Effects of Increased N Deposition on Leaf Functional Traits of Four Contrasting Tree Species in Northeast China
Abstract
:1. Introduction
- LMA and its components leaf thickness and density;
- Leaf anatomical and morphological traits, and;
- Leaf traits and plant biomass allocation patterns.
2. Results
2.1. Seedlings’ Biomass and Biomass Distribution per Organ
2.2. Leaf Morphology
2.3. Leaf Anatomy
2.4. Correlations of Leaf Functional Traits
3. Discussion
3.1. Effect of N Deposition on LMA and Its Dependency on LT and LD
3.2. Effects of Additional N Deposition on Leaf Anatomical and Hydraulic Traits and Their Relationship with LT and LD
3.3. Correlation of LMA with Biomass Allocation
3.4. Conclusions
4. Materials and Methods
4.1. Research Site
4.2. Experimental Design
4.3. Seedling Biomass Above and Below Ground
4.4. Leaf Morphology
4.5. Leaf Tissue Anatomy and Stomata
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grigal, D. Atmospheric Deposition and Inorganic Nitrogen Flux. Water Air Soil Pollut. 2012, 223. [Google Scholar] [CrossRef]
- Peretti, M.; Piñeiro, G.; Fernandez-Long, M.; Carnelos, D. Influence of the precipitation interval on wet atmospheric deposition. Atmos. Environ. 2020. [Google Scholar] [CrossRef]
- Liu, X.; Duan, L.; Mo, J.; Du, E.; Shen, J.; Xiankai, L.; Zhang, Y.; Zhou, X.; He, C.; Zhang, F. Nitrogen deposition and its ecological impact in China: An overview. Environ. Pollut. 2011, 159, 2251–2264. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Jiang, C.; Ma, Q.; Xu, Y.-G.; Zou, H.; Zhang, S. Observation of the nitrogen deposition in the lower Liaohe River Plain, Northeast China and assessing its ecological risk. Atmos. Res. 2011, 101, 460–468. [Google Scholar] [CrossRef]
- Jia, Y.; Yu, G.; He, N.; Zhan, X.; Fang, H.; Sheng, W.; Zuo, Y.; Zhang, D.; Wang, Q.-F. Spatial and decadal variations in inorganic nitrogen wet deposition in China induced by human activity. Sci. Rep. 2014, 4, 3763. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Zhang, Y.; Han, W.; Tang, A.; Shen, J.; Cui, Z.; Vitousek, P.; Erisman, J.W.; Goulding, K.; Christie, P.; et al. Enhanced Nitrogen Deposition over China. Nature 2013, 494, 459–462. [Google Scholar] [CrossRef]
- Xu, W.; Luo, X.; Pan, Y.; Zhang, L.; Tang, A.; Shen, J.; Zhang, Y.; Li, K.; Wu, Q.; Yang, D.; et al. Quantifying atmospheric nitrogen deposition through a nationwide monitoring network across China. Atmos. Chem. Phys. 2015, 15. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Song, L.; He, C.; Zhang, F. Nitrogen deposition as an important nutrient from the environment and its impact on ecosystems in China. J. Arid Land 2010, 2, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Bobbink, R.; Hicks, K.; Galloway, J.; Spranger, T.; Alkemade, R.; Ashmore, M.; Bustamante, M.; Cinderby, S.; Davidson, E.; Dentener, F.; et al. Global assessment of nitrogen deposition effects on terrestrial plant diversity: A synthesis. Ecol. Appl. 2010, 20, 30–59. [Google Scholar] [CrossRef] [Green Version]
- Yan, G.; Zhou, M.; Wang, M.; Han, S.; Liu, G.; Zhang, X.; Sun, W.; Huang, B.; Wang, H.; Xing, Y.; et al. Nitrogen deposition and decreased precipitation altered nutrient foraging strategies of three temperate trees by affecting root and mycorrhizal traits. Catena 2019, 181, 104094. [Google Scholar] [CrossRef]
- Cooke, J.; Martin, T. Short-term physiological and developmental responses to nitrogen availability in hybrid poplar. New Phytol. 2005, 167, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Kawai, K.; Ahmad, B.; Palle, I.; Okada, N. Variations of leaf and stem traits in relation to altitudinal distributions of 12 Fagaceae species of Mount Kinabalu, Borneo. Tropics 2020. [Google Scholar] [CrossRef]
- Marañón, T.; Fernández, C.M.; Gil-Martinez, M.; Dominguez, M.; Madejón, P.; Villar, R. Variation in morphological and chemical traits of Mediterranean tree roots: Linkage with leaf traits and soil conditions. Plant. Soil 2020. [Google Scholar] [CrossRef]
- Niinemets, Ü. Components of leaf dry mass per area: Thickness and density: Alter leaf photosynthetic capacity in reverse directions in woody plants. New Phytol. 1999, 144, 35–47. [Google Scholar] [CrossRef]
- Li, Y.; Kröber, W.; Bruelheide, H.; Haerdtle, W.; Oheimb, G. Crown and leaf traits as predictors of subtropical tree sapling growth rates. J. Plant. Ecol. 2017, 10, 136–145. [Google Scholar] [CrossRef]
- Patil, M.; Kumar, A.; Kumar, P.; Singh, A. Litter Decomposability Traits and Their Linkage with the Cycling of Nutrients in the Forest Ecosystems under the Lens of Climate Change: A Conceptual Overview. Adv. Zool. Bot. 2020, 8, 269–277. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, W.; Zhang, S.-B. The study of a determinate growth orchid highlights the role of new leaf production in photosynthetic light acclimation. Plant. Ecol. 2017. [Google Scholar] [CrossRef]
- Poorter, H.; Niinemets, Ü.; Poorter, L.; Wright, I.; Villar, R. Causes and Consequences of Variation in Leaf Mass per Area (LMA): A Meta-Analysis. New Phytol. 2009, 182, 565–588. [Google Scholar] [CrossRef]
- de la Riva, E.; Villar, R.; Poorter, H.; Olmo, M.; Ubera, J. Leaf Mass per Area (LMA) and Its Relationship with Leaf Structure and Anatomy in 34 Mediterranean Woody Species along a Water Availability Gradient. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [Green Version]
- Reich, P.B.; Flores-Moreno, H. Peeking beneath the hood of the leaf economics spectrum. New Phytol. 2017, 214, 1395–1397. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Azofeifa, G.A.; Castro, K.; Wright, S.J.; Gamon, J.; Kalacska, M.; Rivard, B.; Schnitzer, S.A.; Feng, J.L. Differences in leaf traits, leaf internal structure, and spectral reflectance between two communities of lianas and trees: Implications for remote sensing in tropical environments. Remote Sens. Environ. 2009, 113, 2076–2088. [Google Scholar] [CrossRef] [Green Version]
- Garnier, E.; Cordonnier, P.; Guillerm, J.L.; Sonié, L. Specific leaf area and leaf nitrogen concentration in annual and perennial grass species growing in Mediterranean old-fields. Oecologia 1997, 111, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Integrating species composition and leaf nitrogen content to indicate effects of nitrogen deposition. Environ. Pollut. 2017, 221, 392–397. [CrossRef] [PubMed]
- Franks, P.J.; Beerling, D.J. Maximum leaf conductance driven by CO2 effects on stomatal size and density over geologic time. Proc. Natl. Acad. Sci. USA 2009, 106, 10343. [Google Scholar] [CrossRef] [Green Version]
- Sack, L.; Scoffoni, C. Leaf venation: Structure, function, development, evolution, ecology and applications in the past, present and future. New Phytol. 2013, 198, 983–1000. [Google Scholar] [CrossRef]
- Scoffoni, C.; Albuquerque, C.; Cochard, H.; Buckley, T.N.; Fletcher, L.R.; Caringella, M.A.; Bartlett, M.; Brodersen, C.R.; Jansen, S.; McElrone, A.J.; et al. The Causes of Leaf Hydraulic Vulnerability and Its Influence on Gas Exchange in Arabidopsis thaliana. Plant. Physiol. 2018, 178, 1584. [Google Scholar] [CrossRef] [Green Version]
- Cai, Q.; Ji, C.; Yan, Z.; Jiang, X.; Fang, J. Anatomical responses of leaf and stem of Arabidopsis thaliana to nitrogen and phosphorus addition. J. Plant. Res. 2017, 130. [Google Scholar] [CrossRef]
- Lu, Y.; Duursma, R.A.; Farrior, C.E.; Medlyn, B.E.; Feng, X. Optimal stomatal drought response shaped by competition for water and hydraulic risk can explain plant trait covariation. New Phytol. 2020, 225, 1206–1217. [Google Scholar] [CrossRef]
- Sack, L.; Scoffoni, C.; John, G.P.; Poorter, H.; Mason, C.M.; Mendez-Alonzo, R.; Donovan, L.A. Leaf mass per area is independent of vein length per area: Avoiding pitfalls when modelling phenotypic integration (reply to Blonder et al. 2014). J. Exp. Bot. 2014, 65, 5115–5123. [Google Scholar] [CrossRef] [Green Version]
- Givnish, T. Ecological constraints on the evolution of plasticity in plants. Evol. Ecol. 2002, 16, 213–242. [Google Scholar] [CrossRef]
- Sack, L.; Scoffoni, C.; John, G.; Poorter, H.; Mason, C.; Méndez-Alonzo, R.; Donovan, L. How do leaf veins influence the worldwide leaf economic spectrum? Review and synthesis. J. Exp. Bot. 2013, 64, 4053–4080. [Google Scholar] [CrossRef] [Green Version]
- McCulloh, K.; Sperry, J.S.; Lachenbruch, B.; Meinzer, F.C.; Reich, P.B.; Voelker, S. Moving water well: Comparing hydraulic efficiency in twigs and trunks of coniferous, ring-porous, and diffuse-porous saplings from temperate and tropical forests. New Phytol. 2010, 186, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Lusk, C.H. Leaf functional trait variation in a humid temperate forest, and relationships with juvenile tree light requirements. PeerJ. 2019, 7, e6855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiflett, S.; Liáng, L.; Crum, S.; Feyisa, G.L.; Wang, J.; Jenerette, D. Variation in the urban vegetation, surface temperature, air temperature nexus. Sci. Total Environ. 2017, 579, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Li, K.; Zhu, X.; Wang, F. The worldwide leaf economic spectrum traits are closely linked with mycorrhizal traits. Fungal Ecol. 2020, 43, 100877. [Google Scholar] [CrossRef]
- Jokela, A.; Bäck, J.; Huttunen, S.; Jalkanen, R. Excess nitrogen fertilization and the structure of Scots-pine needles. Eur. J. For. Pathol. 1995, 25, 109–124. [Google Scholar] [CrossRef]
- Zhu, K.; Wang, A.; Wu, J.; Yuan, F.; Guan, D.; Jin, C.; Zhang, Y.; Gong, C. Effects of nitrogen additions on mesophyll and stomatal conductance in Manchurian ash and Mongolian oak. Sci. Rep. 2020, 10, 10038. [Google Scholar] [CrossRef]
- Zheng, Y.; Xiao, X.; Guo, Z.; Howard, T. A County-Level Analysis of the Spatial Distribution of Forest Resources in China. J. For. Plan. 2001, 7, 69–78. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, D.; Wang, X.; Gu, J.; Mei, L. Fine root architecture, morphology, and biomass of different branch orders of two Chinese temperate tree species. Plant. Soil 2006, 288, 155–171. [Google Scholar] [CrossRef]
- Zhang, H.; Shi, L.; Fu, S. Effects of nitrogen deposition and increased precipitation on soil phosphorus dynamics in a temperate forest. Geoderma 2020, 380, 114650. [Google Scholar] [CrossRef]
- Zhang, W.; Shen, W.; Zhu, S.; Wan, S.; Luo, Y.; Yan, J.; Wang, K.; Liu, L.; Dai, H.; Li, P. Can canopy addition of nitrogen better illustrate the effect of atmospheric nitrogen deposition on forest ecosystem? Sci. Rep. 2015, 5, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Block, C.; Knoepp, J.; Fraterrigo, J. Interactive effects of disturbance and nitrogen availability on phosphorus dynamics of southern Appalachian forests. Biogeochemistry 2012, 112. [Google Scholar] [CrossRef]
- Kramer-Walter, K.R.; Laughlin, D.C. Root nutrient concentration and biomass allocation are more plastic than morphological traits in response to nutrient limitation. Plant. Soil 2017, 416, 539–550. [Google Scholar] [CrossRef]
- Yu, H.; Li, B.; Xu, C.; Zhang, Z.; Zhong, Y.; Zhong, Q.-L. Short-Term Nitrogen Addition Does Not Significantly Alter the Effects of Seasonal Drought on Leaf Functional Traits in Machilus pauhoi Kanehira Seedlings. Forests 2019, 10, 78. [Google Scholar] [CrossRef] [Green Version]
- Mao, R.; Zhang, X.-H.; Song, C. Effects of nitrogen addition on plant functional traits in freshwater wetland of Sanjiang Plain, Northeast China. Chinese Geogr. Sci. 2014, 24, 674–681. [Google Scholar] [CrossRef]
- Villar, R.; Ruiz-Robleto, J.; Ubera, J.; Poorter, H. Exploring variation in leaf mass per area (LMA) from leaf to cell: An anatomical analysis of 26 woody species. Am. J. Bot. 2013, 100. [Google Scholar] [CrossRef] [PubMed]
- Castro-Díez, P.; Puyravaud, J.-P.; Cornelissen, J. Leaf structure and anatomy as related to leaf mass per area variation in seedlings of a wide range of woody plant species and types. Oecologia 2000, 124, 476–486. [Google Scholar] [CrossRef]
- De la Riva, E.; Tosto, A.; Perez-Ramos, I.; Fernández, C.M.; Olmo, M.; Anten, N.; Marañón, T.; Villar, R. A plant economics spectrum in Mediterranean forests along environmental gradients: Is there coordination among leaf, stem and root traits? J. Veg. Sci. 2015, 27. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Dudley, T.; Chen, B.-M.; Chang, X.; Liáng, L.; Peng, S. Responses of tree and insect herbivores to elevated nitrogen inputs: A meta-analysis. Acta Oecol. 2016, 77. [Google Scholar] [CrossRef]
- Selaya, N.G.; Oomen, R.; Netten, J.; Werger, M.; Anten, N. Biomass allocation and leaf life span in relation to light interception by tropical forest plants during the first years of secondary succession. J. Ecol. 2008, 96, 1211–1221. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, C.; Zhou, Z.; Gu, J. Contrasting responses of hydraulic traits between leaf and branch to 16-year nitrogen addition in a larch plantation. For. Ecol. Manag. 2020, 475, 118461. [Google Scholar] [CrossRef]
- Coble, A.; Cavaleri, M. Light drives vertical gradients of leaf morphology in a sugar maple (Acer saccharum) forest. Tree Physiol. 2014, 34. [Google Scholar] [CrossRef] [PubMed]
- Niinemets, Ü. Global-Scale Climatic Controls of Leaf Dry Mass per Area, Density, and Thickness in Trees and Shrubs. Ecology 2001, 82, 453–469. [Google Scholar] [CrossRef]
- Oguchi, R.; Hikosaka, K.; Hirose, T. Does the change in light acclimation need leaf anatomy? Plant. Cell Environ. 2003, 26, 505–512. [Google Scholar] [CrossRef]
- Bourne, A.; Creek, D.; Peters, J.; Ellsworth, D.; Choat, B. Species climate range influences hydraulic and stomatal traits in Eucalyptus species. Ann. Bot. 2017, 120. [Google Scholar] [CrossRef]
- He, N.; Liu, C.; Tian, M.; Li, M.; Yang, H.; Yu, G.; Guo, D.; Smith, M.; Yu, Q.; Jihua, H. Variation in leaf anatomical traits from tropical to cold-temperate forests and linkage to ecosystem functions. Funct. Ecol. 2017. [Google Scholar] [CrossRef] [Green Version]
- Baoyin, C.; Wang, C.; Tian, Y.; Chu, Q.; Hu, C. Anatomical characteristics of young stems and mature leaves of dwarf pear. Sci. Hort. 2015, 186. [Google Scholar] [CrossRef]
- Witkowski, E.; Lamont, B. Leaf specific mass confounds leaf density and thickness. Oecologia 1991, 88, 486–493. [Google Scholar] [CrossRef]
- Chabot, B.; Hicks, D. The Ecology of Leaf Life Spans. Annu. Rev. Ecol. Syst. 1982, 13, 229–259. [Google Scholar] [CrossRef]
- John, G.; Scoffoni, C.; Sack, L. Allometry of cells and tissues within leaves. Am. J. Bot. 2013, 100. [Google Scholar] [CrossRef] [Green Version]
- Edwards, E.; Chatelet, D.; Sack, L.; Donoghue, M. Leaf life span and the leaf economic spectrum in the context of whole plant architecture. J. Ecol. 2014, 102. [Google Scholar] [CrossRef] [Green Version]
- Reich, P.; Walters, M.; Tjoelker, M.; Vanderklein, D.; Buschena, C. Photosynthesis and respiration rates depend on leaf and root morphology and nitrogen concentration in nine boreal tree species varying in RGR. Funct. Ecol. 1998, 12, 395–405. [Google Scholar] [CrossRef]
- Krokene, P.; Nagy, N.; Krekling, T. Traumatic Resin Ducts and Polyphenolic Parenchyma Cells in Conifers. In Induced Plant Resistance to Herbivory; Springer: Dordrecht, The Netherlands, 2008; pp. 147–169. [Google Scholar] [CrossRef]
- Duursma, R.; Falster, D. Leaf mass per area, not total leaf area, drives differences in above-ground biomass distribution among woody plant functional types. New Phytol. 2016, 212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nardini, A.; Luglio, J. Leaf hydraulic capacity and drought vulnerability: Possible trade-offs and correlations with climate across three major biomes. Funct. Ecol. 2014, 28. [Google Scholar] [CrossRef]
- Blackman, C.; Brodribb, T.; Jordan, G. Leaf hydraulic vulnerability is related to conduit dimensions and drought resistance across a diverse range of woody angiosperms. New Phytol. 2010, 188, 1113–1123. [Google Scholar] [CrossRef]
- Guo, D.; Xia, M.; Wei, X.; Chang, W.; Liu, Y.; Wang, Z. Anatomical traits associated with absorption and mycorrhizal colonization are linked to root branch order in twenty-three Chinese temperate tree species. New Phytol. 2008, 180, 673–683. [Google Scholar] [CrossRef]
- Mei, L.; Gu, J.; Zhang, Z.; Wang, Z. Responses of fine root mass, length, production and turnover to soil nitrogen fertilization in Larix gmelinii and Fraxinus mandshurica forests in Northeastern China. J. For. Res. 2010, 15, 194–201. [Google Scholar] [CrossRef]
- Poorter, H.; Jagodzinski, A.M.; Ruiz-Peinado, R.; Kuyah, S.; Luo, Y.; Oleksyn, J.; Usoltsev, V.A.; Buckley, T.N.; Reich, P.B.; Sack, L. How does biomass distribution change with size and differ among species? An analysis for 1200 plant species from five continents. New Phytol. 2015, 208, 736–749. [Google Scholar] [CrossRef]
- Coble, A.; Cavaleri, M. Vertical leaf mass per area gradient of mature sugar maple reflects both height-driven increases in vascular tissue and light-driven increases in palisade layer thickness. Tree Physiol. 2017, 37. [Google Scholar] [CrossRef]
- Waldhoff, D.; Parolin, P. Morphology and Anatomy of Leaves. In Amazonian Floodplain Forests; Ecophysiology, Biodiversity and Sustainable Management; Springer: Dordrecht, The Netherlands, 2010; pp. 179–202. [Google Scholar] [CrossRef]
- Beikircher, B.; Cesare, C.; Mayr, S. Hydraulics of high-yield orchard trees: A case study of three Malus domestica cultivars. Tree Physiol. 2013, 33. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
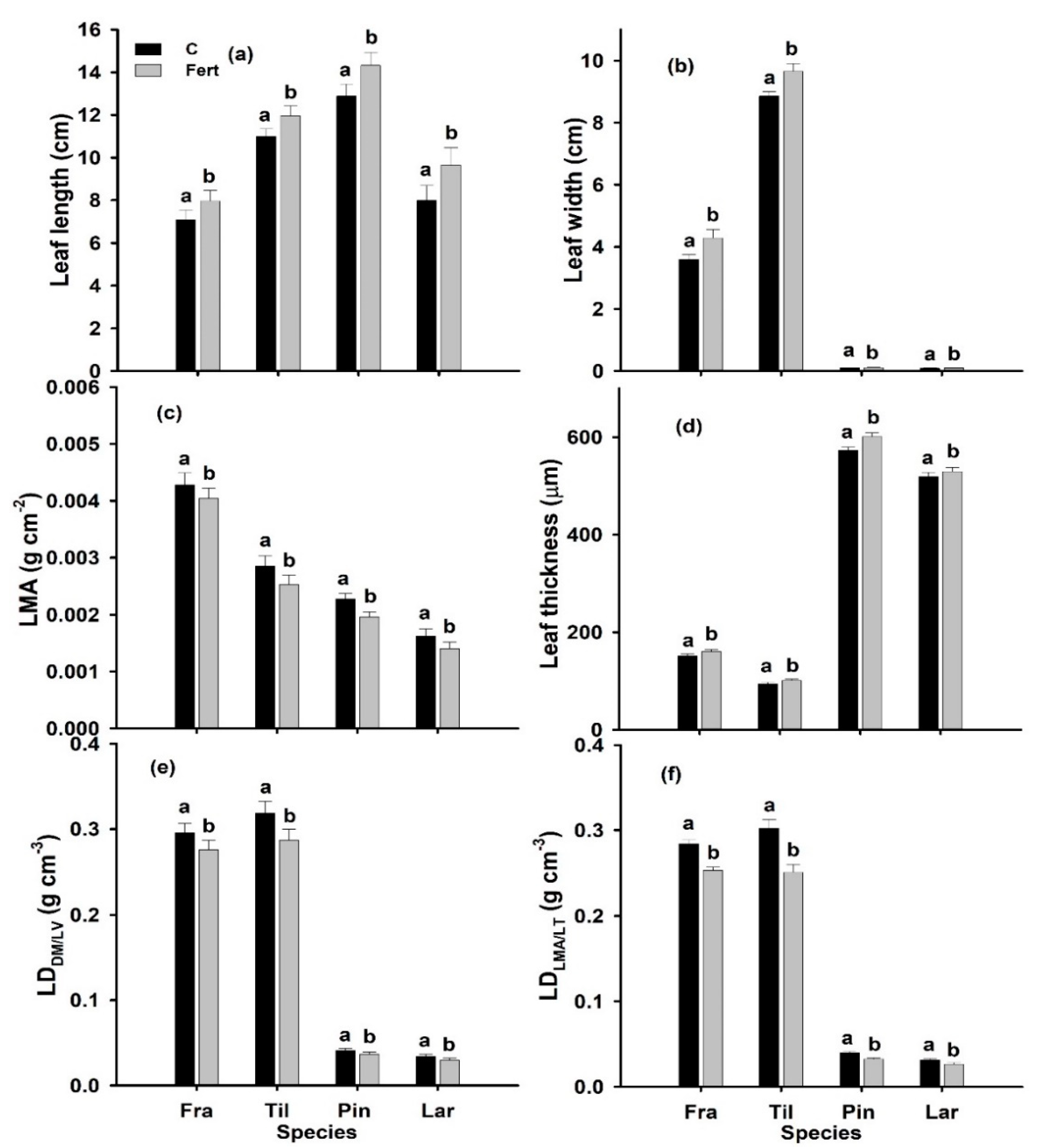
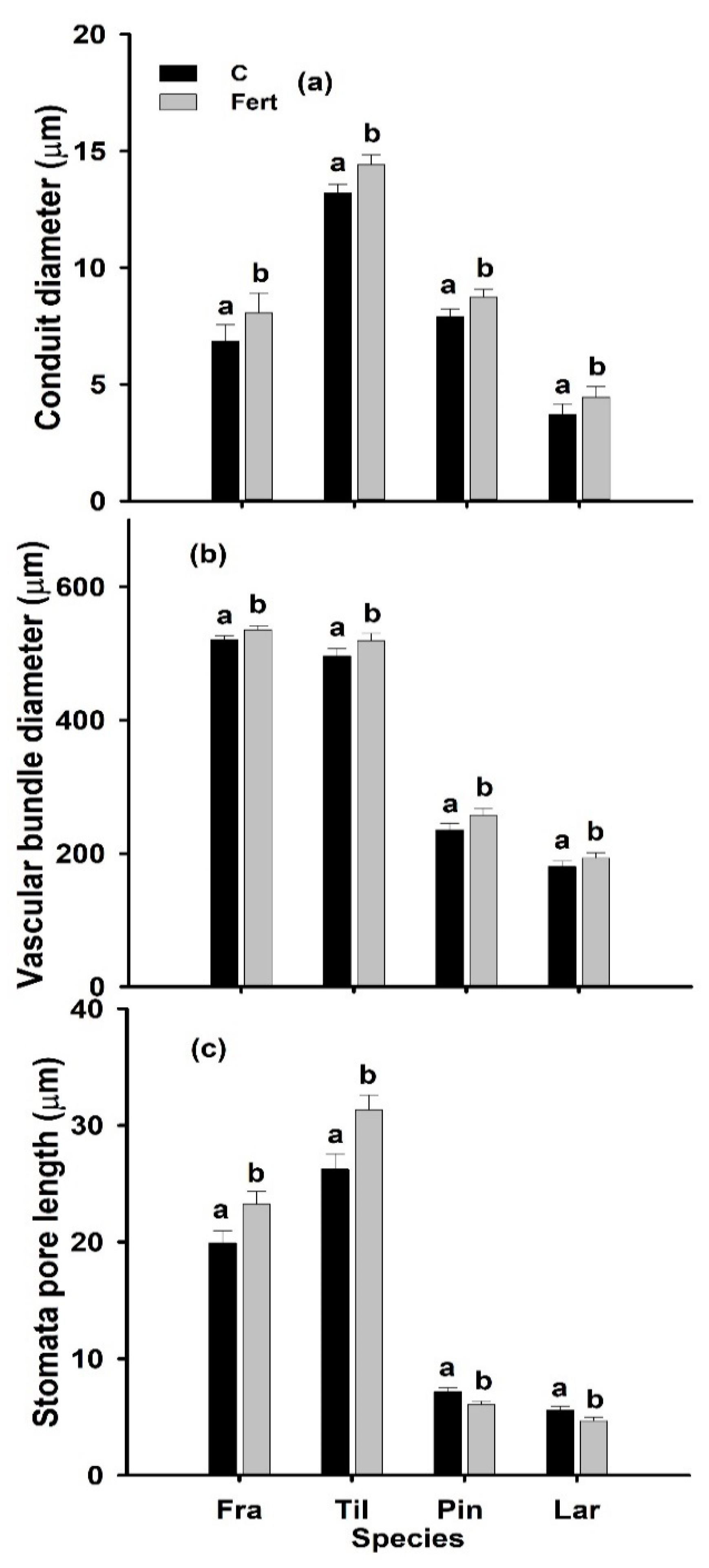
| Source of Variation | df | Leaf Length (cm) | Leaf Width (cm) | LMA (g cm−2) | LT (µm) | LDDM/LV (g cm−3) | LDLMA/LT (g cm−3) | CD (µm) | VBD (µm) | SL (µm) |
|---|---|---|---|---|---|---|---|---|---|---|
| Species | 3 | ˂0.001 | ˂0.001 | ˂0.001 | ˂0.001 | ˂0.001 | ˂0.001 | ˂0.001 | ˂0.001 | ˂0.001 |
| N Deposition | 1 | 0.008 | 0.007 | 0.021 | 0.009 | 0.030 | 0.001 | 0.015 | 0.012 | 0.020 |
| Spec × N | 3 | 0.891 | 0.057 | 0.980 | 0.357 | 0.386 | 0.002 | 0.950 | 0.918 | 0.005 |
| Species | N Deposition Level | Palisade- | Spongy- | Adaxial | Abaxial | |
|---|---|---|---|---|---|---|
| Mesophyll Thickness (µm) | Epidermis Thickness (µm) | |||||
| F. mandshurica | C | 81.5 ± 2.4 a | 57.4 ± 1.8 a | 17.8 ± 0.8 a | 7.9 ± 0.5 a | |
| Fert | 89.3 ± 2.6 b | 63.6 ± 1.5 b | 18.8 ± 0.7 b | 8.5 ± 0.4 b | ||
| T. amurensis | C | 24.7 ± 1.0 a | 46.3 ± 2.1 a | 15.7 ± 0.7 a | 8.1 ± 0.3 a | |
| Fert | 28.9 ± 1.2 b | 52.6 ± 2.3 b | 16.8 ± 0.7 b | 8.8 ± 0.3 b | ||
| Mesophyll thickness (µm) | Epi-hypodermis thickness (µm) | Resin duct diameter (µm) | ||||
| P. koraiensis | C | 25.4 ± 1.3 a | 12.5 ± 1.1 a | 46.7 ± 1.2 a | ||
| Fert | 27.8 ± 0.6 b | 13.4 ± 1.6 a | 49.6 ± 1.2 b | |||
| L. gmelinii | C | 26.9 ± 0.8 a | 21.9 ± 1.0 a | 25.5 ± 0.9 a | ||
| Fert | 30.1 ± 1.3 b | 23.8 ± 1.4 b | 28.0 ± 0.5 b | |||
| Leaf Length | Leaf Width | LMA | LT | LDDM/LV | CD | VBD | SL | Biomass | RMF | SMF | LMF | Root:shoot | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | Fert | C | Fert | C | Fert | C | Fert | C | Fert | C | Fert | C | Fert | C | Fert | C | Fert | C | Fert | C | Fert | C | Fert | C | Fert | |
| Leaf length | 1 | 1 | ||||||||||||||||||||||||
| Leaf width | 0.055 | −0.066 | 1 | 1 | ||||||||||||||||||||||
| LMA | −0.294 | −0.426 | 0.432 | 0.451 | 1 | 1 | ||||||||||||||||||||
| LT | 0.308 | 0.427 | −0.894 ** | −0.907 ** | −0.720 ** | −0.708 ** | 1 | 1 | ||||||||||||||||||
| LDDM/LV | −0.217 | −0.341 | 0.883 ** | 0.890 ** | 0.792 ** | 0.799 ** | −0.986 ** | −0.981* | 1 | 1 | ||||||||||||||||
| CD | 0.547 | 0.420 | 0.856 ** | 0.863 ** | 0.305 | 0.302 | −0.614 * | −0.632 * | 0.665 * | 0.678 * | 1 | 1 | ||||||||||||||
| VBD | −0.202 | −0.289 | 0.816 ** | 0.846 ** | 0.867 ** | 0.850 ** | −0.958 ** | −0.949 ** | 0.986 ** | 0.988 ** | 0.630 * | 0.680 * | 1 | 1 | ||||||||||||
| SL | −0.055 | −0.188 | 0.953 ** | 0.967 ** | 0.683 * | 0.662 * | −0.960 ** | −0.965 ** | 0.978 ** | 0.974 ** | 0.793 ** | 0.804 ** | 0.945 ** | 0.951 ** | 1 | 1 | ||||||||||
| Biomass | −0.757 ** | −0.770 ** | 0.520 | 0.549 | 0.633 * | 0.319 | −0.787 ** | −0.715 ** | 0.740 ** | 0.596 * | 0.080 | 0.105 | 0.706 * | 0.502 | 0.632 * | 0.564 | 1 | 1 | ||||||||
| RMF | −0.686 * | −0.637 * | 0.150 | −0.246 | 308 | 0.500 | −0.386 | −0.085 | 339 | 0.127 | −0.230 | −0.506 | 0.295 | 0.119 | 0.240 | −0.065 | 0.562 | 0.159 | 1 | 1 | ||||||
| SMF | 0.791 ** | 0.926 ** | −0.324 | −0.259 | −0.721 ** | −0.579 * | 0.672 * | 0.592 * | −0.651 * | −0.535 | 0.93 | 0.217 | −0.653 * | −0.480 | −0.506 | −0.393 | −0.868 ** | −0.780 ** | −0.753 ** | −0.688 * | 1 | 1 | ||||
| LMF | −0.591 * | −0.708 * | 0.350 | 0.576 | 0.800 ** | 0.352 | −0.664 * | −0.739 ** | 0.672 * | 0.623 * | 0.053 | 0.152 | 0.712 ** | 0.554 | 0.543 | 0.598* | 0.807 ** | 0.932 ** | 0.279 | 0.057 | −0.841 ** | −0.764 ** | 1 | 1 | ||
| Root: Shoot | −0.677 * | −0.642 * | 0.128 | −0.207 | 0.328 | 0.537 | −0.374 | −0.126 | 0.332 | 0.170 | −0.238 | −0.470 | 0.294 | 0.163 | 0.229 | −0.023 | 0.546 | 0.179 | 0.998 ** | 0.997 ** | −0.752 ** | −0.700 * | 0.277 | 0.076 | 1 | 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, A.; Sun, J.; Zarif, N.; Khan, K.; Jamil, M.A.; Yang, L.; Clothier, B.; Rewald, B. Effects of Increased N Deposition on Leaf Functional Traits of Four Contrasting Tree Species in Northeast China. Plants 2020, 9, 1231. https://doi.org/10.3390/plants9091231
Khan A, Sun J, Zarif N, Khan K, Jamil MA, Yang L, Clothier B, Rewald B. Effects of Increased N Deposition on Leaf Functional Traits of Four Contrasting Tree Species in Northeast China. Plants. 2020; 9(9):1231. https://doi.org/10.3390/plants9091231
Chicago/Turabian StyleKhan, Attaullah, Jingjue Sun, Nowsherwan Zarif, Kashif Khan, Muhammad Atif Jamil, Lixue Yang, Brent Clothier, and Boris Rewald. 2020. "Effects of Increased N Deposition on Leaf Functional Traits of Four Contrasting Tree Species in Northeast China" Plants 9, no. 9: 1231. https://doi.org/10.3390/plants9091231
APA StyleKhan, A., Sun, J., Zarif, N., Khan, K., Jamil, M. A., Yang, L., Clothier, B., & Rewald, B. (2020). Effects of Increased N Deposition on Leaf Functional Traits of Four Contrasting Tree Species in Northeast China. Plants, 9(9), 1231. https://doi.org/10.3390/plants9091231









