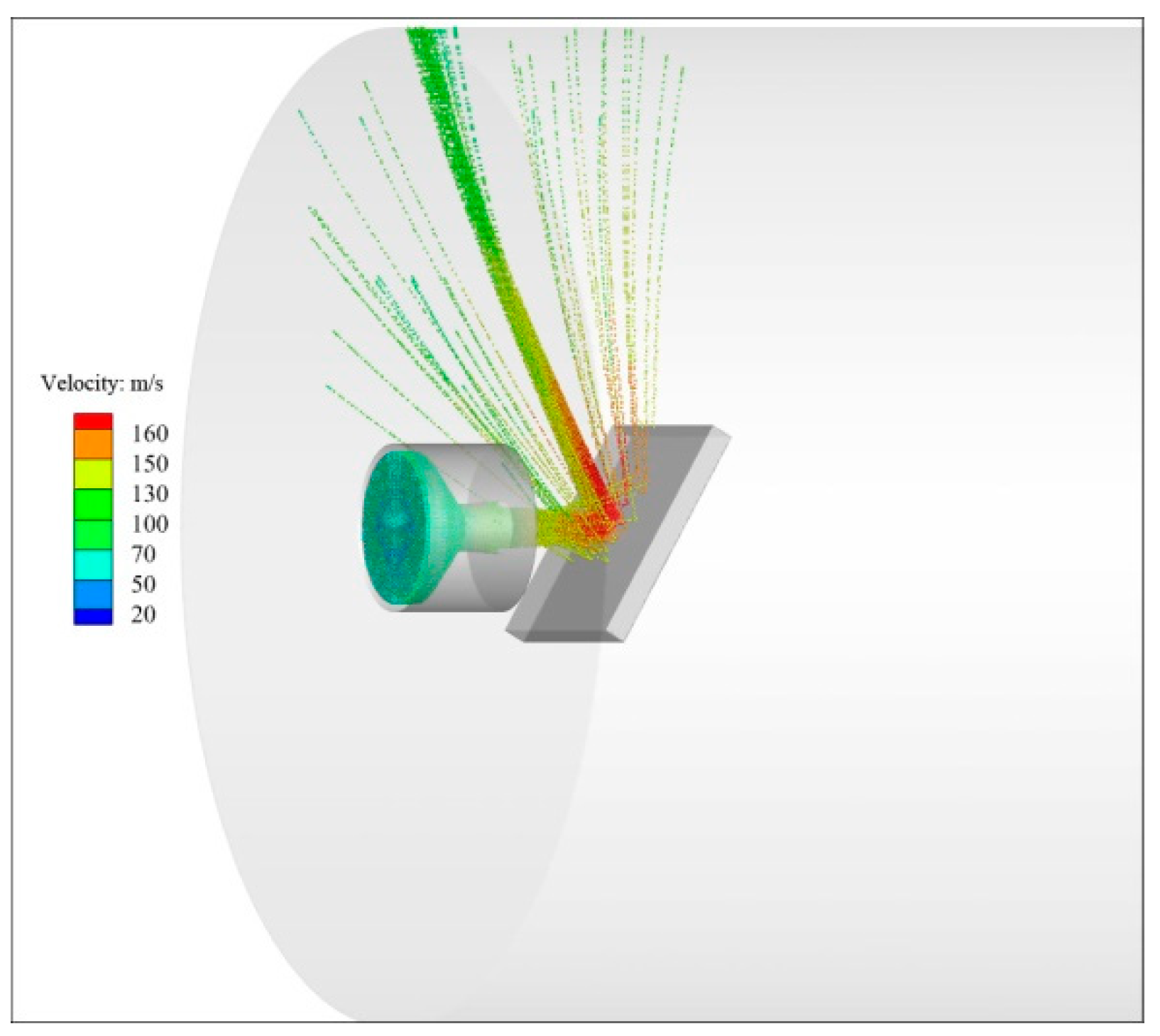
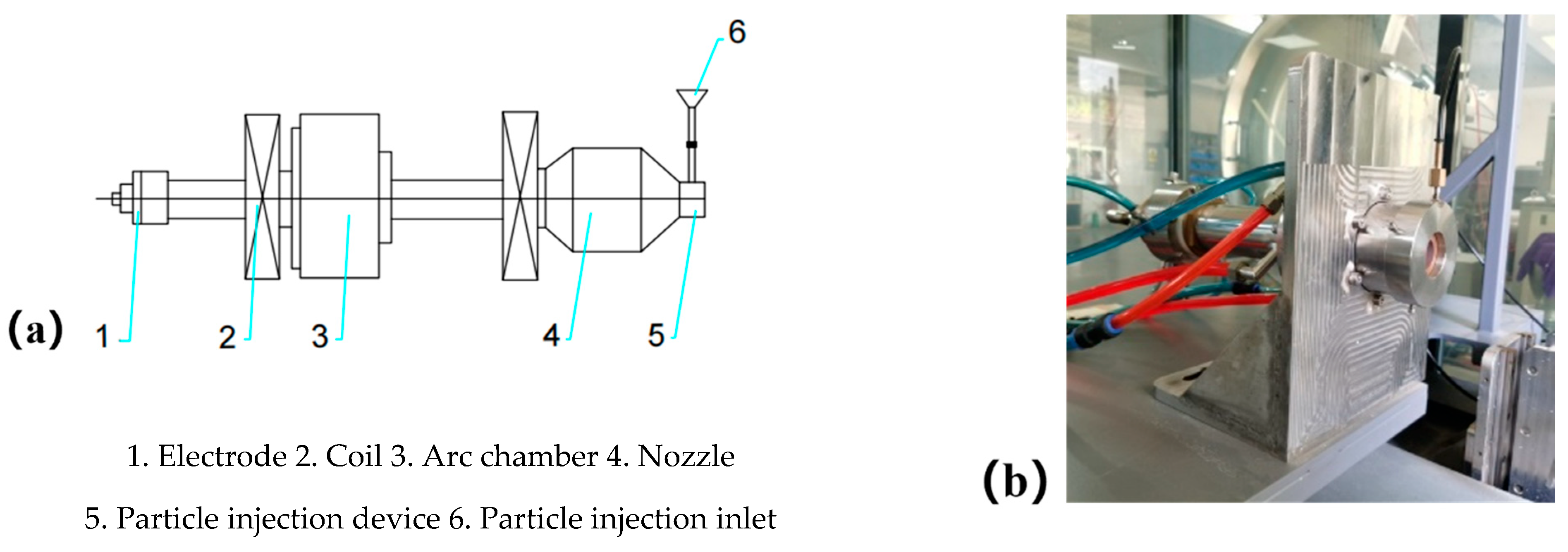
The ablation device described earlier was used to replicate the thermal conditions of the SFRJ chamber. K-type thermocouples were attached to the back of each sample to record temperature profiles during testing.
4.1. Role of Functional Fillers
As previously noted, the SFRJ combustion chamber operates under high-temperature, oxygen-rich conditions. Previous studies have demonstrated that incorporating inorganic fillers (e.g., zirconium-containing compounds or silicon carbide) into silicone matrices facilitates the formation of solid or liquid antioxidant films on the material surface [
17]. This protective layer acts as a barrier, reducing direct contact between oxidizing gases and the pristine matrix to mitigate ablation.
Silicon carbide (SiC) promotes the formation of a liquid SiO
2 film under high-temperature oxidative conditions. In comparison, zirconium diboride (ZrB
2) reacts to form liquid B
2O
3 and a solid ZrO
2 skeleton. However, at temperatures exceeding 2073 K, B
2O
3 evaporates faster than it can form; in contrast, SiO
2 exhibits viscous flow at around 2000 K, enabling the formation of a continuous protective layer while retaining its ceramic properties and protective function up to 3000 K [
32].
Thus, combining SiC and ZrB2 can provide effective ablation resistance across a broad temperature range. Additionally, silica (SiO2) fillers contribute to mechanical reinforcement. This section examines the synergistic effects of SiC, ZrB2, and SiO2 fillers.
In this study, 50 phr (parts per hundred rubber) of SiC, ZrB
2, or SiO
2 was individually incorporated into a silicone rubber (SR) matrix to evaluate their effects on thermal stability.
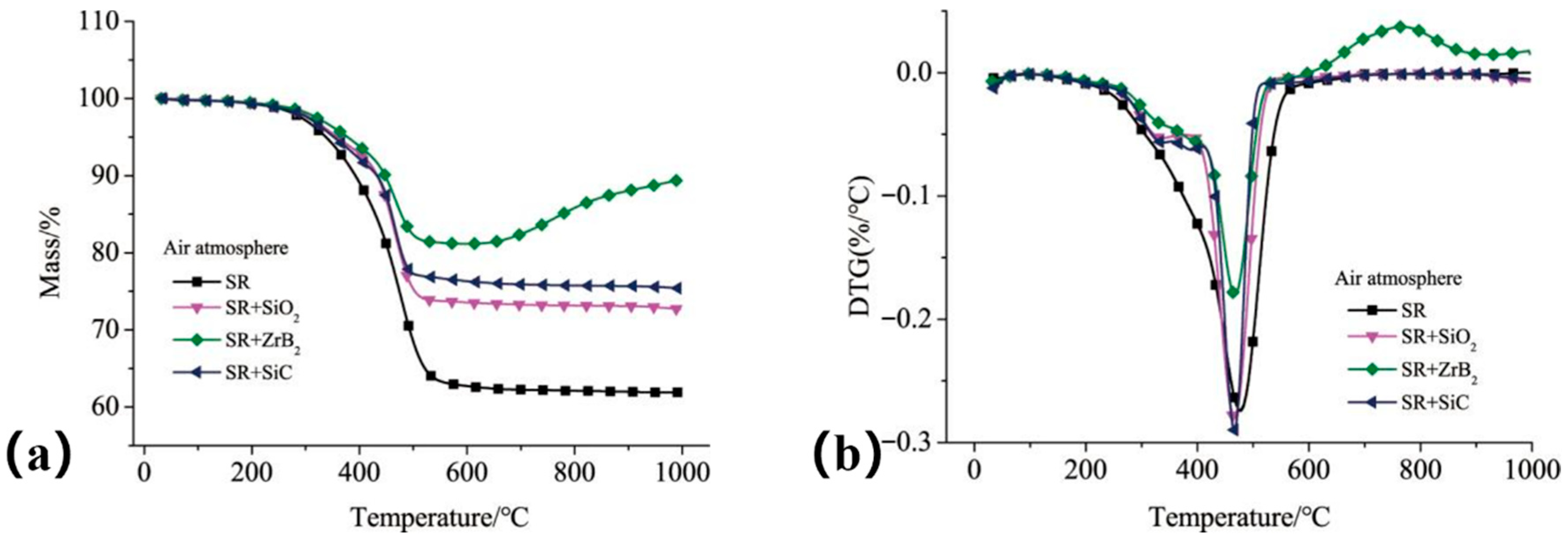
Figure 14 shows the TGA and derivative thermogravimetric (DTG) curves. The composite containing ZrB
2 exhibited the lowest mass loss, with a residual mass of 89.4% at 1000 °C. In contrast, the composite with SiO
2 filler showed the highest mass loss. This is because, at the 5 K/min heating rate, the inert SiO
2 filler primarily dilutes the polymer matrix without actively contributing to the formation of a protective ceramic residue (as ZrB
2 does) or promoting stable char formation (as SiC can). Consequently, the overall residual mass fraction is lower. However, it is critical to emphasize that this TGA result does not capture the potential beneficial role of SiO
2 under dynamic ablation conditions: at higher temperatures (>1800 °C), SiO
2 melts and flows viscously, which can significantly contribute to forming a continuous, erosion-resistant protective layer—an effect demonstrated in subsequent ablation tests (see Figure 16 and the discussion of Formulation S3). The mass increase observed above 780 °C (
Figure 14b) is attributed to the oxidation of ZrB
2, which produces a porous ZrO
2 framework and a liquid B
2O
3 layer that collectively inhibit oxidative degradation of the matrix.
To further explore the thermal stability and ablation behavior of composites containing ZrB
2 with different filler combinations, additional experiments were conducted. In each composite formulation, the content of ZrB
2 and SiC was maintained at 50 phr, while SiO
2 was fixed at 30 phr—a proportion previously found to optimize the balance between mechanical properties and ablation resistance. The specific compositions of these composites are listed in
Table 7.
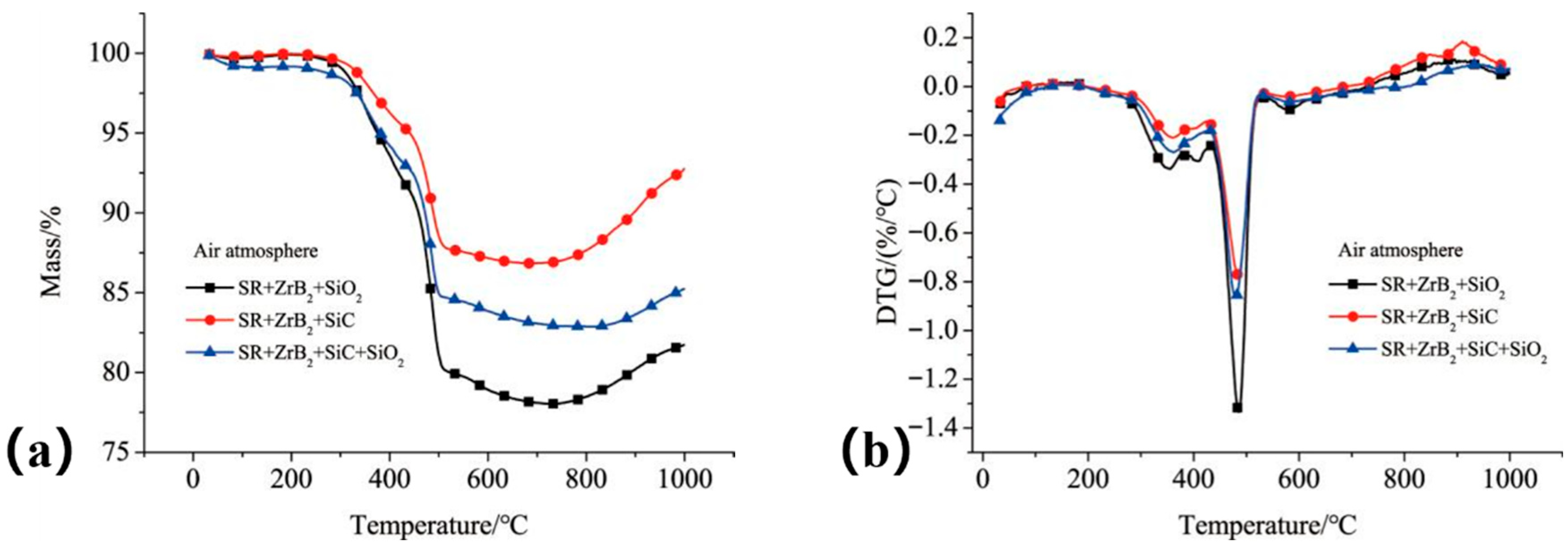
As shown in
Figure 15, Composite S1—formulated with only ZrB
2 and SiO
2—exhibited the highest residual mass (92.69%,
Figure 15a) and the lowest maximum mass loss rate among the three composites (
Figure 15b). However, results from dynamic simulated ablation tests (
Figure 16) revealed that Composite S3 (incorporating SiC, ZrB
2, and SiO
2) had the lowest mass ablation rate.
The SiO2 filler provides mechanical reinforcement, and its melting behavior at high temperatures contributes to forming a continuous liquid antioxidant layer. This layer effectively resists two-phase flow erosion and works in tandem with the ceramic fillers to establish a stable antioxidative film during ablation. Consequently, Composite S3 exhibits superior dynamic ablation resistance compared to the other formulations.
4.2. Influence of Fibers
All fiber-reinforced composites (S4, S5, S6) were tested under standardized ablation conditions to ensure comparable results. The test parameters were set as follows: surface temperature maintained at 2000 ± 50 K (controlled via infrared pyrometer feedback); O
2 mass fraction of 17 ± 1% in the test stream; chamber pressure of 130 ± 5 kPa; alumina particle (mean diameter: 70 μm) mass flow rate of 2.0 ± 0.1 g/s; and test duration fixed at 180 s. These conditions were selected to replicate the severe environment identified in Region 4 of the SFRJ chamber simulation (
Table 6).
To further enhance the composites’ erosion resistance, fibrous reinforcements were incorporated into the S3 baseline formulation. Two types of short fibers—aramid fibers (AF) and carbon fibers (CF)—were added to investigate their effects on the composites’ thermal stability and erosion resistance. The specific formulations are summarized in
Table 8.
These fibrous reinforcements are expected to improve both the mechanical properties and oxidation resistance of the silicone rubber matrix—critical for withstanding gas–solid two-phase flow conditions in the SFRJ chamber. Aramid fibers possess a ternary copolymer aromatic heterocyclic amide structure, whereas carbon fibers are composed of carbonaceous material. Both fiber types act as a skeletal framework within the matrix, enhancing the composite’s erosion resistance.
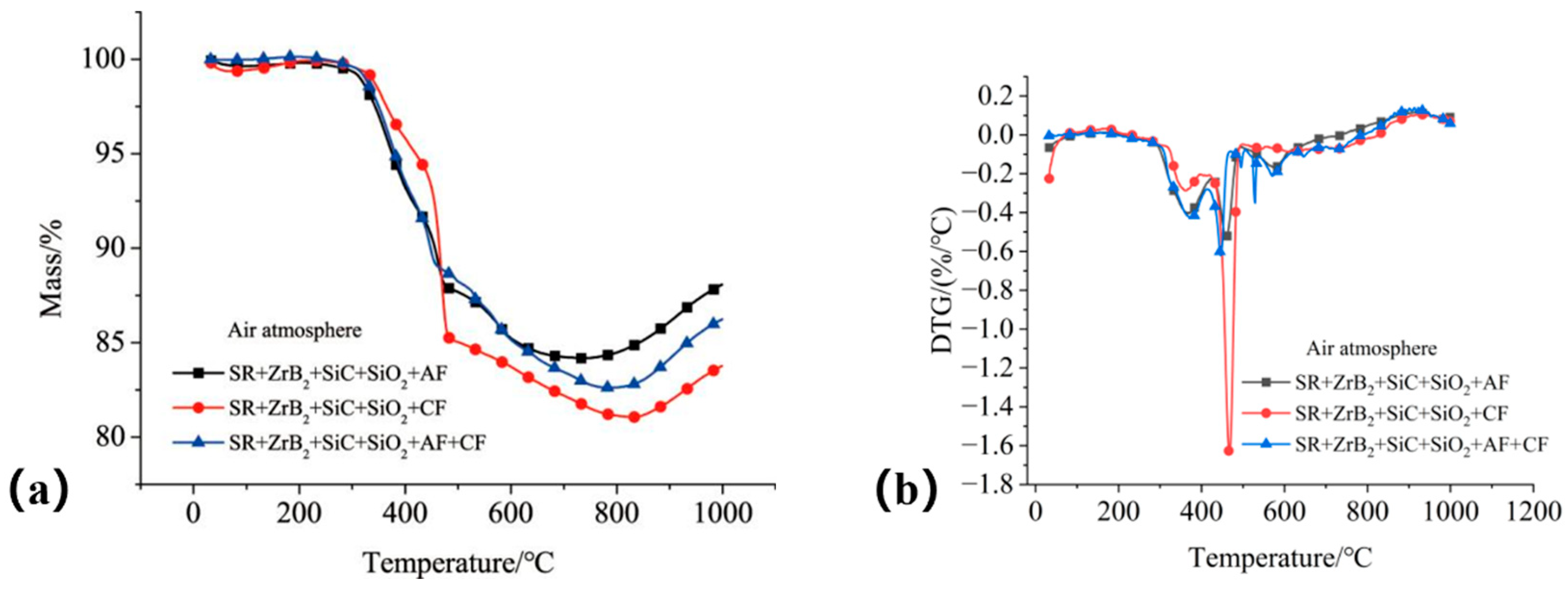
The thermal stability of the fiber-reinforced composites was evaluated via TGA and DTG analyses, as shown in
Figure 17. Composites incorporating aramid fibers (S4 and S6) exhibited the lowest mass loss, with residual masses of 87.6% and 85.2% at 1000 °C, respectively, indicating that aramid fibers improve thermal stability. All fiber-reinforced composites displayed a distinct DTG peak in the range of 430–450 °C, suggesting a significant degradation process occurs within this temperature interval.
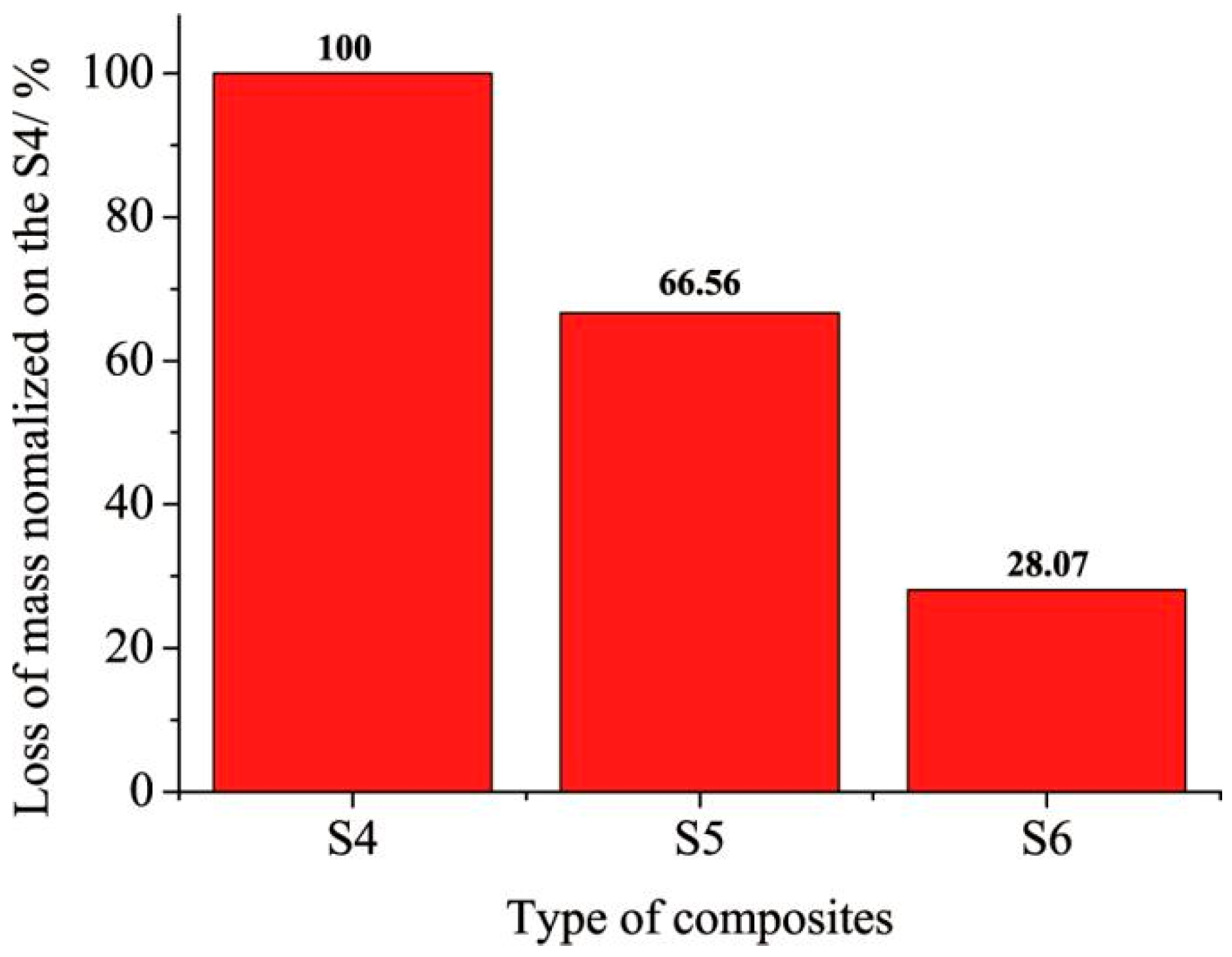
However, under simulated ablation conditions (180 s duration), composites reinforced with carbon fibers (S5) showed superior erosion and oxidation resistance, with the lowest normalized mass loss (
Figure 18). Although the hybrid fiber composite (S6) visually exhibited a more compact char structure (Figure 20c), its mass loss was slightly higher than that of S5. This can be attributed to the oxidative decomposition of the aramid fiber component in S6 under the high-temperature, oxygen-rich environment—an effect schematized in
Figure 19. In contrast, the carbon fibers in S5 are fully carbonized and inorganic, giving them superior intrinsic thermo-oxidative stability compared to organic aramid fibers. This explains why S5 retained more mass under these severe conditions. The contrast between thermal stability (from TGA) and ablation performance highlights that different fibers enhance distinct aspects of the composite’s behavior: aramid fibers improve thermal stability, while carbon fibers boost ablation resistance under erosive and oxidative conditions.
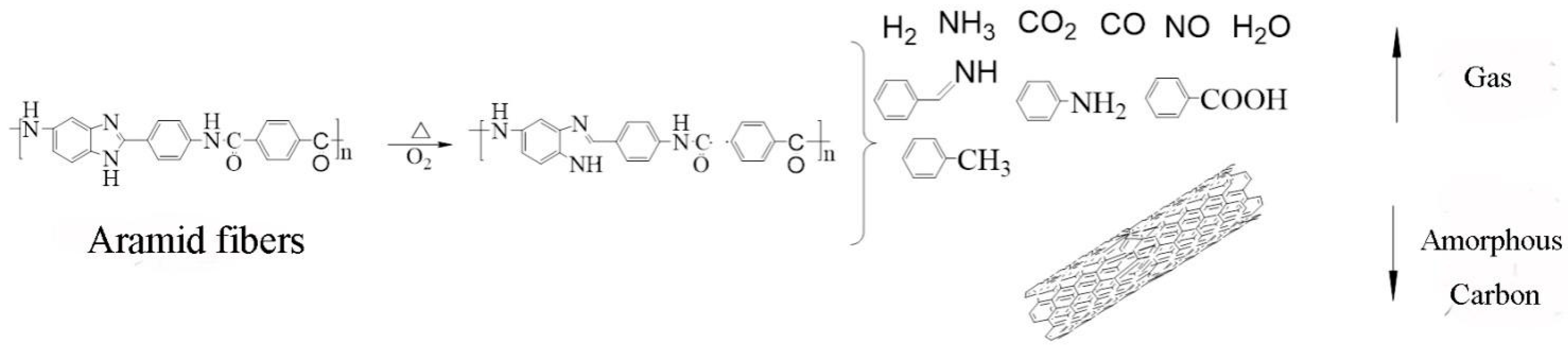
In high-temperature, oxygen-rich environments, aramid fibers undergo oxidative decomposition, as illustrated in
Figure 19. According to the literature [
33], aramid fibers undergo thermal degradation at approximately 500 °C in air, releasing gases such as CO
2, H
2O, CO, HCN, and NO
2. Weak aromatic heterocyclic bonds break within this temperature range; between 600 °C and 700 °C, complex depolymerization and restructuring reactions lead to nearly complete decomposition. In contrast, carbon fibers—being fully carbonized inorganic materials—react with oxidizing gases only under more extreme temperature conditions.
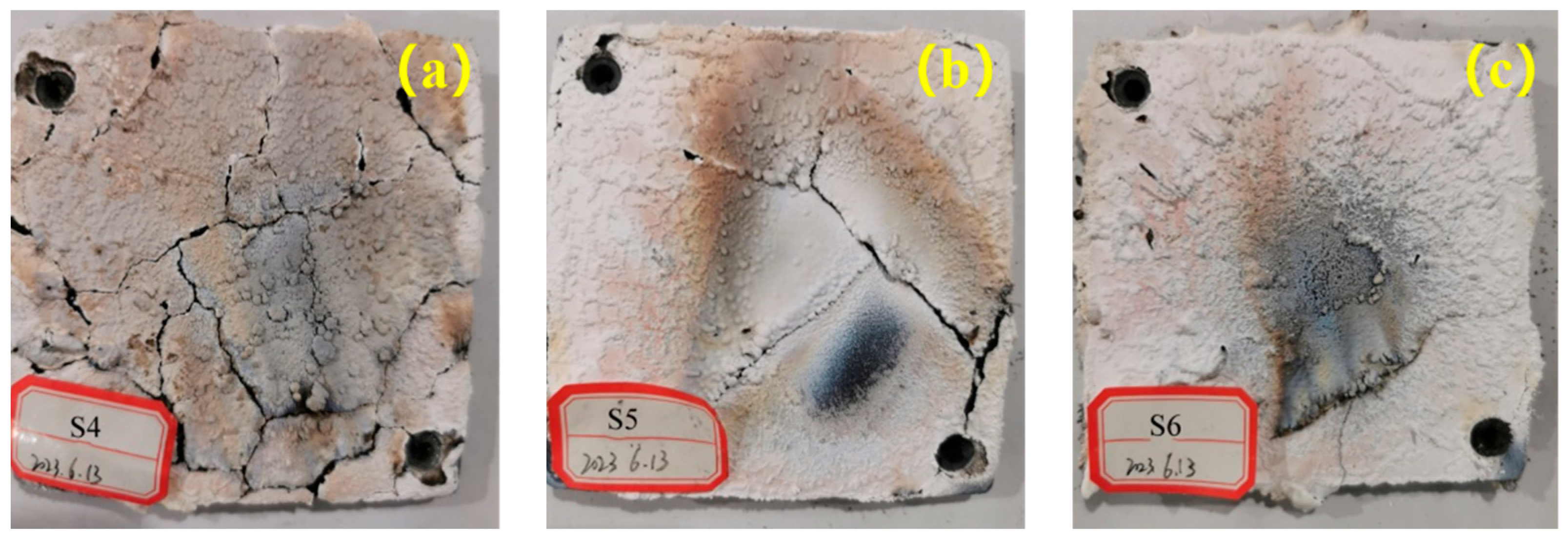
Figure 20 shows post-ablation images of the charred surfaces of Composites S4, S5, and S6. Visually, the composite reinforced with carbon fibers (S5) had a compact, flat char surface with no macrocracks—unlike S4. The hybrid fiber composite S6 (
Figure 20c) also displayed a highly coherent, defect-free surface that appeared structurally superior to S5. This excellent structural integrity of S6 is likely due to a synergistic effect: aramid fibers pyrolyze and expand to fill voids, while carbon fibers provide a stable skeletal framework. However, as evidenced by the mass loss data (
Figure 18), this superior macroscopic structure does not directly translate to optimal ablation resistance under the specific thermo-chemical stress of the oxygen-rich environment—where the chemical stability of the carbon fibers in S5 dominates. TGA results indicated that composites containing carbon fibers (S5 and S6) produced a higher char yield, contributing to the formation of a stronger char skeleton.
SEM analysis (
Figure 21) showed that S4 had numerous voids and cracks in its char layer—indicative of a weak skeletal structure caused by pyrolysis and gas release from aramid fibers. In contrast, S5 exhibited a denser, more continuous char structure, attributed to the supporting role of carbon fibers. S6—incorporating both fiber types—also showed a compact char structure: inorganic carbon fibers acted as a skeleton, while pyrolytic products from aramid fibers filled voids, further improving ablation resistance.
These microstructural observations align with the macroscopic images: S6 possesses a very compact, integrated char layer. However, the critical difference lies in the chemical nature of the reinforcing skeleton. In S5, the carbon fiber skeleton remains largely intact, providing continuous mechanical reinforcement against erosion. In S6, while the initial structure is excellent, the decomposition of aramid fibers can lead to localized weakening upon prolonged exposure, making it slightly more susceptible to mass loss under combined thermo-chemical and mechanical attack. This explains why S5, with its chemically inert fibrous skeleton, achieved the lowest ablation rate.
In summary, incorporating either carbon fibers (S5) or a hybrid of organic and inorganic fibers (S6) significantly improved the compactness and mechanical integrity of the char layer, leading to markedly enhanced ablation resistance.