Phosphorous Nanofertilizers for Precise Application in Rice Cultivation as an Adaptation to Climate Change
Abstract
1. Introduction
2. Environmental Impact of Phosphorus Use
3. Nanofertilizers with Phosphorus-Controlled Release of Phosphorus
4. Benefits of Nanofertilizers for Agroecosystems
5. Conclusions
6. Future Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAO. World Cereal Production, Utilization, Stocks, and Trade All Likely to Contract in 2022/23; FAO: Rome, Italy, 2022; Available online: https://www.fao.org/worldfoodsituation/csdb/en/ (accessed on 3 June 2022).
- Olad, A.; Zebhi, H.; Salari, D.; Mirmohseni, A.; Tabar, A.R. Slow-release NPK fertilizer encapsulated by carboxymethyl cellulose-based nanocomposite with the function of water retention in soil. Mater. Sci. Eng. C 2018, 90, 333–340. [Google Scholar] [CrossRef] [PubMed]
- World Bank. Commodities Price Data 2022; World Bank: Washington DC, USA, 2022; Available online: https://thedocs.worldbank.org/ (accessed on 2 August 2022).
- Schachtman, D.P.; Reid, R.J.; Ayling, S.M. Phosphorus uptake by plants: From soil to cell. Plant Physiol. 1998, 116, 447–453. [Google Scholar] [CrossRef]
- Amanullah, A.; Zakirullah, M.; Khalil, S.K. Timing and rate of phosphorus application influence maize phenology, yield and proftability in Northwest Pakistan. Int. J. Plant Prod. 2012, 4, 281–292. [Google Scholar]
- Goldstein, A.H.; Baertlein, D.A.; McDaniel, R.G. Phosphate starvation inducible metabolism in Lycopersicon esculentum: I. Excretion of acid phosphatase by tomato plants and suspension-cultured cells. Plant Physiol. 1988, 87, 711–715. [Google Scholar] [CrossRef]
- Conley, D.J.; Paerl, H.W.; Howarth, R.W.; Boesch, D.F.; Seitzinger, S.P.; Havens, K.E.; Lancelot, C.; Likens, G.E. Ecology—Controlling eutrophication: Nitrogen and phosphorus. Science 2009, 323, 1014–1015. [Google Scholar] [CrossRef]
- Beaulieu, J.J.; DelSontro, T.; Downing, J.A. Eutrophication will increase methane emissions from lakes and impoundments during the 21st century. Nat. Commun. 2019, 10, 1375. [Google Scholar] [CrossRef]
- Webb, J.R.; Leavitt, P.R.; Simpson, G.L.; Baulch, H.M.; Haig, H.A.; Hodder, K.R.; Finlay, K. Regulation of carbon dioxide and methane in small agricultural reservoirs: Optimizing potential for greenhouse gas uptake. Biogeosciences 2019, 16, 4211–4227. [Google Scholar] [CrossRef]
- Hayes, N.M.; Vanni, M.J. Microcystin concentrations can be predicted with phytoplankton biomass and watershed morphology. Inland Waters 2018, 8, 273–283. [Google Scholar] [CrossRef]
- Vaughan, I.P.; Gotelli, N.J. Water quality improvements offset the climatic debt for stream macroinvertebrates over twenty years. Nat. Commun. 2019, 10, 1956. [Google Scholar] [CrossRef] [PubMed]
- Shuvo, A.; O’Reilly, C.M.; Blagrave, K.; Ewins, C.; Filazzola, A.; Gray, D.; Mahdiyan, O.; Moslenko, L.; Quinlan, R.; Sharma, S. Total phosphorus and climate are equally important predictors of water quality in lakes. Aquat. Sci. 2021, 83, 16. [Google Scholar] [CrossRef]
- Quinlan, R.; Filazzola, A.; Mahdiyan, O.; Shuvo, A.; Blagrave, K.; Ewins, C.; Moslenko, L.; Gray, D.K.; O’Reilly, C.M.; Sharma, S. Relationships of total phosphorus and chlorophyll in lakes worldwide. Limnol. Oceanogr. 2020, 66, 392–404. [Google Scholar] [CrossRef]
- EU Commission. Farm to Fork Strategy; EU Commission: Brussels, Belgium, 2020; Available online: https://ec.europa.eu/food/farm2fork_en (accessed on 30 November 2020).
- Ndaba, B.; Roopnarain, A.; Rama, H.; Maaza, M. Biosynthesized metallic nanoparticles as fertilizers: An emerging precision agriculture strategy. J. Integr. Agric. 2022, 21, 1225–1242. [Google Scholar] [CrossRef]
- Priyam, A.; Yadav, N.; Reddy, P.M.; Afonso, L.O.B.; Schultz, A.G.; Singh, P.P. Fertilizing benefits of biogenic phosphorous nanonutrients on Solanum lycopersicum in soils with variable pH. Heliyon 2022, 8, e09144. [Google Scholar] [CrossRef]
- Banerjee, S.; Mazumder, S.; Chatterjee, D.; Bose, S.; Majee, S.B. Chapter 7—Nanotechnology for cargo delivery with a special emphasis on pesticide, herbicide, and fertilizer. In Nano-Enabled Agrochemicals in Agriculture; Ghorbanpour, M., Shahid, M.A., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 105–144. ISBN 9780323910095. [Google Scholar]
- Bhardwaj, A.K.; Arya, G.; Kumar, R.; Hamed, L.; Pirasteh-Anosheh, H.; Jasrotia, P.; Kashyap, P.L.; Singh, G.P. Switching to nanonutrients for sustaining agroecosystems and environment: The challenges and benefits in moving up from ionic to particle feeding. J. Nanobiotechnol. 2022, 20, 19. [Google Scholar] [CrossRef]
- Anjum, M.; Pradhan, S.N.; Narayana Pradhan, S. Application of nanotechnology in precision farming: A review. Int. J. Chem. Stud. 2018, 6, 755–760. [Google Scholar]
- Steffen, W.; Richardson, K.; Rockström, J.; Cornell, S.E.; Fetzer, I.; Bennett, E.M.; Biggs, R.; Carpenter, S.R.; de Vries, W.; de Wit, C.A.; et al. Planetary boundaries: Guiding human development on a changing planet. Science 2015, 347, 1259855. [Google Scholar] [CrossRef]
- ESPP. European Sustainable Phosphorus Platform; ESPP: Bruxelles, Belgium, 2022; Available online: https://phosphorusplatform.eu/links-and-resources/p-facts/p-fact-4 (accessed on 4 May 2022).
- Naher, U.; Othman, R.; Panhwar, Q.; Ismail, M. Biofertilizer for Sustainable Rice Production and Reduction of Environmental Pollution. In Crop Production and Global Environmental Issues; Springer: Cham, Switzerland, 2015; pp. 283–291. [Google Scholar]
- Amaral, A.; Carvalho, D. Avaliação Do Desenvolvimento da Fava de Indústria (Vicia faba L.) na Condições do Vale do Tejo. Revista da UI_IPSantarém-Unidade de Investigação do Instituto Politécnico de Santarém 2018, 6, 14–25. [Google Scholar]
- Varennes, A. Produtividade dos solos e Ambiente; Escolar Editora: Lisboa, Portugal, 2003; 490p. [Google Scholar]
- Tiessen, H. Phosphorus in the global environment. In The Ecophysiology of Plant-Phosphorus Interactions; White, P.J., Hammond, J.P., Eds.; Plant Ecophysiology; Springer: Dordrecht, The Netherlands, 2008; Volume 7. [Google Scholar]
- Choudhury, A.; Kennedy, I.R.; Ahmed, M.F.; Kecskés, M.L. Phosphorus Fertilization for Rice and Control of Environmental Pollution Problems. Pak. J. Biol. Sci. 2007, 10, 2098–2105. [Google Scholar] [CrossRef] [PubMed]
- Malmaeus, J.M.; Blenckner, T.; Markensten, H.; Persson, I. Lake phosphorus dynamics and climate warming: A mechanistic model approach. Ecol. Model. 2006, 190, 1–14. [Google Scholar] [CrossRef]
- Rockström, J.; Klum, M. Big World, Small Planet: Abundance within Planetary Boundaries; Bokförlaget Max Ström: Stockholm, Sweden, 2015; pp. 1–207. [Google Scholar]
- Lychuk, T.E.; Moulin, A.P.; Lemke, R.L.; Izaurralde, R.C.; Johnson, E.N.; Olfert, O.O.; Brandt, S.A. Modelling the effects of climate change, agricultural inputs, cropping diversity, and environment on soil nitrogen and phosphorus: A case study in Saskatchewan, Canada. Agric. Water Manag. 2021, 252, 106850. [Google Scholar] [CrossRef]
- Asensio, D.; Zuccarinia, P.; Ogaya, R.; Maranón-Jiménez, S.; Sardans, J.; Penuelas, J. Simulated climate change and seasonal drought increase carbon and phosphorus demand in Mediterranean forest soil. Soil Biol. Biochem. 2021, 163. [Google Scholar] [CrossRef]
- Wang, W.; Li, Y.; Guan, P.; Chang, L.; Zhu, X.; Zhang, P.; Wua, D. How do climate warming affect soil aggregate stability and aggregate-associated phosphorus storage under natural restoration? Geoderma 2022, 420, 115891. [Google Scholar] [CrossRef]
- Bjørn, A.; Chandrakumar, C.; Boulay, A.M.; Doka, G.; Fang, K.; Gondran, N.; Hauschild, M.; Kerkhof, A.; King, H.; Margni, M.; et al. Review of life-cycle based methods for absolute environmental sustainability assessment and their applications. Environ. Res. Lett. 2020, 15, 083001. [Google Scholar] [CrossRef]
- Fang, K. Assessing the natural capital use of eleven nations: An application of a revised three-dimensional model of ecological footprint. Acta Ecol. Sin. 2015, 35, 3766–3777. [Google Scholar]
- Li, M.; Wiedmann, T.; Hadjikakou, M. Towards meaningful consumption-based planetary boundary indicators: The phosphorus exceedance footprint. Glob. Environ. Chang. 2019, 54, 227–238. [Google Scholar] [CrossRef]
- Li, M.; Wiedmann, T.; Liu, J.; Wang, Y.; Hu, Y.; Zhang, Z.; Hadjikakou, M. Exploring consumption-based planetary boundary indicators: An absolute water footprinting assessment of Chinese provinces and cities. Water Res. 2020, 184, 116163. [Google Scholar] [CrossRef]
- Li, M.; Wiedmann, T.; Fang, K.; Hadjikakou, M. The role of planetary boundaries in assessing absolute environmental sustainability across scale. Environ. Int. 2021, 152, 106475. [Google Scholar] [CrossRef] [PubMed]
- Dearing, J.A.; Wang, R.; Zhang, K.; Dyke, J.G.; Haberl, H.; Hossain, M.S.; Langdon, P.G.; Lenton, T.M.; Raworth, K.; Brown, S.; et al. Safe and just operating spaces for regional social-ecological systems. Glob. Environ. Chang. 2014, 28, 227–238. [Google Scholar] [CrossRef]
- Rockström, J.; Edenhofer, O.; Gaertner, J.; DeClerck, F. Planet-proofing the global food system. Nat. Food 2020, 1, 3–5. [Google Scholar] [CrossRef]
- Ramirez-Rodriguez, G.B.; Dal Sasso, G.; Carmona, F.J.; Miguel-Rojas, C.; Perez-de-Luque, A.; Masciocchi, N.; Guagliardi, A.; Delgado-Lopez, J.M. Engineering biomimetic calcium phosphate nanoparticles: A green synthesis of slow-release multinutrient (NPK) nanofertilizers. ACS Appl. Bio Mater. 2020, 3, 1344–1353. [Google Scholar] [CrossRef]
- El-Azeim, M.M.A.; Sherif, M.A.; Hussien, M.S.; Tantawy, I.A.A.; Bashandy, S.O. Impacts of nano- and non-nanofertilizers on potato quality and productivity. Acta Ecol. Sin. 2020, 40, 388–397. [Google Scholar] [CrossRef]
- Basavegowda, N.; Baek, K.H. Current and future perspectives on the use of nanofertilizers for sustainable agriculture: The case of phosphorus nanofertilizer. 3 Biotech 2021, 11, 357. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Kumar, V.; Kumar, S.; Kumar Shahi, S. Plant development and crop protection using phytonanotechnology: A new window for sustainable agriculture. Chemosphere 2022, 299, 134465. [Google Scholar] [CrossRef]
- Cheng, Y.; Yin, L.; Lin, S.; Wiesner, M.; Bernhardt, E.; Liu, J. Toxicity reduction of polymer-stabilized silver nanoparticles by sunlight. J. Phys. Chem. C 2011, 115, 4425–4432. [Google Scholar] [CrossRef]
- Adhikari, T.; Kundu, S.; Meena, V.; Rao, A.S. Utilization of Nano Rock Phosphate by Maize (Zea mays L.) Crop in a Vertisol of Central India. J. Agric. Sci. Technol. 2014, 4, 384–394. [Google Scholar]
- Mikhak, A.; Sohrabi, A.; Kassaee, M.Z.; Feizian, M. Synthetic nanozeolite/nanohydroxyapatite as a phosphorus fertilizer for German chamomile (Matricariachamomilla L.). Ind. Crops Prod. 2017, 95, 444–452. [Google Scholar] [CrossRef]
- Xiong, L.; Wang, P.; Hunter, M.N.; Kopittke, P.M. Bioavailability and movement of hydroxyapatite nanoparticles (HA-NPs) applied as a phosphorus fertiliser in soils. Environ. Sci. Nano 2018, 5, 2888–2898. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Miranda-Villagómez, E.; Trejo-Téllez, L.I.; Gómez-Merino, F.C.; Sandoval-Villa, M.; Sánchez-García, P.; Aguilar-Méndez, M.A. Nanophosphorus Fertilizer Stimulates Growth and Photosynthetic Activity and Improves P Status in Rice. J. Nanomater. 2019, 2019, 5368027. [Google Scholar]
- Shylaja, S.; Prashanthi, Y.; Rao, T.N. Synthesis and evaluating the effects of nano hydroxyapatite on germination, growth and yield of cluster beans. Mater. Today Proc. 2022, 64, 917–921. [Google Scholar] [CrossRef]
- Liu, R.; Lal, R. Synthetic apatite nanoparticles as a phosphorus fertilizer for soybean (Glycine max). Sci. Rep. 2014, 4, 5686. [Google Scholar] [CrossRef]
- Taşkın, M.B.; Şahin, O.; Taskin, H.; Atakol, O.; Inal, A.; Gunes, A. Effect of synthetic nano-hydroxyapatite as an alternative phosphorus source on growth and phosphorus nutrition of lettuce (Lactuca sativa L.) plant. J. Plant Nutr. 2018, 41, 1148–1154. [Google Scholar] [CrossRef]
- Pradhan, S.; Durgam, M.; Mailapalli, D.R. Urea loaded hydroxyapatite nanocarrier for efficient delivery of plant nutrients in rice. Arch. Agron. Soil Sci. 2021, 67, 371–382. [Google Scholar] [CrossRef]
- Rajonee, A.; Zaman, S.; Huq, S. Preparation, Characterization and Evaluation of Efficacy of Phosphorus and Potassium Incorporated Nano Fertilizer. Adv. Nanopart. 2017, 6, 62–74. [Google Scholar] [CrossRef]
- Yasmeen, T.; Arif, M.S.; Shahzad, S.M.; Riaz, M.; Tufail, M.A.; Mubarik, M.S.; Ahmad, A.; Ali, S.; Albasher, G.; Shakoor, A. Abandoned agriculture soil can be recultivated by promoting biological phosphorus fertility when amended with nano-rock phosphate and suitable bacterial inoculant. Ecotoxicol. Environ. Saf. 2022, 234, 113385. [Google Scholar] [CrossRef]
- Saraiva, R.; Ferreira, Q.; Rodrigues, G.; Oliveira, M. Nanofertilizantes—A precisão na cultura do arroz. In Proceedings of the Encontro Ciência 2021, Lisboa, Portugal, 28–30 June 2021. [Google Scholar]
- Saraiva, R.; Rodrigues, G.; Ferreira, Q.; Oliveira, M. The use of nanofertilizers to increase precision in rice production. In 16th SDEWES2021 Conference: Book Abstracts; Faculty of Mechanical Engineering and Naval Architecture: Zagreb, Croatia, 2021; p. 545. [Google Scholar]
- Chen, J.; Peng, H.; Wang, X.; Shao, F.; Yuan, Z.; Han, H. Graphene oxide exhibits broad-spectrum antimicrobial activity against bacterial phytopathogens and fungal conidia by intertwining and membrane perturbation. Nanoscale 2014, 6, 1879–1889. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Kang, J.; Lu, K.; Zhou, R.; Mu, L.; Zhou, O. Graphene oxide amplifies the phytotoxicity of arsenic in wheat. Sci. Rep. 2014, 4, 6122. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wei, H.; Li, Z.; Li, S.; Yan, H.; He, Y.; Tian, Z. Effects of graphene on germination and seedling morphology in rice. J. Nanosci. Nanotechnol. 2015, 15, 2695–2701. [Google Scholar] [CrossRef]
- Mukherjee, A.; Majumdar, S.; Servin, A.D.; Pagano, L.; Dhankher, O.P.; White, J.C. Carbon Nanomaterials in Agriculture A Critical Review. Front. Plant Sci. 2016, 7, 172. [Google Scholar] [CrossRef]
- Chen, J.; Yang, L.; Li, S.; Ding, W. Various Physiological Response to Graphene Oxide and Amine-Functionalized Graphene Oxide in Wheat (Triticum aestivum). Molecules 2018, 23, 1104. [Google Scholar] [CrossRef]
- Chen, X.; Tongxin, W. Preparation and characterization of atrazine-loaded biodegradable PLGA nanospheres. J. Integr. Agric. 2019, 18, 1035–1041. [Google Scholar] [CrossRef]
- Kulikov, S.N.; Chirkov, S.N.; Ilina, A.V.; Lopatin, S.A.; Varlamov, V.P. Effect of the molecular weight of chitosan on its antiviral activity in plants. Appl. Biochem. Microbiol. 2006, 42, 200–203. [Google Scholar] [CrossRef]
- Sathiyabama, M.G.; Akila, R.; Einstein, C. Chitosan-induced defense responses in tomato plants against early blight disease caused by Alternariasolani (Ellis and Martin) Sorauer. Arch. Phytopathol. Plant Protect. 2013, 47, 1777–1787. [Google Scholar] [CrossRef]
- Silva, M.; Nunes, D.; Cardoso, A.R.; Ferreiral, D.; Britol, M.; Pintadol, M.E.; Vasconcelos, M.W. Chitosan as a biocontrol agent against the pinewood nematode (Bursaphelenchus xylophilus). For. Pathol. 2014, 44, 420–423. [Google Scholar] [CrossRef]
- Hossain, M.S.; Iqbal, A. Effect of shrimp chitosan coating on postharvest quality of banana (Musa sapientum L.) fruits. Int. Food Res. J. 2016, 23, 277–283. [Google Scholar]
- Pandey, P.; Verma, M.; De, N. Chitosan in agricultural context—A review. Bull. Environ. Pharmacol. Life Sci. 2018, 7, 87–96. [Google Scholar]
- Angelim, A.L.; Costa, S.P.; Farias, B.C.; Aquino, L.F.; Melo, V.M. An innovative bioremediation strategy using a bacterial consortium entrapped in chitosan beads. J. Environ. Manag. 2013, 127, 10–17. [Google Scholar] [CrossRef]
- Liu, L.; Luo, X.B.; Ding, L.; Luo, S.L. Application of Nanotechnology in the Removal of Heavy Metal from Water. In Nanomaterials for the Removal of Pollutants and Resource Reutilization Micro and Nano Technologies; Luo, X., Deng, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 83–147. [Google Scholar]
- Saraiva, R.; Ferreira, Q.; Rodrigues, G.; Oliveira, M. A fitotoxicidade de óxido de grafeno como base para nanofertilizantes. In Proceedings of the Encontro Ciência 2022, Lisboa, Portugal, 16–18 May 2022. [Google Scholar]
- Chen, J.; Mu, Q.; Tian, X. Phytotoxicity of graphene oxide on rice plants is concentration dependent. Mater. Express 2019, 9, 635–640. [Google Scholar] [CrossRef]
- Arora, S.; Murmu, G.; Mukherjee, K.; Saha, S.; Maity, D. A comprehensive overview of nanotechnology in sustainable agriculture. J. Biotechnol. 2022, 355, 21–41. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Rana, V.S.; Kumari, M.; Mishra, P. Biofertilizers: Boon for fruit production. J. Pharm. Phytochem. 2018, 7, 3244–3247. [Google Scholar]
- Verma, K.K.; Song, X.-P.; Joshi, A.; Rajput, V.D.; Singh, M.; Sharma, A.; Singh, R.K.; Li, D.-M.; Arora, J.; Minkina, T.; et al. Nanofertilizer Possibilities for Healthy Soil, Water, and Food in Future: An Overview. Front. Plant Sci. 2022, 13, 865048. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Navarro, M.; Ashraf, M.; Akram, N.A.; Munné-Bosch, S. Nanofertilizer use for sustainable agriculture: Advantages and limitations. Plant Sci. 2019, 289, 110270. [Google Scholar] [CrossRef]
- Ashour, H.A.; Mahmoud, A.W.M. Response of Jatropha integerrima plants irrigated with different levels of saline water to nano silicon and gypsum. J. Agric. Stud. 2017, 5, 136–160. [Google Scholar]
- Mahmood-ur-Rahman; Ijaz, M.; Qamar, S.; Bukhari, S.A.; Malik, K. Chapter 27—Abiotic Stress Signaling in Rice Crop. In Advances in Rice Research for Abiotic Stress Tolerance; Hasanuzzaman, M., Fujita, M., Nahar, K., Biswas, J.K., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 551–569. [Google Scholar]
- Prakash, V.; Rai, P.; Sharma, N.C.; Pratap, S.V.; Kumar, T.D.; Sharma, S.; Sahi, S. Application of zinc oxide nanoparticles as fertilizer boosts growth in rice plant and alleviates chromium stress by regulating genes involved in regulating oxidative stress. Chemosphere 2022, 303, 134554. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.K.; Singh, V.P.; Prasad, S.M.; Chauhan, D.K.; Dubey, N.K. Silicon nanoparticles (SiNp) alleviate chromium (VI) phytotoxicity in Pisum sativum (L.) seedlings. Plant Physiol. Biochem. 2015, 96, 189–198. [Google Scholar] [CrossRef]
- López-Luna, J.; Silva-Silva, M.J.; Martinez-Vargas, S.; Mijangos-Ricardez, O.F.; González-Chávez, M.C.; Solís-Domínguez, F.A.; Cuevas-Díaz, M.C. Magnetite nanoparticle (NP) uptake by wheat plants and its effect on cadmium and chromium toxicological behavior. Sci. Total Environ. 2016, 565, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Zhou, Y.; Ma, C.X.; Feng, Y.; Hao, Y.; Rui, Y.; Wu, W.; Gui, X.; Le, V.N.; Han, Y.; et al. Jointed toxicity of TiO2 NPs and Cd to rice seedlings: NPs alleviated Cd toxicity and Cd promoted NPs uptake. Plant Physiol. Biochem. 2017, 110, 82–93. [Google Scholar] [CrossRef]
- Rossi, L.; Sharifan, H.; Zhang, W.L.; Schwab, A.P.; Ma, X. Mutual effects and in planta accumulation of coexisting cerium oxide nanoparticles and cadmium in hydroponically grown soybean (Glycine max L. Merr.). Environ. Sci.-Nano 2018, 5, 150–157. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, C.B.; Zhao, Y.L.; Huang, Y.C.; Liu, Z.Q. Foliar application with nano-silicon reduced cadmium accumulation in grains by inhibiting cadmium translocation in rice plants. Environ. Sci. Pollut. Res. 2018, 25, 2361–2368. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, M.; Chen, W.; Zhu, S.; Liu, N.; Zhu, L. Immobilization of lead and cadmium from aqueous solution and contaminated sediment using nano-hydroxyapatite. Environ. Pollut. 2010, 158, 514–519. [Google Scholar] [CrossRef]
- Haghighi, M.; Da Silva, J.A.T.; Mozafarian, M.; Afifipour, Z. Can Si and nano-Si alleviate the effect of drought stress induced by PEG in seed germination and seedling growth of tomato? Minerva Biotechnol. 2013, 25, 17–22. [Google Scholar]
- Djanaguiraman, M.; Nair, R.; Giraldo, J.P.; Prasad, P.V.V. Cerium oxide nanoparticles decrease drought induced oxidative damage in sorghum leading to higher photosynthesis and grain yield. ACS Omega 2018, 3, 14406–14416. [Google Scholar] [CrossRef]
- Kaur, H.; Kalia, A.; Singh, S.J.; Singh, D.G.; Kaur, G.; Pathania, S. Interaction of TiO2 nanoparticles with soil: Effect on microbiological and chemical traits. Chemosphere 2022, 301, 134629. [Google Scholar] [CrossRef] [PubMed]
- Babu, S.; Singh, R.; Yadav, D.; Rathore, S.S.; Raj, R.; Avasthe, R.; Yadav, S.K.; Das, A.; Yadav, V.; Yadav, B.; et al. Nanofertilizers for agricultural and environmental sustainability. Chemosphere 2022, 292, 133451. [Google Scholar] [CrossRef] [PubMed]
- Sangawe, V.; Inamdar, A.; Adhapure, N. Chapter 18—Rhizospheric health management through nanofertilizers. In Rhizosphere Engineering; Dubey, R.C., Kumar, P., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 329–353. [Google Scholar]
- Boregowda, N.; Jogigowda, S.C.; Bhavya, G.; Sunilkumar, C.R.; Geetha, N.; Udikeri, S.S.; Chowdappa, S.; Govarthanan, M.; Jogaiah, S. Recent advances in nanoremediation: Carving sustainable solution to clean-up polluted agriculture soils. Environ. Pollut. 2022, 297, 118728. [Google Scholar] [CrossRef]
- Mahapatra, D.M.; Satapathy, K.C.; Panda, B. Biofertilizers and nanofertilizers for sustainable agriculture: Phycoprospects and challenges. Sci. Total Environ. 2022, 803, 149990. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, R.C.; Kumaraswamy, R.V.; Kumari, S.; Sharma, S.S.; Pal, A.; Raliya, R.; Biswas, P.; Saharan, V. Cu-chitosan nanoparticle boost defense responses and plant growth in maize (Zea mays L.). Sci. Rep. 2017, 7, 9754. [Google Scholar] [CrossRef]
- Prajapati, D.; Pal, A.; Dimkpa, C.; Singh, U.; Devi, K.A.; Choudhary, J.L.; Saharan, V. Chitosan nanomaterials: A prelim of next-generation fertilizers; existing and future prospects. Carbohydr. Polym. 2022, 288, 119356. [Google Scholar] [CrossRef] [PubMed]
- Batley, G.E.; Kirby, J.K.; McLaughlin, M.J. The fate and risks of nanomaterials in aquatic and terrestrial environments. Acc. Chem. Res. 2013, 46, 842–862. [Google Scholar] [CrossRef]
- Hyland, C.; Ketterings, Q.; Geohring, L.; Stockin, K.; Dewing, D.; Czymmek, K.; Albrecht, G. Agronomy Fact Sheet Series—Fact Sheet 13; Cornell University Cooperative Extension: Ithaca, NY, USA, 2005. [Google Scholar]
- Zhan, X.Y.; Zhang, Q.W.; Zhang, H.; Hussain, H.A.; Shaaban, M.; Yang, Z.L. Pathways of nitrogen loss and optimized nitrogen management for a rice cropping system in arid irrigation region, northwest China. J. Environ. Manag. 2020, 268, 110702. [Google Scholar]
- Liu, J.; Zuo, Q.; Zhai, L.; Luo, C.; Liu, H.; Wang, H.; Liu, S.; Zou, G.; Ren, T. Phosphorus losses via surface runoff in rice-wheat cropping systems as impacted by rainfall regimes and fertilizer applications. J. Integr. Agric. 2016, 15, 667–677. [Google Scholar] [CrossRef]
- Hua, L.L.; Liu, J.; Zhai, L.M.; Xi, B.; Zhang, F.L.; Wang, H.Y.; Liu, H.; Chen, A.; Fu, B. Risks of phosphorus runoff losses from five Chinese paddy soils under conventional management practices. Agric. Ecosyst. Environ. 2017, 245, 112–123. [Google Scholar] [CrossRef]
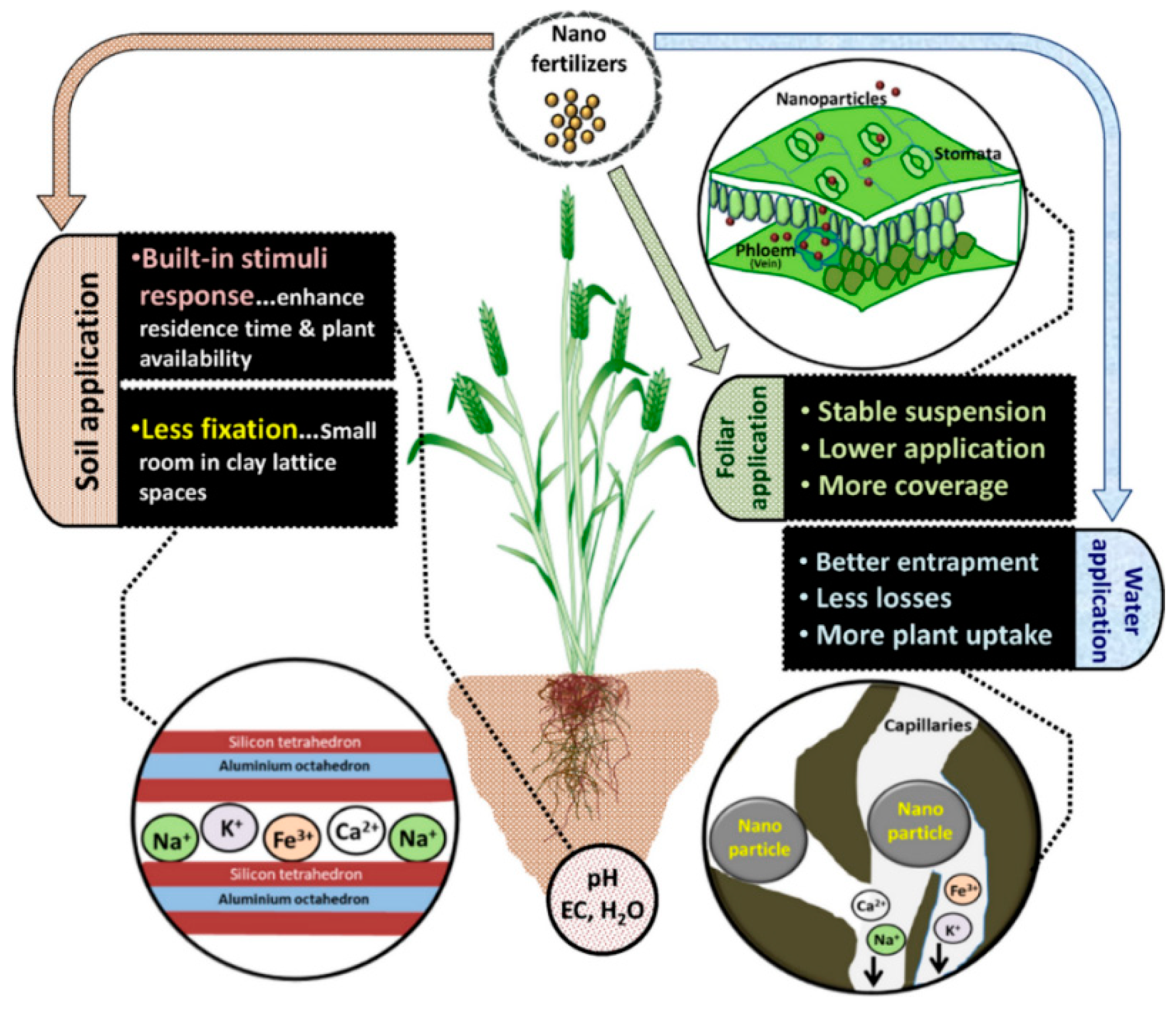
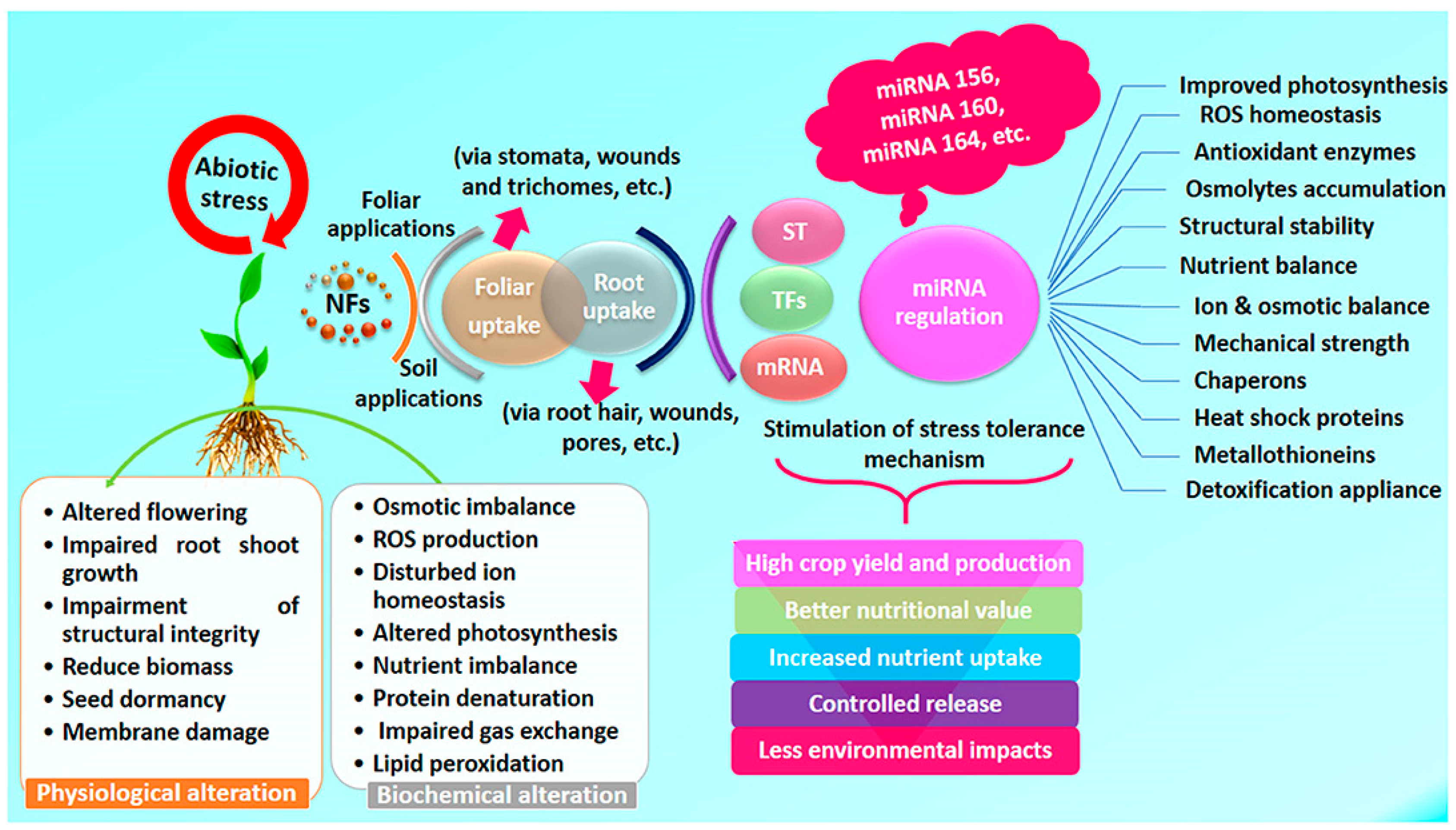
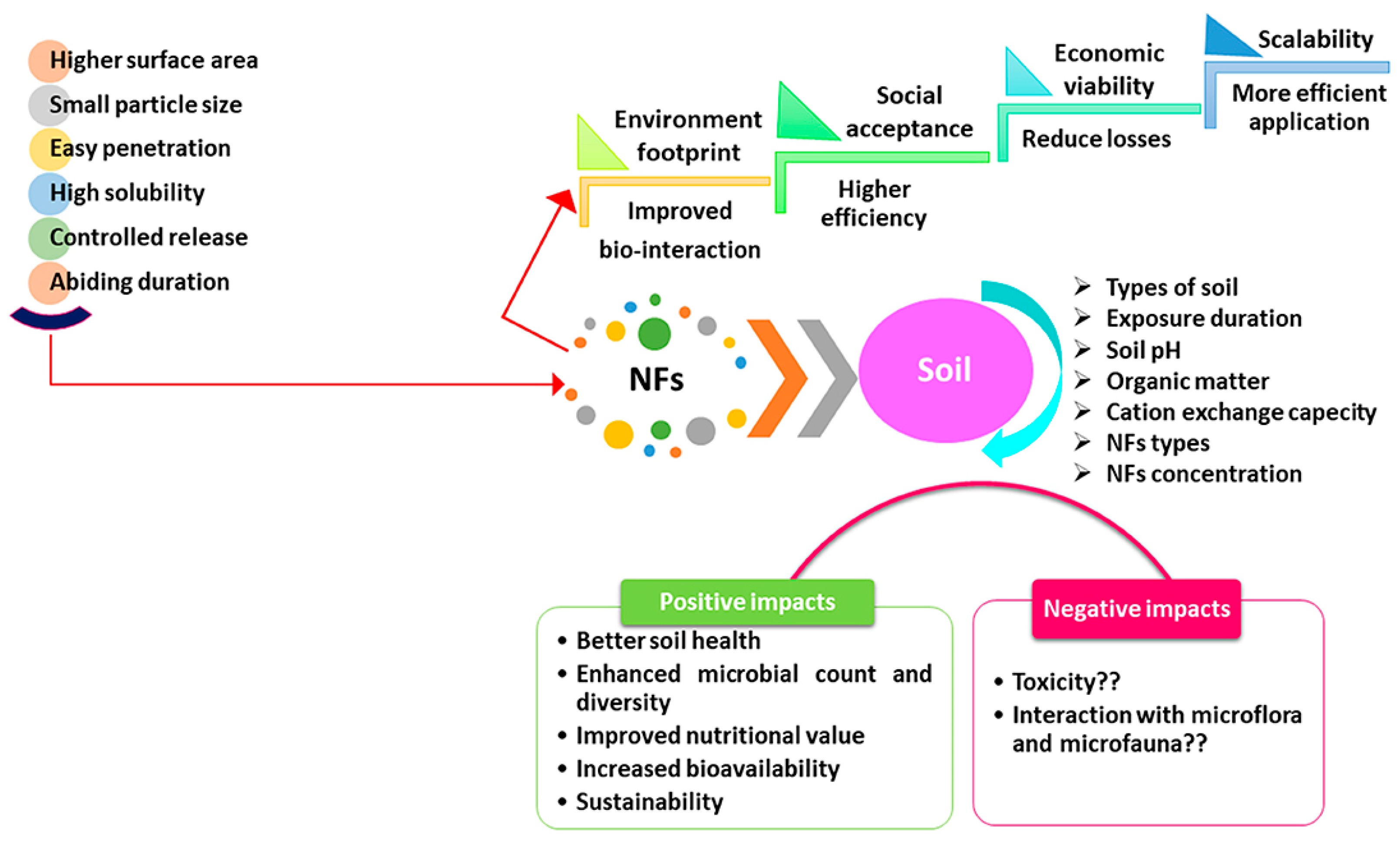
| Effect | Impact at Micro Level | Impact at Macro Level |
|---|---|---|
| P leaching and soil erosion | Contamination of waterbodies | Endangerment of public health; food security, biodiversity and ecosystems services |
| Precipitation or adsorption in soil particles | Soil P enrichment but non-available to plants | More resources needed every year |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saraiva, R.; Ferreira, Q.; Rodrigues, G.C.; Oliveira, M. Phosphorous Nanofertilizers for Precise Application in Rice Cultivation as an Adaptation to Climate Change. Climate 2022, 10, 183. https://doi.org/10.3390/cli10110183
Saraiva R, Ferreira Q, Rodrigues GC, Oliveira M. Phosphorous Nanofertilizers for Precise Application in Rice Cultivation as an Adaptation to Climate Change. Climate. 2022; 10(11):183. https://doi.org/10.3390/cli10110183
Chicago/Turabian StyleSaraiva, Raquel, Quirina Ferreira, Gonçalo C. Rodrigues, and Margarida Oliveira. 2022. "Phosphorous Nanofertilizers for Precise Application in Rice Cultivation as an Adaptation to Climate Change" Climate 10, no. 11: 183. https://doi.org/10.3390/cli10110183
APA StyleSaraiva, R., Ferreira, Q., Rodrigues, G. C., & Oliveira, M. (2022). Phosphorous Nanofertilizers for Precise Application in Rice Cultivation as an Adaptation to Climate Change. Climate, 10(11), 183. https://doi.org/10.3390/cli10110183










