Abstract
Climate change is rapidly progressing as the carbon budget balance is broken due to excessive energy and land use. This study was conducted to find and quantify new carbon sinks to implement the carbon neutrality policy prepared by the international community to solve these problems. To reach this goal, an allometric equation of the willow community, which dominates riparian vegetation, was developed and applied to calculate the net primary productivity of the willow community. Furthermore, after the amount of carbon emitted via soil respiration was quantified, the net ecosystem production was calculated by subtracting the amount of soil respiration from the net primary productivity. In comparisons of the results obtained via this process with those obtained from forest vegetation, the willow community, representative of riparian vegetation, showed a much higher carbon sequestration rate than forest vegetation. Considering these results comprehensively, the willow community could be a new and significant carbon absorption source. In this context, proper river restoration should be realized to contribute to carbon neutrality and secure various ecosystem service functions.
1. Introduction
Extreme weather events, such as droughts, heat waves, heavy rains, floods, and landslides, are becoming more frequent worldwide due to the effects of climate change. Other effects of the rapidly changing climate include rising sea levels, ocean acidification, and biodiversity loss [1,2].
Annual global CO2 emissions reached 37.8 G tons in 2021, and natural sinks are estimated to remove between 9.5 and 11 G tons of CO2 per year [3]. In the Republic of Korea, annual CO2 emissions reached 0.690 G tons in 2022, and natural sinks are estimated to remove 0.045 G tons of CO2 per year [4]. CO2 is left behind in this process, and the CO2 concentration, which traps heat in the atmosphere and contributes to climate change, continues to rise. Therefore, it is more urgent than ever to reduce carbon emissions and enhance carbon sinks [5].
In this reality, carbon neutrality is essential by the mid-21st century to limit the global temperature rise threshold to 1.5 °C, which the IPCC suggests is safe. This goal is also enshrined in the Paris Convention, which has been signed by 195 countries. Carbon neutrality means having a balance between emitting and absorbing carbon from the atmosphere. Carbon sequestration signifies removing carbon oxide from the atmosphere and then storing it. All worldwide greenhouse gas (GHG) emissions must be counterbalanced via carbon sequestration to achieve net zero emissions. A carbon sink is any system that absorbs more carbon than it emits. The main natural carbon sinks are soil, forests, and oceans [6,7,8]. Forests are crucial in capturing atmospheric carbon dioxide that would otherwise contribute to global warming. The international community is preparing measures to increase carbon sinks, such as the IUCN’s Nature-Based Solutions [9] and the UN Decade on Ecosystem.
The carbon cycle, which involves carbon exchange between the atmosphere, vegetation, and soil, is important in shaping future climate trends [10,11,12,13]. Vegetation plays a role in fixing carbon in the atmosphere via photosynthesis, and the carbon moves to the soil through processes such as decomposition. This process determines the ecosystem’s net ecosystem production (NEP), which quantifies the amount of carbon absorbed or released. In addition, it is necessary to analyze the carbon flux within the ecosystem to understand the carbon cycle and NEP across different vegetation types [14,15,16,17].
Carbon emissions from human activity are causing rapid climate change by disrupting the balance of global carbon cycles [6,18,19,20,21]. As a result, efforts are being made to reduce carbon emissions worldwide. The discovery of new carbon sinks that use plants’ carbon dioxide absorption capacity is drawing attention [22,23,24]. In addition to carbon dioxide absorption, vegetation exerts various ecosystem service functions in the background [25,26,27].
Functional changes within ecosystems due to rapid climate change are expected to significantly impact the carbon budget between the atmosphere, vegetation, and soil, ultimately affecting future climate trends [15,28,29,30]. Therefore, understanding the carbon cycle mechanisms and quantification processes for various ecosystems may be key in future climate change research. As a result, related researchers are putting a lot of attention and effort into strategically clearly identifying the carbon budget of ecosystems to respond to future climate change system predictions and results [15,17,31,32].
Wetlands account for only 5–8% of the world’s land surface, but the carbon stored in wetlands accounts for 20–30% of the world’s carbon reserves. Therefore, wetlands are known as an important carbon sink in the biosphere [33,34,35] while also being a source of greenhouse gases (GHGs), such as carbon dioxide (CO2) and methane (CH4) [36,37,38,39,40]. Consequently, wetlands play an important role in regulating carbon cycles on a global scale. Quantifying wetlands’ carbon balance, including carbon storage, absorption, and emissions, has become the main topic of carbon research [41].
The EU’s Green Deal and Biodiversity Strategy include goals to restore degraded ecosystems such as wetlands [42]. The Water Framework Directive and Habitat Directive also protect wetland areas to safeguard their biodiversity and carbon sink functions [43,44].
Rewetting initiatives focus on rewetting peatlands that were previously drained for agriculture or other uses. Rewetting restores the natural water levels, which helps prevent the release of CO2 and promotes carbon storage by allowing peatlands to accumulate organic material again. Restored wetlands can emit methane (CH4), a potent greenhouse gas, which presents a trade-off. However, the net effect remains positive for climate mitigation due to the high carbon storage potential [45,46,47].
Some European countries, such as the UK and Germany, have introduced carbon farming practices that incentivize landowners to restore wetlands. This approach aligns with carbon offset schemes, where landowners are compensated for sequestering carbon via wetland restoration [48,49,50].
Wetlands hold significant promise for natural climate solutions and contribute to national and global carbon reduction goals. By investing in their restoration and preservation, countries worldwide are not only enhancing carbon sinks but also protecting biodiversity, improving water quality, and creating resilience to climate impacts, such as flooding. Wetlands, thus, represent a multifunctional ecosystem that supports both climate mitigation and ecological health on a global scale [51,52,53,54].
In Asian countries, including the Republic of Korea, most wetlands have been converted into agricultural land to produce rice, a staple food. The wetlands are greatly damaged as a result, making it difficult for them to perform their normal functions. In particular, the river was reduced to a simple waterway by removing riparian vegetation that interfered with rice growth by creating shade. However, the level of environmental awareness has significantly increased in these regions due to recent social changes caused by economic growth, and the restoration of damaged wetlands, including rivers, is actively progressing. Nonetheless, the level of restoration does not significantly exceed the level of aesthetic refinement, so the contribution to environmental improvement is not significant [55,56].
The Miho River, where this study was conducted, is a habitat for an endemic South Korean fish called the Miho spined loach (Cobitis choii Kim and Son) and has received the attention of environmental activists and academia to minimize interference with the river, allowing it to be passively restored via natural processes. As cultivation was stopped in this area, which had been covered with agricultural land, various riparian vegetation was established. Thus, depending on the distance from the waterway, annual herb-dominated vegetation, perennial herb-dominated vegetation, shrubby willow communities, and tree willow communities have formed the typical riparian vegetation, unlike the artificially restored area [55,57].
Willows, representative riparian vegetation, grow fast, have high productivity, and are artificially afforested and utilized [58,59,60,61]. Young willows or shrubby willows show very fast growth rates initially and a high carbon absorption capacity [62,63]. When the carbon absorption capacity decreases after growth, it is also logged and used as renewable energy [60,61].
Willow stands cultivated using a short-term circulation method in Italy showed a carbon absorption capacity of 12.4 tonC·ha−1·yr−1 [64]. Twelve-year-old willow stands in Poland showed a carbon absorption capacity of 13.5 tonC·ha−1·yr−1 [65]. Willow plantations in Sweden showed a low carbon absorption capacity of 0.97 to 2.77 tonC·ha−1·yr−1, while carbon accumulation in the soil showed a high level of 61 to 157 tonC·ha−1·yr−1 [66].
This study aimed to calculate the net primary productivity (NPP) of willow communities, which are major riparian vegetation, considering the river ecosystem’s carbon budget and carbon circulation system. Simultaneously, this study evaluated the net ecosystem production (NEP) by measuring the amount of carbon dioxide emitted from the soil into the atmosphere. Furthermore, this study aimed to estimate the carbon absorption capacity by assuming that South Korean rivers are properly restored based on this study’s results.
2. Materials and Methods
2.1. Study Site
This study was carried out in the riparian forest established in the Miho Bridge reach of the Miho River (Figure 1), which contained rice paddies before 2006. However, agricultural activities were stopped when the Miho Bridge was installed after the construction of Sejong City, and riparian vegetation was restored via passive restoration (Figure 2 and Table 1). Most of the riparian zone in this area was covered with agricultural land before the construction of the Miho Bridge, and natural grasslands and willow communities were mainly distributed on the sandbar and partially distributed in the riparian zone. After constructing the Miho Bridge, as agricultural activities were suspended in this area, agricultural land was passively restored to a typical riparian zone dominated by willow, natural grasslands, and bare grounds.
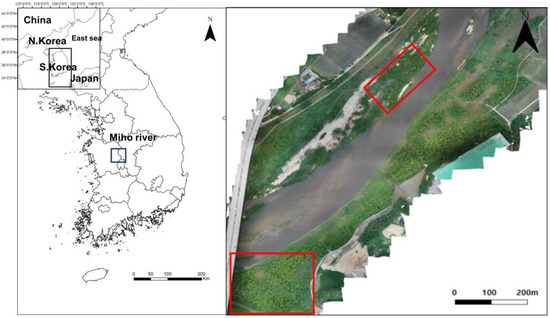
Figure 1.
Map showing the study area in Miho River. Red boxes indicate survey sites.
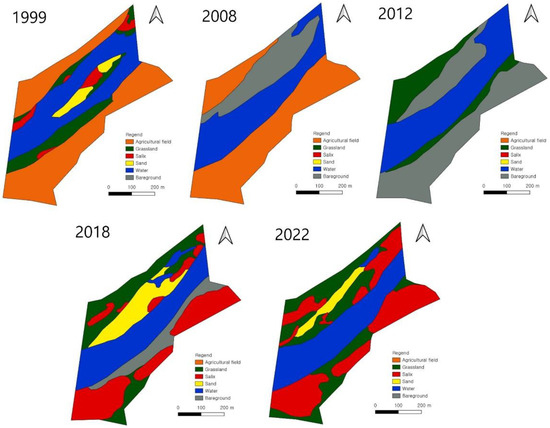
Figure 2.
Maps showing changes in landscape elements before and after construction of Sejong City (2006).

Table 1.
Changes in area of landscape elements before and after construction of Sejong City (2006).
The Miho River belongs to the tributary of the Geum River. Its length is 89.2 km, and the watershed area is 1860.9 km2. The mean annual temperature of this area is 10.8 to 13.1 °C, and the mean annual precipitation is 1200 to 1400 mm (Figure 3). The riparian vegetation in the reach where this study was conducted is dominated by Salix pierotii Miq.
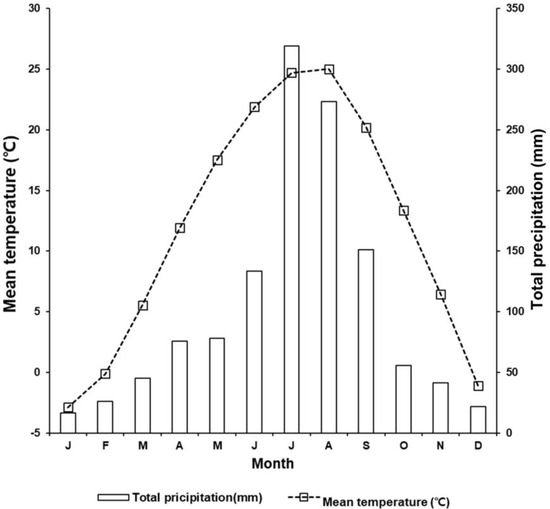
Figure 3.
Seasonal variation in observed total monthly precipitation and monthly mean temperature measured from 2001 to 2023 in Cheongju Meteorological Station located 16 km away from the study site.
2.2. Derivation of Allometric Equation
A total of 20 sample trees of various sizes were selected and excavated to derive the allometric equation of the S. pierotii community. The diameter at breast height (DBH) for the selected trees was measured, and the fresh weight of the excavated individuals was measured by dividing them into stems, branches, leaves, and roots in the field. Then, a part of each organ was sampled, transported to a laboratory, and oven-dried at 80 °C to a constant weight. The moisture content was calculated from the difference between the fresh and dry weights. The allometric equation was derived from the relationship between the DBH and the dry weight of the stem, branch, leaf, and root (Equation (1)).
Y = aXb
log Y = log a + b log X
log Y = log a + b log X
Y: estimated standing crop;
X: DBH;
a: regression coefficient;
b: slope of the regression line.
2.3. Measurement of Net Primary Productivity (NPP)
Two permanent quadrats of 100 m2 (10 m × 10 m) were installed to measure the NPP by selecting a site to represent the entire study area. The NPP was measured using the allometric method [67,68,69]. DBH and height of each tree located in the permanent quadrat were measured in May 2022 and May 2023 to obtain the biomass. The biomasses in 2021 (W1) and 2022 (W2) were calculated by putting these measurements into the allometric equation. The NPP was calculated from the biomass increase in 2022 compared with that in 2021 (∆W = W2 − W1). The amount of carbon and CO2 was estimated by applying the carbon fraction of the IPCC [70].
2.4. Measurement of Soil Respiration
The rate of soil respiration (SR) in the S. pierotii Miq. stand was measured using a portable infrared CO2 analyzer (EGM-5, PP Systems, Amesbury, MA, USA) and a static closed chamber with a surface area of 78 cm2 and a volume of 1171 mL (SRC-2, PP Systems, Amesbury, MA, USA) with a sensor (STP-2, PP Systems, Amesbury, MA, USA) for soil temperature measurements [71]. The closed dynamic chamber method was employed to measure the SR in the field, which is based on measuring the rate of increase in the CO2 concentration inside the chamber with a known soil surface area over a recommended period [72,73]. Six soil collars of cylindrical polyvinyl chloride (PVC) with a diameter of 10 cm and a height of 8 cm were installed randomly at a soil depth of 6 cm at each study site to measure the RS rate. The RS rates were measured once a month in situ from May 2022 to December 2023. All measurements were made between 9:00 a.m. and 13:00 p.m. local time to avoid diurnal fluctuations in temperature [74]. Living plants within the collars were removed at least one day before each measurement to eliminate CO2 concentrations from aboveground plant parts. We measured the rate of increase in the CO2 concentration emitted from soil surfaces every second over 120 s by placing the SRC-2 chamber top on the collars installed in the ground and sealed with rubber gaskets. An EGM-5 portable gas analyzer connected to the SRC-2 chamber calculated the quadratic and linear match of the measured data [71,75]. During each measurement event in the field, the quantification of RS was conducted using the software built into the EGM-5, which assumes a quadratic relationship between the CO2 concentration within the chamber and the time to correct non-linearities caused by leakage. The RS rate was calculated using the following equation:
where Cn represents the CO2 concentration at a given time Tn; C0 represents the CO2 concentration at the chamber installation time (T = 0); V represents the total chamber volume (m3); and A is the area of exposed soil (m2).
SR rate (mg CO2 m−2 h−1) = [(Cn − C0)/Tn]∙[V/A]
During the soil respiration measurements, the air temperature (°C) at 1.5 m above the ground surface and the soil temperature (°C) at a 5 cm soil depth were continuously measured and recorded at 1-h intervals for one year from May 2022 to December 2023 using a temperature logger with a soil probe (HOBO Pro Air/Soil Temp., Bourne, MA, USA) at each study site. The daily mean values of the soil respiration and temperature (air and soil) were calculated by averaging all the measured data in each study site. The amount of soil respiration of the heterotrophic organisms was calculated by applying the respiratory coefficient (0.5), which is usually cited for forest soil [76,77].
Optimal regression equations were derived to examine the relationships between the SR, Ta (1.5 m aboveground), and Ts (5 cm belowground). The dependence of the SR rate on the temperature (Ta and Ts) was modeled using the following regression equation:
where SR rate is the measured SR (mg CO2 m−2 h−1); t is the measured Ta (°C) or Ts (°C); α is the coefficient of the basic respiration rate at a reference temperature of 0 °C; and b is the sensitivity of the SR to temperature (Ta and Ts). The sensitivity (b) is related to Q10, which is derived as follows:
where Q10 is the SR rate increase for a 10 °C rise in temperature (T). The a, b, and Q10 parameter values derived from the observed field data reflect the effects of T and other variables on SR. Thus, Q10 is generally used to describe the temperature dependence of SR.
SR rate = α·exp(b∙t)
Q10 = exp10b
2.5. Calculation of Carbon Absorption Capacity
The net ecosystem production (NEP) was obtained from the difference between the CO2 absorption (NPP) by vegetation and CO2 emission via the respiration of soil microorganisms and animals, i.e., heterotrophic respiration [15].
2.6. Statistical Test of Allometric Equation
The measured data, DBH and biomass, are nonlinear data and need to be converted into linear data. Therefore, the measured values were converted into linear data by taking logs (Equation (1)), and normality verification was performed through the Shapiro–Wilk test. The accuracy verification of the calculated regression equation indicated the explanatory rate of the regression equation through R2. The difference and variance between predicted and measured values were verified through root mean squared error (RMSE) and mean absolute error (MAE). In order to verify the overestimation and underestimation of the measured values, it was verified by using the mean percentage error (MPE) value.
n: number of sample trees;
y: measured biomass of sample trees;
ŷ: estimated biomass of sample trees by allometric equation.
3. Results
3.1. Verification of the Fitness of the Derived Regression
Table 2 shows the variables derived to evaluate the fitness of the allometric equations derived in this study. The explanation rate of the equation derived by applying the allometric method was 88%, and the RMSE and MAE of the equation showed 0.08 and 0.07, respectively. The MPE values derived from the regression equation depended on the organ, and the predicted values were overestimated for stem, leaf, and root, while underestimated for branch and total relative to the measured values.

Table 2.
The results of the regression equation’s fitness verification.
3.2. Net Primary Productivity (NPP) of S. pierotii Community
The allometric equations derived from the correlation between the diameter at breast height and the dry weight of stems, branches, leaves, and roots, and the total dry combined weight of each organ, are shown in Figure 4. The DBH was significantly correlated with each organ and total dry weight.
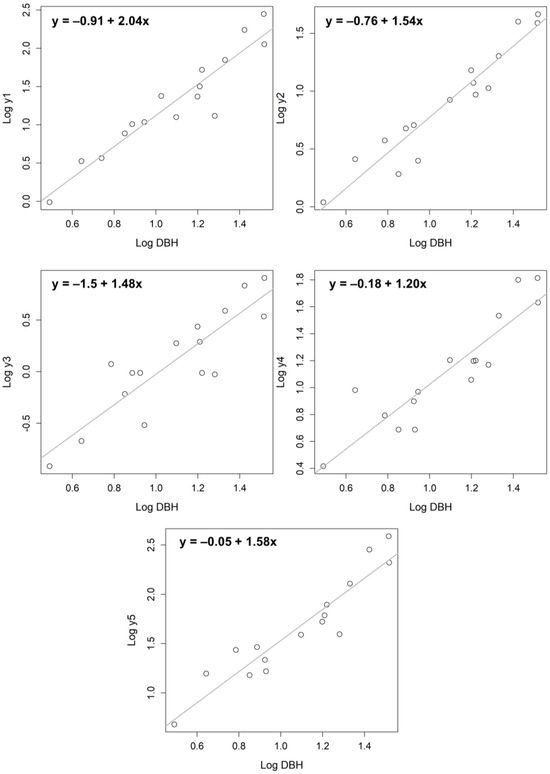
Figure 4.
The allometric equations derived from the correlation between the diameter at breast height (DBH) and the dry weight of stems, branches, leaves, roots, and the total dry weight of each organ. The circles are observed data. y1 = stem; y2 = branch; y3 = leaf; y4 = root; y5 = total biomass.
The NPP obtained by substituting the changes in the DBH that occurred via growth for one year along the Miho River into these allometric equations was 40.8 tonC·ha−1·yr−1.
The NPP and heterotrophic respiration were 40.8 tonC·ha−1·yr−1 and 7.1 tonC·ha−1·yr−1, respectively. Hence, the NEP obtained from the difference between the carbon absorption (NPP) by vegetation and carbon emission via the respiration of heterotrophic organisms was calculated as 33.6 tonC·ha−1·yr−1 (Table 3).

Table 3.
The net primary productivity (NPP), heterotrophic respiration, and net ecosystem production (NEP) measured in the Salix pierotii community established along the Miho River.
3.3. Seasonal Changes in Soil Respiration and Amount of Annual Soil Respiration
The soil respiration rate showed the typical seasonal change pattern. The soil respiration rate was lowest in January and began to gradually increase, peaking in August. Then, it decreased until December (Figure 5).
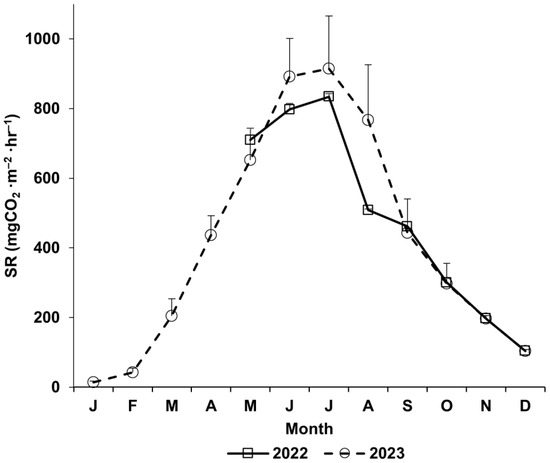
Figure 5.
Seasonal variation in monthly mean soil respiration of S. pierotii community established along Miho River. Bars indicate standard errors of mean soil respiration.
The total amount of soil respiration in the S. pierotii community established along the Miho River was calculated as 14.2 tonC·ha−1·yr−1, and the amount of heterotrophic respiration was 7.1 tonC·ha−1·yr−1 (Table 3).
Optimal regression equations using the exponential form of Equation (2) were derived from the relationships between the monthly mean SR rates and the temperatures (Ta and Ts) observed at 1.5 m aboveground and a 5 cm soil depth for the entire study period to elucidate the dependence of SR on the temporal variabilities in temperature in the S. pierotii stand (Figure 6). The temperature at a 5 cm soil depth correlated more than the air temperature with the soil respiration. The Q10 values for the air and soil temperatures were 2.25 and 3.15, respectively (Figure 6).
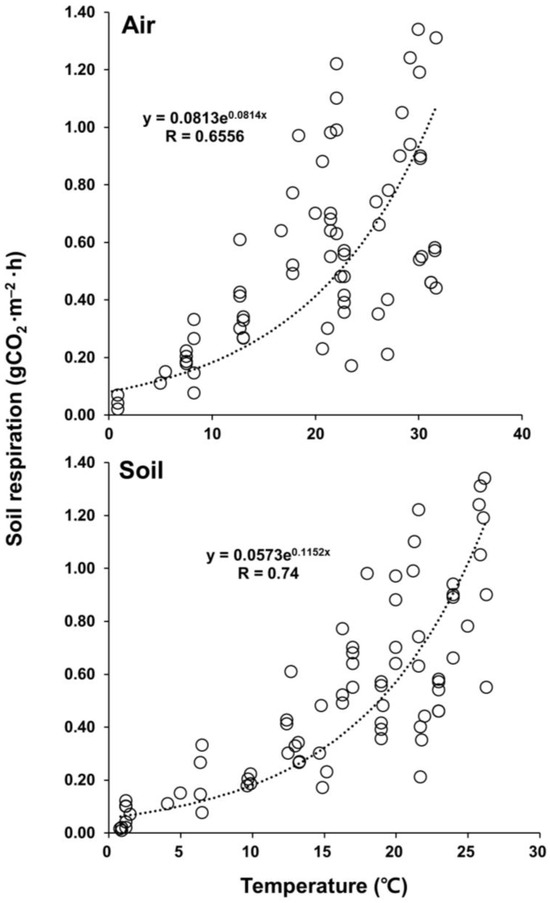
Figure 6.
The scatter plots of observed soil respiration vs. temperatures (°C) measured in air (1.5 m height) and soil (0.5 cm depth) for the S. pierotii community along the Miho River. The circles are observed data. Q10 = 2.25 and 3.15, respectively.
3.4. NEP of S. pierotii Community
The NPP and heterotrophic respiration were 40.8 tonC·ha−1·yr−1, and 7.1 tonC·ha−1·yr−1, respectively. As a result, the NEP obtained from the difference between the carbon absorption (NPP) by vegetation and carbon emission via the respiration of heterotrophic organisms was calculated as 33.6 tonC·ha−1·yr−1 (Table 3).
4. Discussion
4.1. Carbon Dynamics in Riparian Forest
Wetlands are known to have relatively high accumulation and absorption rates of carbon compared with terrestrial ecosystems [78,79]. Biological and environmental factors, such as the rate of plant decomposition, groundwater, and temperature, determine the amount of carbon sequestration. Many studies have been conducted on this topic as wetlands’ carbon storage capacity has recently emerged [80,81].
The carbon dioxide flow is more active at high temperatures and well-drained conditions with an active oxygen supply [81,82]. The amount of carbon dioxide absorbed in wetlands greatly fluctuates depending on weather conditions. Sometimes, the carbon dioxide absorption source is converted into an emission source. Therefore, longer-term measurements must be performed to understand the general trend of the carbon dioxide flow in wetlands and the effect of the carbon dioxide flow throughout the entire wetland carbon cycle [33].
A study conducted on the backwater wetlands in the eastern United States determined that wetland soils can store 1.6 to 2.2 tonC·ha−1 of carbon per year [83]. In addition, the amount of carbon absorption in grassland wetlands in the northeastern United States was calculated at 3.0 tonC·ha−1 per year [83]. Based on the carbon dioxide flow data measured in temperate wetlands in Japan, the production of wetland plants was 5.56 tonC·ha−1 per year, and the ecosystem respiration was 4.92 tonC·ha−1 per year. Consequently, the net carbon dioxide flow was calculated to be 0.64 tonC·ha−1 per year [84]. In addition, studies on CO2 flow in wetlands in various regions of North America and Europe have shown that wetlands absorb less than 1.00 tonC·ha−1 of CO2 per year [78,79].
However, wetlands are reported as carbon emission sources in the Republic of Korea [85]. This is because the carbon budget was considered by focusing on methane gas emissions from water bodies without considering the carbon storage of soil and the carbon absorption capacity of riparian vegetation. In addition, wetlands with short or damaged formation periods can emit greenhouse gases such as carbon dioxide and methane gas from the soil at higher levels than general soil [86,87]. However, this study’s results indicate that wetlands function as carbon sinks if the carbon adsorption amount of the vegetation and the carbon storage amount of the soil are accurately measured in stable wetlands.
The net primary productivity of the S. pierotii community calculated in this study was very high, at 40.8 tonC·ha−1·yr−1 (Table 3). Flooding, a representative disturbance factor of the riparian ecosystem, increases the moisture content, bulk density, and concentration of ammonium ions (NH4+) in riparian soil [88]. In addition, the soil’s nutrient content tends to increase with the frequency of flooding [89], which may change the structure and abundance of soil microbial communities in the riparian ecosystem [90,91].
The net primary productivity of Salix genus plant communities measured in many countries was 1.2 to 23.9 tonC·ha−1·yr−1 (Table 4). However, since most of the studies focused on aboveground production, further studies on underground production are required. According to Sleight et al. [92], the ratio of underground to aboveground biomass increases as the community age increases in Salix genus plant communities. Considering the characteristics of Salix genus plants, where the growth of the underground part increases to up to double that of the aboveground part with increasing age, the net primary productivity of the underground part was considered to be underestimated in these results [92,93].
The carbon sequestration capacity of willow stands in riparian ecosystems can be influenced and optimized depending on changing climate conditions. Willows are fast-growing, highly adaptable trees, making them promising candidates for enhancing carbon sequestration in response to climate change. Establishing buffer zones of willow stands around vulnerable water bodies can protect them against climate-related stresses, such as increased water temperatures and contamination, and increase carbon sequestration. These buffer zones act as carbon sinks alongside other ecosystem services. Willow stands in riparian forests support ecological health via erosion control, water quality improvement, habitat provision, and microclimate regulation. Hence, their presence is crucial for sustaining the biodiversity and functionality of riparian ecosystems [64].
However, regular monitoring of local temperatures, precipitation, and soil conditions is required to elucidate how climate change is affecting willow growth and carbon sequestration rates. Adaptive management could be implemented as climate patterns evolve, including adjusting fertilization, pest management, or irrigation practices [94,95].

Table 4.
A comparison of the net primary productivity (NPP) and net ecosystem production (NEP) among Salix spp.
Table 4.
A comparison of the net primary productivity (NPP) and net ecosystem production (NEP) among Salix spp.
| Scientific Name | NPP (tonC·ha−1·yr−1) | NEP (tonC·ha−1·yr−1) | Reference |
|---|---|---|---|
| Salix pierotii | 40.8 | 33.6 | Observed data |
| S. pierotii | 11.2 | 9.2 | [95] |
| S. pierotii | 11.3 | [96] | |
| Salix chaenomeloides | 5.1 | [97] | |
| Salix triandra L. subsp. nipponica | 1.2 | [97] | |
| S. triandra L. subsp. nipponica | 17.6 | [98] | |
| S. triandra L. subsp. nipponica | 23.9 | [99] | |
| Salix spp. | 8.4 | [60] |
4.2. Soil Respiration of Riparian Forests
The correlation between soil respiration and temperature and water content is usually used to explain differences in soil respiration between sites [100,101]. The Q10 value is effective in estimating the correlation between soil respiration and temperature in soil respiration studies [102,103,104]. The Q10 values calculated for air and soil temperatures in this study ranged from 2.25 to 3.15, similar to the range (1.3 to 3.3) reported in previous studies on wetlands [102,104]. However, the Q10 value for wetlands is higher than that for forest soil due to the anaerobic environment, which is believed to be because the higher the temperature, the greater the effect on microorganisms’ metabolic activity [103,105]. Like temperature, the water content, which strongly affects soil respiration, is affected by precipitation, which can increase the decomposition rate of microorganisms and the amount of plant photosynthesis [106,107,108]. However, an increased water content due to precipitation in water-rich wetlands can lead to lower soil respiration, as oxygen saturation and microbial activity can be restricted [109,110].
In Jung et al.’s [95] results, the amounts of soil respiration for riparian vegetation were 19.8 tonC·ha−1·yr−1, 30.1 tonC·ha−1·yr−1, and 22.0 tonC·ha−1·yr−1 in the Phragmites japonica, Miscanthus sacchariflorus, and S. pierotii communities, respectively, which were higher than the values measured in this study. In that study, the amount of soil respiration was calculated based on the values measured from July to September, when the values were highest, which is judged to be due to overestimating the values. The soil respiration of plant communities dominated by Poplar genus plants, another representative riparian vegetation, was 6.81 to 14.88 tonC·ha−1·yr−1 [111] and 1.98 tonC·ha−1·yr−1 [112], which was similar to this study’s results. The soil respiration measured in the forest ecosystem was 10.0 to 13.4 tonC·ha−1·yr−1, 6.1 to 14.1 tonC·ha−1·yr−1, and 6.0 to 8.0 tonC·ha−1·yr−1 in broad-leaved, mixed, and coniferous forests, respectively. The soil respiration of broad-leaved and mixed forests showed similar values to those of the S. pierotii community measured in this study, but those of coniferous forests were lower [113,114]. This is believed to be related to the differences in environmental factors between rivers and forests, including soil moisture content [81].
Pacific et al. [115] interpreted the high carbon dioxide emissions in willow communities as resulting from the frequent inflow of organic carbon from floods and the soil texture of the site where the community was established, allowing optimal gas diffusion [116].
4.3. Preparation to Realize Carbon Neutrality
Meeting the goal of the Paris Climate Accord to limit global warming to 2 °C requires adopting multiple strategies to rapidly reduce and mitigate carbon emissions. These strategies include an array of negative emission technologies that result in the net removal of greenhouse gases from the atmosphere, including the capture and storage of carbon in vegetation and soil via reforestation, afforestation, and changes in agricultural practices [117,118]. Restoring degraded landscapes will also improve ecological integrity, providing many additional benefits to biodiversity and human well-being [9]. As a result, 56 countries have pledged to restore 168.4 million ha of deforested and degraded land through the Bonn Challenge, which will sequester an estimated 15.7 Gt of CO2 and generate USD 48.4 billion in economic activity [119]. These efforts’ effectiveness can be improved with information on how much and quickly carbon can be stored in forest vegetation and soil.
Riparian forests located along water channels may be crucial to these efforts. Reference rates of carbon stock accumulation have been compiled for many forest types [120] but do not typically distinguish between riparian and upland forests. Despite their relatively small spatial footprint, riparian forests usually have more favorable growing conditions (e.g., soil moisture) and may accumulate carbon stocks at a higher rate than upland forests [62,63,121], contributing more to rapid, short-term carbon sequestration. Furthermore, riparian ecosystems are widely recognized to provide numerous ecosystem services [63,122,123], having the potential to mitigate the effects of climate change [124] and be biodiversity hotspots that provide critical habitats for fish and wildlife [63,125]. Because riparian ecosystems have been severely degraded worldwide [126,127,128], riparian forest restoration may be a valuable strategy for providing both rapid carbon sequestration value and long-term ecosystem service returns.
4.4. Ecological Restoration as a Strategy to Ensure New Carbon Sinks
Restoring degraded landscapes is a globally recognized strategy to sequester carbon, improve ecological integrity, conserve biodiversity, and provide additional benefits to human health and well-being. Investment in riparian forest restoration has received relatively little attention, partly due to their relatively small spatial extent. Nevertheless, the restoration of riparian forests may be a valuable strategy due to their potential for rapid carbon sequestration and to provide biodiversity hotspots and numerous valuable ecosystem services [129].
However, most wetlands in Asian countries, including South Korea, have been converted into agricultural land to produce rice, a staple food. Furthermore, many of these rice fields have been transformed into urban areas in recent years [55,56]. Consequently, the wetlands have been greatly damaged, making it difficult for them to perform their normal functions. In particular, the river was reduced to a very simple waterway by removing riparian vegetation that interfered with the growth of rice by creating shade. However, the level of environmental awareness has significantly increased in these regions due to recent social changes caused by economic growth, and the restoration of damaged wetlands, including rivers, is progressing. Nonetheless, the level of restoration does not significantly exceed the level of aesthetic refinement; thus, the contribution to environmental improvement is not significant [55,56]. The naturalness of the restored rivers is minimally improved, and the riparian vegetation, which plays an important role as a carbon sink, is very poor [55,56].
However, the Miho Bridge area along the Miho River—which was left to natural processes after agricultural activities were stopped in the riparian zone during the construction of a new city, Sejong City—has been restored passively, differing from these restoration projects. Reflecting the flooding regime, bare ground has established on the waterfront, and a vegetation zone dominated by herbaceous plants, a vegetation zone dominated by shrubs, and a vegetation zone dominated by trees have developed, as well as sandbars of various scales and backwater wetlands, making it an important ecological space for wildlife [130]. A willow community, which has been attracting attention as a new carbon absorption source, has been established in a large area along with various vegetation types dominated by herbaceous plants. In evaluations of the carbon budget, this willow community was determined to have a very high carbon absorption capacity (Table 4). In this respect, the study area presents a new direction for environmental policy to realize carbon neutrality alongside river restoration.
Evaluating the carbon budget revealed that the carbon dioxide absorption capacity per unit area of the willow community was more than four times the amount absorbed by forest vegetation such as pine and oak colonies [15]. The amount of carbon dioxide absorbed by the willow community in the Republic of Korea was estimated to be approximately 1.84 million tons by substituting the amount of carbon dioxide absorption per unit area into the currently established willow community area [131]. Furthermore, assuming the river restoration was properly carried out, such as the passive restoration conducted in the study area [55,56], the absorption amount was approximately 15 million tons. This amount is equivalent to 60% of South Korea’s carbon dioxide absorption by 2050, the year of South Korea’s carbon neutralization goal [4], and is expected to play an important role as a new carbon sink in achieving the country’s carbon neutrality goal.
5. Conclusions
Wetlands have functioned as carbon sinks over extended periods. The carbon sequestration capacity of riparian vegetation is higher than that of forest vegetation in the same area. However, recent climate changes have altered the carbon cycle processes in riparian ecosystems. Degraded riparian ecosystems may be assessed as sources that release stored carbon dioxide and methane. Conversely, well-preserved or stable riparian ecosystems can function as carbon sinks by storing significant amounts of carbon in the soil and water bodies and via vegetation photosynthesis. Additionally, wetlands are valuable for carbon sequestration alongside providing diverse ecosystem services that contribute to environmental sustainability.
As such, the restoration of degraded riparian ecosystems is urgent. Restoring deforested and degraded landscapes is a globally recognized strategy to sequester carbon, improve ecological integrity, conserve biodiversity, and provide additional benefits to human health and well-being. Investment in riparian forest restoration could be a valuable strategy, as riparian forests have the potential for rapid carbon sequestration, are hotspots of biodiversity, and provide numerous valuable ecosystem services. However, the restoration method must be carefully selected. Restoration must follow passive restoration first rather than active restoration in riparian zones with frequent disturbances and high intensity due to the influence of floods. Then, assisted restoration may be added if insufficient parts are found during subsequent monitoring [132,133].
Finding intact riparian ecosystems in the Republic of Korea is challenging due to their conversion into agricultural land, urbanization, or structural simplification for flood control purposes. As a result, degraded wetlands are often evaluated as sources of greenhouse gas emissions rather than sinks. Moreover, previous studies on carbon sequestration in wetland vegetation are scarce, making accurate carbon budget assessments difficult.
In this study, we developed an allometric equation of willow communities and measured the net primary productivity of willow communities by applying this equation. Furthermore, the amount of carbon emitted via soil respiration was measured and converted into heterotrophic respiration based on previous research results. Then, the net ecosystem production was obtained by subtracting the amount of heterotrophic respiration from the net primary productivity. The results obtained through this process were compared with those obtained from forest vegetation, revealing that the willow community, a representative riparian vegetation, sequestered a significantly higher level of carbon than the forest vegetation. Considering these results comprehensively, the willow community as riparian vegetation is a new and crucial carbon absorption source.
Author Contributions
Conceptualization, B.-S.L. and C.-S.L.; methodology, B.-S.L. and S.-J.J.; software, B.-S.L.; validation, B.-S.L. and J.-E.S.; formal analysis, B.-S.L.; investigation, B.-S.L. and J.-E.S.; data curation, B.-S.L. and J.-E.S.; writing—original draft preparation, B.-S.L.; writing—review and editing, B.-S.L., S.-J.J. and C.-S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Korea Environment Industry & Technology Institute (KEITI) through the Wetland Ecosystem Value Evaluation and Carbon Absorption Value Promotion Technology Development Project, funded by the Korea Ministry of Environment (2022003630002).
Data Availability Statement
The data are included in the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Furtak, K.; Wolińska, A. The Impact of Extreme Weather Events as a Consequence of Climate Change on the Soil Moisture and on the Quality of the Soil Environment and Agriculture—A Review. Catena 2023, 231, 107378. [Google Scholar] [CrossRef]
- Seneviratne, S.; Nicholls, N.; Easterling, D.; Goodess, C.; Kanae, S.; Kossin, J.; Luo, Y.; Marengo, J.; Mcinnes, K.; Rahimi, M.; et al. Changes in Climate Extremes and Their Impacts on the Natural Physical Environment: An Overview of the IPCC SREX Report. In Managing the Risks of Extreme Events and Disasters to Advance Climate Change Adaptation; Cambridge University Press: Cambridge, UK, 2012; p. 12566. [Google Scholar]
- What Is Carbon Neutrality and How Can It Be Achieved by 2050? Available online: https://www.europarl.europa.eu/topics/en/article/20190926STO62270/what-is-carbon-neutrality-and-how-can-it-be-achieved-by-2050 (accessed on 21 September 2024).
- The Government of the Republic of Korea. 2050 Carbon Neutral Strategy of the Republic of Korea Towards a Sustainable and Green Society; The Government of the Republic of Korea: Seoul, Republic of Korea, 2020.
- McLaughlin, H.; Littlefield, A.A.; Menefee, M.; Kinzer, A.; Hull, T.; Sovacool, B.K.; Bazilian, M.D.; Kim, J.; Griffiths, S. Carbon Capture Utilization and Storage in Review: Sociotechnical Implications for a Carbon Reliant World. Renew. Sustain. Energy Rev. 2023, 177, 113215. [Google Scholar] [CrossRef]
- Prajapati, S.; Choudhary, S.; Kumar, V.; Dayal, P.; Srivastava, R.; Gairola, A.; Borate, R. Carbon Sequestration: A Key Strategy for Climate Change Mitigation towards a Sustainable Future. Emrg. Trnd. Clim. Chng. 2023, 2, 1–14. [Google Scholar]
- Masson-Delmotte, V.; Zhai, P.; Pörtner, H.-O.; Roberts, D.; Skea, J.; Shukla, P.; Pirani, A.; Moufouma-Okia, W.; Péan, C.; Pidcock, R.; et al. (Eds.) Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; IPCC: Geneva, Switzerland, 2018; ISBN 978-1-00-915795-7. [Google Scholar]
- Nunes; Meireles, C.; Gomes, P.; Ribeiro, A. Forest Contribution to Climate Change Mitigation: Management Oriented to Carbon Capture and Storage. Climate 2020, 8, 21. [Google Scholar] [CrossRef]
- IUCN. International Union for Conservation of Nature Annual Report 2016; IUCN: Gland, Switzerland, 2016. [Google Scholar]
- Cox, P.M.; Betts, R.A.; Jones, C.D.; Spall, S.A.; Totterdell, I.J. Acceleration of Global Warming Due to Carbon-Cycle Feedbacks in a Coupled Climate Model. Nature 2000, 408, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Prentice, I.; Farquhar, G.; Fasham, M.; Goulden, M.; Heimann, M.; Jaramillo, V.; Kheshgi, H.; Le Quéré, C.; Scholes, R.; Wallace, D. The Carbon Cycle and Atmospheric Carbon Dioxide. In Climate Change 2001: The Scientific Basis; Houghton, J.T., Ding, Y., Griggs, D.J., Noguer, M., van der Linden, P.J., Dai, X., Maskell, K., Johnson, C.A., Eds.; Cambridge University Press: Cambridge, UK, 2001; pp. 183–237. ISBN 978-0-521-80767-8. [Google Scholar]
- Joo, S.J.; Park, M.-S.; Kim, G.-S.; Lee, C.S. CO2 Flux in a Cool-Temperate Deciduous Forest (Quercus Mongolica) of Mt. Nam in Seoul, Korea. J. Ecol. Field Biol. 2011, 34, 95–106. [Google Scholar] [CrossRef]
- Rodrigues, C.I.D.; Brito, L.M.; Nunes, L.J.R. Soil Carbon Sequestration in the Context of Climate Change Mitigation: A Review. Soil Syst. 2023, 7, 64. [Google Scholar] [CrossRef]
- Kim, G.S.; Joo, S.J.; Lee, C.S. Seasonal Variation of Soil Respiration in the Mongolian Oak (Quercus Mongolica Fisch. Ex Ledeb). For. Cool. Temp. Zone Korea. For. 2020, 11, 984. [Google Scholar]
- Kim, G.S.; Kim, A.R.; Lim, B.S.; Seol, J.; An, J.H.; Lim, C.H.; Joo, S.J.; Lee, C.S. Assessment of the Carbon Budget of Local Governments in South Korea. Atmosphere 2022, 13, 342. [Google Scholar] [CrossRef]
- Nayak, N.; Mehrotra, R.; Mehrotra, S. Carbon Biosequestration Strategies: A Review. Carbon Capture Sci. Technol. 2022, 4, 100065. [Google Scholar] [CrossRef]
- Liu, M.; Bai, X.; Tan, Q.; Luo, G.; Zhao, C.; Wu, L.; Chen, F.; Li, C.; Yang, Y.; Ran, C.; et al. Climate Change Enhanced the Positive Contribution of Human Activities to Net Ecosystem Productivity from 1983 to 2018. Front. Ecol. Evol. 2023, 10, 1101135. [Google Scholar] [CrossRef]
- Kabir, M.; Habiba, U.E.; Khan, W.; Shah, A.; Rahim, S.; los Rios-Escalante, P.R.D.; Farooqi, Z.-U.-R.; Ali, L.; Shafiq, M. Climate Change Due to Increasing Concentration of Carbon Dioxide and Its Impacts on Environment in 21st Century; a Mini Review. J. King Saud Univ. Sci. 2023, 35, 102693. [Google Scholar] [CrossRef]
- Rackley, S.A.; Ming, T.; Li, W.; Tyka, M.; Sewel, A.; Clery, D.; Dowson, G.; Styring, P.; Andrews, G.; McCord, S.; et al. Negative Emissions Technologies for Climate Change Mitigation; Elsevier: Amsterdam, The Netherlands, 2023; ISBN 978-0-12-823167-8. [Google Scholar]
- Nakane, K. Quantitative Evaluation of Atmospheric CO2 Sink into Forest Soils from the Tropics to the Boreal Zone during the Past Three Decades. Ecol. Res. 2001, 16, 671–685. [Google Scholar] [CrossRef]
- Canadell, J.G.; Le Quéré, C.; Raupach, M.R.; Field, C.B.; Buitenhuis, E.T.; Ciais, P.; Conway, T.J.; Gillett, N.P.; Houghton, R.A.; Marland, G. Contributions to Accelerating Atmospheric CO2 Growth from Economic Activity, Carbon Intensity, and Efficiency of Natural Sinks. Proc. Natl. Acad. Sci. USA 2007, 104, 18866–18870. [Google Scholar] [CrossRef] [PubMed]
- Onyeaka, H.; Miri, T.; Obileke, K.; Hart, A.; Anumudu, C.; Al-Sharify, Z.T. Minimizing Carbon Footprint via Microalgae as a Biological Capture. Carbon Capture Sci. Technol. 2021, 1, 100007. [Google Scholar] [CrossRef]
- Heimann, M.; Reichstein, M. Terrestrial Ecosystem Carbon Dynamics and Climate Feedbacks. Nature 2008, 451, 289–292. [Google Scholar] [CrossRef]
- Yang, W.; Min, Z.; Yang, M.; Yan, J. Exploration of the Implementation of Carbon Neutralization in the Field of Natural Resources under the Background of Sustainable Development-An Overview. Int. J. Environ. Res. Public Health 2022, 19, 14109. [Google Scholar] [CrossRef]
- Barbosa, V.; Damasceno da Silva, R.M.; Dias, M.; Castelhano, F.; Roig, H.; Requia, W. Ecosystem Services Provided by Green Areas and Their Implications for Human Health in Brazil. Ecol. Indic. 2024, 161, 111975. [Google Scholar] [CrossRef]
- Ravindranath, N.H.; Ostwald, M. Methods for Estimating Above-Ground Biomass. In Carbon Inventory Methods Handbook for Greenhouse Gas Inventory, Carbon Mitigation and Roundwood Production Projects; Ravindranath, N.H., Ostwald, M., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 113–147. ISBN 978-1-4020-6547-7. [Google Scholar]
- Lal, R. Sequestering Atmospheric Carbon Dioxide. Crit. Rev. Plant Sci. 2009, 28, 90–96. [Google Scholar] [CrossRef]
- Abbass, K.; Qasim, M.Z.; Song, H.; Murshed, M.; Mahmood, H.; Younis, I. A Review of the Global Climate Change Impacts, Adaptation, and Sustainable Mitigation Measures. Environ. Sci. Pollut. Res. 2022, 29, 42539–42559. [Google Scholar] [CrossRef]
- Salimi, S.; Almuktar, S.A.A.A.N.; Scholz, M. Impact of Climate Change on Wetland Ecosystems: A Critical Review of Experimental Wetlands. J. Environ. Manag. 2021, 286, 112160. [Google Scholar] [CrossRef] [PubMed]
- Qubaja, R.; Tatarinov, F.; Rotenberg, E.; Yakir, D. Partitioning of Canopy and Soil CO2 Fluxes in a Pine Forest at the Dry Timberline across a 13-Year Observation Period. Biogeosciences 2020, 17, 699–714. [Google Scholar] [CrossRef]
- Global Carbon Project; Friedlingstein, P.; O’Sullivan, M.; Jones, M.W.; Andrew, R.M.; Gregor, L.; Hauck, J.; Le Quéré, C.; Luijkx, I.T.; Olsen, A.; et al. Global Carbon Budget 2022. Earth Syst. Sci. Data 2022, 14, 4811–4900. [Google Scholar] [CrossRef]
- Yanbin, H.; Zhang, Q.; Hu, S.; Xiao, G.; Chen, X.; Wang, J.; Qi, Y.; Zhang, L.; Han, L. Research Progress and Prospects of Ecosystem Carbon Sequestration under Climate Change (1992–2022). Ecol. Indic. 2022, 145, 109656. [Google Scholar] [CrossRef]
- Mitsch, W.; Bernal, B.; Nahlik, A.; Mander, Ü.; Zhang, L.; Anderson, C.; Jørgensen, S.E.; Brix, H. Wetlands, Carbon, and Climate Change. Landsc. Ecol. 2012, 28, 583–597. [Google Scholar] [CrossRef]
- Nahlik, A.M.; Fennessy, M.S. Carbon Storage in US Wetlands. Nat. Commun. 2016, 7, 13835. [Google Scholar] [CrossRef]
- Han, L.; Wan, Z.; Guo, Y.; Song, C.; Jin, S.; Zuo, Y. Estimation of Soil Organic Carbon Storage in Palustrine Wetlands, China. Int. J. Environ. Res. Public Health 2020, 17, 4646. [Google Scholar] [CrossRef] [PubMed]
- Bloom, A.A.; Bowman, K.W.; Lee, M.; Turner, A.J.; Schroeder, R.; Worden, J.R.; Weidner, R.; McDonald, K.C.; Jacob, D.J. A Global Wetland Methane Emissions and Uncertainty Dataset for Atmospheric Chemical Transport Models (WetCHARTs Version 1.0). Geosci. Model Dev. 2017, 10, 2141–2156. [Google Scholar] [CrossRef]
- Bousquet, P.; Ringeval, B.; Pison, I.; Dlugokencky, E.J.; Brunke, E.-G.; Carouge, C.; Chevallier, F.; Fortems-Cheiney, A.; Frankenberg, C.; Hauglustaine, D.A.; et al. Source Attribution of the Changes in Atmospheric Methane for 2006–2008. Atmos. Chem. Phys. 2011, 11, 3689–3700. [Google Scholar] [CrossRef]
- Ma, K.; Zhang, Y.; Tang, S.; Liu, J. Spatial Distribution of Soil Organic Carbon in the Zoige Alpine Wetland, Northeastern Qinghai–Tibet Plateau. Catena 2016, 144, 102–108. [Google Scholar] [CrossRef]
- Kolka, R.; Trettin, C.; Windham-Myers, L. The Importance of Wetland Carbon Dynamics to Society. In Wetland Carbon and Environmental Management; American Geophysical Union (AGU): Washington, DC, USA, 2021; pp. 421–436. ISBN 978-1-119-63930-5. [Google Scholar]
- Liu, X.; Lu, X.; Yu, R.; Sun, H.; Hao, X.; Qi, Z.; Cao, Z.; Zhang, Z. Greenhouse Gases Emissions from Riparian Wetlands: An Example from the Inner Mongolia Grassland Region in China. Biogeosciences 2021, 18, 4855–4872. [Google Scholar] [CrossRef]
- Lu, W.; Xiao, J.; Liu, F.; Zhang, Y.; Liu, C.; Lin, G. Contrasting Ecosystem CO2 Fluxes of Inland and Coastal Wetlands: A Meta-Analysis of Eddy Covariance Data. Glob. Change Biol. 2017, 23, 1180–1198. [Google Scholar] [CrossRef]
- European Commission Nature Restoration Law. Supporting the Restoration of Ecosystems for People, the Climate and the Planet. Available online: https://environment.ec.europa.eu/topics/nature-and-biodiversity/nature-restoration-law_en (accessed on 23 September 2024).
- Van Rees, C.B.; Waylen, K.A.; Schmidt-Kloiber, A.; Thackeray, S.J.; Kalinkat, G.; Martens, K.; Domisch, S.; Lillebø, A.I.; Hermoso, V.; Grossart, H.-P.; et al. Safeguarding freshwater life beyond 2020: Recommendations for the new global biodiversityframework from the European experience. Conserv. Lett. 2021, 14, e12771. [Google Scholar] [CrossRef]
- Ryfisch, S.; Seeger, I.; McDonald, H.; Lago, M.; Blicharska, M. Opportunities and Limitations for Nature-Based Solutions in EU Policies—Assessed with a Focus on Ponds and Pondscapes. Land Use Policy 2023, 135, 106957. [Google Scholar] [CrossRef]
- Premrov, A.; Wilson, D.; Saunders, M.; Yeluripati, J.; Renou-Wilson, F. CO2 Fluxes from Drained and Rewetted Peatlands Using a New ECOSSE Model Water Table Simulation Approach. Sci. Total Environ. 2021, 754, 142433. [Google Scholar] [CrossRef]
- Makrickas, E.; Manton, M.; Angelstam, P.; Grygoruk, M. Trading Wood for Water and Carbon in Peatland Forests? Rewetting Is Worth More than Wood Production. J. Environ. Manag. 2023, 341, 117952. [Google Scholar] [CrossRef]
- Balode, L.; Blumberga, D. Comparison of the Economic and Environmental Sustainability for Different Peatland Strategies. Land 2024, 13, 518. [Google Scholar] [CrossRef]
- European Commission. Commission Staff Working Document Sustainable Carbon Cycles—Carbon Farming Accompanying the Communication from the Commission to the European Parliament and the Council Sustainable Carbon Cycles. Available online: https://climate.ec.europa.eu/ (accessed on 21 September 2024).
- Li, X.; Martino, S. Assessing the Economic Feasibility of Voluntary Carbon Markets in Land Use Management Scenarios for Scottish Saltmarshes. Ocean. Coast. Manag. 2024, 251, 107099. [Google Scholar] [CrossRef]
- Peters, J.; Beltrán, E.; Wilson, S.; Salm, J.-O.; Ozola, I.; Zableckis, N. Remuneration Schemes for Paludiculture and Carbon Farming Guidance; Michael Succow Foundation: Greifswald, Germany, 2024. [Google Scholar]
- Fennessy, S.; Lei, G. Wetland Restoration for Climate Change Resilience; Ramsar Convention Secretariat: Gland, Switzerland, 2018. [Google Scholar]
- Wang, F.; Liu, J.; Qin, G.; Zhang, J.; Zhou, J.; Wu, J.; Zhang, L.; Thapa, P.; Sanders, C.J.; Santos, I.R.; et al. Coastal Blue Carbon in China as a Nature-Based Solution toward Carbon Neutrality. Innovation 2023, 4, 100481. [Google Scholar] [CrossRef]
- Ellis, P.W.; Page, A.M.; Wood, S.; Fargione, J.; Masuda, Y.J.; Carrasco Denney, V.; Moore, C.; Kroeger, T.; Griscom, B.; Sanderman, J.; et al. The Principles of Natural Climate Solutions. Nat. Commun. 2024, 15, 547. [Google Scholar] [CrossRef]
- World Economic Forum. Wetlands, the Forgotten Carbon Sink That Can Help Mitigate Impact of Climate Change; World Economic Forum: Geneva, Switzerland, 2023. [Google Scholar]
- Lim, B.S.; Kim, D.U.; Kim, A.R.; Seol, J.W.; Lee, C.S. Analysis of Ecodiversity as the Foundation for Conserving Biodiversity and Its Restoration Strategy. Korean J. Ecol. Environ. 2020, 53, 408–426. [Google Scholar] [CrossRef]
- An, J.H.; Lim, B.S.; Seol, J.; Kim, A.R.; Lim, C.H.; Moon, J.S.; Lee, C.S. Evaluation on the Restoration Effects in the River Restoration Projects Practiced in South Korea. Water 2022, 14, 2739. [Google Scholar] [CrossRef]
- Lim, C.H.; Pi, J.H.; Kim, A.R.; Cho, H.J.; Lee, K.S.; You, Y.H.; Lee, K.H.; Kim, K.D.; Moon, J.S.; Lee, C.S. Diagnostic Evaluation and Preparation of the Reference Information for River Restoration in South Korea. Int. J. Environ. Res. Public Health 2021, 18, 1724. [Google Scholar] [CrossRef] [PubMed]
- Gigler, J.K.; Meerdink, G.; Hendrix, E.M.T. Willow Supply Strategies to Energy Plants. Biomass Bioenergy 1999, 17, 185–198. [Google Scholar] [CrossRef]
- Pulford, I.D.; Riddell-Black, D.; Stewart, C. Heavy Metal Uptake by Willow Clones from Sewage Sludge-Treated Soil: The Potential for Phytoremediation. Int. J. Phytoremediation 2002, 4, 59–72. [Google Scholar] [CrossRef]
- Szczukowski, S.; Stolarski, M.; Tworkowski, J.; Przyborowski, J.A.; Klasa, A. Productivity of Willow Coppice Plants Grown in Short Rotations. Plant Soil Environ. 2005, 51, 423. [Google Scholar] [CrossRef]
- Gorobets, A.; Silva, M. Willow Communities, Optimal Absorption of Carbon Dioxide from the Atmosphere. IOP Conf. Ser. Earth Environ. Sci. 2020, 595, 012038. [Google Scholar] [CrossRef]
- Matzek, V.; Stella, J.; Ropion, P. Development of a Carbon Calculator Tool for Riparian Forest Restoration. Appl. Veg. Sci. 2018, 21, 584–594. [Google Scholar] [CrossRef]
- Naiman, R.J.; Decamps, H.; McClain, M.E. Riparia: Ecology, Conservation, and Management of Streamside Communities; Elsevier: Amsterdam, The Netherlands, 2010; ISBN 978-0-08-047068-9. [Google Scholar]
- Riccioli, F.; Guidi Nissim, W.; Masi, M.; Palm, E.; Mancuso, S.; Azzarello, E. Modeling the Ecosystem Services Related to Phytoextraction: Carbon Sequestration Potential Using Willow and Poplar. Appl. Sci. 2020, 10, 8011. [Google Scholar] [CrossRef]
- Pietrzykowski, M.; Woś, B.; Tylek, P.; Kwaśniewski, D.; Juliszewski, T.; Walczyk, J.; Likus-Cieślik, J.; Ochał, W.; Tabor, S. Carbon Sink Potential and Allocation in Above- and below-Ground Biomass in Willow Coppice. J. For. Res. 2021, 32, 349–354. [Google Scholar] [CrossRef]
- Rytter, R.-M.; Rytter, L.; Högbom, L. Carbon Sequestration in Willow (Salix Spp.) Plantations on Former Arable Land Estimated by Repeated Field Sampling and C Budget Calculation. Biomass Bioenergy 2015, 83, 483–492. [Google Scholar] [CrossRef]
- KFRI (Korea Forest Research Institute). Study on the Basis of Forest Carbon Accounting in Korea; Korea Forest Research Institute: Seoul, Republic of Korea, 2010. [Google Scholar]
- Návar, J. Allometric Equations for Tree Species and Carbon Stocks for Forests of Northwestern Mexico. For. Ecol. Manag. 2009, 257, 427–434. [Google Scholar] [CrossRef]
- Whittaker, R.H.; Marks, P.L. Methods of Assessing Terrestrial Productivty. In Primary Productivity of the Biosphere; Springer: Berlin/Heidelberg, Germany, 1975; pp. 55–118. [Google Scholar]
- IPCC. A Report of Working Group of the Intergovernmental Panel on Climate Change; Intergovernmental Panel on Climate Change: Cambridge, UK, 2001. [Google Scholar]
- PP Systems. 2018. EGM-5 Operation Manual V. 1.03. Available online: https://ppsystems.com/download/technical_manuals/80109-1-EGM5_Operation_V103.pdf (accessed on 20 May 2020).
- Bekku, Y.; Koizumi, H.; Oikawa, T.; Iwaki, H. Examination of Four Methods for Measuring Soil Respiration. Appl. Soil Ecol. 1997, 5, 247–254. [Google Scholar] [CrossRef]
- Lucian, L.; Monica, M.; Mădălina, B.; György, D.; Ioana, C.; Natalia, E.; Rahim, N. Measurements and Statistical Analysis of CO2 Efflux and Related Parameters from Crop and Forested Lands. IOP Conf. Ser.Earth Environ. Sci. 2023, 1216, 012005. [Google Scholar] [CrossRef]
- Davidson, E.A.; Janssens, I.A.; Luo, Y. On the Variability of Respiration in Terrestrial Ecosystems: Moving beyond Q10. Glob. Chang. Biol. 2006, 12, 154–164. [Google Scholar] [CrossRef]
- Madalina, V.; Valentina, C.; Natalia, E.; Lucian, L.; Monica, M.; Anda, R.; Norbert, B.; Madalina, B.; Silvius, S.; György, D. Experimental Determination of Carbon Dioxide Flux in Soil and Correlation with Dependent Parameters. IOP Conf. Ser. Earth Environ. Sci. 2020, 616, 012010. [Google Scholar] [CrossRef]
- Lee, M.-S. Method for Assessing Forest Carbon Sinks by Ecological Process-Based Approach—A Case Study for Takayama Station, Japan. Korean J. Ecol. 2003, 26, 289–296. [Google Scholar] [CrossRef]
- Raich, J.W.; Tufekciogul, A. Vegetation and Soil Respiration: Correlations and Controls. Biogeochemistry 2000, 48, 71–90. [Google Scholar] [CrossRef]
- Abdul Malak, D.; Marín, A.; Trombetti, M.; Sonsoles, S. Carbon Pools and Sequestration Potential of Wetlands in the European Union. In European Topic Centre on Urban, Land and Soil Systems; Similarities and Diversity of European Cities A Typology Tool to Support Urban Sustainability; European Union: Brussels, Belgium, 2021. [Google Scholar]
- Xiao, D.; Deng, L.; Kim, D.G.; Huang, C.; Tian, K. Carbon Budgets of Wetland Ecosystems in China. Glob. Chang. Biol. 2019, 25, 2061–2076. [Google Scholar] [CrossRef]
- Cabezas, A.; Comín, F.A. Carbon and Nitrogen Accretion in the Topsoil of the Middle Ebro River Floodplains (NE Spain): Implications for Their Ecological Restoration. Ecol. Eng. 2010, 36, 640–652. [Google Scholar] [CrossRef]
- Samaritani, E.; Shrestha, J.; Fournier, B.; Frossard, E.; Gillet, F.; Guenat, C.; Niklaus, P.; Tockner, K.; Mitchell, E.; Luster, J. Heterogeneity of Soil Carbon Pools and Fluxes in a Channelized and a Restored Floodplain Section (Thur River, Switzerland). Hydrol. Earth Syst. Sci. Discuss. 2011, 8, 1757–1769. [Google Scholar] [CrossRef]
- Tockner, K.; Stanford, J.A. Riverine Flood Plains: Present State and Future Trends. Environ. Conserv. 2002, 29, 308–330. [Google Scholar] [CrossRef]
- Kayranli, B.; Scholz, M.; Mustafa, A.; Hedmark, Å. Carbon Storage and Fluxes within Freshwater Wetlands: A Critical Review. Wetlands 2010, 30, 111–124. [Google Scholar] [CrossRef]
- Suzuki, S.; Yokozawa, M.; Inubushi, K.; Hara, T.; Kimura, M.; Tsuga, S.; Tako, Y.; Nakamura, Y. Evaluation of CO2 Exchange Rates in a Wetland Ecosystem Using the Closed Geosphere Experiment Facility. J. Hydrometeorol. 2012, 13, 966–980. [Google Scholar] [CrossRef]
- Greenhouse Gas Inventory & Research Center of Korea. National Greenhouse Gas Inventory Report of Korea; Ministry of Environment: Sejong, Republic of Korea, 2022. [Google Scholar]
- Roulet, N.T.; Ash, R.; Quinton, W.; Moore, T. Methane Flux from Drained Northern Peatlands: Effect of a Persistent Water Table Lowering on Flux. Glob. Biogeochem. Cycles 1993, 7, 749–769. [Google Scholar] [CrossRef]
- Turetsky, M.R.; Wieder, R.K.; Vitt, D.H. Boreal Peatland C Fluxes under Varying Permafrost Regimes. Soil Biol. Biochem. 2002, 34, 907–912. [Google Scholar] [CrossRef]
- Ye, C.; Cheng, X.; Zhang, K.; Du, M.; Zhang, Q. Hydrologic Pulsing Affects Denitrification Rates and Denitrifier Communities in a Revegetated Riparian Ecotone. Soil Biol. Biochem. 2017, 115, 137–147. [Google Scholar] [CrossRef]
- Ye, C.; Cheng, X.; Zhang, Y.; Wang, Z.; Zhang, Q. Soil Nitrogen Dynamics Following Short-Term Revegetation in the Water Level Fluctuation Zone of the Three Gorges Reservoir, China. Ecol. Eng. 2012, 38, 37–44. [Google Scholar] [CrossRef]
- Shi, P.; Li, Z.; Li, P.; Zhang, Y.; Li, B. Trade-Offs Among Ecosystem Services after Vegetation Restoration in China’s Loess Plateau. Nat. Resour. Res. 2021, 30, 2703–2713. [Google Scholar] [CrossRef]
- Shen, S.; Pu, J.; Xu, C.; Wang, Y.; Luo, W.; Wen, B. Effects of Human Disturbance on Riparian Wetland Landscape Pattern in a Coastal Region. Remote Sens. 2022, 14, 5160. [Google Scholar] [CrossRef]
- Sleight, N.J.; Volk, T.A.; Eisenbies, M. Belowground Biomass and Root:Shoot Ratios of Three Willow Cultivars at Two Sites. Forests 2023, 14, 525. [Google Scholar] [CrossRef]
- Walter, M.; Brzozowski, B.; Adamczak, M. Effect of Supercritical Extract from Black Poplar and Basket Willow on the Quality of Natural and Probiotic Drinkable Yogurt. Animals 2021, 11, 2997. [Google Scholar] [CrossRef]
- Skendžić, S.; Zovko, M.; Pajač Živković, I.; Lešić, V.; Lemic, D. The Impact of Climate Change on Agricultural Insect Pests. Insects 2021, 12, 440. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Yi, J.; Lee, J.; Shim, K.; Lee, J. Characteristics of Accumulated Soil Carbon and Soil Respiration on Vegetation in Namhangang Basin. Korean J. Environ. Biol. 2014, 32, 363–370. [Google Scholar] [CrossRef]
- Han, S.J.; Kim, H.W.; Kim, H.R.; Kim, H.J.; Han, D.U.; Park, S.K.; You, Y.H. Net Primary Production, Annual Accumulation of Organic Carbon and Leaf Decomposition in Salix Plant Community. J. Wetl. Res. 2010, 12, 15–22. [Google Scholar]
- Kim, T.G.; Lee, P.H.; Oh, K. The Actual Vegetation Map, Standing Crop Biomass and Primary Productivity of Salix spp. in the Upo Wetland. J. Wetl. Res. 2007, 9, 33–43. [Google Scholar]
- Kim, C.S.; Lee, P.H.; Oh, K. Productivity and Production Structure of Salix nipponica. J. Wetl. Res. 1999, 1, 61–69. [Google Scholar]
- Han, D.U.; Yoo, J.W.; Yoo, Y.H.; Lee, E.J.; Park, S.K. Aboveground Primary Productivity of Salix nipponica and Secondary Productivity of Sesarma dehaani at Janghang Wetland in Han River Estuary. Korean J. Ecol. Environ. 2010, 43, 298–306. [Google Scholar]
- Lloyd, J.; Taylor, J.A. On the Temperature Dependence of Soil Respiration. Funct. Ecol. 1994, 8, 315–323. [Google Scholar] [CrossRef]
- Buchmann, N. Biotic and Abiotic Factors Controlling Soil Respiration Rates in Picea Abies Stands. Soil Biol. Biochem. 2000, 32, 1625–1635. [Google Scholar] [CrossRef]
- Raich, J.W.; Schlesinger, W.H. The Global Carbon Dioxide Flux in Soil Respiration and Its Relationship to Vegetation and Climate. Tellus B 1992, 44, 81–99. [Google Scholar] [CrossRef]
- Mo, W.; Lee, M.S.; Uchida, M.; Inatomi, M.; Saigusa, N.; Mariko, S.; Koizumi, H. Seasonal and Annual Variations in Soil Respiration in a Cool-Temperate Deciduous Broad-Leaved Forest in Japan. Agric. For. Meteorol. 2005, 134, 81–94. [Google Scholar] [CrossRef]
- Chen, Y.; Li, S.; Zhang, Y.; Li, T.; Ge, H.; Xia, S.; Gu, J.; Zhang, H.; Lü, B.; Wu, X.; et al. Rice Root Morphological and Physiological Traits Interaction with Rhizosphere Soil and Its Effect on Methane Emissions in Paddy Fields. Soil Biol. Biochem. 2019, 129, 191–200. [Google Scholar] [CrossRef]
- Pumpanen, J.; Ilvesniemi, H.; Hari, P. A Process-Based Model for Predicting Soil Carbon Dioxide Efflux and Concentration. Soil Sci. Soc. Am. J. 2003, 67, 402–413. [Google Scholar] [CrossRef]
- Knapp, A.K.; Beier, C.; Briske, D.D.; Classen, A.T.; Luo, Y.; Reichstein, M.; Smith, M.D.; Smith, S.D.; Bell, J.E.; Fay, P.A.; et al. Consequences of More Extreme Precipitation Regimes for Terrestrial Ecosystems. BioScience 2008, 58, 811–821. [Google Scholar] [CrossRef]
- Afreen, T.; Singh, H. Does Change in Precipitation Magnitude Affect the Soil Respiration Response? A Study on Constructed Invaded and Uninvaded Tropical Grassland Ecosystem. Ecol. Indic. 2019, 102, 84–94. [Google Scholar] [CrossRef]
- Wang, C.; Fu, B.; Zhang, L.; Xu, Z. Soil Moisture–Plant Interactions: An Ecohydrological Review. J. Soils Sediments 2019, 19, 1–9. [Google Scholar] [CrossRef]
- Zhou, X.; Sherry, R.A.; An, Y.; Wallace, L.L.; Luo, Y. Main and Interactive Effects of Warming, Clipping, and Doubled Precipitation on Soil CO2 Efflux in a Grassland Ecosystem. Glob. Biogeochem. Cycles 2006, 20, 1–12. [Google Scholar] [CrossRef]
- Han, G.; Sun, B.; Chu, X.; Xing, Q.; Song, W.; Xia, J. Precipitation Events Reduce Soil Respiration in a Coastal Wetland Based on Four-Year Continuous Field Measurements. Agric. For. Meteorol. 2018, 256–257, 292–303. [Google Scholar] [CrossRef]
- Yan, M.; Zhang, X.; Zhou, G.; Gong, J.; You, X. Temporal and Spatial Variation in Soil Respiration of Poplar Plantations at Different Developmental Stages in Xinjiang, China. J. Arid. Environ. 2011, 75, 51–57. [Google Scholar] [CrossRef]
- Verlinden, M.S.; Broeckx, L.S.; Wei, H.; Ceulemans, R. Soil CO2 Efflux in a Bioenergy Plantation with Fast-Growing Populus Trees—Influence of Former Land Use, Inter-Row Spacing and Genotype. Plant Soil 2013, 369, 631–644. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Joo, S.J.; Park, S.U.; Park, M.S.; Lee, C.S. Estimation of Soil Respiration Using Automated Chamber Systems in an Oak (Quercus Mongolica) Forest at the Nam-San Site in Seoul, Korea. Sci. Total Environ. 2012, 416, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Lee, C.M.; Yang, S.A.; Jung, H.J.; Lee, J.M.; Min, Y.G.; Kim, J.W.; Myung, H.H.; Park, H.C. Estimation of Soil Microbiological Respiration Volume in Forest Ecosystem in the Sobaeksan National Park of Korea. J. Korean Soc. Environ. Restor. Technol. 2023, 26, 19–28. [Google Scholar] [CrossRef]
- Pacific, V.J.; McGlynn, B.L.; Riveros-Iregui, D.A.; Welsch, D.L.; Epstein, H.E. Variability in Soil Respiration across Riparian-Hillslope Transitions. Biogeochemistry 2008, 91, 51–70. [Google Scholar] [CrossRef]
- Doering, M.; Uehlinger, U.; Ackermann, T.; Woodtli, M.; Tockner, K. Spatiotemporal Heterogeneity of Soil and Sediment Respiration in a River-Floodplain Mosaic (Tagliamento, NE Italy). Freshw. Biol. 2011, 56, 1297–1311. [Google Scholar] [CrossRef]
- Hansen, J.; Sato, M.; Kharecha, P.; von Schuckmann, K.; Beerling, D.J.; Cao, J.; Marcott, S.; Masson-Delmotte, V.; Prather, M.J.; Rohling, E.J.; et al. Young People’s Burden: Requirement of Negative CO2 Emissions. Earth Syst. Dyn. 2017, 8, 577–616. [Google Scholar] [CrossRef]
- Smith, P.; Davis, S.J.; Creutzig, F.; Fuss, S.; Minx, J.; Gabrielle, B.; Kato, E.; Jackson, R.B.; Cowie, A.; Kriegler, E.; et al. Biophysical and Economic Limits to Negative CO2 Emissions. Nat. Clim. Chang. 2016, 6, 42–50. [Google Scholar] [CrossRef]
- International Union for the Conservation of Nature (IUCN). Bonn Challenge; IUCN: Gland, Switzerland, 2018. [Google Scholar]
- Intergovernmental Panel on Climate Change (IPCC). 2006 IPCC Guidelines for National Greenhouse Gas Inventories; IUCN: Gland, Switzerland, 2006. [Google Scholar]
- Sutfin, N.A.; Wohl, E.E.; Dwire, K.A. Banking Carbon: A Review of Organic Carbon Storage and Physical Factors Influencing Retention in Floodplains and Riparian Ecosystems. Earth Surf. Process. Landf. 2016, 41, 38–60. [Google Scholar] [CrossRef]
- Daigneault, A.J.; Eppink, F.V.; Lee, W.G. A National Riparian Restoration Programme in New Zealand: Is It Value for Money? J. Environ. Manag. 2017, 187, 166–177. [Google Scholar] [CrossRef]
- O’Brien, J.M.; Warburton, H.J.; Graham, S.E.; Franklin, H.M.; Febria, C.M.; Hogsden, K.L.; Harding, J.S.; McIntosh, A.R. Leaf Litter Additions Enhance Stream Metabolism, Denitrification, and Restoration Prospects for Agricultural Catchments. Ecosphere 2017, 8, e02018. [Google Scholar] [CrossRef]
- Capon, S.J.; Chambers, L.E.; Mac Nally, R.; Naiman, R.J.; Davies, P.; Marshall, N.; Pittock, J.; Reid, M.; Capon, T.; Douglas, M.; et al. Riparian Ecosystems in the 21st Century: Hotspots for Climate Change Adaptation? Ecosystems 2013, 16, 359–381. [Google Scholar] [CrossRef]
- Knopf, F.L.; Johnson, R.R.; Rich, T.; Samson, F.B.; Szaro, R.C. Conservation of Riparian Ecosystems in the United States. Wilson Bull. 1988, 100, 272–284. [Google Scholar]
- Nilsson, C.; Berggren, K. Alterations of Riparian Ecosystems Caused by River Regulation: Dam Operations Have Caused Global-Scale Ecological Changes in Riparian Ecosystems. How to Protect River Environments and Human Needs of Rivers Remains One of the Most Important Questions of Our Time. BioScience 2000, 50, 783–792. [Google Scholar] [CrossRef]
- Perry, L.G.; Andersen, D.C.; Reynolds, L.V.; Nelson, S.M.; Shafroth, P.B. Vulnerability of Riparian Ecosystems to Elevated and Climate Change in Arid and Semiarid Western North America. Glob. Chang. Biol. 2012, 18, 821–842. [Google Scholar] [CrossRef]
- Zedler, J.B.; Kercher, S. WETLAND RESOURCES: Status, Trends, Ecosystem Services, and Restorability. Annu. Rev. Environ. Resour. 2005, 30, 39–74. [Google Scholar] [CrossRef]
- Dybala, K.E.; Matzek, V.; Gardali, T.; Seavy, N.E. Carbon Sequestration in Riparian Forests: A Global Synthesis and Meta-Analysis. Glob. Chang. Biol. 2019, 25, 57–67. [Google Scholar] [CrossRef]
- Lee, C. Ecology of the Miho River. Available online: https://m.ecomedia.co.kr/news/newsview.php?ncode=1065578017109847 (accessed on 23 September 2024).
- Ministry of Environment. Stream/River Ecosystem Survey and Health Assessment (2022~2024); Ministry of Environment: Seoul, Republic of Korea, 2022. [Google Scholar]
- Gann, G.D.; McDonald, T.; Walder, B.; Aronson, J.; Nelson, C.R.; Jonson, J.; Hallett, J.G.; Eisenberg, C.; Guariguata, M.R.; Liu, J. International Principles and Standards for the Practice of Ecological Restoration. Restor. Ecol. 2019, 27, S1–S46. [Google Scholar] [CrossRef]
- Atkinson, J.; Bonser, S.P. “Active” and “Passive” Ecological Restoration Strategies in Meta-analysis. Restor. Ecol. 2020, 28, 1032–1035. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).