Abstract
The chemical composition of cuticular waxes and pigments and the morphological features of cork oak (Quercus suber) leaves were determined for six samples with seeds of different geographical origins covering the natural distribution of the species. The leaves of all samples exhibited a hard texture and oval shape with a dark green colour on the hairless adaxial surface, while the abaxial surface was lighter, with numerous stomata and densely covered with trichomes in the form of stellate multicellular hairs. The results suggest an adaptive role of leaf features among samples of different provenance and the potential role of such variability in dealing with varying temperatures and rainfall regimes through local adaptation and phenotypic plasticity, as was seen in the trial site, since no significant differences in leaf traits among the various specimens were found, for example, specific leaf area 55.6–67.8 cm2/g, leaf size 4.6–6.8 cm2 and photosynthetic pigment (total chlorophyll, 31.8–40.4 µg/cm2). The leaves showed a substantial cuticular wax layer (154.3–235.1 µg/cm2) composed predominantly of triterpenes and aliphatic compounds (61–72% and 17–23% of the identified compounds, respectively) that contributed to forming a nearly impermeable membrane that helps the plant cope with drought conditions. These characteristics are related to the species and did not differ among trees of different seed origin. The major identified compound was lupeol, indicating that cork oak leaves may be considered as a potential source of this bioactive compound.
1. Introduction
The extracellular surface of plant leaves is covered by a hydrophobic layer known as the cuticle, which is composed primarily of cutin, an insoluble polyester of hydroxyfatty acids, glycerol and complex mixtures of waxes that are deposited within and above the structural cutin matrix [,,]. Cuticular waxes vary between plant species and are composed of different organic solvent-soluble lipids, consisting of very long chain fatty acids and their derivatives including aldehydes, primary alcohols and alkanes; in some species they also contain significant amounts of pentacyclic triterpenoids [,,]. They show a high degree of crystallinity, low chemical reactivity and hydrophobicity [,]. The composition of cuticular waxes is influenced by plant genotype, leaf side and age. Waxes differ in their functions and responses to biotic and abiotic environments.
Cuticular waxes confer properties upon the leaf surface such as nonstomatal water loss and gas exchange control, as well as protection from the external environment. Wax deposition is often a response to water stress and therefore stress resistant plants adapted to arid conditions often have thicker wax layers than those from more temperate locations or those that are susceptible to stress [,,,,,]. Content is not the only factor determining the properties and function of the waxy layer; knowledge of its composition is also important. However, the relationship among cuticular wax content and composition, leaf morphology and the response to different environmental conditions remains unclear.
Cork oak (Quercus suber L.) is an evergreen sclerophyllous tree species distributed in the western Mediterranean Basin, with a natural range including Algeria, France, Italy, Morocco, Portugal, Spain and Tunisia. Cork oak forests play an important ecological role in terms of carbon sequestration, soil protection, hydrological cycle regulation and ecosystem sustainability, while they are also of critical economic importance due to their production of cork that feeds a dedicated industrial chain []. However, climate change scenarios involving enhanced water deficits in the Mediterranean region threatens the ecosystem, even if cork oak shows considerable adaptability to environmental conditions and its genetic variability allows it to cope with climatic variation [].
An approach to simulating a shift of climatic characteristics is provenance analysis, which enables an assessment of the phenotypic response of various populations and the identification of populations growing well and resistant to adverse environmental factors through the use of indicators or estimators of plant responses to environmental factors, that is, morphological and physiological characteristics []. Since the natural distribution of Q. suber encompasses significant environmental and geographic gradients, it can be expected that long-term natural selection and genetic drift have resulted in a high level of genetic variation among populations concerning adaptive traits such as leaf morphological area and the accumulation of cuticular wax on leaf surfaces.
However, little information is available for Q. suber regarding natural variation in leaf morphology, cuticular features and phytochemical data, despite the fact that it has been demonstrated that the species possesses several mechanisms for drought tolerance including small and thick leaves, sclerophyllicity and deep tap-rooting [,,,]. Only one work was found in the literature regarding the cuticular waxes of Q. suber leaves []; for a sampling of two trees in spring and summer, the report described a composition including mainly n-alkyl esters (25–45% of the wax extract) and alkanols (18–50%), as well as alkanes, alkanals and alkanoic acids. The paper also noted significant variability in the content of cuticular waxes.
The aim of this study is to develop further knowledge of the features of Q. suber leaves and their variability, mainly in terms of cuticular wax quantity and composition, by using samples with different seed geographical origins that represent the area of the species’ natural distribution. Sampling was performed in the Cork Oak Provenance and Progeny Trial established in Portugal by the European Network for the Evaluation of Genetic Resources of Cork Oak for Appropriate Use in Breeding and Gene Conservation Strategies [,,]. Leaves from trees with six different geographical origins (from Portugal, Spain, Italy, France, Morocco and Tunisia) were collected and the morphological parameters, pigments and cuticular wax characteristics were analysed, looking for variations which may be related to genetic or edaphoclimatic issues. This will be the first detailed study of the leaf features and cuticular wax composition of a broad sample of cork oak of different provenance, thereby providing insights into the species’ variability.
2. Results
The leaves from all of the Quercus suber samples showed a hard texture and oval shape with a dark green colour on the adaxial surface and a lack of trichomes, while the abaxial surface was lighter with numerous stomata and was densely covered with epidermal hairs (trichomas) in the form of stellate multicellular hairs (Figure 1). Figure 2 shows an exemplary Q. suber leaf cross-section observed using optical microscopy, showing the thick cuticular membrane covering the adaxial leaf side deposited on the epidermal cell layer with between-cell indentations. The trichomes and stomata are clearly visible on the abaxial side.
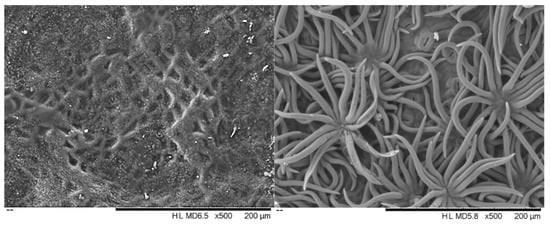
Figure 1.
Scanning electron micrographs of the adaxial (left) and abaxial (right) surface of Quercus suber leaves.
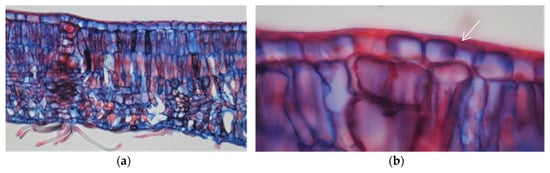
Figure 2.
Optical microscopy photographs of a cross-section of a Quercus suber leaf (a) and an enlargement of the adaxial side showing the cuticular membrane (b). Arrows indicate the cuticular structures covering the epidermal cell layer.
The average leaf features of the six cork oak provenances are presented in Table 1. Leaf size (LS) differed significantly among provenances (p = 0.018), ranging between 4.6 cm2 in the French sample (small leaves) and 6.8 cm2 in the Italian sample (larger leaves), with a mean coefficient of variation of leaf area of 0.39 (between 0.23 and 0.42). The mean specific leaf area (SLA) was 64.1 cm2/g, ranging between 55.6 cm2/g (Spanish sample) and 67.8 cm2/g (Italian sample), with a coefficient of variation in all provenances between 0.15 and 0.9 and lacking statistically significant differences (p = 0.243).

Table 1.
Morphological and physiological characterization of leaves of cork oak (Quercus suber) from six provenances. The provenances are Portugal (PT35), Spain (ES11), Italy (IT3), France (FR3), Morocco (MA27) and Tunisia (TU32). Mean and standard deviation of 20 leaves per provenance.
The total chlorophyll content expressed on a unit per leaf area basis or per unit dry weight ranged between 40.4 µg/cm2 (2.53 mg/g) (Moroccan provenance) and 31.8 µg/cm2 (2.06 mg/g) (French provenance) with similar ratios of chlorophyll a to chlorophyll b between provenances (1.9 to 2.0). The total carotenoids concentration ranged from 8.7 µg/cm2 (Moroccan provenance) to 7.2 µg/cm2 (French provenance). Data on content of total chlorophyll and carotenoids within provenances expressed by mg/g and µg/cm2 differed significantly (p < 0.001). The average values of total chlorophylls and carotenoids in leaves of Portuguese, Moroccan and Tunisian provenance were higher than the average values of other provenances.
The average extracted yield of cuticular wax was 189.4 µg/cm2, with some variation among provenances, although differences were not statistically significant (p = 0.449), ranging from the lowest value of 136.4 µg/cm2 (Tunisia) to the highest value of 231.5 µg/cm2 (France), with coefficients of variation between 0.27 and 0.43. On a dry leaf mass basis, the cuticular waxes represented on average 24.6 mg/g, ranging between 20.7 mg/g (Spain) and 29.7 mg/g (France). Based on the driving force, the cuticular transpiration rate of the adaxial leaf surface ranged from 2.7 × 10−4 g/m2s to 5.5 × 10−4 g/m2s and the cuticular water permeance ranged from 1.6 × 10−5 m/s to 2.5 × 10−5 m/s.
The results obtained for the cuticular wax composition of the Q. suber leaves are summarized in Table 2 regarding chemical families and detailed for the six provenances of Q. suber in Table 3. On average, the cuticular wax extracts were composed of terpenes (60.1 % of peak area), fatty acids (12.7%) and alkanes (6.1%), with a small proportion of alkanols (1.1%) and aromatics compounds (1.9%). The average proportion of identified compounds in the chromatograms was 91.2%.

Table 2.
Chemical class composition of the cuticular wax of leaves from six Quercus suber provenances, as a percentage of the total peak areas in the GC-MS chromatograms. Mean and standard deviation of 12 samples.

Table 3.
Chemical composition (% of all chromatogram peak areas) of the cuticular waxes of leaves from six Quercus suber provenances (mean of two trees per provenance). The provenances are Portugal (PT35), Spain (ES11), Italy (IT3), France (FR3), Morocco (MA27) and Tunisia (TU32).
A principal component analysis (PCA) and cluster analysis (CA) were performed on the seven chemical families of epicuticular wax compounds (alkanols, alkanes, fatty acids, aromatic compounds, sterols, terpenes and others) found in 12 Q. suber leave samples obtained from six provenances. From the analysis of the eigenvalues of the principal components and of the scree plot, the initial hyperspace defined by seven dimensions (one for each group of compounds) was reduced to a plane defined by the first two principal components. This plane explained 56.5% of the information contained in the original data. Figure 3 shows the projections of both compound groups and samples on this plane. The first dimension (Factor 1) is positively correlated with n-alkanols and n-alkanes, while terpenes are correlated with the negative side of this axis. This indicates that samples ES-1 and Pt-2 show the highest contents of n-alkanols and n-alkanes, while samples FR-2 and MA-1 show the highest amounts of terpenes and lowest amounts of n-alkanols and n-alkanes. The second principal component (Factor 2) is highly correlated with aromatic compounds, which increases along this axis. Therefore, samples ES-2 and TU-1 show the highest content of aromatic compounds. From the analysis of Figure 3, it is difficult to identify groups of samples. Since Figure 3 shows the projections of samples on a plane and not in their original position in the hyperspace, a cluster analysis was performed on the same data to identify groups of samples (Figure 4). Again, no clear group separation was observed by CA. This confirms that there is a similar pattern for the chemical profile of the cuticular waxes of all provenances regarding chemical families.
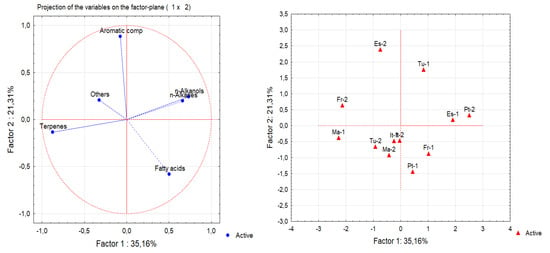
Figure 3.
Principal component analysis (PCA) of the chemical classes of epicuticular wax compounds from leaves of 12 samples from six provenances: (left) score plots of the original variables; (right) sample plot (ES—Spain; Fr—France; IT—Italy; MA—Morocco; PT—Portugal; TU—Tunisia).
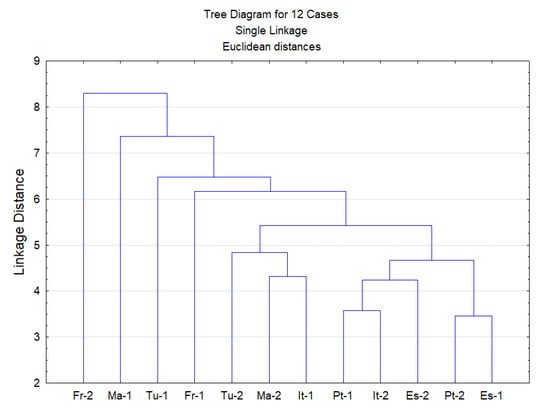
Figure 4.
Dendrogram obtained by cluster analysis of the family classes of epicuticular wax compounds from 12 leave samples from six provenances (ES—Spain; FR—France; IT—Italy; MA—Morocco; PT—Portugal; TU—Tunisia).
The chemical compounds identified in the cuticular waxes of leaves from the six Q. suber provenances are presented in Table 3 in proportion of the total chromatogram area and are grouped by chemical family.
Triperpenes represented between 54.7% (Portuguese provenance) and 64.3% (Moroccan provenance) of the total peak area. Lupeol was the major triterpene found in all the provenances (33–40% of the compounds). Other abundant triterpenes were friedooleanan-3-ol (6.8% on average), friedelin (4.1%) and β-amyrin (5.7%), followed by smaller amounts of betulin and betulinic acid. Oleanolic and ursolic acids were also identified but in small amounts.
Sterols were present in amounts varying between 3.2% and 6.6% in the Spanish and Maroccan provenances, respectively. β-Sitosterol was the major compound from this family in all cases, while tocopherols (α, β and γ tocopherol) were present in smaller amounts.
The aliphatic compounds constituted an abundant group including fatty acids (9.2–15.2% of the compounds), n-alkanes (5.6–7.5%) and primary alcohols (0.4–2.0%), which together accounted for 17–23% of the total compounds. Among the fatty acids, C30, C28 and C16 acids were predominant, representing 36.8%, 22.7% and 12.9% of the total fatty acids, respectively. The homologous series of saturated acids with an even number of C was present from C8 to C30. Unsaturated acids only represented on average 6.9% of the total fatty acids. The chain length of the n-alkanes varied between C15 and C31, with n-octacosane (C28) and hentriacontane (C31) as the major compounds. Alkanols comprised only 0.4–1.3% of all compounds, with tretracosanol (C24OH) and octacosanol (C28OH) as the major compounds in the wax extracts. Glycerol was present in minor amounts, as well as one monoacylglycerol. Aromatic compounds comprised 1.4–2.6% of all compounds, with kaempferol, pinoresinol and hexadecy-(E)-p-coumarate as the major compounds.
3. Discussion
The study was conducted in a Q. suber provenance trial where 35 cork oak provenances covering the natural distribution range are represented, which is of utmost importance to assess the effect of seed geographic origin on adaptive traits [,]. In the present study, six provenances were selected that cover the broad geographic area of cork oak distribution (Portugal, Spain, France and Italy in southern Europe and Morocco and Tunisia in northern Africa).
The cork oak leaves in the trees of all the provenances showed a clear schlerophytic character (Figure 1 and Figure 2) and their morphological features (Table 1) were within the range of values reported for the species. Leaf sizes (4.6–6.8 cm2) were similar to those reported by Mediavilla et al. [] for Q. suber leaves taken from different orientations in the canopy (5.5–7.4 cm2) and the 7.1 cm2 reported by Prats et al. []. The specific leaf area values (55.6–67.8 cm2/g) were of the same order of magnitude as those obtained in adult leaves of Q. suber growing under contrasting environments and located in different positions and orientations of the canopy (50.0 to 126.0 cm2/g) [,,,,].
When comparing the six cork oak provenances, two sets could be defined in relation to leaf area (Table 1), with ES, FR and TU provenances characterized by smaller leaves and PT, IT and MA provenances characterized by larger leaves. Specific leaf area (SLA) for the six provenances showed a low coefficient of variation (CV 0.14), suggesting that they developed a similar degree of leaf sclerophylly. This finding is in line with most of the few available studies on Q. suber. Rzigui et al. [] observed that there was no difference in SLA values between two provenances in Tunisia (Gafour and Feija) with contrasting environments (125 and 126 cm2/g) and Daoudi et al. [] reported that SLA did not differ significantly between three humid, semi-arid and sub-humid provenances in Algeria, although SLA was lower under conditions of water deficiency. Lobo-do-Vale et al. [] reported an adaptive response to drought with an SLA reduction (85 cm2/g in a mild year and 65 cm2/g in a dry year), while Aranda et al. [] observed that, while irrigation had no significant effect, SLA decreased with increasing irradiance, thereby improving the potential for carbon uptake relative to transpiration water loss. SLA plays an important role in linking plant carbon and water cycles and is sensitive to environmental change [,], with leaf traits being influenced by a combination of species, climate and soil factors [,,].
In the present study, the observed amounts of the total chlorophyll, expressed per unit of leaf area or per unit of dry weight (Table 1), were consistent with those reported in the available studies for Q. suber, for example, the results of Ramírez-Valiente et al. [] for leaves of open-pollinated trees from populations in Morocco, Portugal and Spain (2.68 mg/g, 2.64 mg/g and 2.74 mg/g respectively) and 55.7 µg/cm2 and 56.0 µg/cm2 total chlorophyll in sun and shade leaves of 40-year-old Q. suber trees []. Mediavilla et al. [] also observed that the chlorophyll content differed among canopy orientations, with lower values on the west side (80 vs. 110 µg/cm2), probably due to a direct effect of excess radiation at the time of the day with highest leaf temperatures and lowest water potential on the west facing leaves.
A substantial average wax layer of 189.4 µg/cm2 (or 24.6 mg/g) covered the leaves of the six Q. suber provenances (Table 1) without any provenance effect. The values are slightly above the 125 µg/cm2 reported by Martins et al. [] for the cuticular wax of young leaves of Q. suber. A higher amount of cuticular wax is observed in Q. suber leaves than in other Quercus species. For instance, young leaves of Q. ilex (holm oak, a persistent leaf oak) have 71 µg/cm2 of cuticular wax [], Q. robur (pedunculate oak, deciduous tree growing in cooler climates with less hydric stress) has 59 µg/cm2 [] and Q. petraea (sessile oak) has 101.5–134.5 µg/ cm2 []. Similar values were also reported for Q. polymorpha (Mexican white oak) leaves, with a wax layer of 199.4 µg/cm2 [].
The high wax content of the cork oak leaves suggests that cuticular characteristics may be associated with adaptation to the local environmental conditions of high temperatures and water deficit. Accumulation of cuticular wax is considered an important strategy against drought in many plant species, providing an essential barrier to protect plants from drought stress [,]. In this study, the cork oak did not differ in their responses to environmental conditions by provenance, indicating that the accumulation of cuticular waxes on the leaves, albeit depending on the species, mainly responds to the specific environmental conditions.
Despite the role of the cuticle as a water barrier, there is still a movement of water through the cuticle between the outer cell wall of the epidermis and the surrounding atmosphere, giving the cuticle some permeability []. This movement is based on a simple diffusion process along a gradient of the water’s chemical potential with the water molecules being absorbed at one interface, following a random path through the cuticle in a mainly lipophilic chemical environment and desorbed at the other interface []. The degree to which cuticles transferred water was measured in the adaxial leaf surface of the Q. suber samples, with an average cuticular permeance of 1.8 × 10−5 m/s (Table 2). No information is available on the cuticular permeance of Q. suber leaves but comparison with other Quercus species shows that this value is quite low, in agreement with its adaptation to hot and dry environments. The available values for cuticular permeances range from 3.6 × 10−5 m/s for Q. ilex, 7.3 × 10−5 m/s for Q. rubra, 10 × 10−5 m/s for Q. coccifera, to 27 × 10−5 m/s for Q. sessiliflora []. The permeances determined with isolated cuticular membranes were from 3.8 × 10−5 m/s to 7.4 × 10−5 m/s for Q. petraea [].
Our results on the chemical composition of the dichloromethane extract of Q. suber leaves (Table 2 and Table 3) show that the majority of compounds are pentacyclic triterpenoids (mainly lupeol) and that long-chain aliphatic components are mainly fatty acids (mainly in C30, C28 and C16). The extraction method may have a determining role in the extent of compound solubilization, namely, on the amount of terpenes in relation to aliphatic compounds. Since the objective in the present study was to extract the total wax layer, including epicuticular and intracuticular waxes from both sides of the leaf, an intensive 6 h extraction with dichloromethane was conducted.
The only work to our knowledge regarding the composition of cuticular waxes in young Q. suber leaves [] applied a quick surface extraction method by dipping and shaking the leaves for a few seconds with chloroform and this explains the compositional differences to our results. In fact, this extract contained only small amounts of triterpenoids (triterpenone, friedelin), although the aliphatic composition was similar to that found in the present study: 4–27% n-alkanes; 18–50% even chain amphiphilic compounds (n-alkan-1-ols); up to 25% n-alkanals; <5% n-alkanoic acids; and 25–45% n-alkyl esters []. It was reported that triterpenoids are located almost exclusively in the intracuticular wax compartment and therefore require a more intensive extraction procedure [,].
The cuticular wax composition appears to be related to the species, since no statistical significant differences were found between cork oak provenances and there was no clustering of provenances by chemical families, based on PCA and cluster analysis (Figure 3 and Figure 4).
Q. suber leaf wax contains the same lipid classes as Q. ilex and Q. robur but with different distribution patterns. In a Q. ilex leaf, the most abundant wax components are C22 and C24 n-alkanoic acids (38%) and n-alkan-1-ols (43–54%), with small amounts of the triterpenols α- and β-amyrin []. In a Q. robur leaf wax, the dominating classes are alcohols (about 70% of the wax with chain lengths ranging from C16 to C34, with tetracosanol as a main component), fatty acids (20% of the wax, especially with chain length of C14 and C22), aldehydes (28%, of the wax with chain lengths from C20 to C32 with C26 and C28 as the main components) and several triperpenoids (8% of the wax, taraxerol, β-amyrin, α-amyrin and lupeol were identified) [,].
A comparison with Fagus and Castanea species, also members of the Fagaceae family to which Q. suber belongs, shows that the epicuticular leaf wax of Castanea sativa consists of a homologous series of wax lipids (wax esters, aldehydes, primary alcohols and fatty acids) and large amounts of triterpenoids (α- and β-amyrin and lupeol) [], while that of Fagus sylvatica contains only wax lipids, without any triterpenoids [].
The cuticular wax of Q. suber leaves is rich in triterpenoids that can be obtained as an extract after solubilization. Over the last three decades, extensive research has revealed important pharmacological applications of triterpenoids with potential uses in new functional foods, drugs, cosmetics and healthcare products. Lupeol was studied for the treatment of various diseases, including skin wounds and various medicinal properties of lupeol have been reported, including anti-inflammatory, antioxidant, anti-diabetic and anti-mutagenic effects [,,].
Quercus suber leaves are a promising and highly available source of triterpenes, since large quantities of leaves are generated each year from silvicultural practices (e.g., pruning), which can be used to produce chemicals using environmentally friendly extraction processes []. Our results estimate a potential extraction yield per kg of dry leaves of Q. suber of 15 g triterpenes, of which 9 g is lupeol.
4. Material and Methods
4.1. Sampling
The study was carried out on a provenance trial of Quercus suber L. at Herdade do Monte Fava, Santiago do Cacém, near Setúbal, in central Portugal (38° 00′ N, 08°07′ W, altitude 79 m). The site has a Mediterranean climate, with hot and dry summers (total year rainfall, 556.6 mm; summer accumulated rainfall, 19.4 mm; annual average temperature, 15.8 °C; average minimum temperature of the coldest month, 4.3 °C; average maximum temperature of the hottest month, 31.3 °C) and the soil has a sandy texture []. The trial was established in March 1998, as part of an international network on cork oak genetic resources funded by an EU Concerted Action Fair 202 []. The plant material included in this field trial resulted from a seed collection conducted during the autumn of 1996 from 35 cork oak populations that covered the species’ natural range; seedlings were raised from these seeds with a common protocol in one nursery, planted in the trial field and the trees were allowed to grow until leaves were sampled [,].
The sampling carried out for the present study included 21-year-old trees from six provenances: Portugal, Spain, Italy, France, Morocco and Tunisia. Detailed information on the location and climate data from the original seed collection sites of the studied provenances is given in Table 4 as well as that of the trees’ growing site. Leaves were randomly sampled from two trees of each provenance in March 2019. The leaves were collected from different branches on the southern exposed side of the crown, in the lower part of the canopy up to a height of approximately 2 m, making up a total sample per tree of about 100 leaves.

Table 4.
Characterization of the trial site and identification of the Quercus suber provenances with collection site location, Tm annual average air temperature (°C) and the long-term annual average precipitation (PPT, mm) (adapted from Varela []) and characterization of the provenance trial of Quercus suber (Herdade do Monte Fava).
4.2. Morphological Variables
The morphological variables were measured in 20 leaves that were randomly selected from leaves sampled for each of the two trees of each provenance. Leaves were digitalized and analysed using WinSEEDLETM 2011. The leaves were oven-dried at 70 °C to a constant mass and the total dry mass per leaf was determined. Specific leaf area (SLA, cm2/g) was calculated as the ratio between the measured leaf area and dry weight; this was used as an indirect index of sclerophylly. The sclerophylly index was calculated as (IE) = leaf dry mass (g)/2 × leaf area (dm2) and defines sclerophylly as IE > 0.6 and mesophylly as IE < 0.6, according to Rizzini [].
4.3. Determination of Leaf Pigment Contents
Chlorophyll a and b were determined in six disks of known area taken from leaves of each of the two trees per provenance. The tissue samples were homogenized in the dark during 24 to 36 h, with 3 mL of dimethylformamide solution. Chlorophyll a and b, carotenoids and total chlorophyll were quantified in the supernatant phase by using a spectrophotometer and readings were taken at 663.8, 646.8 and 480.0 nm, respectively, according to the methodology described by Lichthenthaler [] and using the equations and specific absorption in the wavelength reported by Wellburn []. The leaf pigments chlorophyll a and b, total chlorophyll and carotenoids were expressed as µg/cm2 of leaf area.
4.4. Cuticle Permeability Assay
The cuticular permeance was determined by the measurement of water loss through the adaxial, astomatous leaf surface. Leaf samples were rehydrated by submerging the petioles of the leaves in water at room temperature for 12 h. The leaf petioles and the abaxial leaf surface were sealed with paraffin wax to ensure that water transpiration only occurred via the stomata-free adaxial leaf surface. Leaf weight was measured immediately afterwards. Leaf samples were then placed in boxes over silica gel and placed in an incubator with control the surrounding temperature (25 °C) and weighed repeatedly over a 4 h period. The transpiration rate (flux of water vapor; J, in g m−2 s−1) was obtained from the change in fresh weight of the samples (ΔW, in g) over time (Δt, in s) and surface area (A, in m2):
The leaf surface area (A leaf) of the adaxial surface was obtained by scanning the leaf surface.
The cuticular permeance P (in m s−1) and minimum leaf conductance gmin can be obtained from the transpiration rate J and the driving force Δc of water across the cuticle according to
The driving force (Δc) is the difference between the concentration of water vapour in the leaf interior and the surrounding atmosphere (g m–3), resulting in permeability parameters in m s–1. Since during cuticular transpiration the diffusion of water occurs in the solid phase of the cuticle, atmospheric pressure has no influence and, consequently, using the concentration-based driving force is appropriate. The resulting permeability parameters were described in m s–1. The water activity of leaf samples (aleaf) was assumed to be unity []. The humidity of the air was controlled by silica gel, resulting in a water activity (aair) close to zero. Therefore, the driving force for water loss through transpiration was identical to the density of water vapour at saturation in the air (cWV at 25 °C was 23.07 g m−3; []) [,].
4.5. Extraction of Cuticular Waxes
Cuticular wax was extracted from whole fresh leaves with dichloromethane in a Soxhlet apparatus over 6 h using a sample of 20 leaves per tree. After extraction, the solvent was evaporated. This extraction method yields a total wax mixture containing both epicuticular and intracuticular waxes that cover both sides of the leaf.
The amount of soluble cuticular lipids was determined from the mass difference of the extracted leaves after drying at 105 °C and was expressed on a leaf surface area and dry weight basis (the ratio between wax in μg and the two-sided leaf surface area in cm2, obtained by digitalization).
4.6. Cuticular Wax Composition
The cuticular wax (obtained as dichloromethane extracts from 20 leaves per tree) was analyzed using gas-chromatography mass spectrometry (GC-MS). Two mg of each leaf extract was taken and derivatized in 120 μL of pyridine; the compounds with hydroxyl and carboxyl groups were trimethylsilylated into trimethylsilyl (TMS) ethers and esters, respectively, by adding 80 μL of bis(trimethylsily)-trifluoroacetamide (BSTFA). The reaction mixture was heated at 60 °C for 30 min in an oven. The derivatized extracts (1 μL) were immediately analyzed by GC-MS (EMIS, Agilent 5973 MSD, Palo Alto, CA, USA), with an ionization energy of 70 eV and the MS source was kept at 220 °C under the following GC conditions: Zebron 7HGG015-02 column (30 m, 0.25 mm; ID, 0.1 μm film thickness), with injector at 280 °C. The column temperature was initially held at 50 °C for 1 min, raised to 150 °C at a rate of 10 °C min−1, then to 300 °C at 4 °C min−1, to 370 °C at 5 °C min−1 and at 8 °C min−1 until it reached 380 °C; this was followed by an isothermal period of 5 min. Compounds were identified as TMS derivatives by matching their mass spectra with a GC-MS spectral library (Wiley, NIST) and by comparing their fragmentation profiles with published data [,]. Two replicates were made per extract.
4.7. Structural and Anatomical Observations
For scanning electron microscopy (SEM), small pieces of fresh- and air-dried leaves were fixed to sample holders and observed with a scanning electron microscope (TM 3030 Plus Hitachi).
The cuticular membrane was observed in a thin leaf cross section by optical microscopy. The leaves were impregnated with DP 1500 polyethylene glycol and cross sections of approximately 10 µm thickness were cut with a rotary microtome (Medite M530). The sections were stained with safranin and astral blue and were mounted in Euparal. Observations were made using a light microscope (Leica DM LA) and the photomicrographs were taken with a Nikon Microphot-FXA.
4.8. Statistical Analyses
To compare leaf functional traits among provenances, one-way analysis of variance (ANOVA) was performed. Duncan’s post-hoc tests were used to analyse pairwise differences between provenances. Statistical significance was set at p < 0.05. All statistical analyses were performed using the Sigmaplot® (Version 11.0, Systat Software, Inc., Chicago, IL, USA).
Principal component analysis (PCA) and cluster analysis (CA) were performed to analyse the chemical composition of cuticular wax from leaves of Quercus suber trees of different provenance. Using PCA, the hyperspace was defined by the original variables and dimension reduction was carried out using the significant principal components (new axes). These new axes were correlated with the original variables. Thus, the samples were plotted onto a reduced space where similar samples could be grouped. Agglomerative hierarchical CA, using the Euclidean distance and single-linkage method, was carried out to assess the existence of groups of samples suggested by PCA []. PCA and CA were performed with the StatisticaTM software, version 6, from Statsoft (Tulsa, OK, USA).
5. Conclusions
The results obtained for Quercus suber leaves from six different provenances suggest the adaptive value of leaf features under a Mediterranean climate, namely, specific leaf area, leaf size and photosynthetic pigment, highlighting their potential role in dealing with varying temperatures and rainfall regimes through local adaptation and phenotypic plasticity. Cork oak leaves possess a substantial cuticular wax layer that forms a nearly impermeable membrane which is a tool to cope with adverse drought related with environmental conditions. The composition of the cuticular wax also favours the hydrophobicity of the layer by inclusion of very long-chain alkanes and alkanoic acids (e.g., C28 and C30). These characteristics are species specific and did not differ across provenances.
Triterpenes are the major component of the cuticular wax complex and contain a high proportion of lupeol. Thus, the leaves of cork oak represent a potential source for this interesting bioactive compound. The chemical composition of the cuticular wax did not differ among provenances, which may be ascribed to similar water stress conditions in the Mediterranean region, which probably has a stronger effect than genetic variability in Q. suber.
Author Contributions
H.P. and I.M. conceived the study and carried out the experimental design; S.F.-D. performed data curation; R.S. and A.R. performed the experiments and data interpretation; I.M. wrote the first manuscript draft; H.P., I.M. and S.F.-D. were responsible for data validation and manuscript revisions. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Portuguese Foundation for Science and Technology (FCT) through the funding of Centro de Estudos Florestais (UID/AGR/00239/2019) and LEAF (UID/AGR/04129/2020). Rita Simões acknowledges a doctoral scholarship from FCT with the SUSFOR Doctoral Programme (PD/BD/128259/2016). The cork oak provenance field trials were funded by the European Commission (FAIR1-CT-95-0202) and national programs (PBIC/AGR/2282/95, PAMAF 4027, PRAXIS/3/3.2/Flor/2110/95).
Acknowledgments
We are particularly indebted to Helena Almeida in providing plant materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pollard, M.; Beisson, F.; Li, Y.; Ohlrogge, J.B. Building lipid barriers: Biosynthesis of cutin and suberin. Trends Plant Sci. 2008, 13, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, E.; Heredia-Guerrero, J.A.; Heredia, A. The biophysical design of plant cuticles: An overview. New Phytol. 2011, 189, 938–949. [Google Scholar] [CrossRef] [PubMed]
- Ingram, G.; Nawrath, C. The Roles of the Cuticle in Plant Development: Organ Adhesions and Beyond. J. Exp. Bot. 2017, 68, 5307–5321. [Google Scholar] [CrossRef] [PubMed]
- Samuels, L.; Kunst, L.; Jetter, R. Sealing Plant Surfaces: Cuticular Wax Formation by Epidermal Cells. Annu. Rev. Plant Biol. 2008, 59, 683–707. [Google Scholar] [CrossRef] [PubMed]
- Kunst, L.; Samuels, L. Plant cuticles shine: Advances in wax biosynthesis and export. Curr. Opin. Plant Biol. 2009, 12, 721–727. [Google Scholar] [CrossRef]
- Jetter, R.; Kunst, L.; Samuels, A.L. Composition of plant cuticular waxes. In Biology of the Plant Cuticle, Annual Plant Reviews; Riederer, M., Müller, C., Eds.; Blackwell: Oxford, UK, 2006; Volume 23, pp. 145–181. [Google Scholar]
- Reynhardt, E.C.; Riederer, M. Structures and molecular dynamics of plant waxes. II. Cuticular waxes from leaves of Fagus sylvatica L. and Hordeum vulgare L. Eur. Biophys. J. 1994, 23, 59–70. [Google Scholar]
- Zeisler-Diehl, V.; Müller, Y.; Schreiber, L. Epicuticular wax on leaf cuticles does not establish the transpiration barrier, which is essentially formed by intracuticular wax. J. Plant Physiol. 2018, 227, 66–74. [Google Scholar] [CrossRef]
- Sharma, P.; Kothari, S.L.; Rathore, M.S.; Gour, V.S. Properties, variations, roles and potential applications of epicuticular wax: A review. Turk. J. Bot. 2018, 42, 135–149. [Google Scholar] [CrossRef]
- Shepherd, T.; Griffiths, D.W. The effects of stress on plant cuticular waxes. New Phytol. 2006, 171, 469–499. [Google Scholar] [CrossRef]
- Kosma, D.K.; Jenks, M. Eco-physiological and molecular-genetic determinants of plant cuticle function in drought and salt stress tolerance. In Advances in Molecular Breeding toward Drought and Salt Tolerant Crops; Jenks, M.A., Hasegawa, P.M., Jain, S.M., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 91–120. [Google Scholar]
- Serrano, M.; Coluccia, F.; Torres, M.; L’Haridon, F.; Métraux, J.-P. The cuticle and plant defense to pathogens. Front. Plant Sci. 2014, 5, 274. [Google Scholar] [CrossRef]
- Domínguez, E.; Heredia-Gerrero, J.A.; Heredia, A. The plant cuticle: Old challenges, new perspectives. J. Exp. Bot. 2017, 68, 5251–5255. [Google Scholar] [CrossRef] [PubMed]
- Schuster, A.C.; Burghardt, M.; Riederer, M. The ecophysiology of leaf cuticular transpiration: Are cuticular water permeabilities adapted to ecological conditions? J. Exp. Bot. 2017, 68, 5271–5279. [Google Scholar] [CrossRef] [PubMed]
- Pereira, H. Cork: Biology, Production and Uses; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Leite, C.; Oliveira, V.; Lauw, A.; Pereira, H. Cork rings suggest how to manage Quercus suber to mitigate the effects of climate changes. Agric. For. Meteorol. 2019, 266, 12–19. [Google Scholar] [CrossRef]
- Pérez-Harguindeguy, N.; Díaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 2013, 61, 167–234. [Google Scholar] [CrossRef]
- Oliveira, G.; Correia, O.; Martins Loução, M.; Catarino, F.M. Phenological and growth-patterns of the Mediterranean oak Quercus suber L. Trees Struct. Funct. 1994, 9, 41–46. [Google Scholar] [CrossRef][Green Version]
- Vaz, M.; Pereira, J.S.; Gazarini, L.C.; David, T.S.; David, J.S.; Rodrigues, A.; Moroco, J.; Chaves, M.M. Drought-induced photosynthetic inhibition and autumn recovery in two Mediterranean oak species (Quercus ilex and Quercus suber). Tree Physiol. 2010, 30, 946–956. [Google Scholar] [CrossRef] [PubMed]
- Prats, K.A.; Brodersen, C.R.; Ashton, M.S. Influence of dry season on Quercus suber L. leaf traits in the Iberian Peninsula. Am. J. Bot. 2019, 106, 656–666. [Google Scholar] [CrossRef]
- Costa-e-Silva, F.; Correia, A.C.; Piayda, A.; Dubbert, M.; Rebmenn, C.; Cuntz, M.; Werner, C.; David, J.S.; Pereira, J.S. Effects of an extremely dry winter on net ecosystem carbon exchange and tree phenology at a cork oak woodland. Agric. For. Meteorol. 2015, 204, 48–57. [Google Scholar] [CrossRef]
- Martins, C.M.C.; Mesquita, S.M.M.; Vaz, W.L.C. Cuticular Waxes of the Holm (Quercus ilex L. subsp. ballota (Desf.) Samp.) and Cork (Q. suber L.) Oaks. Phytochem. Anal. 1999, 10, 1–5. [Google Scholar] [CrossRef]
- Varela, M.C. European Network for the Evaluation of Genetic Resources of Cork Oak for Appropriate Use in Breeding and Gene Conservation Strategies; Handbook; INIA: Lisbon, Portugal, 2000. [Google Scholar]
- Eriksson, G. Quercus suber—Recent Genetic Research; European Forest Genetic Resources Programme (EUFORGEN), Bioversity International: Rome, Italy, 2017; 30p, Available online: http://www.euforgen.org/fileadmin/templates/euforgen.org/upload/Publications/Thematic_publications/Quercus_suber_OpenSourceCR_web.pdf (accessed on 25 June 2020).
- Sampaio, T.; Branco, M.; Guichoux, E.; Petit, R.J.; Pereira, J.S.; Varela, M.C.; Almeida, M.H. Does the geography of cork oak origin influence budburst and leaf pest damage? For. Ecol. Manag. 2016, 373, 33–43. [Google Scholar] [CrossRef]
- Sampaio, T.; Gonçalves, E.; Patrício, M.S.; Cota, T.M.; Almeida, M.H. Seed origin drives differences in survival and growth traits of cork oak (Quercus suber L.) populations. For. Ecol. Manag. 2019, 448, 267–277. [Google Scholar] [CrossRef]
- Varela, M.C.; Tessier, C.; Ladier, J.; Dettori, S.; Filigheddu, M.; Bellarosa, R.; Vessella, F.; Almeida, M.H.; Sampaio, T.; Patrício, M.S. Characterization of the International Network Fair 202 of provenance and progeny trials of cork oak on multiple sites for further use on forest sustainable management and conservation of genetic resources. In Proceedings of the Second International Congress of Silviculture, Designing the Future of the Forestry Sector, Florence, Italy, 26–29 November 2014. [Google Scholar]
- Mediavilla, S.; Martín, I.; Babiano, J.; Escudero, A. Foliar plasticity related to gradients of heat and drought stress across crown orientations in three Mediterranean Quercus species. PLoS ONE 2019, 14, e0224462. [Google Scholar] [CrossRef] [PubMed]
- Vaz, M.; Moroco, J.; Ribeiro, N.; Gazarini, L.C.; Pereira, J.S.; Chaves, M.M. Leaf-level responses to light in two co-occurring Quercus (Quercus ilex and Quercus suber): Leaf structure, chemical composition and photosynthesis. Agrofor. Syst. 2011, 82, 173–181. [Google Scholar] [CrossRef]
- Ramírez-Valiente, J.A.; Valladares, F.; Delgado, A.; Granados, S.; Aranda, I. Factors affecting cork oak growth under dry conditions: Local adaptation and contrasting additive genetic variance within populations. Tree Genet. Genomes 2011, 7, 285–295. [Google Scholar] [CrossRef]
- Rzigui, T.; Jazzar, L.; Ben Baaziz, K.; Fkiri, S.; Nasr, Z. Drought tolerance in cork oak is associated with low leaf stomatal and hydraulic conductances. iForest 2018, 11, 728–733. [Google Scholar] [CrossRef]
- Lobo-do-Val, R.; Besson, C.K.; Caldeira, M.C.; Chaves, M.M.; Pereira, J.S. Drought reduces tree growing season length but increases nitrogen resorption efficiency in a Mediterranean ecosystem. Biogeosciences 2019, 16, 1265–1279. [Google Scholar] [CrossRef]
- Daoudi, H.; Derridj, A.; Hannachi, L.; Mévy, J.P. Comparative drought responses of Quercus suber seedlings of three algerian provenances under greenhouse conditions. Revue d’Ecologie (Terre et Vie) 2018, 73, 57–70. [Google Scholar]
- Aranda, I.; Pardos, M.; Puértolas, J.; Jiménez, M.D.; Pardos, J.A. Water use efficiency in cork oak (Quercus suber L.) is modified by the interaction of water and light availabilities. Tree Physiol. 2007, 27, 671–677. [Google Scholar] [CrossRef]
- Gunn, S.; Farrar, J.F.; Collis, B.E.; Nason, M. Specific leaf area in barley: Individual leaves versus whole plants. New Phytol. 1999, 143, 45–51. [Google Scholar] [CrossRef]
- Pierce, L.L.; Running, S.W.; Walker, J. Regional-scale relationships of leaf-area index to specific leaf-area and leaf nitrogen-content. Ecol. Appl. 1999, 4, 313–321. [Google Scholar] [CrossRef]
- Reich, P.B.; Wright, I.J.; Lusk, C. Predicting leaf physiology from simple plant and climate attributes: A global glopnet analysis. Ecol. Appl. 2007, 17, 1982–1988. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Kabeya, D.; Günter, H. Leaf traits, shoot growth and seed production in mature Fagus sylvatica trees after 8 years of CO2 enrichment. Ann. Bot. 2011, 107, 1405–1411. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, Y.; Li, X.; Chen, Z.; He, N. Variation in leaf morphological, stomatal, and anatomical traits and their relationships in temperate and subtropical forests. Sci. Rep. 2019, 9, 5803. [Google Scholar] [CrossRef] [PubMed]
- Faria, T.; García-Plazaola, J.I.; Abadía, A.; Cerasoli, S.; Pereira, J.S.; Chaves, M.M. Diurnal changes in photoprotective mechanisms in leaves of cork oak (Quercus suber L.) during summer. Tree Physiol. 1996, 16, 115–123. [Google Scholar] [CrossRef]
- Prasad, R.B.N.; Gülz, P.G. Surface structure and chemical composition of leaf waxes from Quercus robur L., Acer pseudoplatanus L. and JugIans regia L. Z. Naturforsch. C 1990, 45, 813–817. [Google Scholar] [CrossRef]
- Bahamonde, H.A.; Gil, L.; Fernández, V. Surface properties and permeability to calcium chloride of Fagus sylvatica and Quercus petraea leaves of different canopy heights. Front. Plant Sci. 2018, 9, 494. [Google Scholar] [CrossRef]
- Maiti, R.; Rodriguez, H.G.; Sarkar, N.C.; Kumari, A. Biodiversity in leaf chemistry (Pigments, epicuticular wax and leaf nutrients) in woody plant species in north-eastern Mexico, a synthesis. Forest Res. 2016, 5, 170. [Google Scholar]
- Sánchez, F.J.; Manzanares, M.; de Andrés, E.F.; Tenorio, J.L.; Ayerbe, L. Residual transpiration rate, epicuticular wax load and leaf colour of pea plants in drought conditions. Influence on harvest index and canopy temperature. Eur. J. Agron. 2001, 15, 57–70. [Google Scholar] [CrossRef]
- Xue, D.; Zhang, X.; Lu, X.; Chen, G.; Chen, Z.-H. Molecular and Evolutionary Mechanisms of Cuticular Wax for Plant Drought Tolerance. Front. Plant Sci. 2017, 8, 621. [Google Scholar] [CrossRef]
- Riederer, M.; Schreiber, L. Protecting against water loss: Analysis of the barrier properties of plant cuticles. J. Exp. Bot. 2001, 52, 2023–2032. [Google Scholar] [CrossRef]
- Kerstiens, G. Cuticular water permeability and its physiological significance. J. Exp. Bot. 1996, 47, 1813–1832. [Google Scholar] [CrossRef]
- Burghardt, M.; Riederer, M. Ecophysiological relevance of cuticular transpiration of deciduous and evergreen plants in relation to stomatal closure and leaf water potential. J. Exp. Bot. 2003, 54, 1941–1949. [Google Scholar] [CrossRef] [PubMed]
- Jetter, R.; Schäffer, S. Chemical composition of the Prunus laurocerasus leaf surface. Dynamic changes of the epicuticular wax film during leaf development. Plant. Physiol. 2001, 126, 1725–1737. [Google Scholar] [CrossRef] [PubMed]
- Buschhaus, C.; Jetter, R. Composition differences between epicuticular and intracuticular wax substructures: How do plants seal their epidermal surfaces? J. Exp. Bot. 2011, 62, 841–853. [Google Scholar] [CrossRef]
- Gülz, P.G.; Müller, E. Seasonal variation in the composition of epicuticular waxes of Quercus robur leaves. Z. Naturforsch. C 1992, 47, 800–806. [Google Scholar] [CrossRef]
- Gülz, P.G.; Müller, E.; Herrmann, T. Chemical composition and surface structures of epicuticular leaf waxes from Castanea sativa and Aesculus hippocastanum. Z. Naturforsch. C 1992, 47, 661–666. [Google Scholar] [CrossRef]
- Gülz, P.G.; Prasad, R.B.N.; Müller, E. Surface structures and chemical composition of epicuticular waxes during leaf development of Fagus sylvatica L. Z. Naturforsch. C 1992, 47, 190–196. [Google Scholar] [CrossRef]
- Fernández, M.A.; de las Heras, B.; García, M.D.; Sáenz, M.T.; Villar, A. New insights into the mechanism of action of the anti-inflammatory triterpene lupeol. J. Pharm. Pharmacol. 2001, 53, 1533–1539. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; Zhang, L.; Yang, X.; Lv, Z. Lupeol, a dietary triterpene, inhibited growth and induced apoptosis through down-regulation of DR3 in SMMC7721 cells. Cancer Investig. 2009, 27, 163–170. [Google Scholar] [CrossRef]
- Beserra, F.P.; Xue, M.; Maia, G.L.A.; Leite Rozza, A.L.; Pellizzon, C.H.; Jackson, C.J. Lupeol, a Pentacyclic Triterpene, Promotes Migration, Wound Closure and Contractile Effect In Vitro: Possible Involvement of PI3K/Akt and p38/ERK/MAPK Pathways. Molecules 2018, 23, 2819. [Google Scholar] [CrossRef]
- Paulo, J.A.; Crous-Duran, J.; Firmino, P.N.; Faias, S.P.; Palma, J.H.N. System Report: Cork Oak Silvopastoral Systems in Portugal; The AGFORWARD Research Project WP 2; AGFORWARD: Bedfordshire, UK, 2006. [Google Scholar]
- Rizzini, C.T. Tratado de Fitogeografia do Brasil; HUCITEC, USP: São Paulo, Brazil, 1976. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Meth. Enzymol. 1987, 148, 350–382. [Google Scholar]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Nobel, P.S. Physicochemical and Environmental Plant Physiology; Academic Press: Oxford, UK, 2009. [Google Scholar]
- Bueno, A.; Alfarhan, A.; Arand, K.; Burghardt, M.; Deininger, A.-C.; Hedrich, R.; Leide, J.; Seufert, P.; Staiger, S.; Riederer, M. Temperature effects on the cuticular transpiration barrier of two desert plants with water-spender and water-saver life strategies. J. Exp. Bot. 2019, 70, 1613–1625. [Google Scholar] [CrossRef] [PubMed]
- Kolattukudy, P.E.; Agrawal, V.P. Structure and composition of aliphatic constituents of potato tuber skin (suberin). Lipids 1974, 9, 682–691. [Google Scholar] [CrossRef]
- Ferreira, J.P.; Miranda, I.; Sen, A.; Pereira, H. Chemical and cellular features of virgin and reproduction cork from Quercus variabilis. Ind. Crops Prod. 2016, 94, 638–648. [Google Scholar] [CrossRef]
- Alvin, C.R. Methods of Multivariate Analysis; Wiley: New York, NY, USA, 2002. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).