Transient Gene Expression is an Effective Experimental Tool for the Research into the Fine Mechanisms of Plant Gene Function: Advantages, Limitations, and Solutions
Abstract
1. Introduction
2. Transient Expression of Heterologous Genes: Plant Systems, Gene Constructs, and Their Delivery to Plant Cells
3. Main Research Areas Utilizing Transient Gene Expression in Plants
3.1. Localization of the Protein Products in Plant Cells
3.2. Study of the Physiological Role of Gene Products Involved in Plant Growth and Development
3.3. Study of the Physiological Role of Gene Products Involved in Plant Responses to Biotic Environmental Factors
3.4. Study of the Mechanisms of Regulation of Plant Metabolic Pathways
3.5. Study of Protein–Protein Interactions (PPI) and Their Subcellular Localization
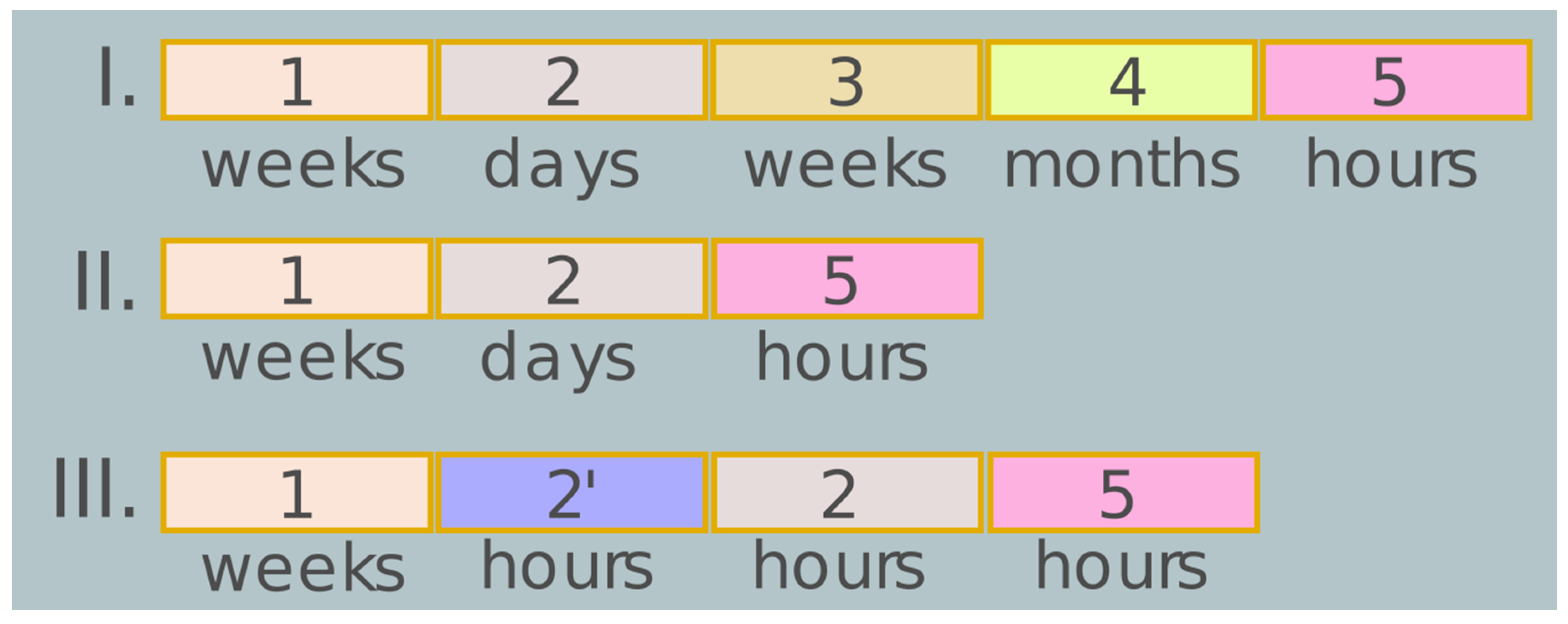
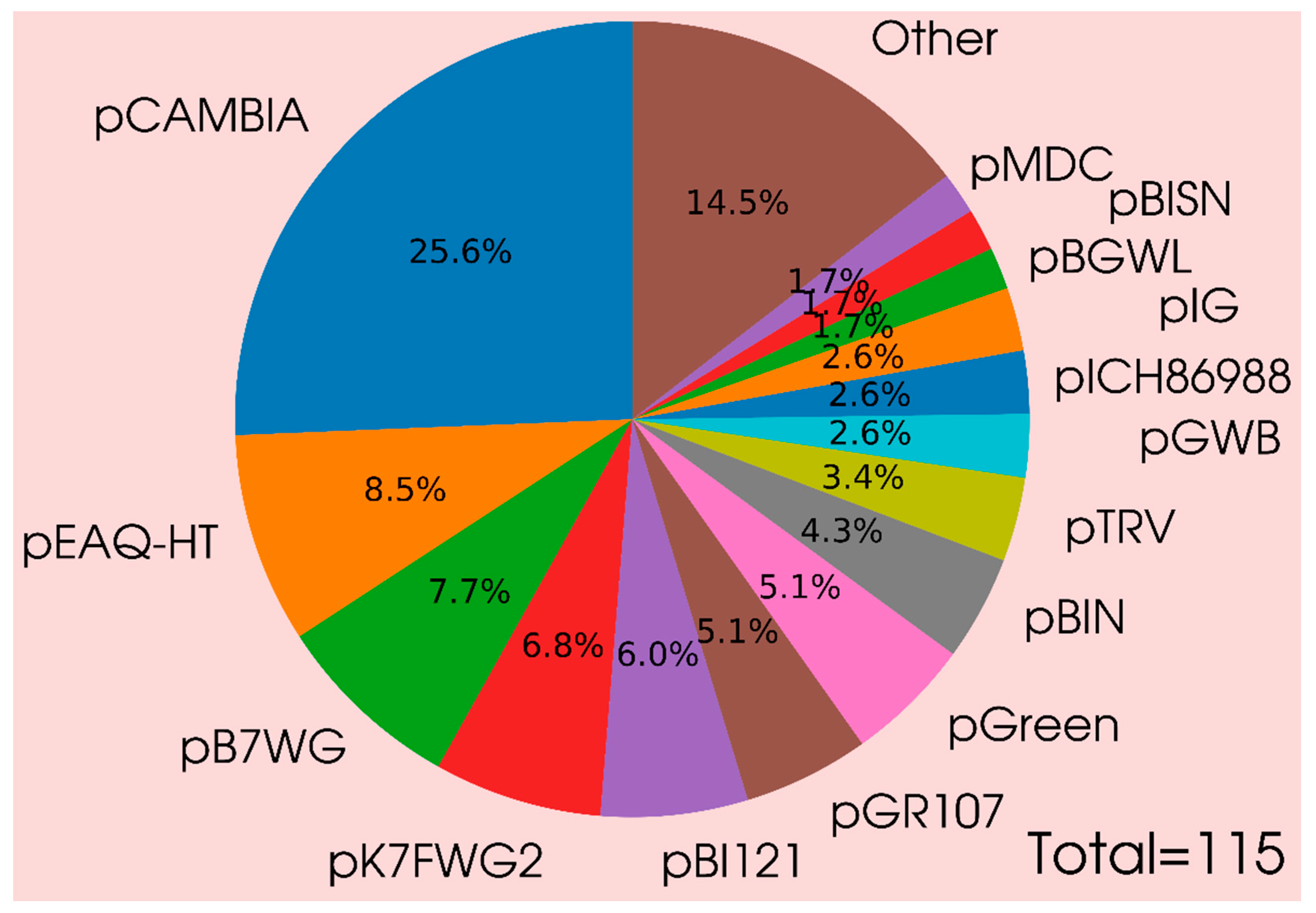
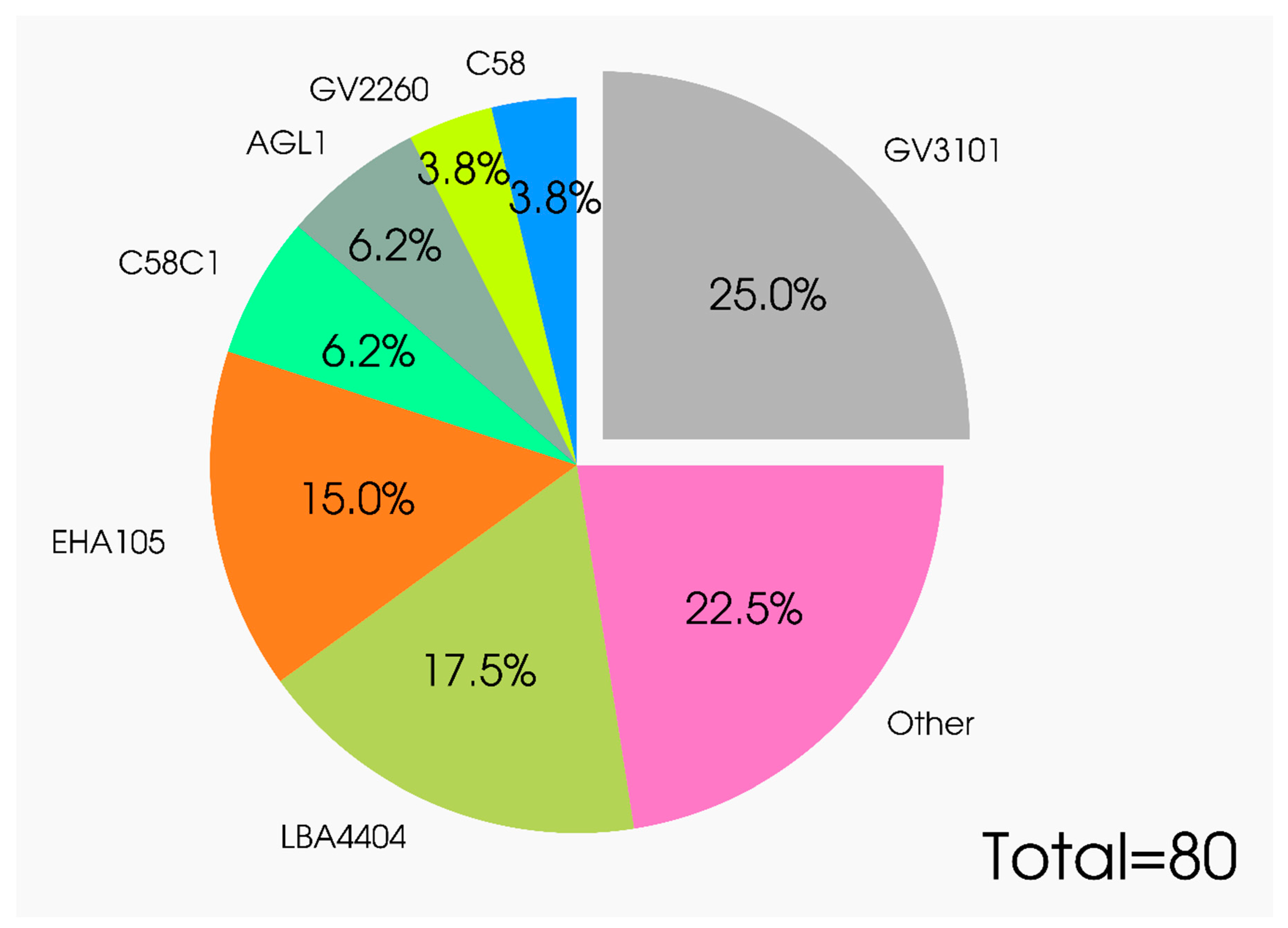
4. Specific Features of Vector Constructs, Agrobacterial Strains, and Nuances of Conditions for Effective Expression of Gene Products in Plants
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Goldenkova-Pavlova, I.V.; Pavlenko, O.S.; Mustafaev, O.N.; Deyneko, I.V.; Kabardaeva, K.V.; Tyurin, A.A. Computational and Experimental Tools to Monitor the Changes in Translation Efficiency of Plant mRNA on a Genome-Wide Scale: Advantages, Limitations, and Solutions. Int. J. Mol. Sci. 2018, 20, 33. [Google Scholar] [CrossRef] [PubMed]
- Kabardaeva, K.V.; Tyurin, A.A.; Pavlenko, O.S.; Gra, O.A.; Deyneko, I.V.; Kouchoro, F.; Mustafaev, O.N.; Goldenkova-Pavlova, I.V. Fine Tuning of Translation: A Complex Web of Mechanisms and Its Relevance to Plant Functional Genomics and Biotechnology. Russ. J. Plant Physiol. 2019, 66, 835–849. [Google Scholar] [CrossRef]
- Guidarelli, M.; Baraldi, E. Transient transformation meets gene function discovery: The strawberry fruit case. Front. Plant Sci. 2015, 6, 444. [Google Scholar] [CrossRef] [PubMed]
- Jelly, N.S.; Valat, L.; Walter, B.; Maillot, P. Transient expression assays in grapevine: A step towards genetic improvement. Plant Biotechnol. J. 2014, 12, 1231–1245. [Google Scholar] [CrossRef]
- Vyacheslavova, A.O.; Berdichevets, I.N.; Tyurin, A.A.; Shimshilashvili, K.R.; Mustafaev, O.N.; Goldenkova-Pavlova, I.V. Expression of heterologous genes in plant systems: New possibilities. Russ. J. Genet. 2012, 48, 1067–1079. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, M.; Siemiatkowska, B.; Toleco, M.R.; Jing, Y.; Strotmann, V.; Zhang, J.; Stahl, Y.; Fernie, A.R. A Highly Efficient Agrobacterium-Mediated Method for Transient Gene Expression and Functional Studies in Multipe Plant Species. Plant Commun. 2020, 100028. [Google Scholar] [CrossRef]
- Tsuda, K.; Qi, Y.; Nguyen, L.V.; Bethke, G.; Tsuda, Y.; Glazebrook, J.; Katagiri, F. An efficient Agrobacterium-mediated transient transformation of Arabidopsis. Plant J. 2012, 69, 713–719. [Google Scholar] [CrossRef]
- Zagorskaya, A.A.; Deineko, E.V. Suspension-cultured plant cells as a platform for obtaining recombinant proteins. Russ. J. Plant Physiol. 2017, 64, 795–807. [Google Scholar] [CrossRef]
- Donini, M.; Marusic, C. Current state-of-the-art in plant-based antibody production systems. Biotechnol. Lett. 2019, 41, 335–346. [Google Scholar] [CrossRef]
- Chen, Q.; Lai, H. Gene delivery into plant cells for recombinant protein production. Biomed. Res. Int. 2015, 2015, 932161. [Google Scholar] [CrossRef]
- Komakhin, R.A.; Vysotskii, D.A.; Shukurov, R.R.; Voblikova, V.D.; Komakhina, V.V.; Strelnikova, S.R.; Vetchinkina, E.M.; Babakov, A.V. Novel strong promoter of antimicrobial peptides gene pro-SmAMP2 from chickweed (Stellaria media). BMC Biotechnol. 2016, 16, 43. [Google Scholar] [CrossRef] [PubMed]
- Saur, I.M.L.; Bauer, S.; Lu, X.; Schulze-Lefert, P. A cell death assay in barley and wheat protoplasts for identification and validation of matching pathogen AVR effector and plant NLR immune receptors. Plant Methods 2019, 15, 118. [Google Scholar] [CrossRef]
- Kim, N.H.; Hwang, B.K. Pepper pathogenesis-related protein 4c is a plasma membrane-localized cysteine protease inhibitor that is required for plant cell death and defense signaling. Plant J. 2015, 81, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.-G.; Ma, Y.-Y.; Bi, P.-P.; Wei, W.; Liu, J.; Hu, Y.; Gou, Y.-J.; Zhu, D.; Wen, Y.-Q.; Feng, J.-Y. Transcription factor FvTCP9 promotes strawberry fruit ripening by regulating the biosynthesis of abscisic acid and anthocyanins. Plant Physiol. Biochem. 2020, 146, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Tang, X.; Liu, W.; Fu, X.; Luo, H.; Ghimire, S.; Zhang, N.; Si, H. A potato RING-finger protein gene StRFP2 is involved in drought tolerance. Plant Physiol. Biochem. 2020, 146, 438–446. [Google Scholar] [CrossRef]
- Xu, Q.; Tang, C.; Wang, X.; Sun, S.; Zhao, J.; Kang, Z.; Wang, X. An effector protein of the wheat stripe rust fungus targets chloroplasts and suppresses chloroplast function. Nat. Commun. 2019, 10, 5571. [Google Scholar] [CrossRef]
- Lin, H.Y.; Chen, J.C.; Fang, S.C. A Protoplast Transient Expression System to Enable Molecular, Cellular, and Functional Studies in Phalaenopsis orchids. Front. Plant Sci. 2018, 9, 843. [Google Scholar] [CrossRef]
- Jia, N.; Zhu, Y.; Xie, F. An Efficient Protocol for Model Legume Root Protoplast Isolation and Transformation. Front. Plant Sci. 2018, 9, 670. [Google Scholar] [CrossRef]
- Scott, A.; Wyatt, S.; Tsou, P.L.; Robertson, D.; Allen, N.S. Model system for plant cell biology: GFP imaging in living onion epidermal cells. BioTechniques 1999, 26, 1128–1132. [Google Scholar] [CrossRef]
- Tan, B.; Xu, M.; Chen, Y.; Huang, M. Transient expression for functional gene analysis using Populus protoplasts. Plant Cell Tissue Organ Cult. (PCTOC) 2013, 114, 11–18. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, L. Transient expression and analysis of fluorescent reporter proteins in plant pollen tubes. Nat. Protoc. 2011, 6, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Yao, D.; Lin, F.; Jiang, M. PEG-mediated transient gene expression and silencing system in maize mesophyll protoplasts: A valuable tool for signal transduction study in maize. Acta Physiol. Plant. 2014, 36, 1271–1281. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, J.; Duan, S.; Ao, Y.; Dai, J.; Liu, J.; Wang, P.; Li, Y.; Liu, B.; Feng, D.; et al. A highly efficient rice green tissue protoplast system for transient gene expression and studying light/chloroplast-related processes. Plant Methods 2011, 7, 30. [Google Scholar] [CrossRef]
- Vyacheslavova, A.O.; Mustafaev, O.N.; Tyrin, A.A.; Shimshilashvili, K.R.; Berdichevets, I.N.; Shayakhmetova, D.M.; Goldenkov, M.A.; Fadeev, V.S.; Sheludko, Y.V.; Goldenkova-Pavlova, I.V. Set of module vectors for stable or transient expression of heterologous genes in plants. Russ. J. Genet. 2012, 48, 892–901. [Google Scholar] [CrossRef]
- Kabardaeva, K.V.; Turin, A.; Kouchoro, F.; Mustafaev, O.N.; Deineko, I.V.; Fadeev, V.S.; Goldenkova-Pavlova, I.V. Regulatory Contexts in the 5’-Region of mRNA from Arabidopsis thaliana Plants and Their Role in Translation Efficiency. Russ. J. Plant Physiol. 2020, 67, 425–434. [Google Scholar] [CrossRef]
- Burris, K.P.; Dlugosz, E.M.; Collins, A.G.; Stewart, C.N.; Lenaghan, S.C. Development of a rapid, low-cost protoplast transfection system for switchgrass (Panicum virgatum L.). Plant Cell Rep. 2016, 35, 693–704. [Google Scholar] [CrossRef]
- Fujikawa, Y.; Nakanishi, T.; Kawakami, H.; Yamasaki, K.; Sato, M.H.; Tsuji, H.; Matsuoka, M.; Kato, N. Split luciferase complementation assay to detect regulated protein-protein interactions in rice protoplasts in a large-scale format. Rice 2014, 7, 11. [Google Scholar] [CrossRef][Green Version]
- Li, C.; Lin, H.; Dubcovsky, J. Factorial combinations of protein interactions generate a multiplicity of florigen activation complexes in wheat and barley. Plant J. 2015, 84, 70–82. [Google Scholar] [CrossRef]
- Nakashima, K.; Yamaguchi-Shinozaki, K.; Shinozaki, K. The transcriptional regulatory network in the drought response and its crosstalk in abiotic stress responses including drought, cold, and heat. Front. Plant Sci. 2014, 5, 170. [Google Scholar] [CrossRef]
- Pruneda-Paz, J.L.; Breton, G.; Nagel, D.H.; Kang, S.E.; Bonaldi, K.; Doherty, C.J.; Ravelo, S.; Galli, M.; Ecker, J.R.; Kay, S.A. A Genome-Scale Resource for the Functional Characterization of Arabidopsis Transcription Factors. Cell Rep. 2014, 8, 622–632. [Google Scholar] [CrossRef]
- Tyurin, A.A.; Kabardaeva, K.V.; Berestovoy, M.A.; Sidorchuk, Y.V.; Fomenkov, A.A.; Nosov, A.V.; Goldenkova-Pavlova, I.V. Simple and reliable system for transient gene expression for the characteristic signal sequences and the estimation of the localization of target protein in plant cell. Russ. J. Plant Physiol. 2017, 64, 672–679. [Google Scholar] [CrossRef]
- Wang, H.; Wang, W.; Zhan, J.; Huang, W.; Xu, H. An efficient PEG-mediated transient gene expression system in grape protoplasts and its application in subcellular localization studies of flavonoids biosynthesis enzymes. Sci. Hortic. 2015, 191, 82–89. [Google Scholar] [CrossRef]
- Schmidt, M.A.; Herman, E.M. Characterization and functional biology of the soybean aleurone layer. BMC Plant Biol. 2018, 18, 354. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Huang, X.; Wu, M.; Wang, Y.; Chang, Y.; Liu, K.; Zhang, J.; Zhang, Y.; Zhang, F.; Yi, L.; et al. A rapid, highly efficient and economical method of Agrobacterium-mediated in planta transient transformation in living onion epidermis. PLoS ONE 2014, 9, e83556. [Google Scholar] [CrossRef]
- Guo, Y.; Song, X.; Zhao, S.; Lv, J.; Lu, M. A transient gene expression system in Populus euphratica Oliv. protoplasts prepared from suspension cultured cells. Acta Physiol. Plant. 2015, 37, 160. [Google Scholar] [CrossRef]
- Yoo, S.-D.; Cho, Y.-H.; Sheen, J. Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nat. Protoc. 2007, 2, 1565–1572. [Google Scholar] [CrossRef] [PubMed]
- Vaghchhipawala, Z.; Rojas, C.M.; Senthil-Kumar, M.; Mysore, K.S. Agroinoculation and agroinfiltration: Simple tools for complex gene function analyses. Methods Mol. Biol. 2011, 678, 65–76. [Google Scholar] [CrossRef]
- Shoji, T. Analysis of the Intracellular Localization of Transiently Expressed and Fluorescently Labeled Copper-Containing Amine Oxidases, Diamine Oxidase and N-Methylputrescine Oxidase in Tobacco, Using an Agrobacterium Infiltration Protocol. Methods Mol. Biol. 2018, 1694, 215–223. [Google Scholar] [CrossRef]
- Wang, Y.C.; Yu, M.; Shih, P.Y.; Wu, H.Y.; Lai, E.M. Stable pH Suppresses Defense Signaling and is the Key to Enhance Agrobacterium-Mediated Transient Expression in Arabidopsis Seedlings. Sci. Rep. 2018, 8, 17071. [Google Scholar] [CrossRef]
- Rolland, V. Determining the Subcellular Localization of Fluorescently Tagged Proteins Using Protoplasts Extracted from Transiently Transformed Nicotiana benthamiana Leaves. Methods Mol. Biol. 2018, 1770, 263–283. [Google Scholar] [CrossRef]
- Olmedo, P.; Moreno, A.A.; Sanhueza, D.; Balic, I.; Silva-Sanzana, C.; Zepeda, B.; Verdonk, J.C.; Arriagada, C.; Meneses, C.; Campos-Vargas, R. A catechol oxidase AcPPO from cherimoya (Annona cherimola Mill.) is localized to the Golgi apparatus. Plant Sci. 2018, 266, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-S.; Ni, R.; Wang, P.-Y.; Zhu, T.-T.; Sun, C.-J.; Lou, H.-X.; Cheng, A.-X. Isolation and functional characterization of two Caffeoyl Coenzyme A 3-O-methyltransferases from the fern species Polypodiodes amoena. Plant Physiol. Biochem. 2019, 136, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Cao, X.; Liu, H.; Zhu, C.; Klee, H.; Zhang, B.; Chen, K. UDP-glucosyltransferase PpUGT85A2 controls volatile glycosylation in peach. J. Exp. Bot. 2019, 70, 925–936. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Yu, G.; Li, J.; Liu, J.; Wang, X.; Zhu, G.; Zhang, X.; Pan, H. AcERF2, an ethylene-responsive factor of Atriplex canescens, positively modulates osmotic and disease resistance in Arabidopsis thaliana. Plant Sci. 2018, 274, 32–43. [Google Scholar] [CrossRef]
- Guo, Y.F.; Shan, W.; Liang, S.M.; Wu, C.J.; Wei, W.; Chen, J.Y.; Lu, W.J.; Kuang, J.F. MaBZR1/2 act as transcriptional repressors of ethylene biosynthetic genes in banana fruit. Physiol. Plant. 2019, 165, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Hua-Ying, M.; Wen-Ju, W.; Wei-Hua, S.; Ya-Chun, S.; Feng, L.; Cong-Na, L.; Ling, W.; Xu, Z.; Li-Ping, X.; You-Xiong, Q. Genome-wide identification, phylogeny, and expression analysis of Sec14-like PITP gene family in sugarcane. Plant Cell Rep. 2019, 38, 637–655. [Google Scholar] [CrossRef]
- Cheng, J.; Wen, S.; Xiao, S.; Lu, B.; Ma, M.; Bie, Z. Overexpression of the tonoplast sugar transporter CmTST2 in melon fruit increases sugar accumulation. J. Exp. Bot. 2018, 69, 511–523. [Google Scholar] [CrossRef]
- Wang, B.; Wang, G.; Shen, F.; Zhu, S. A Glycine-Rich RNA-Binding Protein, CsGR-RBP3, Is Involved in Defense Responses Against Cold Stress in Harvested Cucumber (Cucumis sativus L.) Fruit. Front. Plant Sci. 2018, 9, 540. [Google Scholar] [CrossRef]
- Sasaki, N.; Takashima, E.; Nyunoya, H. Altered Subcellular Localization of a Tobacco Membrane Raft-Associated Remorin Protein by Tobamovirus Infection and Transient Expression of Viral Replication and Movement Proteins. Front. Plant Sci. 2018, 9, 619. [Google Scholar] [CrossRef]
- Norkunas, K.; Harding, R.; Dale, J.; Dugdale, B. Improving agroinfiltration-based transient gene expression in Nicotiana benthamiana. Plant Methods 2018, 14, 71. [Google Scholar] [CrossRef]
- Bertazzon, N.; Raiola, A.; Castiglioni, C.; Gardiman, M.; Angelini, E.; Borgo, M.; Ferrari, S. Transient silencing of the grapevine gene VvPGIP1 by agroinfiltration with a construct for RNA interference. Plant Cell Rep. 2012, 31, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Ji, K.; Sun, Y.; Luo, H.; Wang, H.; Leng, P. The role of FaBG3 in fruit ripening and B. cinerea fungal infection of strawberry. Plant J. 2013, 76, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Guidarelli, M.; Zoli, L.; Orlandini, A.; Bertolini, P.; Baraldi, E. The mannose-binding lectin gene FaMBL1 is involved in the resistance of unripe strawberry fruits to Colletotrichum acutatum. Mol. Plant Pathol. 2014, 15, 832–840. [Google Scholar] [CrossRef]
- Zeng, H.; Xie, Y.; Liu, G.; Lin, D.; He, C.; Shi, H. Molecular identification of GAPDHs in cassava highlights the antagonism of MeGAPCs and MeATG8s in plant disease resistance against cassava bacterial blight. Plant Mol. Biol. 2018, 97, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Berestovoy, M.; Pavlenko, O.; Tyurin, A.; Gorshkova, E.; GOLDENKOVA-PAVLOVA, I. Altered fatty acid composition of Nicotiana benthamiana and Nicotiana excelsior leaves under transient overexpression of the cyanobacterial desC gene. Biol. Plant. 2020, 64, 167–177. [Google Scholar] [CrossRef]
- Harris, N.N.; Luczo, J.M.; Robinson, S.; Walker, A. Transcriptional regulation of the three grapevine chalcone synthase genes and their role in flavonoid synthesis in Shiraz. Aust. J. Grape Wine Res. 2013, 19, 221–229. [Google Scholar] [CrossRef]
- Deluc, L.; Bogs, J.; Walker, A.R.; Ferrier, T.; Decendit, A.; Merillon, J.-M.; Robinson, S.P.; Barrieu, F. The Transcription Factor VvMYB5b Contributes to the Regulation of Anthocyanin and Proanthocyanidin Biosynthesis in Developing Grape Berries. Plant Physiol. 2008, 147, 2041–2053. [Google Scholar] [CrossRef]
- Walker, A.R.; Lee, E.; Bogs, J.; McDavid, D.A.; Thomas, M.R.; Robinson, S.P. White grapes arose through the mutation of two similar and adjacent regulatory genes. Plant J. 2007, 49, 772–785. [Google Scholar] [CrossRef]
- Bogs, J.; Jaffé, F.W.; Takos, A.M.; Walker, A.R.; Robinson, S.P. The Grapevine Transcription Factor VvMYBPA1 Regulates Proanthocyanidin Synthesis during Fruit Development. Plant Physiol. 2007, 143, 1347–1361. [Google Scholar] [CrossRef]
- Czemmel, S.; Stracke, R.; Weisshaar, B.; Cordon, N.; Harris, N.N.; Walker, A.R.; Robinson, S.P.; Bogs, J. The Grapevine R2R3-MYB Transcription Factor VvMYBF1 Regulates Flavonol Synthesis in Developing Grape Berries. Plant Physiol. 2009, 151, 1513–1530. [Google Scholar] [CrossRef]
- Anwar, M.; Wang, G.; Wu, J.; Waheed, S.; Allan, A.C.; Zeng, L. Ectopic Overexpression of a Novel R2R3-MYB, NtMYB2 from Chinese Narcissus Represses Anthocyanin Biosynthesis in Tobacco. Molecules 2018, 23, 781. [Google Scholar] [CrossRef]
- Zhai, R.; Zhao, Y.; Wu, M.; Yang, J.; Li, X.; Liu, H.; Wu, T.; Liang, F.; Yang, C.; Wang, Z.; et al. The MYB transcription factor PbMYB12b positively regulates flavonol biosynthesis in pear fruit. BMC Plant Biol. 2019, 19, 85. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.J.; Mao, X.; Hao, Q.T.; Liu, B.L.; Xue, J.A.; Li, R.Z. Splice Variants of the Castor WRI1 Gene Upregulate Fatty Acid and Oil Biosynthesis When Expressed in Tobacco Leaves. Int. J. Mol. Sci. 2018, 19, 146. [Google Scholar] [CrossRef]
- Nogueira, M.; Enfissi, E.M.A.; Welsch, R.; Beyer, P.; Zurbriggen, M.D.; Fraser, P.D. Construction of a fusion enzyme for astaxanthin formation and its characterisation in microbial and plant hosts: A new tool for engineering ketocarotenoids. Metab. Eng. 2019, 52, 243–252. [Google Scholar] [CrossRef]
- Kerppola, T.K. Bimolecular Fluorescence Complementation (BiFC) Analysis as a Probe of Protein Interactions in Living Cells. Annu. Rev. Biophys. 2008, 37, 465–487. [Google Scholar] [CrossRef] [PubMed]
- Kudla, J.; Bock, R. Lighting the Way to Protein-Protein Interactions: Recommendations on Best Practices for Bimolecular Fluorescence Complementation Analyses. Plant Cell 2016, 28, 1002–1008. [Google Scholar] [CrossRef]
- Miller, K.E.; Kim, Y.; Huh, W.-K.; Park, H.-O. Bimolecular Fluorescence Complementation (BiFC) Analysis: Advances and Recent Applications for Genome-Wide Interaction Studies. J. Mol. Biol. 2015, 427, 2039–2055. [Google Scholar] [CrossRef]
- Xing, S.; Wallmeroth, N.; Berendzen, K.W.; Grefen, C. Techniques for the Analysis of Protein-Protein Interactions in Vivo. Plant Physiol. 2016, 171, 727–758. [Google Scholar] [CrossRef]
- Liu, T.Y.; Chou, W.C.; Chen, W.Y.; Chu, C.Y.; Dai, C.Y.; Wu, P.Y. Detection of membrane protein-protein interaction in planta based on dual-intein-coupled tripartite split-GFP association. Plant J. 2018, 94, 426–438. [Google Scholar] [CrossRef]
- Horstman, A.; Tonaco, I.A.; Boutilier, K.; Immink, R.G. A cautionary note on the use of split-YFP/BiFC in plant protein-protein interaction studies. Int. J. Mol. Sci. 2014, 15, 9628–9643. [Google Scholar] [CrossRef]
- He, F.; Zhang, F.; Sun, W.; Ning, Y.; Wang, G.-L. A Versatile Vector Toolkit for Functional Analysis of Rice Genes. Rice 2018, 11, 27. [Google Scholar] [CrossRef]
- Lukan, T.; Machens, F.; Coll, A.; Baebler, Š.; Messerschmidt, K.; Gruden, K. Plant X-tender: An extension of the AssemblX system for the assembly and expression of multigene constructs in plants. PLoS ONE 2018, 13, e0190526. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yin, X.; Kong, X.; Li, W.; Ma, L.; Sun, X.; Guan, Y.; Todd, C.D.; Yang, Y.; Hu, X. A series of TA-based and zero-background vectors for plant functional genomics. PLoS ONE 2013, 8, e59576. [Google Scholar] [CrossRef] [PubMed]
- Miki, D.; Shimamoto, K. Simple RNAi vectors for stable and transient suppression of gene function in rice. Plant Cell Physiol. 2004, 45, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Ning, Y.; Shi, X.; He, F.; Zhang, C.; Fan, J.; Jiang, N.; Zhang, Y.; Zhang, T.; Hu, Y.; et al. Immunity to Rice Blast Disease by Suppression of Effector-Triggered Necrosis. Curr. Biol. 2016, 26, 2399–2411. [Google Scholar] [CrossRef]
- Yamamoto, T.; Hoshikawa, K.; Ezura, K.; Okazawa, R.; Fujita, S.; Takaoka, M.; Mason, H.S.; Ezura, H.; Miura, K. Improvement of the transient expression system for production of recombinant proteins in plants. Sci. Rep. 2018, 8, 4755. [Google Scholar] [CrossRef]
- Diaz-Pendon, J.A.; Li, F.; Li, W.-X.; Ding, S.-W. Suppression of Antiviral Silencing by Cucumber Mosaic Virus 2b Protein in Arabidopsis Is Associated with Drastically Reduced Accumulation of Three Classes of Viral Small Interfering RNAs. Plant Cell 2007, 19, 2053–2063. [Google Scholar] [CrossRef]
- Thomas, C.L.; Leh, V.; Lederer, C.; Maule, A.J. Turnip crinkle virus coat protein mediates suppression of RNA silencing in Nicotiana benthamiana. Virology 2003, 306, 33–41. [Google Scholar] [CrossRef]
- Shamloul, M.; Trusa, J.; Mett, V.; Yusibov, V. Optimization and utilization of Agrobacterium-mediated transient protein production in Nicotiana. J. Vis. Exp. 2014. [Google Scholar] [CrossRef]
- Vargas-Guevara, C.; Vargas-Segura, C.; Villalta-Villalobos, J.; Pereira, L.F.P.; Gatica-Arias, A. A simple and efficient agroinfiltration method in coffee leaves (Coffea arabica L.): Assessment of factors affecting transgene expression. 3 Biotech. 2018, 8, 471. [Google Scholar] [CrossRef]
- Munoz, A.; Castellano, M.M. Coimmunoprecipitation of Interacting Proteins in Plants. Methods Mol. Biol. 2018, 1794, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Vinocur, B.; Shoseyov, O.; Altman, A. Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci. 2004, 9, 244–252. [Google Scholar] [CrossRef] [PubMed]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tyurin, A.A.; Suhorukova, A.V.; Kabardaeva, K.V.; Goldenkova-Pavlova, I.V. Transient Gene Expression is an Effective Experimental Tool for the Research into the Fine Mechanisms of Plant Gene Function: Advantages, Limitations, and Solutions. Plants 2020, 9, 1187. https://doi.org/10.3390/plants9091187
Tyurin AA, Suhorukova AV, Kabardaeva KV, Goldenkova-Pavlova IV. Transient Gene Expression is an Effective Experimental Tool for the Research into the Fine Mechanisms of Plant Gene Function: Advantages, Limitations, and Solutions. Plants. 2020; 9(9):1187. https://doi.org/10.3390/plants9091187
Chicago/Turabian StyleTyurin, Alexander A., Alexandra V. Suhorukova, Ksenia V. Kabardaeva, and Irina V. Goldenkova-Pavlova. 2020. "Transient Gene Expression is an Effective Experimental Tool for the Research into the Fine Mechanisms of Plant Gene Function: Advantages, Limitations, and Solutions" Plants 9, no. 9: 1187. https://doi.org/10.3390/plants9091187
APA StyleTyurin, A. A., Suhorukova, A. V., Kabardaeva, K. V., & Goldenkova-Pavlova, I. V. (2020). Transient Gene Expression is an Effective Experimental Tool for the Research into the Fine Mechanisms of Plant Gene Function: Advantages, Limitations, and Solutions. Plants, 9(9), 1187. https://doi.org/10.3390/plants9091187





