Effects of Inorganic Salt Solutions on Vigour, Viability, Oxidative Metabolism and Germination Enzymes in Aged Cabbage and Lettuce Seeds
Abstract
:1. Introduction
2. Results
2.1. Effect of the Application of Inorganic Salt Solutions on % Seedling Production and Vigour of Cabbage and Lettuce Seeds
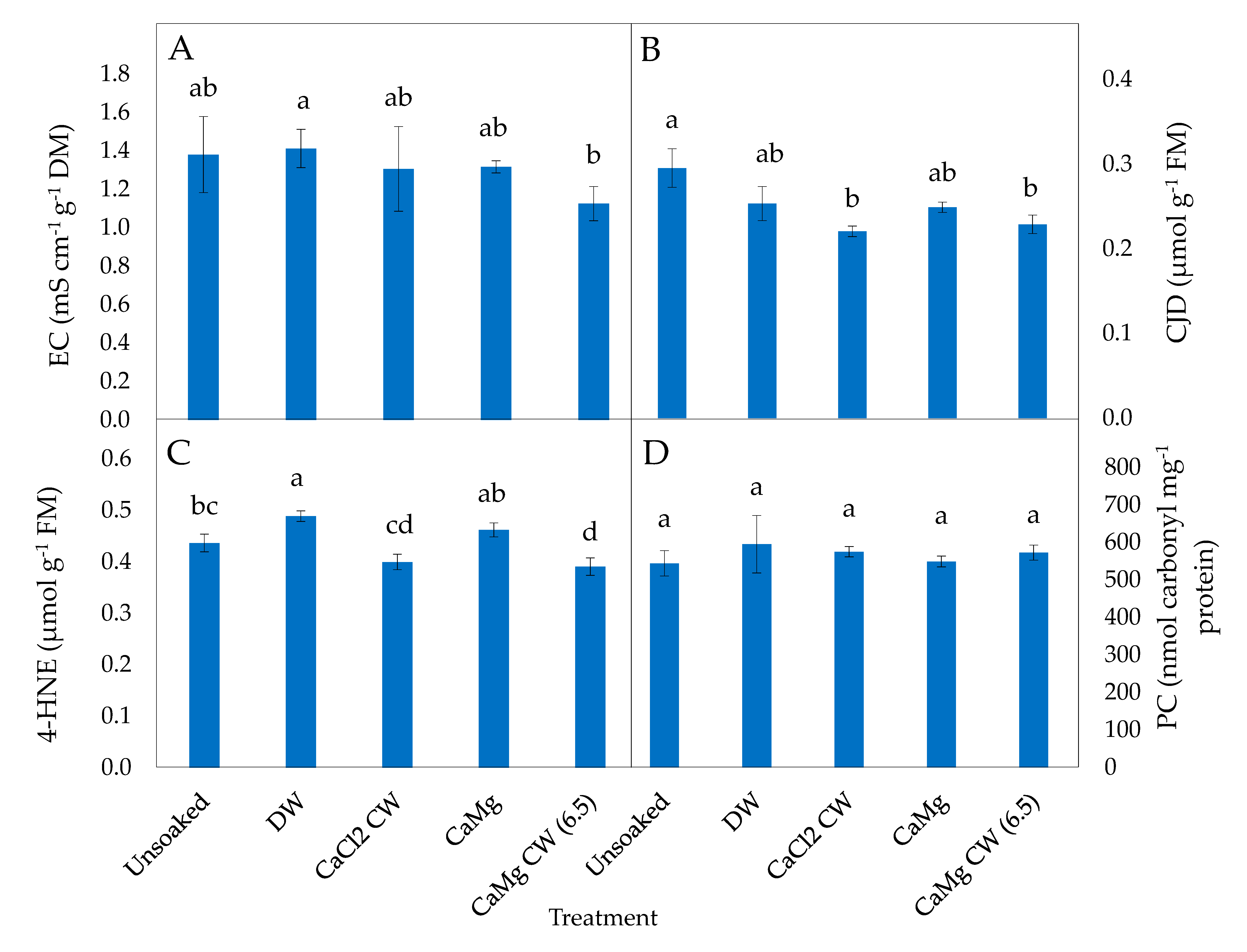
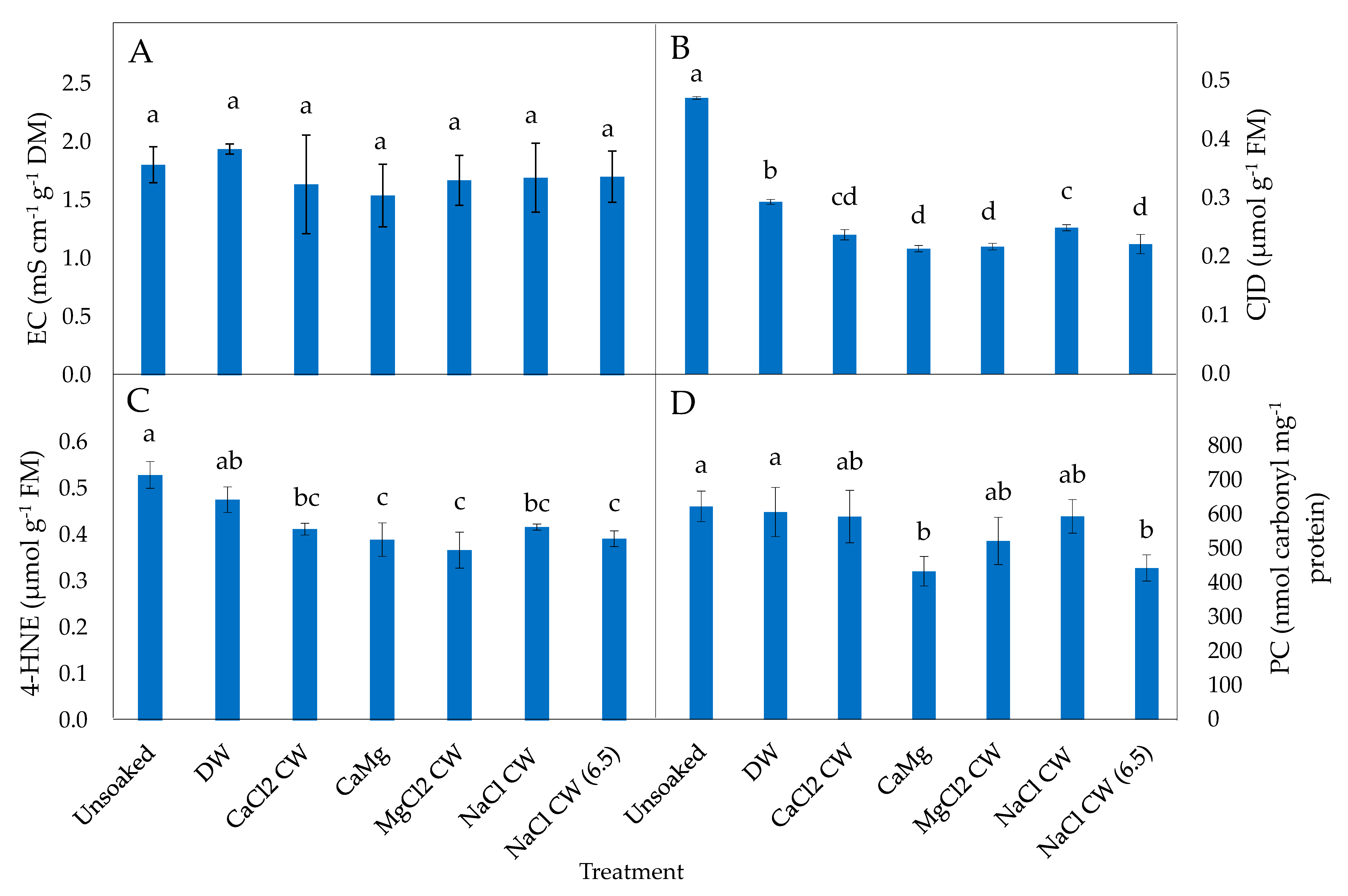
2.2. Effect of the Application of Inorganic Salt Solutions on Biomarkers of Oxidative Stress in Lettuce Seeds
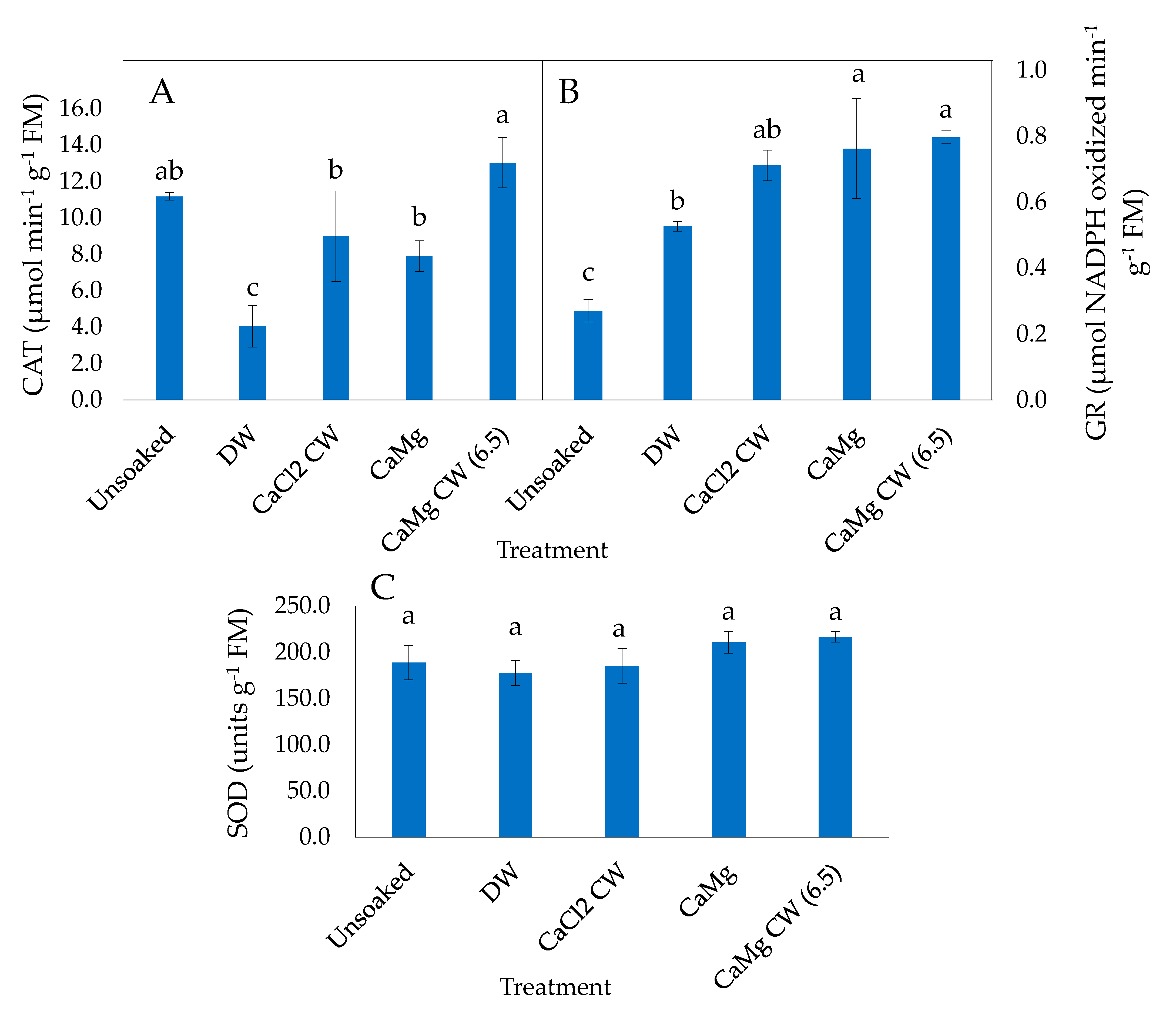
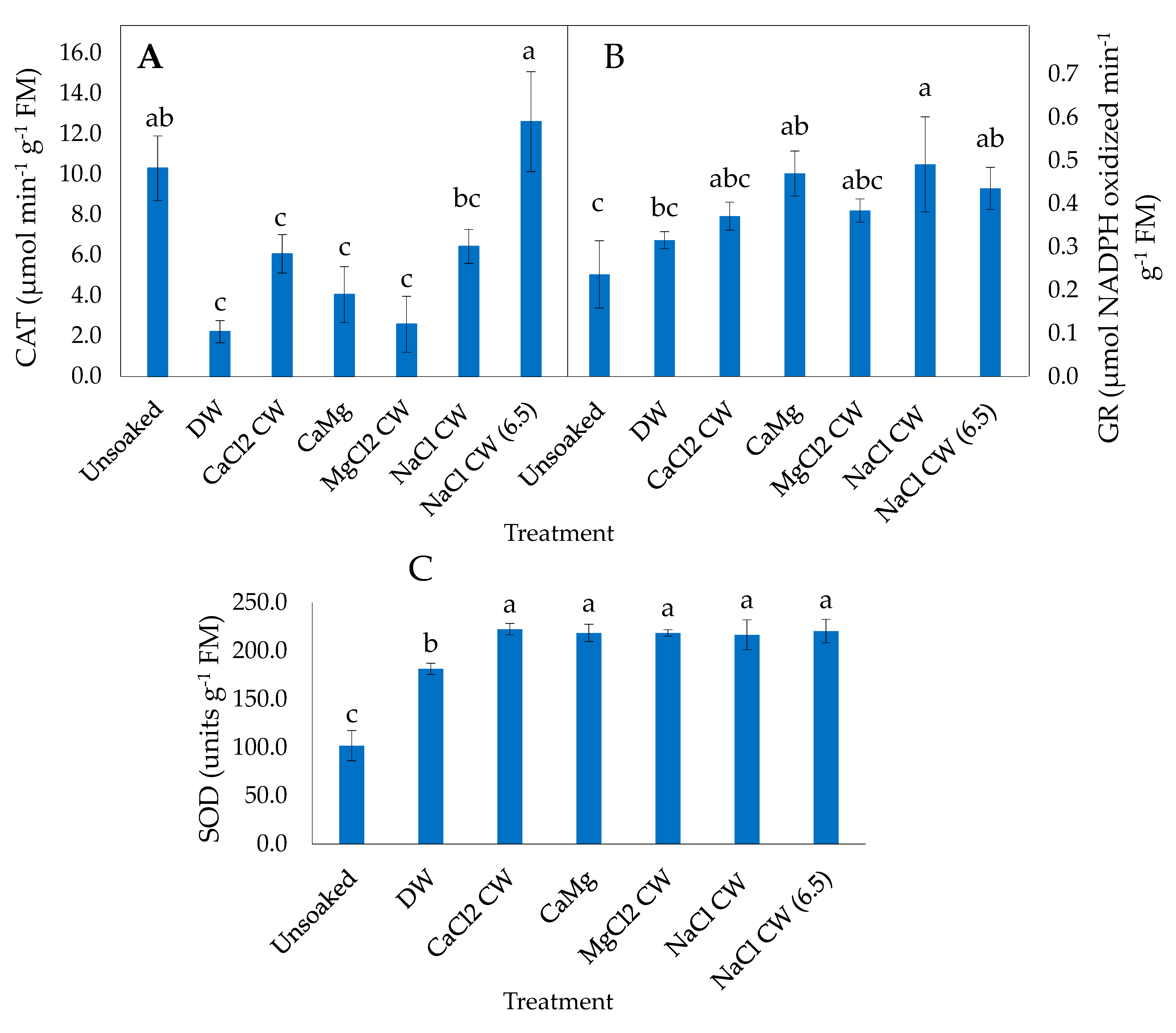
2.3. Effect of the Application of Inorganic Salt Solutions on Enzymatic Antioxidant Activities of Lettuce Seeds
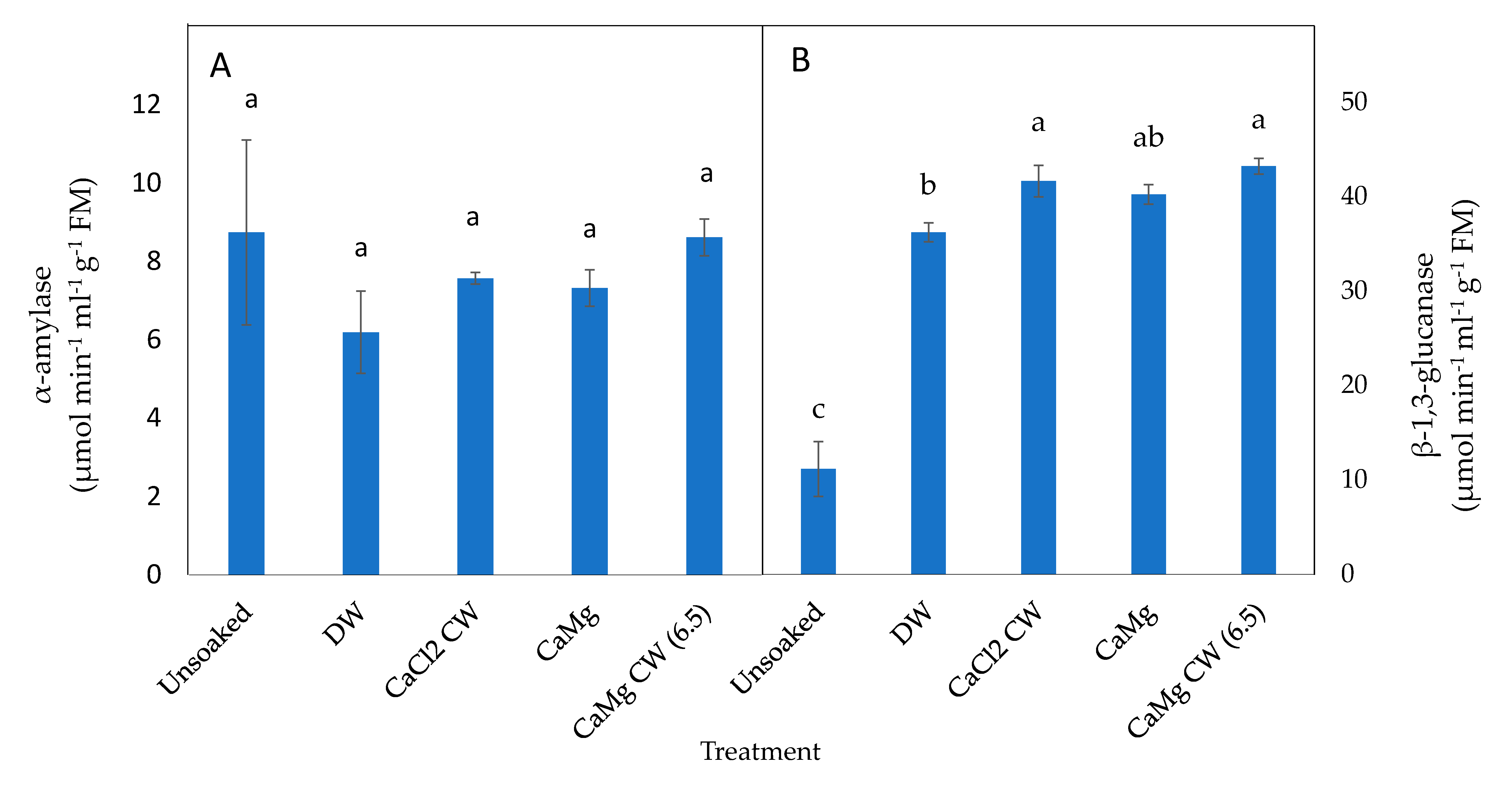
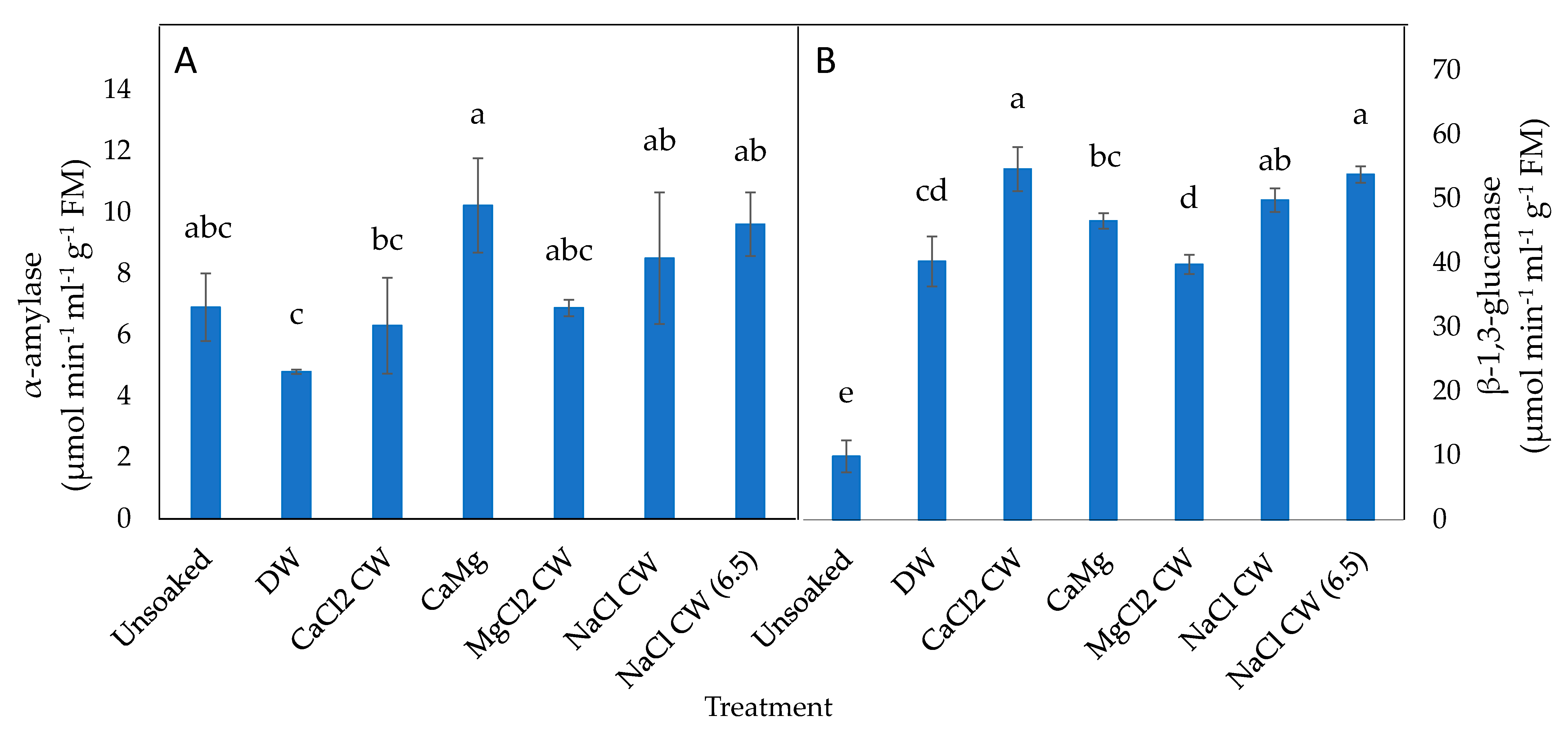
2.4. Effect of the Application of Inorganic Salt Solutions on Germination-Related Enzymes in Lettuce Seeds
3. Discussion
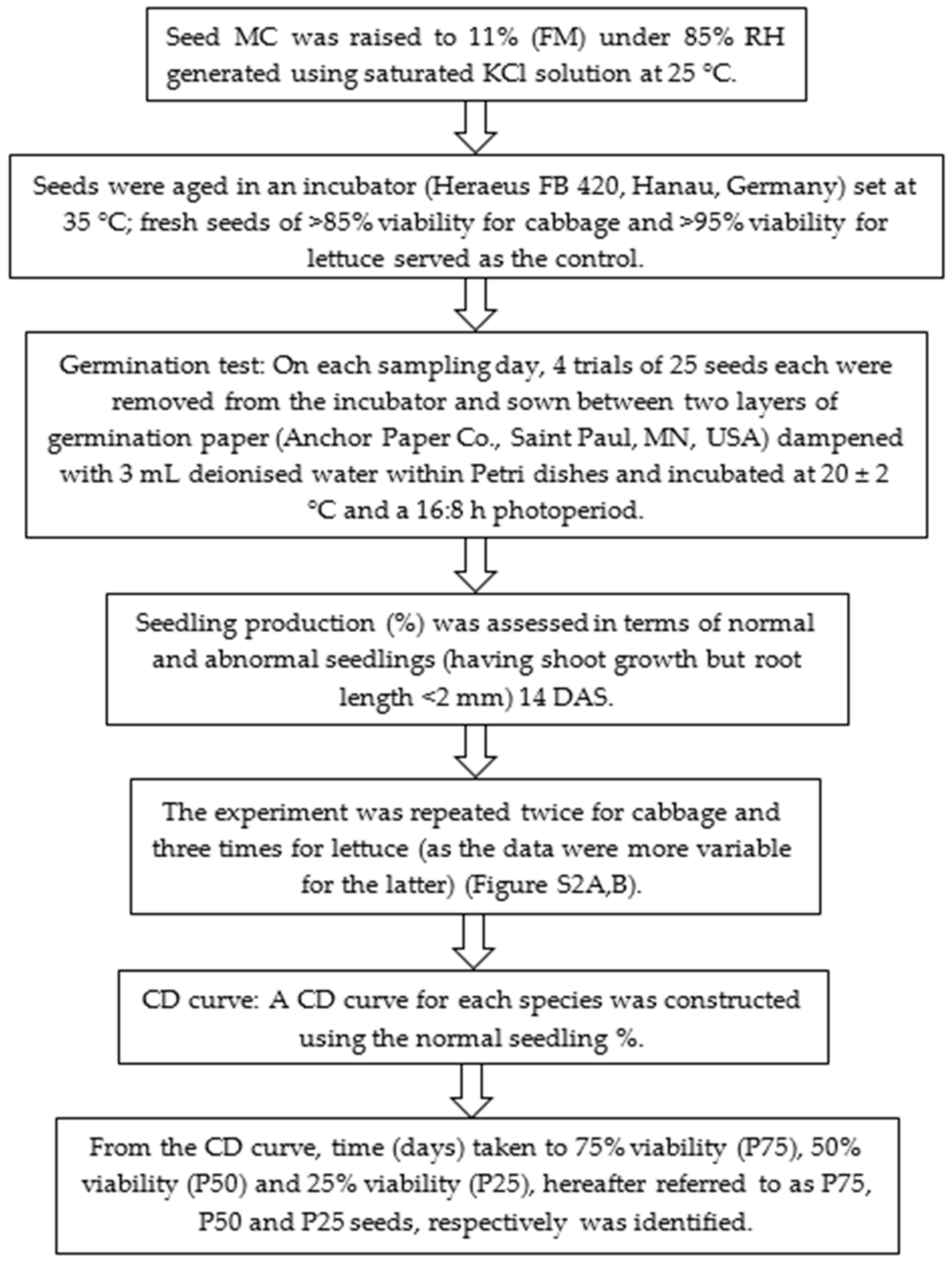
4. Materials and Methods
4.1. Seed Material
4.2. Seed Vigour Assessment
4.3. Controlled Deterioration
4.4. Preparation of Inorganic Salt Solutions
4.5. Application of Inorganic Salt Hydration Treatment
4.5.1. Assessment of Oxidative Stress and Germinability Biomarkers
Electrolyte Conductivity
Conjugated Dienes
4-Hydroxy-2-Nonenal (4-HNE)
Protein Carbonylation Evaluation
4.5.2. Enzymatic Antioxidant Activity
Catalase Activity
Glutathione Reductase
Superoxide Dismutase
4.5.3. Germination Associated Enzymes
α-Amylase Activity
β-1,3-Glucanase Activity
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tekrony, D.M.; Egli, D.B. Accumulation of seed vigour during development and maturation. Basic and applied aspects of seed biology. In Current Plant Science and Biotechnology in Agriculture; Ellis, R.H., Black, M., Murdoch, A.J., Hong, T.D., Eds.; Springer: Dordrecht, The Netherlands, 1997; Volume 30, pp. 369–384. [Google Scholar]
- Dayal, A.; Rangare, N.; Kumar, A.; Kumari, M. Effect of physiological maturity on seed quality of maize (Zea mays L.). Forage Res. 2014, 40, 1–6. [Google Scholar]
- Ibrahim, A.E.; Roberts, E.H. Viability of lettuce seeds: I. Survival in hermetic storage. J. Exp. Bot. 1983, 34, 620–630. [Google Scholar] [CrossRef]
- Walters, C.; Towill, L. Seeds and pollen. In Agriculture Handbook. The Commercial Storage of Fruits, Vegetables, and Florist and Nursery Stocks; Gross, K.C., Wang, C.Y., Saltveit, M., Eds.; USDA-ARS, National Center for Genetic Resources Preservation Preservation of Plant Germplasm Research: Fort Collins, CO, USA, 2004; pp. 735–743. ISBN 3015046128. [Google Scholar]
- Basra, S.M.A.; Ahmad, N.; Khan, M.M.; Iqbal, N.; Cheema, M.A. Assessment of cottonseed deterioration during accelerated ageing. Seed Sci. Technol. 2003, 31, 531–540. [Google Scholar] [CrossRef]
- Poonguzhali, S. Improving vigour and viability of blackgram cv.co 6 [Vigna mungo (L) Hepper] through seed priming with inorganics. Legum. Res.-Int. J. 2016, 39, 820–829. [Google Scholar] [CrossRef]
- Trawatha, S.E.; TeKrony, D.M.; Hildebrand, D.F. Relationship of soybean seed quality to fatty acid and c6-aldehyde levels during storage. Crop Sci. 1995, 35, 1415–1422. [Google Scholar] [CrossRef]
- Shaban, M. Review on Physiological aspects of seed deterioration. Int. J. Agric. Crop Sci. 2013, 6, 627–631. [Google Scholar]
- Kibinza, S.; Vinel, D.; Côme, D.; Bailly, C.; Corbineau, F. Sunflower seed deterioration as related to moisture content during ageing, energy metabolism and active oxygen species scavenging. Physiol. Plant. 2006, 128, 496–506. [Google Scholar] [CrossRef]
- Nagel, M.; Kranner, I.; Neumann, K.; Rolletschek, H.; Seal, C.E.; Colville, L.; Fernández-Marín, B.; Börner, A. Genome-wide association mapping and biochemical markers reveal that seed ageing and longevity are intricately affected by genetic background and developmental and environmental conditions in barley. Plant Cell Environ. 2015, 38, 1011–1022. [Google Scholar] [CrossRef]
- Jatoi, S.A.; Afzal, M.; Nasim, S.; Anwar, R. Seed deterioration study in pea, using accelerated ageing techniques. Pak. J. Biol. Sci. 2001, 4, 1490–1494. [Google Scholar]
- Saxena, O.P.; Singh, G.; Pakeeraiah, T.; Pandey, N. Seed deterioration studies in some vegetable seeds. Acta Hortic. 1987, 39–44. [Google Scholar] [CrossRef]
- Lee, H.-S.; Jeon, Y.-A.; Lee, Y.-Y.; Lee, S.-Y.; Kim, Y.-G. Comparison of Seed Viability Among 42 Species Stored in a Genebank. Korean J. Crop Sci. 2013, 58, 432–438. [Google Scholar] [CrossRef]
- Hill, H.; Bradford, K.J.; Cunningham, J.; Taylor, A.G. Primed Lettuce Seeds Exhibit Increased Sensitivity to Moisture Content During Controlled Deterioration. HortScience 2007, 42, 1436–1439. [Google Scholar] [CrossRef] [Green Version]
- Leprince, O.; Harren, F.J.M.; Buitink, J.; Alberda, M.; Hoekstra, F.A. Metabolic dysfunction and unabated respiration precede the loss of membrane integrity during dehydration of germinating radicles. Plant Physiol. 2000, 122, 597–608. [Google Scholar] [CrossRef] [Green Version]
- Saed-Moucheshi, A.; Shekoofa, A.; Pessarakli, M. Reactive oxygen species (ROS) generation and detoxifying in plants. J. Plant Nutr. 2014, 37, 1573–1585. [Google Scholar] [CrossRef]
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mira, S.; Estrelles, E.; González-Benito, M.E.; Corbineau, F. Biochemical changes induced in seeds of Brassicaceae wild species during ageing. Acta Physiol. Plant. 2011, 33, 1803–1809. [Google Scholar] [CrossRef]
- Feng, J.; Shen, Y.; Shi, F.; Li, C. Changes in seed germination ability, lipid peroxidation and antioxidant enzyme activities of Ginkgo biloba seed during desiccation. Forests 2017, 8, 286. [Google Scholar] [CrossRef]
- Sahu, B.; Sahu, A.K.; Thomas, V.; Naithani, S.C. Reactive oxygen species, lipid peroxidation, protein oxidation and antioxidative enzymes in dehydrating Karanj (Pongamia pinnata) seeds during storage. South African J. Bot. 2017, 112, 383–390. [Google Scholar] [CrossRef]
- Al-maskri, A.; Kharr, M.M.; Ai-mantheriand, O.; Al-habs, K. Effect of accelerated aging on lipid peroxidation, leakage and seedling vigor (RGR) in cucumber (Cucumis sativus L.) seeds. Park. J Agri. Sci 2002, 39, 330–337. [Google Scholar]
- Parkhey, S.; Naithani, S.C.; Keshavkant, S. ROS production and lipid catabolism in desiccating Shorea robusta seeds during aging. Plant Physiol. Biochem. 2012, 57, 261–267. [Google Scholar] [CrossRef]
- Kalemba, E.M.; Pukacka, S. Carbonylated proteins accumulated as vitality decreases during long-term storage of beech (Fagus sylvatica L.) seeds. Trees 2014, 28, 503–515. [Google Scholar] [CrossRef] [Green Version]
- Mirdad, Z.; Powell, A.A.; Matthews, S. Prediction of germination in artificially aged seeds of Brassica spp. using the bulk conductivity test. Seed Sci. Technol. 2006, 34, 273–286. [Google Scholar] [CrossRef]
- Smith, M.T. Membrane Changes and Lipid Peroxidation during Ageing in Seeds of Lactuca sativa L.; University of Natal: Durban, South Africa, 1986. [Google Scholar]
- Mira, S.; González-Benito, M.E.; Hill, L.M.; Walters, C. Characterization of volatile production during storage of lettuce (Lactuca sativa) seed. J. Exp. Bot. 2010, 61, 3915–3924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradford, K.J.; Steiner, J.J.; Trawatha, S.E. Seed priming influence on germination and emergence of pepper seed lots. Crop Sci. 1990, 30, 718–721. [Google Scholar] [CrossRef]
- Jett, L.W.; Welbaum, G.E.; Morse, R.D. Effects of matric and osmotic priming treatments on broccoli seed germination. J. Am. Soc. Hortic. Sci. 1996, 121, 423–429. [Google Scholar] [CrossRef]
- Delian, E.; Bădulescu, L.; Dobrescu, A.; Chira, L.; Lagunovschi-Luchian, V. A brief overview of seed priming benefits in tomato. Rom. Biotechnol. Lett. 2017, 22, 12505–12513. [Google Scholar]
- Mondal, S.; Bose, B. An impact of seed priming on disease resistance: A review. In Microbial Diversity and Biotechnology in Food Security; Kharwar, R.N., Upadhyay, R.S., Dubey, N.K., Raghuwanshi, R., Eds.; Springer India: New Delhi, India, 2014; pp. 193–203. ISBN 978-81-322-1800-5. [Google Scholar]
- Abdolahi, M.; Andelibi, B.; Zangani, E.; Shekari, F.; Jamaati-E-Somarin, S. Effect of accelerated aging and priming on seed germination of rapeseed (Brassica napus L.) cultivars. Int. Res. J. Appl. Basic Sci. 2012, 3, 499–508. [Google Scholar]
- Jisha, K.C.; Vijayakumari, K.; Puthur, J.T. Seed priming for abiotic stress tolerance: An overview. Acta Physiol. Plant. 2013, 35, 1381–1396. [Google Scholar] [CrossRef]
- Varier, A.; Vari, A.K.; Dadlani, M. The subcellular basis of seed priming. Curr. Sci. 2010, 99, 450–456. [Google Scholar]
- Carrozzi, L.E.; Creus, C.M.; Barassi, C.A.; Monterubbianesi, G.; Di Benedetto, A. Reparation of aged lettuce (Lactuca sativa) seeds by osmotic priming and Azospirillum brasilense inoculation. Botany 2012, 90, 1093–1102. [Google Scholar] [CrossRef] [Green Version]
- Khan, H.A.; Ayub, C.; Pervez, M.A.; Bilal, R.M.; Shahid, M.; Zaif, K. Effect of seed priming with NaCl on salinity tolerance of hot pepper (Capsicum annuum L.) at seedling stage. Soil Environ. 2009, 28, 81–87. [Google Scholar]
- Batool, A.; Ziaf, K.; Amjad, M. Effect of halo-priming on germination and vigor index of cabbage (Brassica oleracea var. capitata). J. Environ. Agric. Sci. 2015, 2. [Google Scholar]
- Ashraf, M.; Rauf, H. Inducing salt tolerance in maize (Zea mays L.) through seed priming with chloride salts: Growth and ion transport at early growth stages. Acta Physiol. Plant. 2001, 23, 407–414. [Google Scholar] [CrossRef]
- Pasqua, G.; Manes, F.; Monacelli, B.; Natale, L.; Anselmi, S. Effects of the culture medium pH and ion uptake in in vitro vegetative organogenesis in thin cell layers of tobacco. Plant Sci. 2002, 162, 947–955. [Google Scholar] [CrossRef]
- Berjak, P.; Sershen; Varghese, B.; Pammenter, N.W. Cathodic amelioration of the adverse effects of oxidative stress accompanying procedures necessary for cryopreservation of embryonic axes of recalcitrant-seeded species. Seed Sci. Res. 2011, 21, 187–203. [Google Scholar] [CrossRef]
- Hanaoka, K. Antioxidant effects of reduced water produced by electrolysis of sodium chloride solutions. J. Appl. Electrochem. 2001, 31, 1307–1313. [Google Scholar] [CrossRef]
- Gondwe, D.S.B.; Berjak, P.; Pammenter, N.W.; Sershen; Varghese, B. Effect of priming with cathodic water and subsequent storage on invigoration of Pisum sativum, Cucurbita maxima and Lycopersicon esculentum seeds. Seed Sci. Technol. 2016, 44, 370–381. [Google Scholar] [CrossRef]
- Hanaoka, K.; Sun, D.; Lawrence, R.; Kamitani, Y.; Fernandes, G. The mechanism of the enhanced antioxidant effects against superoxide anion radicals of reduced water produced by electrolysis. Biophys. Chem. 2004, 107, 71–82. [Google Scholar] [CrossRef]
- Nawaz, J.; Hussain, M.; Jabbar, A.; Nadeem, G.A.; Sajid, M.; Subtain, M.; Shabbir, I. Seed priming a technique. Int. J. Agric. Crop Sci. 2013, 6, 1373–1381. [Google Scholar]
- Naidoo, C.; Berjak, P.; Pammenter, N.W.; Varghese, B. The role of reactive oxygen species and antioxidants during precooling stages of axis cryopreservation in recalcitrant Trichilia dregeana. Botany 2016, 94, 391–403. [Google Scholar] [CrossRef] [Green Version]
- Sivritepe, N.; Sivritepe, H.Ö. Organic priming with seaweed extract (Ascophyllum nodosum) affects viability of pepper seeds. Asian J. Chem. 2008, 20, 5689–5694. [Google Scholar]
- Tarquis, A.M.; Bradford, K.J. Prehydration and priming treatments that advance germination also increase the rate of deterioration of lettuce seeds. J. Exp. Bot. 1992, 43, 307–317. [Google Scholar] [CrossRef]
- Hawkins, C.L.; Morgan, P.E.; Davies, M.J. Quantification of protein modification by oxidants. Free Radic. Biol. Med. 2009, 46, 965–988. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, M.; Fedorowicz-Strońska, O.; Głowacka, K.; Waśkiewicz, A.; Sadowski, J. CaCl2 treatment improves drought stress tolerance in barley (Hordeum vulgare L.). Acta Physiol. Plant. 2017, 39, 41. [Google Scholar] [CrossRef] [Green Version]
- Larkindale, J.; Knight, M.R. Protection against heat stress-induced oxidative damage in arabidopsis involves calcium, abscisic acid, ethylene, and salicylic acid. Plant Physiol. 2002, 128, 682–695. [Google Scholar] [CrossRef]
- Demidchik, V. Reactive oxygen species, oxidative stress and plant ion channels. In Ion Channels and Plant Stress Responses. Signaling and Communication in Plants; Demidchik, V., Maathuis, F., Eds.; Signaling and Communication in Plants; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2010; pp. 207–232. [Google Scholar]
- Bailly, C. Active oxygen species and antioxidants in seed biology. Seed Sci. Res. 2004, 14, 93–107. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Foreman, J.; Demidchik, V.; Bothwell, J.H.F.; Mylona, P.; Miedema, H.; Torres, M.A.; Linstead, P.; Costa, S.; Brownlee, C.; Jones, J.D.G.; et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 2003, 422, 442–446. [Google Scholar] [CrossRef]
- Demidchik, V.; Shabala, S.N.; Coutts, K.B.; Tester, M.A.; Davies, J.M. Free oxygen radicals regulate plasma membrane Ca2+- and K+-permeable channels in plant root cells. J. Cell Sci. 2003, 116, 81–88. [Google Scholar] [CrossRef] [Green Version]
- Sanders, D.; Brownlee, C.; Harper, J.F. Communicating with calcium. Plant Cell 1999, 11, 691–706. [Google Scholar] [CrossRef] [Green Version]
- Demidchik, V.; Straltsova, D.; Medvedev, S.S.; Pozhvanov, G.A.; Sokolik, A.; Yurin, V. Stress-induced electrolyte leakage: The role of K+-permeable channels and involvement in programmed cell death and metabolic adjustment. J. Exp. Bot. 2014, 65, 1259–1270. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Ashraf, M. Seed preconditioning modulates growth, ionic relations, and photosynthetic capacity in adult plants of hexaploid wheat under salt stress. J. Plant Nutr. 2007, 30, 381–396. [Google Scholar] [CrossRef]
- Ree, J.F.; Guerra, M.P. Exogenous inorganic ions, partial dehydration, and high rewarming temperatures improve peach palm (Bactris gasipaes Kunth) embryogenic cluster post-vitrification regrowth. Plant Cell Tissue Organ Cult. 2020. [Google Scholar] [CrossRef]
- Sathish, S.; Sundareswaran, S. Biochemical evaluation of seed priming in fresh and aged seeds of maize hybrid [COH(M) 5] and its parental lines. Curr. Biot. 2010, 4, 162–170. [Google Scholar]
- Chowdhury, S.R.; Choudhuri, M.A. Effects of CaCl2 and ABA on changes in H2O2, metabolism in two jute species under water deficit stress. J. Plant Physiol. 1989, 135, 179–183. [Google Scholar] [CrossRef]
- Khorshidi, M.; Nojavan, A.M. The effects of abscisic acid and CaCl2 on the activities of antioxidant enzymes under cold stress in maize seedlings in the dark. Pakistan J. Biol. Sci. 2006, 9, 54–59. [Google Scholar] [CrossRef]
- Wang, H.Y.; Chen, C.L.; Sung, J.M. Both warm water soaking and matriconditioning treatments enhance anti-oxidation of bitter gourd seeds germinated at sub-optimal temperature. Seed Sci. Technol. 2003, 31, 47–56. [Google Scholar] [CrossRef]
- Farooq, M.; Basra, S.M.A.; Wahid, A.; Khaliq, A.; Kobayashi, N. Rice seed invigoration: A review. In Organic Farming, Pest Control and Remediation of Soil Pollutants; Sustainable Agriculture Reviews; Lichtfouse, E., Ed.; Springer Netherlands: Dordrecht, The Netherlands, 2009; Volume 1, pp. 137–175. ISBN 978-1-4020-9653-2. [Google Scholar]
- de Oliveira, A.B.; Gomes-Filho, E.; Enéas-Filho, J.; Prisco, J.T.; Alencar, N.L.M. Seed priming effects on growth, lipid peroxidation, and activity of ROS scavenging enzymes in NaCl-stressed sorghum seedlings from aged seeds. J. Plant Interact. 2012, 7, 151–159. [Google Scholar] [CrossRef]
- Mano, J.; Biswas, M.S.; Sugimoto, K. Reactive carbonyl species: A missing link in ROS signaling. Plants 2019, 8, 391. [Google Scholar] [CrossRef] [Green Version]
- Juszczuk, I.M.; Tybura, A.; Rychter, A.M. Protein oxidation in the leaves and roots of cucumber plants (Cucumis sativus L.), mutant MSC16 and wild type. J. Plant Physiol. 2008, 165, 355–365. [Google Scholar] [CrossRef]
- Bailly, C.; El-Maarouf-Bouteau, H.; Corbineau, F. From intracellular signaling networks to cell death: The dual role of reactive oxygen species in seed physiology. Comptes Rendus-Biol. 2008, 331, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Dell’Aquila, A. Wheat seed ageing and embryo protein degradation. Seed Sci. Res. 1994, 4, 293–298. [Google Scholar] [CrossRef]
- Pyngrope, S.; Bhoomika, K.; Dubey, R.S. Oxidative stress, protein carbonylation, proteolysis and antioxidative defense system as a model for depicting water deficit tolerance in Indica rice seedlings. Plant Growth Regul. 2013, 69, 149–165. [Google Scholar] [CrossRef]
- Larson, R.A. Plant defenses against oxidative stress. Arch. Insect Biochem. Physiol. 1995, 29, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Siadat, S.A.; Moosavi, A.; Zadeh, M.S. Effects of seed priming on antioxidant activity and germination characteristics of maize seeds under different ageing treatment. Res. J. Seed Sci. 2012, 5, 51–62. [Google Scholar] [CrossRef]
- Demirkaya, M.; Dietz, K.J.; Sivritepe, H.O. Changes in antioxidant enzymes during ageing of onion seeds. Not. Bot. Horti Agrobot. Cluj-Napoca 2010, 38, 49–52. [Google Scholar] [CrossRef]
- Joshi, R. Role of Enzymes in seed germination. Int. J. Creat. Res. Thoughts 2018, 6, 1–5. [Google Scholar] [CrossRef]
- Simmons, C.R. The Physiology and Molecular Biology of Plant 1,3-β-D-Glucanases and 1,3;1,4-β-D-Glucanases; Taylor & Francis Group: Abingdon, UK, 1994; Volume 13, ISBN 0735268940. [Google Scholar]
- Kotake, T.; Nakagawa, N.; Takeda, K.; Sakurai, N. Auxin-induced elongation growth and expressions of cell wall-bound exo- and endo-β-glucanases in barley coleoptiles. Plant Cell Physiol. 2000, 41, 1272–1278. [Google Scholar] [CrossRef] [Green Version]
- Weir, B.L.; Paulson, K.N.; Lorenz, O. A The effect of ammoniacal nitrogen on lettuce (Lactuca saliva) and radish (Raphanus sativus) Plants. Soil Sci. Soc. Am. 1972, 462–465. [Google Scholar] [CrossRef]
- Komba, C.G.; Brunton, B.J.; Hampton, J.G. Accelerated ageing vigour testing of kale (Brassica oleracea L. var. acephala DC) seed. Seed Sci. Technol. 2006, 34, 205–208. [Google Scholar] [CrossRef]
- Tekrony, D.M. Accelerated aging test: Principles and procedures. Seed Technol. 2005, 27, 135–146. [Google Scholar]
- Mycock, D.J. Addition of calcium and magnesium to a glycerol and sucrose cryoprotectant solution improves the quality of plant embryo recovery from cryostorage. Cryo-Letters 1999, 20, 77–82. [Google Scholar]
- Gebashe, F.C. Studies on the Cryopreservation of Shoot Apices from Recalcitrant-Seeded Trichilia emetica Vahl. and Trichilia dregeana Sond. Master’s Thesis, Durban University of Technology, Durban, South Africa, 2015. [Google Scholar]
- Brocklehurst, P.A.; Dearman, J.; Drew, R.L.K. Effects of osmotic priming on seed germination and seedling growth in leek. Sci. Hortic. (Amsterdam) 1984, 24, 201–210. [Google Scholar] [CrossRef]
- Sershen; Varghese, B.; Naidoo, C.; Pammenter, N.W. The use of plant stress biomarkers in assessing the effects of desiccation in zygotic embryos from recalcitrant seeds: Challenges and considerations. Plant Biol. 2016, 18, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Augustyniak, E.; Adam, A.; Wojdyla, K.; Rogowska-wrzesinska, A.; Willetts, R.; Korkmaz, A.; Atalay, M.; Weber, D.; Grune, T.; Borsa, C.; et al. Validation of protein carbonyl measurement: A multi-centre study. Redox Biol. 2015, 4, 149–157. [Google Scholar] [CrossRef] [Green Version]
- Farrant, J.M.; Bailly, C.; Leymarie, J.; Hamman, B.; Come, D.; Corbineau, F. Wheat seedlings as a model to understand desiccation tolerance and sensitivity. Physiol. Plant. 2004, 120, 563–574. [Google Scholar] [CrossRef]
- Claiborne, A. Catalase activity. In Greenwald EA (ed) CRC Handbook of Methods for Oxygen Radical Research; CRC Press: Boca Raton, FL, USA, 1985; pp. 283–284. [Google Scholar]
- Esterbauer, H.; Grill, D. Seasonal variation of glutathione and glutathione reductase in needles of Picea abies. Plant Physiol. 1978, 61, 119–121. [Google Scholar] [CrossRef] [Green Version]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Varghese, B.; Sershen; Berjak, P.; Varghese, D.; Pammenter, N.W. Differential drying rates of recalcitrant Trichilia dregeana embryonic axes: A study of survival and oxidative stress metabolism. Physiol. Plant. 2011, 142, 326–338. [Google Scholar] [CrossRef]
- Biswas, P.K.; Devi, A.; Roy, P.K.; Paul, K.B. Enzyme activity in dormant and nondormant large crabgrass (Digitaria sanguinalis) seeds following hydration. Weed Sci. 1978, 26, 90–93. [Google Scholar] [CrossRef]
- Farashah, D.H.; Afshari, T.R.; Sharifzadeh, F.; Chavoshinasab, S. Germination improvement and α-amylase and β-1,3-glucanase activity in dormant and nondormant seeds of oregano (Origanum vulgare). Aust. J. Crop Sci. 2011, 5, 421–427. [Google Scholar]
- Bernfeld, P. Amylases, alpha and β. Methods Enzymol. I 1955, I, 149–158. [Google Scholar] [CrossRef]
- Baker, J.E. Purification and partial characterization of α-amylase allozymes from the lesser grain borer, Rhyzopertha dominica. Insect Biochem. 1991, 21, 303–311. [Google Scholar] [CrossRef]
- Celestino, K.R.S.; Cunha, R.B.; Felix, C.R. Characterization of a β-glucanase produced by Rhizopus microsporus var. microsporus, and its potential for application in the brewing industry. BMC Biochem. 2006, 7, 23. [Google Scholar] [CrossRef] [Green Version]
- Subbarao, G.V.; Ito, O.; Berry, W.L.; Wheeler, R.M. Sodium—a functional plant nutrient. CRC. Crit. Rev. Plant Sci. 2003, 22, 391–416. [Google Scholar] [CrossRef]
- Kronzucker, H.J.; Coskun, D.; Schulze, L.M.; Wong, J.R.; Britto, D.T. Sodium as nutrient and toxicant. Plant Soil 2013, 369, 1–23. [Google Scholar] [CrossRef] [Green Version]
| Hydration Treatments | AS (%) for P75 | AS (%) for P50 | AS (%) for P25 | AS (%) for P75 | AS (%) for P50 | AS (%) for P25 |
|---|---|---|---|---|---|---|
| Cabbage Seeds | Cabbage Seeds | Cabbage Seeds | Lettuce Seeds | Lettuce Seeds | Lettuce Seeds | |
| DW | 11.00 ± 8.75 NS | 18.50 ± 10.89 a | 20.00 ± 5.24 a | 6.50 ± 6.74 NS | 11.50 ± 9.18 NS | 9.00 ± 4.14 b |
| CaCl2 | 5.50 ± 5.21 NS | 9.00 ± 8.49 NS | 12.50 ± 8.67 NS | 7.00 ± 5.13 NS | 9.00 ± 7.33 NS | 13.00 ± 5.95 NS |
| CaCl2 CW | 1.50 ± 2.98 NS | 7.50 ± 6.95 NS | 7.50 ±9.18 b | 3.00 ± 4.66 NS | 4.00 ± 5.66 NS | 7.00 ± 7.63 NS |
| CaMg | 6.50 ± 6.39 NS | 12.50 ± 3.34 NS | 8.00 ± 5.24 b | 3.00 ± 04.66 NS | 10.50 ± 5.21 NS | 8.50 ± 6.21 NS |
| CaMg CW | 5.00 ± 5.95 NS | 17.50 ± 7.39 NS | 6.00 ± 5.24 b | 3.50 ± 4.50 NS | 5.50 ± 7.07 NS | 11.00 ± 4.66 NS |
| CaMg CW (6.5) | 7.00 ± 5.55 NS | 14.00 ± 4.78 NS | 10.00 ± 4.78 b | 3.00 ± 3.55 NS | 4.00 ± 3.02 NS | 8.00 ± 6.76 NS |
| MgCl2 | 6.50 ± 6.39 NS | 10.50 ± 4.24 NS | 8.00 ± 4.78 b | 4.00 ± 6.05 NS | 2.50 ± 5.63 NS | 12.00 ± 5.66 NS |
| MgCl2 CW | 7.00 ± 6.32 NS | 13.50 ± 5.21 NS | 14.00 ± 5.66 NS | 1.50 ± 2.98 NS | 6.50 ± 6.39 NS | 11.00 ± 5.13 NS |
| NaCl | 16.00 ± 8.00 NS | 23.50 ± 6.57 NS | 4.75 ± 1.58 b | 2.75 ± 1.83 NS | 18.00 ± 3.70 NS | 19.00 ± 4.14 a |
| NaCl CW | 9.50 ± 5.63 NS | 7.50 ± 4.14 NS | 9.00 ± 8.21 b | 0.50 ± 1.41 NS | 8.50 ± 5.83 NS | 10.50 ± 6.02 NS |
| NaCl CW (6.5) | 8.00 ± 4.78 NS | 6.50 ± 5.95 b | 9.00 ± 5.95 b | 5.00 ± 4.14 NS | 6.00 ± 5.66 NS | 5.00 ± 6.32 NS |
| Hydration Treatments | % Normal Seedlings for Fresh Seeds | % Normal Seedlings for CDd (P75) Seeds | % Normal Seedlings for CDd (P50) Seeds | % Normal Seedlings for CDd (P25) Seeds |
|---|---|---|---|---|
| DW | 89.00 ± 9.26 NS | 72.00 ± 14.81 NS | 43.00 ± 10.64 a | 20.00 ± 3.02 NS |
| CaCl2 | 94.00 ± 6.76 NS | 71.00 ± 9.74 NS | 26.00 ± 7.09 b | 17.00 ± 7.33 NS |
| CaCl2 CW | 94.50 ± 5.21 NS | 78.50 ± 10.01 NS | 21.50 ± 10.24 b | 19.00 ± 6.32 NS |
| CaMg | 92.50 ± 7.54 NS | 63.50 ± 12.91 NS | 36.00 ± 6.05 NS | 23.50 ± 10.13 NS |
| CaMg CW | 91.50 ± 9.43 NS | 72.5 ± 10.99 NS | 49.50 ± 9.30 NS | 22.00 ± 3.70 NS |
| CaMg CW (6.5) | 96.50 ± 5.83 NS | 69.50 ± 12.46 NS | 48.00 ± 7.41 NS | 26.50 ± 3.66 NS |
| MgCl2 | 95.00 ± 7.01 NS | 78.00 ± 8.28 NS | 35.00 ± 9.50 NS | 14.50 ± 8.26 NS |
| MgCl2 CW | 92.50 ± 9.90 NS | 75.50+11.60 NS | 40.00 ± 7.71 NS | 21.50 ± 10.68 NS |
| NaCl | 93.50 ± 5.47 NS | 59.00 ± 4.14 NS | 48.00 ± 9.80 NS | 24.50 ± 7.84 NS |
| NaCl CW | 95.50 ± 4.99 NS | 75.00 ± 5.13 NS | 34.00 ± 10.25 NS | 25.50 ± 8.26 NS |
| NaCl CW (6.5) | 94.00 ± 8.00 NS | 69.50 ± 11.70 NS | 48.50 ± 6.91 NS | 32.00 ± 9.32 NS |
| Hydration Treatments | % Normal Seedling for Fresh Seeds | % Normal Seedling for CDd (P75) Seeds | % Normal Seedling for CDd (P50) Seeds | % Normal Seedling for CDd (P25) Seeds |
|---|---|---|---|---|
| DW | 99.00 ± 1.85 NS | 73.50 ± 9.78 NS | 50.00 ± 10.03 b | 21.00 ± 6.32 b |
| CaCl2 | 100.00 ± 0.00 NS | 70.00 ± 8.28 NS | 58.00 ± 11.51 NS | 32.00 ± 11.31 NS |
| CaCl2 CW | 100.00 ± 0.00 NS | 74.00 ± 10.03 NS | 67.00 ± 12.42 a | 39.00 ± 11.06 a |
| CaMg | 98.50 ± 4.24 NS | 76.50 ± 10.57 NS | 76.50 ± 10.57 a | 40.00 ± 9.56 a |
| CaMg CW | 100.00 ± 0.00 NS | 72.50 ± 4.50 NS | 63.00 ± 5.95 NS | 23.00 ± 6.32 NS |
| CaMg CW (6.5) | 99.00 ± 1.85 NS | 76.50 ± 5.83 NS | 68.00 ± 10.90 a | 29.00 ± 5.13 NS |
| MgCl2 | 99.00 ± 1.85 NS | 83.00 ± 9.50 NS | 68.00 ± 10.90 NS | 28.00 ± 11.11 NS |
| MgCl2 CW | 99.50 ± 1.41 NS | 71.00 ± 11.06 NS | 57.00 ± 12.96 NS | 39.00 ± 10.20 a |
| NaCl | 98.00 ± 2.20 NS | 76.25 ± 5.06 NS | 50.00 ± 9.07 NS | 24.50 ± 6.57 NS |
| NaCl CW | 100.00 ± 0.00 NS | 78.50 ± 6.02 NS | 51.50 ± 11.99 NS | 40.00 ± 14.18 a |
| NaCl CW (6.5) | 99.50 ± 1.41 NS | 74.00 ± 6.76 NS | 68.00 ± 10.90 NS | 48.00 ± 15.57 a |
| Controlled Deterioration Level | Treatments | Seedling Vigour Index | |
|---|---|---|---|
| Cabbage | Lettuce | ||
| P50 | DW | 1621.40 ± 911.97 b | 1056.90 ± 323.25 b |
| CaCl2 | 1564.90 ± 522.56 NS | 2705.20 ± 629.67 a | |
| CaCl2 CW | 1352.90 ± 797.91 NS | 2819.10 ± 580.95 a | |
| CaMg | 2855.00 ± 688.79 NS | 2823.50 ± 585.35 a | |
| CaMg CW | 2284.20 ± 781.18 NS | 1772.80 ± 362.52 NS | |
| CaMg CW (6.5) | 3395.10 ± 1073.31 a | 3493.20 ± 1077.06 a | |
| MgCl2 | 3042.80 ± 1570.89 NS | 2525.20 ± 607.15 NS | |
| MgCl2 CW | 2938.70 ± 776.86 NS | 2755.80 ± 1094.63 a | |
| NaCl | 2644.10 ± 1514.80 NS | 2000.80 ± 510.49 NS | |
| NaCl CW | 2019.30 ± 1042.40 NS | 2717.40 ± 906.07 a | |
| NaCl CW (6.5) | 3520.30 ± 910.90 a | 2981.20 ± 795.30 a | |
| P25 | DW | 535.10 ± 222.14 b | 403.30 ± 199.74 b |
| CaCl2 | 903.00 ± 597.52 NS | 1090.93 ± 404.74 NS | |
| CaCl2 CW | 1198.40 ± 534.58 NS | 1341.80 ± 362.03 a | |
| CaMg | 1679.00 ± 796.61 a | 1144.80 ± 405.07 NS | |
| CaMg CW | 531.50 ± 294.91 NS | 490.40 ± 296.44 NS | |
| CaMg CW (6.5) | 1084.00 ± 321.11 NS | 794.40 ± 395.29 NS | |
| MgCl2 | 785.50 ± 627.33 NS | 747.80 ± 511.32 NS | |
| MgCl2 CW | 1084.00 ± 648.73 NS | 1205.80 ± 282.89 NS | |
| NaCl | 1438.70 ± 426.89 NS | 404.20 ± 147.05 NS | |
| NaCl CW | 1633.10 ± 801.91 a | 1493.80 ± 668.57 a | |
| NaCl CW (6.5) | 2205.18 ± 1003.93 a | 1562.30 ± 1038.11 a | |
| S/n | Pre-Hydration Treatment Solution | Concentration of Constituent(s) | pH |
|---|---|---|---|
| 1 | Control | Deionised water | 5.6 |
| 2 | CaCl2 (non-electrolysed) | 1 mM CaCl2 | 6.0 |
| 3 | CaCl2 generated CW | 1 mM CaCl2 | 10.8 |
| 4 | CaMg (non-electrolysed) | 1 µm CaCl2; 1 mM MgCl2 | 5.9 |
| 5 | CaMg generated CW | 1 µm CaCl2; 1 mM MgCl2 | 11.2 |
| 6 | CaMg generated CW adjusted to pH 6.5 | 1 µm CaCl2; 1 mM MgCl2 | 6.5 |
| 7 | MgCl2 solution (non-electrolysed) | 1 mM MgCl2 | 5.9 |
| 8 | MgCl2 generated CW | 1 mM MgCl2 | 10.6 |
| 9 | NaCl solution (non-electrolysed) | 1 mM NaCl | 5.6 |
| 10 | NaCl generated CW | 1 mM NaCl | 11.2 |
| 11 | NaCl generated CW adjusted to pH 6.5 | 1 mM NaCl | 6.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adetunji, A.E.; Sershen; Varghese, B.; Pammenter, N.W. Effects of Inorganic Salt Solutions on Vigour, Viability, Oxidative Metabolism and Germination Enzymes in Aged Cabbage and Lettuce Seeds. Plants 2020, 9, 1164. https://doi.org/10.3390/plants9091164
Adetunji AE, Sershen, Varghese B, Pammenter NW. Effects of Inorganic Salt Solutions on Vigour, Viability, Oxidative Metabolism and Germination Enzymes in Aged Cabbage and Lettuce Seeds. Plants. 2020; 9(9):1164. https://doi.org/10.3390/plants9091164
Chicago/Turabian StyleAdetunji, Ademola Emmanuel, Sershen, Boby Varghese, and Norman W. Pammenter. 2020. "Effects of Inorganic Salt Solutions on Vigour, Viability, Oxidative Metabolism and Germination Enzymes in Aged Cabbage and Lettuce Seeds" Plants 9, no. 9: 1164. https://doi.org/10.3390/plants9091164






