Impact of Proton Beam Irradiation on the Growth and Biochemical Indexes of Barley (Hordeum vulgare L.) Seedlings Grown under Salt Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design of Plant Material and Growing Conditions
2.1.1. Proton Beam Irradiation
2.1.2. Growth Conditions and Salinity Treatment
2.2. Photosynthetic Pigments Analysis
2.3. Oxidative Stress Indexes
2.4. Antioxidant Defense System
2.4.1. Non-Enzymatic Constituents
Total Polyphenols Content
Flavonoids Content
2.4.2. Antioxidant Enzymes
Preparation of Enzyme Extracts
SOD Activity
POD Activity
CAT Activity
3. Results and Discussions
3.1. Tolerance of Proton Beam Irradiated Barley Seedlings to Salinity Stress
3.2. Effect of Salinity and Proton Irradiation on Photosynthetic Pigments Content of Barley Seedlings
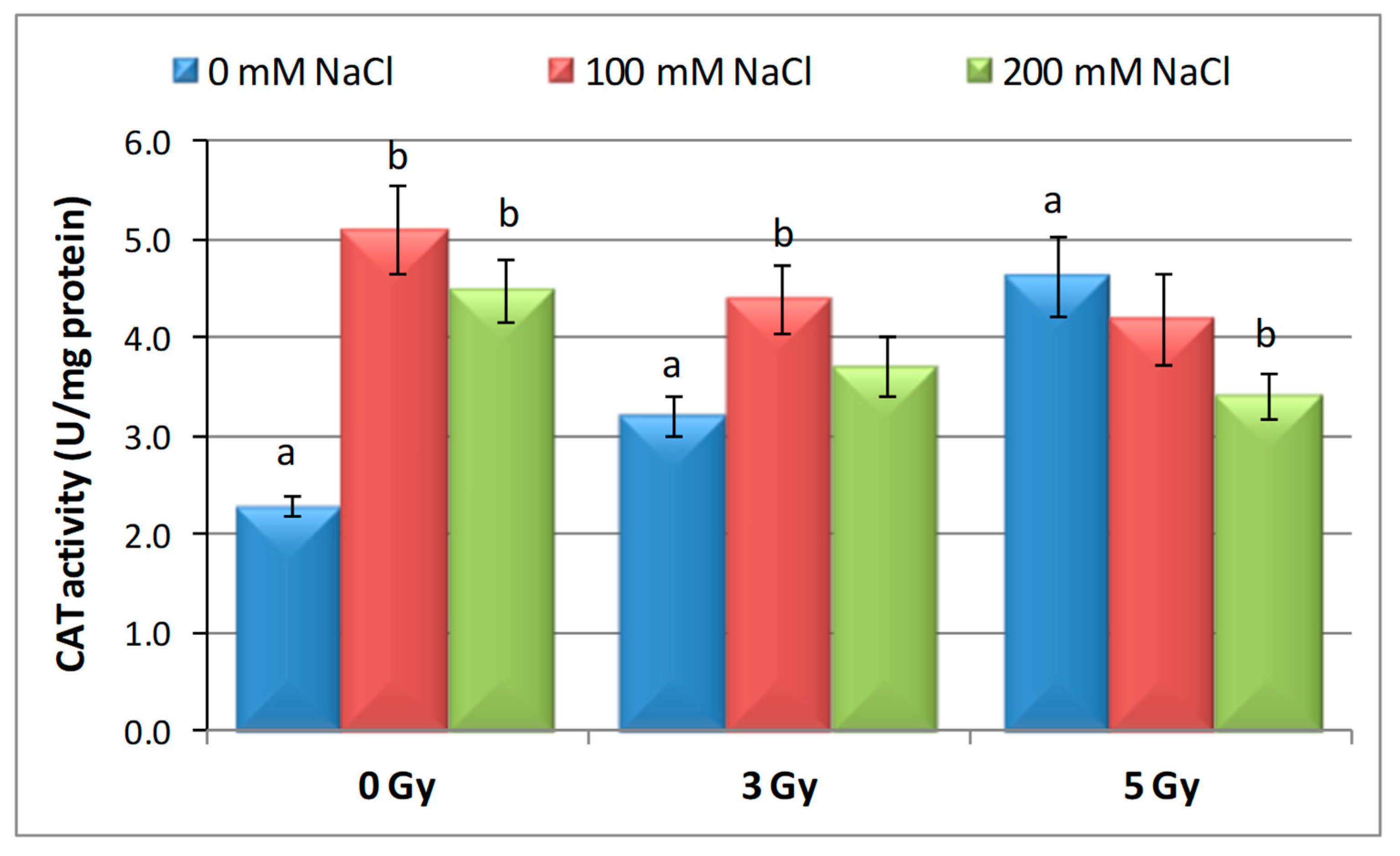
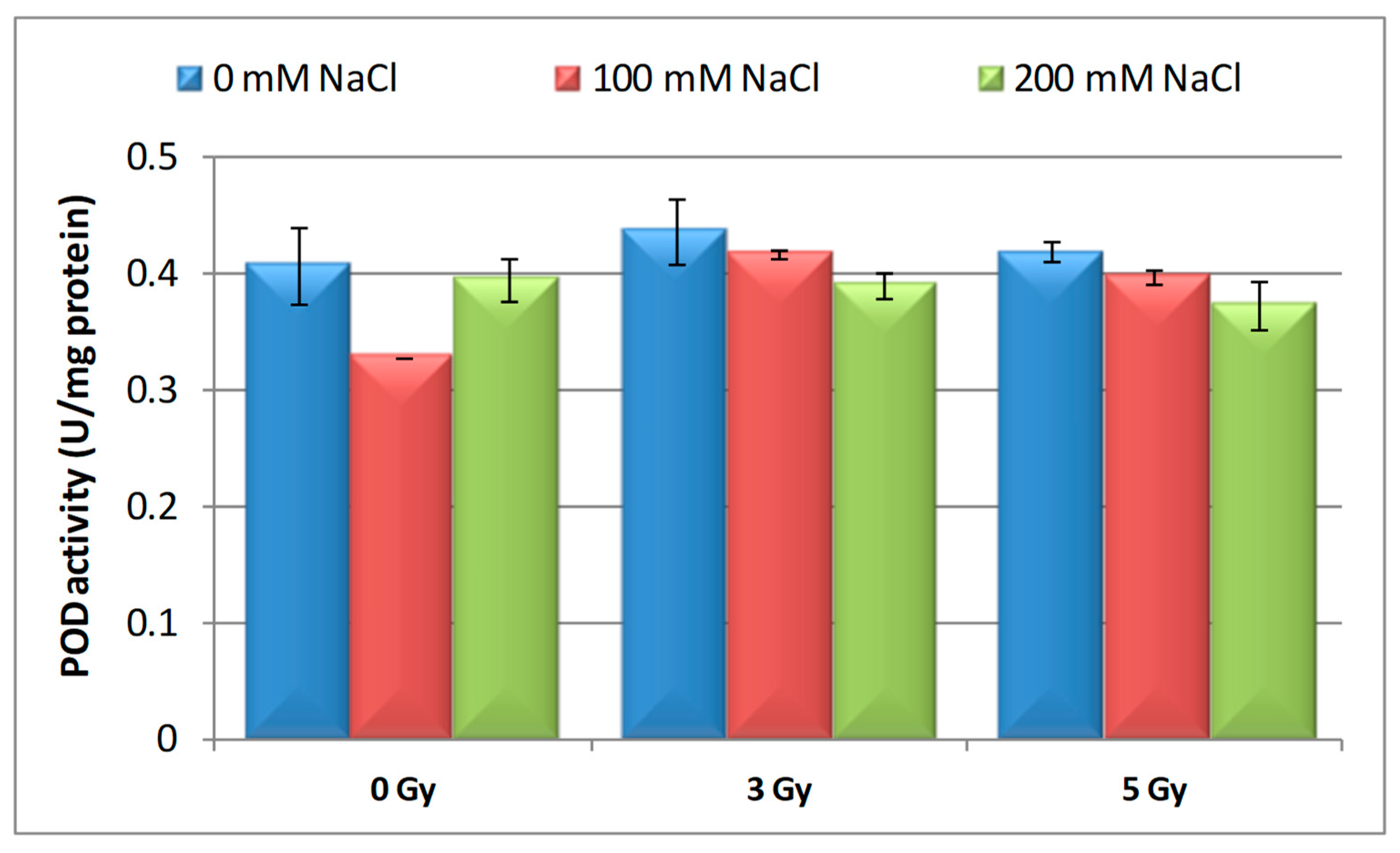
3.3. Effects of Proton Irradiation on ROS Scavenging Systems of Barley Seedling in Response to Salt Stress
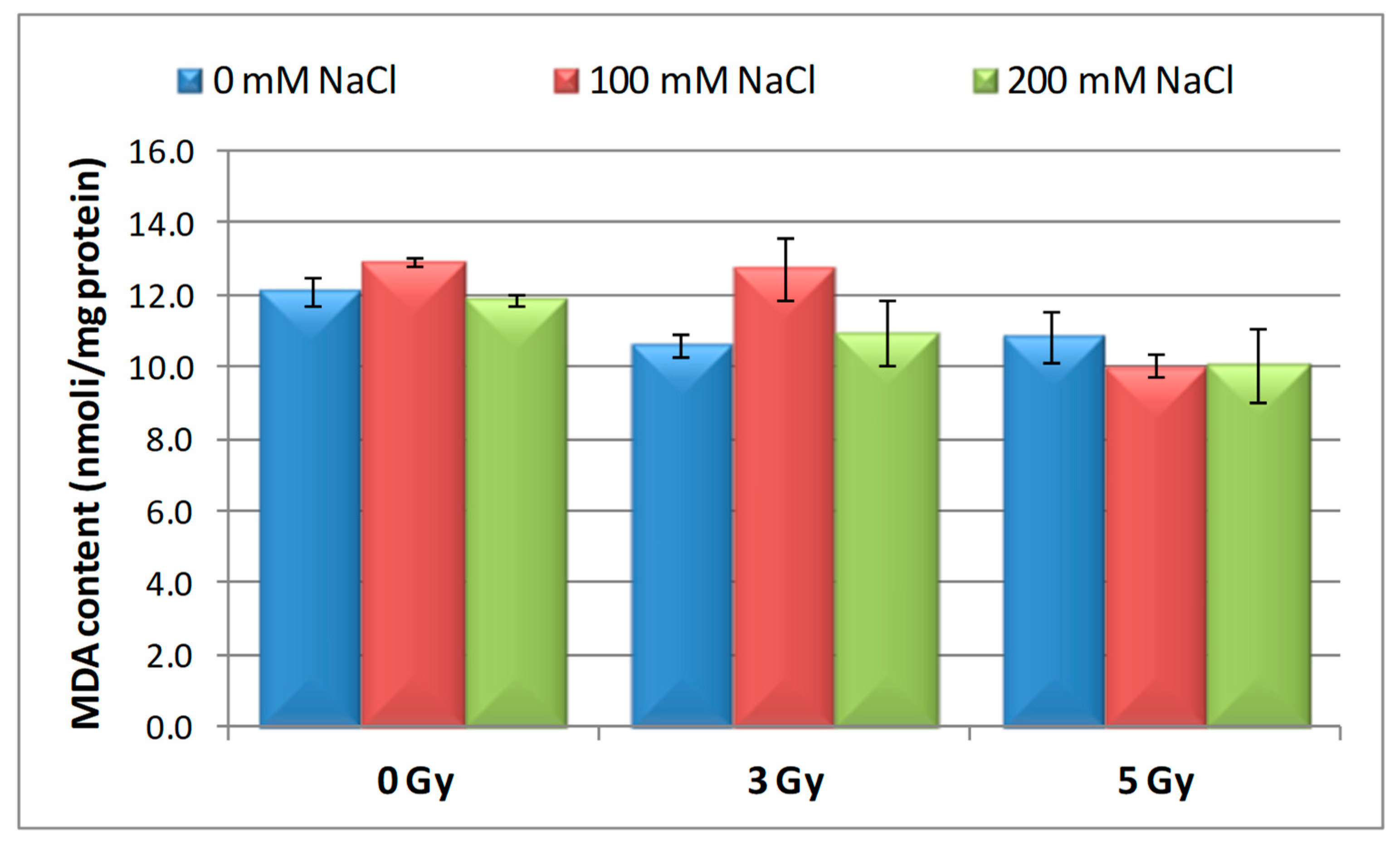
3.4. Effect of Salinity and Proton Irradiation on MDA Content of Barley Seedling
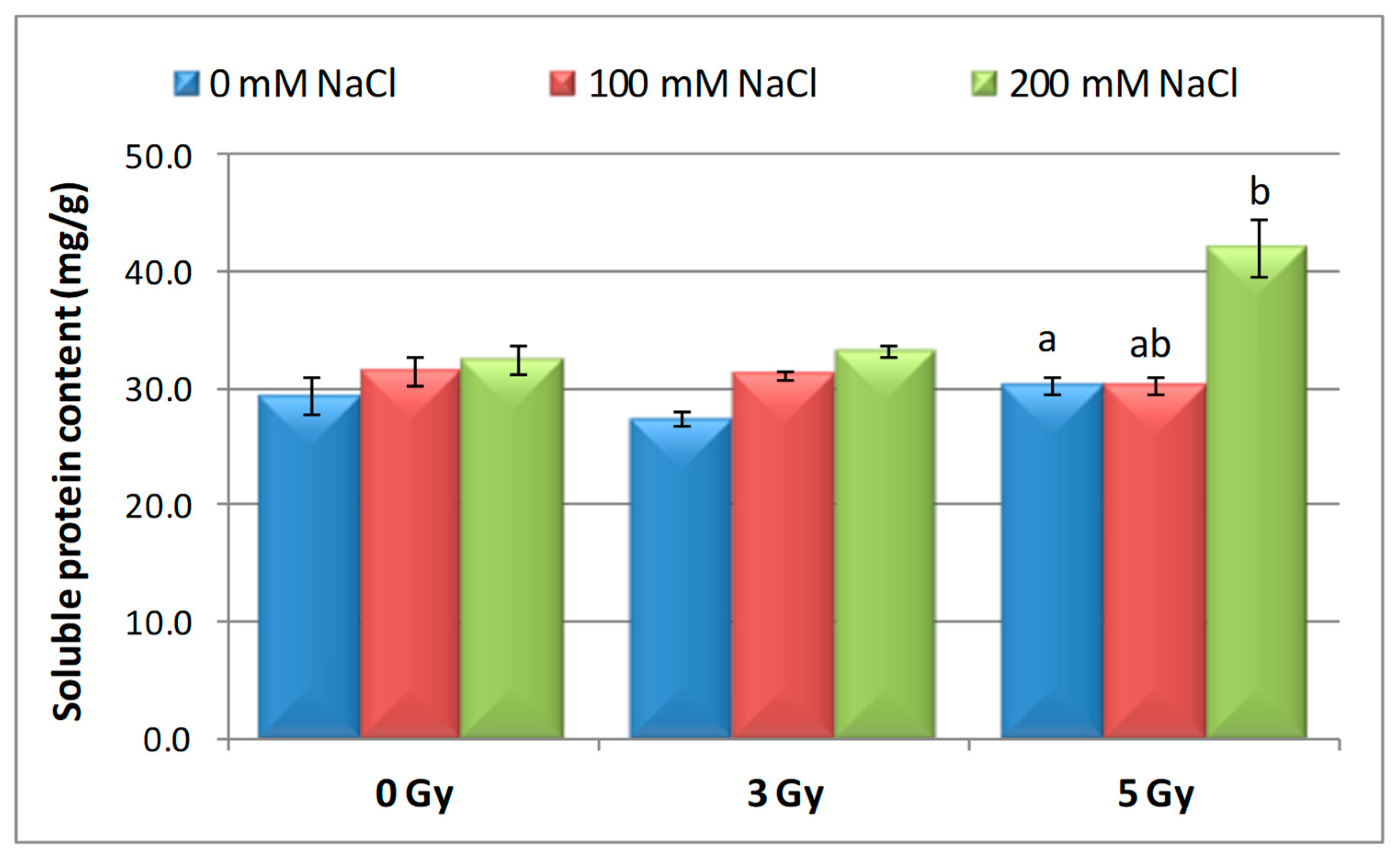
3.5. Effect of Salinity and Proton Irradiation on Soluble Protein Content of Barley Seedling
3.6. Effect of Salinity and Proton Irradiation on Content of Total Polyphenol and Flavonoids in Barley Seedlings
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Akar, T.; Avci, M.; Dusunceli, F. Barley: Post-Harvest Operations; INPhO—Post-Harvest Compendium; Food and Agriculture Organization of the United Nations, AGST/FAO: Ankara, Turkey, 2004; pp. 1–60. [Google Scholar]
- Jebor, M.A.; Al-Saadi, A.; Behjet, R.H.; Al-Terehi, M.; Zaidan, H.K.; Al-Saadi, M.A.K. Characterization and antimicrobial activity of barley grain (Hordeum vulgare) extract. Int. J. Curr. Microbiol. Appl. Sci. 2013, 2, 41–48. [Google Scholar]
- Gupta, B.; Huang, B. Mechanism of Salinity Tolerance in Plants: Physiological, Biochemical, and Molecular Characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef]
- Storey, R.; Wyn Jones, R.G. Salt stress and comparative physiology in the gramineae. I. Ion relations of two salt and water stressed barley cultivars, California Mariout and Arimar. Aust. J. Plant Physiol. 1978, 5, 801–816. [Google Scholar] [CrossRef]
- Mallek-Maalej, E.; Dakhli, R.; Abdi, N.; Slama, A.; Salem, M.B. Evaluation of the Effect of Salt Stress on Some Physiological and Agronomic Traits in Tunisian Durum Wheat Varieties. Eur. J. Sci. Res. 2015, 130, 88–100. [Google Scholar]
- Costa, S.F.; Martins, D.; Agacka-Mołdoch, M.; Czubacka, A.; de Sousa Araújo, S. Strategies to Alleviate Salinity Stress in Plants in Salinity Responses and Tolerance in Plants. In Salinity Responses and Tolerance in Plants; Kumar, V., Wani, S.H., Suprasanna, P., Tran, L.S.P., Eds.; Springer International Publishing AG: Berlin/Heidelberg, Germany, 2018; Volume 1, pp. 307–337. [Google Scholar]
- Abbas, R.; Rasul, S.; Aslam, K.; Baber, M.; Shahid, M.; Mubeen, F.; Naqqash, T. Halotolerant PGPR: A hope for cultivation of saline soils. J. King Saud Univ. Sci. 2019, 31, 1195–1201. [Google Scholar] [CrossRef]
- Kabar, K. Alleviation of salinity stress by plant growth regulators on seed germination. J. Plant Physiol. 1987, 128, 179–183. [Google Scholar] [CrossRef]
- Javid, M.G.; Sorooshzadeh, A.; Moradi, F.; Sanavy, S.A.M.M.; Allahdadi, I. The role of phytohormones in alleviating salt stress in crop plants. Aust. J. Crop. Sci. 2011, 5, 726–734. [Google Scholar]
- Shaddad, M.A.K.; Abd El-Samad, H.M.; Mostafa, D. Role of gibberellic acid (GA3) in improving salt stress tolerance of two wheat cultivars. Int. J. Plant Physiol. Biochem. 2013, 5, 50–57. [Google Scholar]
- Ilangumaran, G.; Smith, D.L. Plant Growth Promoting Rhizobacteria in Amelioration of Salinity Stress: A Systems Biology Perspective. Front. Plant Sci. 2017, 8, 1768. [Google Scholar] [CrossRef]
- Oprica, L.; Stefan, M. Evaluation of morphological and biochemical parameters of soybean seedlings induced by saline stress. Rom. Biotechnol. Lett. 2014, 19, 9615–9624. [Google Scholar]
- Macovei, A.; Garg, B.; Raikwar, S.; Balestrazzi, A.; Carbonera, D.; Buttafava, A.; Bremont, J.F.; Gill, S.S.; Tuteja, N. Synergistic exposure of rice seeds to different doses of γ -ray and salinity stress resulted in increased antioxidant enzyme activities and gene-specific modulation of TC-NER pathway. Biomed. Res. Int. 2014, 170, 780–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dehpour, A.A.; Gholampour, M.; Rahdary, P.; Jafari, T.M.R.; Hamdi, S.M.M. Effect of gamma irradiation and salt stress on germination, callus, protein and proline in rice (Oryza sativa L.). Iran. J. Plant Physiol. 2011, 1, 251–256. [Google Scholar]
- Rejili, M.; Telahigue, D.; Lachiheb, B.; Mrabet, A.; Ferchichi, A. Impact of gamma radiation and salinity on growth and K+/Na+balance in two populations of Medicago sativa (L.) cultivar Gabe. Prog. Nat. Sci. Mater. 2008, 18, 1095–1105. [Google Scholar] [CrossRef]
- Ahmed, A.H.A.; Ghalab, A.R.M.; Hussein, O.S.; El-Hefny, A.M. Effect of Gamma Rays and Salinity on Growth and Chemical Composition of Ambrosia maritima L. Plants. J. Radiat. Res. Appl. Sci. 2011, 4, 1139–1162. [Google Scholar]
- El-Beltagi, H.S.; Mohamed, H.I.; Mohammed, A.H.M.A.; Zaki, L.M.; Mogazy, A.M. Physiological and biochemical effects of γ-irradiation on cowpea plants (Vigna sinensis) under salt stress. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 2013, 41, 104–114. [Google Scholar] [CrossRef] [Green Version]
- Geng, X.; Zhang, Y.; Wang, L.; Yang, X. Pretreatment with High-Dose Gamma Irradiation on Seeds Enhances the Tolerance of Sweet Osmanthus Seedlings to Salinity Stress. Forests 2019, 10, 406. [Google Scholar] [CrossRef] [Green Version]
- Hamideldin, N.; Eliwa, N.E.; Hussein, O.S. Role of jasmonic acid and gamma radiation in alleviating salt stress in moringa. Int. J. Agric. Biol. 2017, 19, 93–98. [Google Scholar] [CrossRef]
- Jan, S.; Parween, T.; Siddiqi, T.O.; Mahmooduzzafar, N. Effect of gamma radiation on morphological, biochemical and physiological aspects of plants and plant products. Environ. Rev. 2012, 20, 17–39. [Google Scholar] [CrossRef] [Green Version]
- Ling, A.P.K.; Ung, Y.C.; Hussein, S.; Harun, A.R.; Tanaka, A.; Yoshihiro, H. Morphological and biochemical responses of Oryza sativa L. (cultivar MR219) to ion beam irradiation. J. Zhejiang Univ. Sci. B 2013, 14, 1132–1143. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Lee, A.R.; Kwon, S.W. Effect of proton beam irradiation on M1 seeds and seedling growth in rice. Plant Breed. Biotechnol. 2015, 3, 384–388. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.M.; Jo, Y.D.; Lee, H.J.; Kim, Y.S.; Kim, D.S.; Kim, J.B.; Kang, S.Y.; Kim, S.H. DNA damage and oxidative stress induced by proton beam in Cymbidium hybrid. Hortic. Environ. Biotechnol. 2015, 56, 240–246. [Google Scholar] [CrossRef]
- Salvi, S.; Druka, A.; Milner, S.G.; Gruszka, D. Induced Genetic Variation, TILLING and NGS-Based Cloning. In Biotechnological Approaches to Barley Improvement; Kumlehn, J., Stein, N., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; Volume 69, pp. 287–310. [Google Scholar]
- Khazaei, H.; Mäkelä, P.S.A.; Stoddard, F.L. Ion beam irradiation mutagenesis in rye (Secale cereale L.), linseed (Linum usitatissimum L.) and faba bean (Vicia faba L.). Agric. Food Sci. 2018, 27, 146–151. [Google Scholar] [CrossRef]
- Kim, W.J.; Ryu, J.; Im, J.; Kim, S.H.; Kang, S.Y.; Lee, J.H.; Jo, H.S.; Ha, B.K. Molecular characterization of proton beam-induced mutations in soybean using genotyping-by-sequencing. Mol. Genet. Genom. 2018, 293, 1169–1180. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Vishwakarma, G.; Chauhan, A.; Shitre, A.; Das, B.K. Use of Proton Beam as a Novel Tool for Mutations in Rice. BARC Newsl. 2018, 366, 5–9. [Google Scholar]
- Bhat, N.N.; Sapra, B.K.; Gupta, S.K. Proton Beam Set-up for Radiobiological Studies. BARC Newsl. 2015, 346, 5–7. [Google Scholar]
- Deoli, N.T.; Hasenstein, K.H. Irradiation effects of MeV protons on dry and hydrated Brassica rapa seeds. Life Sci. Space Res. 2018, 19, 24–30. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophyll and carotenoids: Pigments of photosynthetic bio-membranes. In Methods Enzymology; Packer, L., Douce, R., Eds.; Academic Press: London, UK, 1987; Volume 641, pp. 350–382. [Google Scholar]
- Hodges, D.M.; Delong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef]
- Dhindsa, R.A.; Plumb, D.P.; Thorpe, T.A. Leaf senescence, correlated with increased permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. Exp. Bot. 1981, 126, 93–101. [Google Scholar] [CrossRef]
- Ranieri, A.; Petacco, F.; Castagna, A.; Soldatini, G.F. Redox state and peroxidase system in sunflower plants exposed to ozone. Plant Sci. 2000, 159, 159–167. [Google Scholar] [CrossRef]
- Sinha, A.K. Colorimetric assay of catalase. Anal. Biochem. 1972, 47, 389–394. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Wi, S.G.; Chung, B.Y.; Kim, J.S.; Baek, M.H.; Lee, J.W.; Kim, Y.S. Effects of gamma irradiation on morphological changes and biological responses in plants. Micron 2007, 38, 553–564. [Google Scholar] [CrossRef]
- Singh, B.; Datta, P.S. Gamma irradiation to improve plant vigour, grain development, and yield attributes of wheat. Radiat. Phys. Chem. 2010, 79, 139–143. [Google Scholar] [CrossRef]
- Hanafy, R.S.; Akladious, S.A. Physiological and molecular studies on the effect of gamma radiation in fenugreek (Trigonella foenum-graecum L.) plants. J. Genet. Eng. Biotechnol. 2018, 16, 683–692. [Google Scholar] [CrossRef]
- Koing, A.L.P.; Lai, A.G.; Hussein, S.; Harun, A.R. Physiological responses of Orthosiphon stamineus plantlets to gamma irradiation. Am. Eurasian J. Sustain. Agric. 2008, 2, 135–149. [Google Scholar]
- Qin, H.L.; Wang, Y.G.; Xue, J.M.; Miao, Q.; Ma, L.; Mei, T.; Zhang, W.M.; Guo, W.; Wang, J.Y.; Gu, H.Y. Biological effects of protons targeted to different ranges in Arabidopsis seeds. Int. J. Radiat. Biol. 2007, 83, 301–308. [Google Scholar] [CrossRef]
- Kim, S.K.; Park, S.Y.; Kim, K.R.; Shin, J.H.; Kim, S.Y.; Kim, H.Y.; Lee, I.J. Effect of proton beam irradiation on germination, seedling growth, and pasting properties of starch in rice. J. Crop. Sci. Biotechnol. 2012, 15, 305–310. [Google Scholar] [CrossRef]
- Chauhan, A.; Kumar, V.; Iyer, P.R.; Vishwakarma, G.; Nair, J.P.; Surendran, P.; Sparrow, H.; Gupta, A.K.; Shitre, A.S.; Shinde, A.K.; et al. Effect of proton beam irradiation on survival and seedling growth parameters of Indian rice (Oryza sativa L.) variety ‘Indira Barani Dhan 1′. Electron. J. Plant Breed. 2019, 10, 490–499. [Google Scholar] [CrossRef]
- Bae, C.H.; Lyu, J.I.; Sarantuya, G.; Chai, J.S.; Kim, J.H.; Yahng, T.G.; Lee, M.Y.; Yang, D.C. Efects of Proton Beam Iradiation on Germination and Growth of Tabacco and Rice Plants. Korean J. Plants Res. 2005, 18, 462–469. [Google Scholar]
- Gonzalez, M.C.; Perez, N.; Cristo, E. GINES: A first rice mutant obtained by proton irradiation. Cult. Trop. 2009, 30, 9. [Google Scholar]
- Im, J.; Kim, W.J.; Kim, S.H.; Ha, B.K. Effects of proton beam irradiation on seed germination and growth of soybean, (Glycine max L. Merr.). J. Korean Phys. Soc. 2017, 71, 752–757. [Google Scholar] [CrossRef]
- Oprica, L.; Grigore, M.M.; Vochita, G. Impact of saline stress on growth and biochemical indices of Calendula officinalis seedlings. Rom. Biotechnol. Lett. 2015, 20, 11007–11017. [Google Scholar]
- Baek, M.H.; Kim, J.H.; Chung, B.Y.; Kim, J.S.; Lee, I.S. Alleviation of salt stress by low dose γ-irradiation in rice. Biol. Plant. 2005, 49, 273–276. [Google Scholar] [CrossRef]
- Malanga, G.; Calmanovici, G.; Puntarulo, S. Oxidative damage to chloroplasts from Chlorella vulgaris exposed to ultraviolet-B radiation. Physiol. Plant. 1997, 101, 455–462. [Google Scholar] [CrossRef]
- Kumar, S.G.; Lakshmi, A.; Madhusudhan, K.V.; Ramanjulu, S.; Sudhakar, C. Photosynthesis parameters in two cultivars of mulberry differing in salt tolerance. Photosynthetica 1999, 36, 611–616. [Google Scholar] [CrossRef]
- Sultana, N.; Ikeda, T.; Itoh, R. Effects of NaCl salinity on photosynthesis and dry matter accumulation in developing rice grain. Environ. Exp. Bot. 1999, 42, 211–220. [Google Scholar] [CrossRef]
- De Micco, V.; Paradiso, R.; Aronne, G.; De Pascale, S.; Quarto, M.; Arena, C. Leaf anatomy and photochemical behaviour of Solanum lycopersicum L. plants from seeds irradiated with low-LET ionising radiation. Sci. World J. 2014, 2014, 428141. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Song, M.; Lee, K.J.; Hwang, S.G.; Jang, C.S.; Kim, J.B.; Kim, S.H.; Ha, B.K.; Kang, S.Y.; Kim, D.S. Genome-wide transcriptome profiling of ROS scavenging and signal transduction pathways in rice (Oryza sativa L.) in response to different types of ionizing radiation. Mol. Biol. Rep. 2012, 39, 11231–11248. [Google Scholar]
- Kalimullah, M.; Gaikwad, J.U.; Thomas, S.; Sarma, A.; Vidyasagar, P.B. Assessment of 1H heavy ion irradiation induced effects in the development of rice (Oryza sativa L.) seedlings. Plant. Sci. 2003, 165, 447–454. [Google Scholar] [CrossRef]
- Im, J.; Ko, J.; Kim, H.Y.; Ha, B.K. Biochemical Responses of Soybean (Glycine max L. Merr.) to Proton Beam Irradiation. Plant Breed. Biotechnol. 2017, 5, 97–105. [Google Scholar] [CrossRef] [Green Version]
- Taïbia, K.; Taïbi, F.; Abderrahim, L.A.; Ennajah, A.; Belkhodja, M.; Muletd, J.M. Effect of salt stress on growth, chlorophyll content, lipid peroxidation and antioxidant defense systems in Phaseolus vulgaris L. S. Afr. J. Bot. 2016, 105, 306–312. [Google Scholar] [CrossRef]
- Vanhoudt, N.; Cuypers, A.; Vangronsveld, J.; Horemans, N.; Wannijn, J.; Van Hees, M.; Vandenhove, H. Study of biological effects and oxidative stress related responses in gamma irradiated Arabidopsis thaliana plants. Radioprotection 2011, 46, S401–S407. [Google Scholar] [CrossRef] [Green Version]
- Ozgur, A.; Uzilday, B.; Sekmen, A.H.; Turkan, I. Reactive oxygen species regulation and antioxidant defence in halophytes. Funct. Plant Biol. 2013, 40, 832–847. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef]
- Afzal, F.; Khurshid, R.; Ashraf, N.; Kazi, A.G. Reactive Oxygen Species and Antioxidants in Response to Pathogens and Wounding. In Oxidative Damage to Plants. Antioxidant Networks and Signaling; Ahmad, P., Ed.; Academic Press: London, UK, 2014; pp. 397–424. [Google Scholar]
- Bailey-Serres, J.; Mittler, R. The roles of reactive oxygen species in plant cells. Plant Physiol. 2006, 141, 311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovács, E.; Keresztes, Á. Effect of gamma and UV-B/C radiation on plant cells. Micron 2002, 33, 199–210. [Google Scholar] [CrossRef]
- Qi, W.; Zhang, L.; Xu, H.; Wang, L.; Jiao, Z. Physiological and molecular characterization of the enhanced salt tolerance induced by low-dose gamma irradiation in Arabidopsis seedlings. Biochem. Biophys. Res. Commun. 2014, 450, 1010–1015. [Google Scholar] [CrossRef]
- Arora, A.; Sairam, R.K.; Srivastava, G.C. Oxidative stress and antioxidative system in plants. Curr. Sci. 2002, 82, 1227–1238. [Google Scholar]
- Hong, M.J.; Kim, D.Y.; Ahn, J.W.; Kang, S.Y.; Seo, Y.W.; Kim, J.B. Comparison of radiosensitivity response to acute and chronic gamma irradiation in colored wheat. Genet. Mol. Biol. 2018, 41, 611–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gechev, T.S.; Breusegem, F.V.; Stone, J.M.; Denev, I.; Laloi, C. Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. Bioessays 2006, 28, 1091–1101. [Google Scholar] [CrossRef]
- Zaka, R.; Vandecasteele, C.M.; Misset, M.T. Effects of low chronic doses of ionizing radiation on antioxidant enzymes and G6PDH activities in Stipa capillata Poaceae. J. Exp. Bot. 2002, 53, 1979–1987. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.T.; Willey, N.J.; Hancock, J.T. Low dose ionizing radiation produces too few reactive oxygen species to directly affect antioxidant concentrations in cells. Biol. Lett. 2012, 8, 594–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid. Med. Cell. Longev. 2014, 360438. [Google Scholar] [CrossRef]
- Beyaz, R. Impact of gamma irradiation pretreatment on the growth of common vetch (Vicia sativa L.) seedlings grown under salt and drought stress. Int. J. Radiat. Biol. 2020, 96, 257–266. [Google Scholar] [CrossRef]
- Dionisio-Sese, M.L.; Tobita, S. Antioxidant response of rice seedlings to salinity stress. Plant Sci. 1998, 135, 1–9. [Google Scholar] [CrossRef]
- Katsuhara, M.; Otsuka, T.; Ezaki, B. Salt stress-induced lipid peroxidation is reduced by glutathione S-transferase, but this reduction of lipid peroxides is not enough for a recovery of root growth in Arabidopsis. Plant Sci. 2005, 169, 369–373. [Google Scholar] [CrossRef]
- Song, J.Y.; Kim, D.S.; Lee, M.C.; Lee, K.J.; Kim, J.B.; Kim, S.H.; Ha, B.K.; Yun, S.J.; Kang, S.Y. Physiological characterization of gamma-ray induced salt tolerant rice mutants. Aust. J. Crop. Sci. 2012, 6, 421–429. [Google Scholar]
- Al-Rumaih, M.M.; Al-Rumaih, M.M. Influence of ionizing radiation on antioxidant enzymes in three species of Trigonella. Am. J. Environ. Sci. 2008, 4, 151–156. [Google Scholar] [CrossRef] [Green Version]
- Štajner, D.; Milošević, M.; Popović, B.M. Irradiation effects on phenolic content, lipid and protein oxidation and scavenger ability of soybean seeds. Int. J. Mol. Sci. 2007, 8, 618–627. [Google Scholar] [CrossRef] [Green Version]
- Byun, M.; Kang, I.; Mori, T. Effect of γ-irradiation on the water soluble components of soybeans. Radiat. Phys. Chem. 1996, 47, 155–160. [Google Scholar] [CrossRef]
- Choi, C.W.; Kim, S.C.; Hwang, S.S.; Choi, B.K.; Ahn, H.J.; Lee, M.Y.; Park, S.H.; Kim, S.K. Antioxidant activity and free radical scavenging capacity between Korean medicinal plants and flavonoids by assay-guided comparison. Plant Sci. 2002, 163, 1161–1168. [Google Scholar] [CrossRef]
- Rezk, A.A.; Al-Khayri, J.M.; Al-Bahrany, A.M.; El-Beltagi, H.S.; Mohamed, H.I. X-ray irradiation changes germination and biochemical analysis of two genotypes of okra (Hibiscus esculentus L.). J. Radiat. Res. Appl. Sci. 2019, 12, 393–402. [Google Scholar] [CrossRef] [Green Version]
- Said-Al Ahl, H.A.H.; Sarhan, A.M.Z.; Dahab, A.D.M.A.; Abou-Zeid, E.-S.N.; Ali, M.S.; Naguib, N.Y. Bio-fertilizer and Gamma Radiation Influencing Flavonoids Content in Different Part of Dill Herb. Int. J. Life Sci. Eng. 2015, 1, 145–149. [Google Scholar]


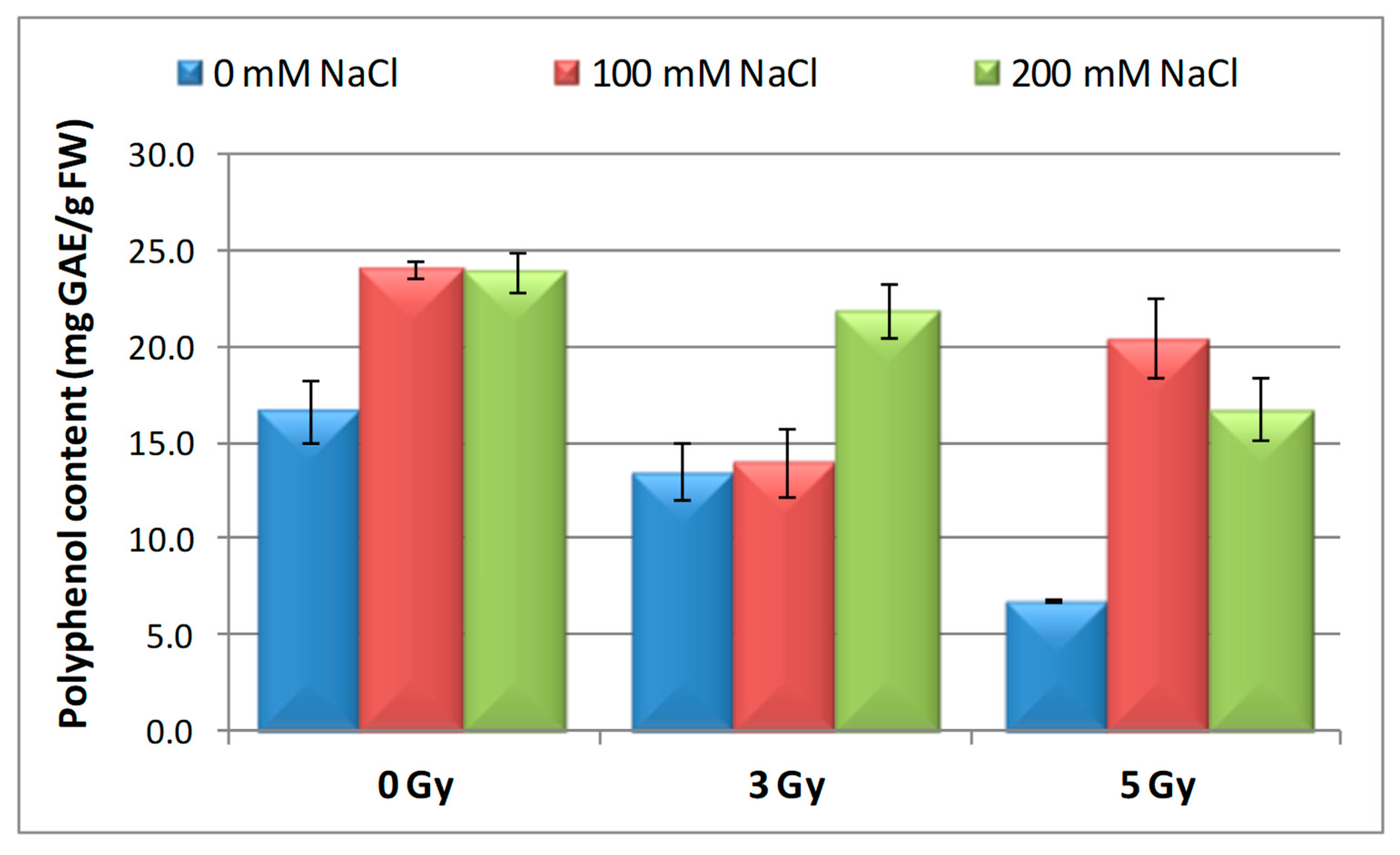
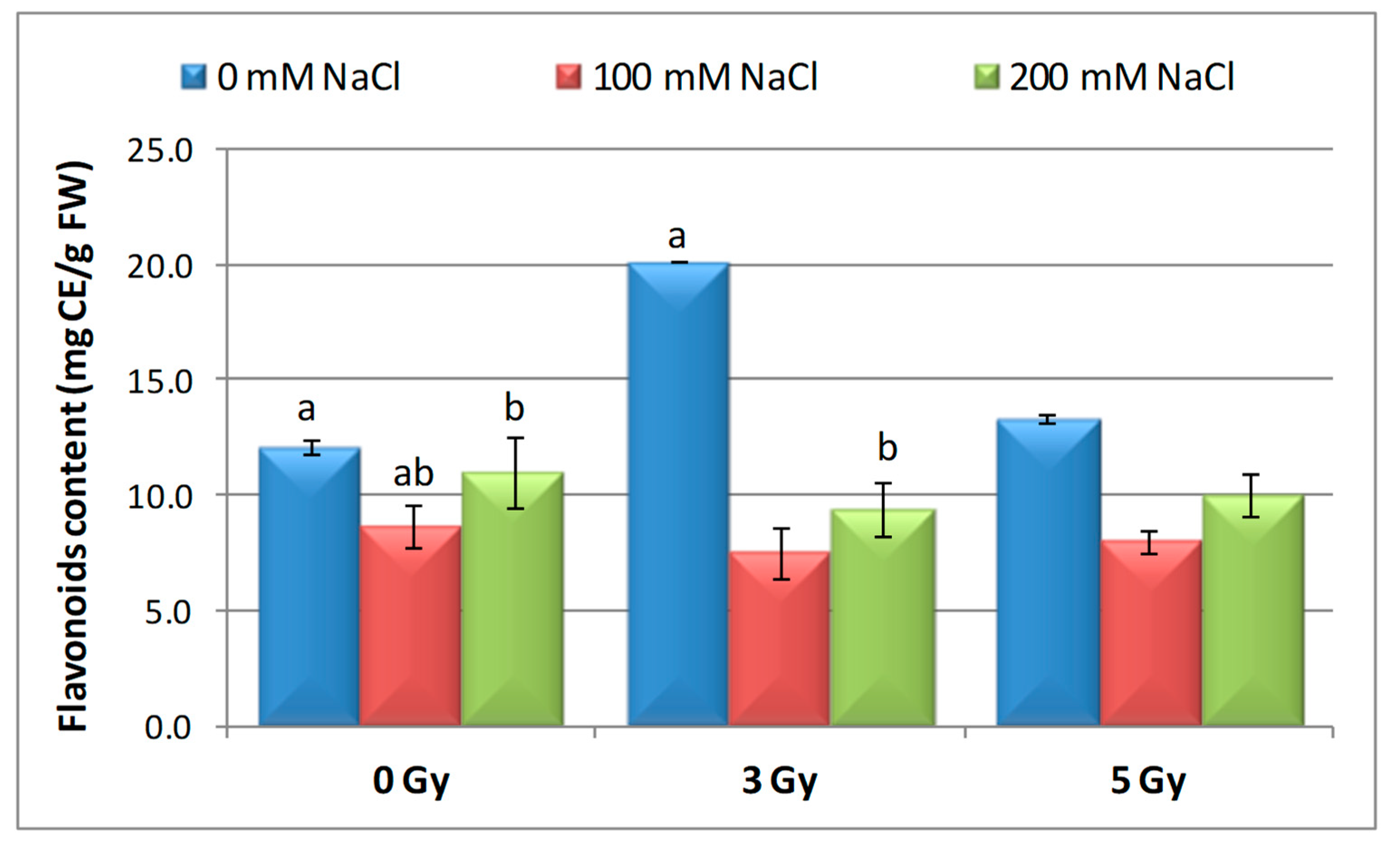
| Samples | Means Values (cm) | P< | % | −/+ Rate (%) |
|---|---|---|---|---|
| Control | 14.50 ± 0.90 | |||
| 3 Gy | 14.30 ± 0.78 | NS | 98.62 | −1.38 |
| 5 Gy | 13.76 ± 0.78 | NS | 94.90 | −5.10 |
| 100 mM NaCl | 15.36 ± 0.99 | NS | 105.93 | 5.93 |
| 200 mM NaCl | 6.00 ± 0.35 | p < 0.001 | 41.38 | −58.62 |
| 3 Gy + 100 mM NaCl | 14.78 ± 0.49 | NS | 101.93 | 1.93 |
| 3 Gy + 200 mM NaCl | 3.93 ± 0.27 | p < 0.001 | 27.10 | −72.90 |
| 5 Gy + 100 mM NaCl | 13.89 ± 0.59 | NS | 95.79 | −4.21 |
| 5 Gy + 200 mM NaCl | 3.78 ± 0.33 | p < 0.001 | 26.07 | −73.93 |
| Sample | Chlorophyll a (mg g−1 FW) | Chlorophyll b (mg g−1 FW) | Carotenoids (mg g−1 FW) |
|---|---|---|---|
| Control | 0.622 ± 0.026 a | 0.160 ± 0.008 a | 0.143 ± 0.005 a |
| 3 Gy | 0.482 ± 0.064 | 0.129 ± 0.018 | 0.095 ± 0.017 |
| 5 Gy | 0.649 ± 0.132 | 0.165 ± 0.029 | 0.130 ± 0.043 |
| 100 mM NaCl | 0.433 ± 0.079 | 0.126 ± 0.015 | 0.111 ± 0.019 |
| 200 mM NaCl | 0.345 ± 0.039 b | 0.101 ± 0.008 b | 0.074 ± 0.009 b |
| 3 Gy + 100 mM NaCl | 0.267 ± 0.013 b | 0.072 ± 0.015 b | 0.061 ± 0.013 |
| 3 Gy + 200 mM NaCl | 0.371 ± 0.002 b | 0.103 ± 0.012 | 0.074 ± 0.006 b |
| 5 Gy + 100 mM NaCl | 0.434 ± 0.092 | 0.239 ± 0.028 | 0.117 ± 0.014 |
| 5 Gy + 200 mM NaCl | 0.570 ± 0.045 | 0.173 ± 0.008 | 0.108 ± 0.009 |
| Samples | SOD Activity | −/+ Rate (%) | CAT Activity | −/+ Rate (%) | POD Activity | −/+ Rate (%) | MDA Content | −/+ Rate (%) | Flavonoid Content | −/+ Rate (%) | Polyphenol Content | −/+ Rate (%) | Soluble Protein Content | −/+ Rate (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 1.10 | 0.00 | 2.30 a | 0.00 | 0.40 | 0.00 | 12.11 | 0.00 | 12.09 a | 0.00 | 16.67 | 0.00 | 29.34 | 0.00 |
| 3 Gy | 1.17 | 6.36 | 2.56 | 11.30 | 0.43 | 7.50 | 10.60 | −12.47 | 20.11 | 66.34 | 13.52 | −18.90 | 27.41 | −6.58 |
| 5 Gy | 1.13 | 2.73 | 5.35 | 132.61 | 0.41 | 2.50 | 10.85 | −10.40 | 13.32 | 10.17 | 6.82 | −59.09 | 30.22 a | 3.00 |
| 100 mM NaCl | 1.00 | −9.09 | 4.90 b | 113.04 | 0.32 | −20.00 | 12.93 | 6.77 | 8.65 ab | −28.45 | 24.04 | 44.21 | 31.56 | 7.57 |
| 200 mM NaCl | 1.02 | −7.27 | 4.85 b | 110.87 | 0.39 | −2.50 | 11.89 | −1.82 | 11.00 b | −9.02 | 23.87 | 43.19 | 32.57 | 11.01 |
| 3 Gy + 100 mM NaCl | 1.03 | −6.36 | 4.69 b | 103.91 | 0.41 | 2.50 | 12.74 | 5.20 | 7.50 | −37.97 | 13.98 | −16.14 | 30.89 | 5.28 |
| 3 Gy + 200 mM NaCl | 1.02 | −7.27 | 3.22 | 40.00 | 0.38 | −5.00 | 10.92 | −9.83 | 9.39 b | −22.33 | 21.87 | 31.19 | 33.30 | 13.50 |
| 5 Gy + 100 mM NaCl | 1.02 | −7.27 | 3.23 | 40.43 | 0.39 | −2.50 | 10.04 | −17.09 | 7.99 | −33.91 | 20.45 | 22.68 | 30.28 a b | 3.20 |
| 5 Gy + 200 mM NaCl | 1.07 | −2.73 | 3.18 b | 38.26 | 0.37 | −7.50 | 10.08 | −16.76 | 10.00 | −17.29 | 16.75 | 0.48 | 40.06 b | 36.53 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oprica, L.; Grigore, M.-N.; Caraciuc, I.; Gherghel, D.; Mihai, C.-T.; Vochita, G. Impact of Proton Beam Irradiation on the Growth and Biochemical Indexes of Barley (Hordeum vulgare L.) Seedlings Grown under Salt Stress. Plants 2020, 9, 1234. https://doi.org/10.3390/plants9091234
Oprica L, Grigore M-N, Caraciuc I, Gherghel D, Mihai C-T, Vochita G. Impact of Proton Beam Irradiation on the Growth and Biochemical Indexes of Barley (Hordeum vulgare L.) Seedlings Grown under Salt Stress. Plants. 2020; 9(9):1234. https://doi.org/10.3390/plants9091234
Chicago/Turabian StyleOprica, Lacramioara, Marius-Nicusor Grigore, Iulia Caraciuc, Daniela Gherghel, Cosmin-Teodor Mihai, and Gabriela Vochita. 2020. "Impact of Proton Beam Irradiation on the Growth and Biochemical Indexes of Barley (Hordeum vulgare L.) Seedlings Grown under Salt Stress" Plants 9, no. 9: 1234. https://doi.org/10.3390/plants9091234
APA StyleOprica, L., Grigore, M.-N., Caraciuc, I., Gherghel, D., Mihai, C.-T., & Vochita, G. (2020). Impact of Proton Beam Irradiation on the Growth and Biochemical Indexes of Barley (Hordeum vulgare L.) Seedlings Grown under Salt Stress. Plants, 9(9), 1234. https://doi.org/10.3390/plants9091234







