Photosynthetic Traits and Nitrogen Uptake in Crops: Which Is the Role of Arbuscular Mycorrhizal Fungi?
Abstract
1. Introduction
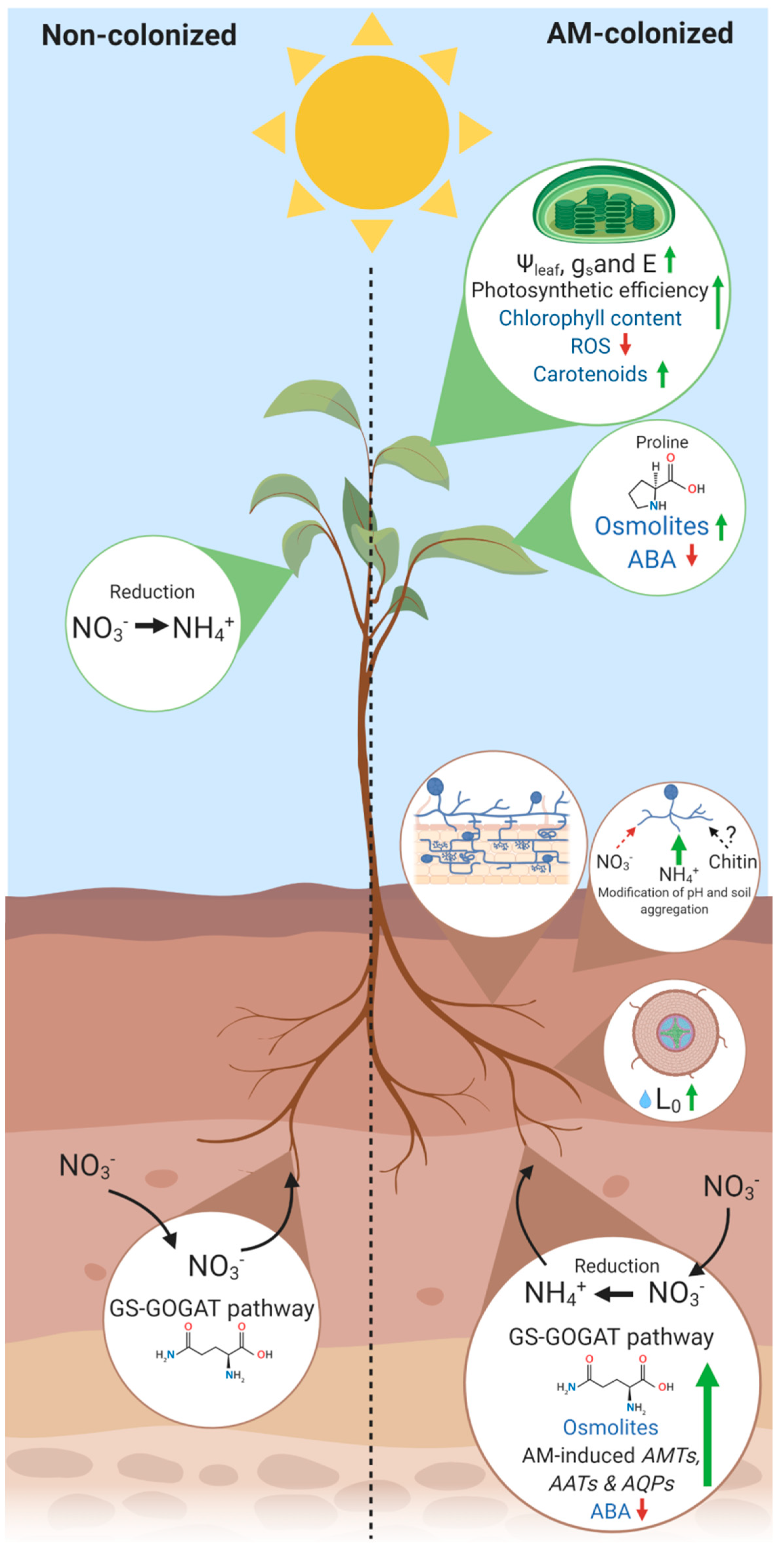
2. AM Symbiosis-Mediated Nitrogen Acquisition in Plant
2.1. Nitrogen Use in Agricultural Practices and Biological Significance in the Plant Life
2.2. AM-Mediated Effects in Soil N-Cycling and Plant Acquisition
3. Linking Root-Colonization by AM Fungi to Plant Water Relations, Biochemical and Photosynthetic Performances
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ort, D.R.; Merchant, S.S.; Alric, J.; Barkan, A.; Blankenship, R.E.; Bock, R.; Croce, R.; Hanson, M.R.; Hibberd, J.M.; Long, S.P. Redesigning photosynthesis to sustainably meet global food and bioenergy demand. Proc. Natl. Acad. Sci. USA 2015, 112, 8529–8536. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Parker, J.E.; Ainsworth, E.A.; Oldroyd, G.E.; Schroeder, J.I. Genetic strategies for improving crop yields. Nature 2019, 575, 109–118. [Google Scholar] [CrossRef]
- Jansson, C.; Vogel, J.; Hazen, S.; Brutnell, T.; Mockler, T. Climate-smart crops with enhanced photosynthesis. J. Exp. Bot. 2018, 69, 3801–3809. [Google Scholar] [CrossRef] [PubMed]
- IPCC 2020 Intergovernmental Panel on Climate Change. Climate Change and Land: Summary for Policymakers. Available online: https://www.ipcc.ch/reports/ (accessed on 1 June 2020).
- Zhang, X.; He, D.; Niu, G.; Yan, Z.; Song, J. Effects of environment lighting on the growth, photosynthesis, and quality of hydroponic lettuce in a plant factory. Int. J. Agric. Biol. Eng. 2018, 11, 33–40. [Google Scholar] [CrossRef]
- Compant, S.; Samad, A.; Faist, H.; Sessitsch, A. A review on the plant microbiome: Ecology, functions, and emerging trends in microbial application. J. Adv. Res. 2019, 19, 29–37. [Google Scholar] [CrossRef]
- Chen, M.; Arato, M.; Borghi, L.; Nouri, E.; Reinhardt, D. Beneficial services of arbuscular mycorrhizal fungi–from ecology to application. Front. Plant Sci. 2018, 9, 1270. [Google Scholar] [CrossRef]
- Wipf, D.; Krajinski, F.; van Tuinen, D.; Recorbet, G.; Courty, P. Trading on the arbuscular mycorrhiza market: From arbuscules to common mycorrhizal networks. New Phytol. 2019, 223, 1127–1142. [Google Scholar] [CrossRef]
- Walder, F.; van der Heijden, M.G. Regulation of resource exchange in the arbuscular mycorrhizal symbiosis. Nature Plants 2015, 1, 1–7. [Google Scholar] [CrossRef]
- Black, K.; Mitchell, D.; Osborne, B. Effect of mycorrhizal-enhanced leaf phosphate status on carbon partitioning, translocation and photosynthesis in cucumber. Plant Cell Environ. 2000, 23, 797–809. [Google Scholar] [CrossRef]
- Churchland, C.; Grayston, S.J. Specificity of plant-microbe interactions in the tree mycorrhizosphere biome and consequences for soil C cycling. Front. Microbiol. 2014, 5, 261. [Google Scholar] [CrossRef] [PubMed]
- Gorzelak, M.A.; Asay, A.K.; Pickles, B.J.; Simard, S.W. Inter-plant communication through mycorrhizal networks mediates complex adaptive behaviour in plant communities. AoB Plants 2015, 7, plv050. [Google Scholar] [CrossRef]
- Kaiser, C.; Kilburn, M.R.; Clode, P.L.; Fuchslueger, L.; Koranda, M.; Cliff, J.B.; Solaiman, Z.M.; Murphy, D.V. Exploring the transfer of recent plant photosynthates to soil microbes: Mycorrhizal pathway vs direct root exudation. New Phytol. 2015, 205, 1537–1551. [Google Scholar] [CrossRef]
- Elliott, A.J.; Daniell, T.J.; Cameron, D.D.; Field, K.J. A commercial arbuscular mycorrhizal inoculum increases root colonization across wheat cultivars but does not increase assimilation of mycorrhiza-acquired nutrients. Plants People Planet 2020. [Google Scholar] [CrossRef]
- Fiorilli, V.; Vannini, C.; Ortolani, F.; Garcia-Seco, D.; Chiapello, M.; Novero, M.; Domingo, G.; Terzi, V.; Morcia, C.; Bagnaresi, P. Omics approaches revealed how arbuscular mycorrhizal symbiosis enhances yield and resistance to leaf pathogen in wheat. Sci. Rep. 2018, 8, 1–18. [Google Scholar] [CrossRef]
- Cervantes-Gámez, R.G.; Bueno-Ibarra, M.A.; Cruz-Mendívil, A.; Calderón-Vázquez, C.L.; Ramírez-Douriet, C.M.; Maldonado-Mendoza, I.E.; Villalobos-López, M.Á.; Valdez-Ortíz, Á.; López-Meyer, M. Arbuscular mycorrhizal symbiosis-induced expression changes in Solanum lycopersicum leaves revealed by RNA-seq analysis. Plant Mol. Biol. Rep. 2016, 34, 89–102. [Google Scholar] [CrossRef]
- Miozzi, L.; Vaira, A.M.; Brilli, F.; Casarin, V.; Berti, M.; Ferrandino, A.; Nerva, L.; Accotto, G.P.; Lanfranco, L. Arbuscular Mycorrhizal Symbiosis Primes Tolerance to Cucumber Mosaic Virus in Tomato. Viruses 2020, 12, 675. [Google Scholar] [CrossRef] [PubMed]
- Balestrini, R.; Chitarra, W.; Antoniou, C.; Ruocco, M.; Fotopoulos, V. Improvement of plant performance under water deficit with the employment of biological and chemical priming agents. J. Agric. Sci. 2018, 156, 680–688. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Chanratana, M.; Kim, K.; Seshadri, S.; Sa, T. Impact of arbuscular mycorrhizal fungi on photosynthesis, water status, and gas exchange of plants under salt stress–a meta-analysis. Front. Plant Sci. 2019, 10, 457. [Google Scholar] [CrossRef]
- Li, S.; Yang, W.; Guo, J.; Li, X.; Lin, J.; Zhu, X. Changes in photosynthesis and respiratory metabolism of maize seedlings growing under low temperature stress may be regulated by arbuscular mycorrhizal fungi. Plant Physiol. Biochem. 2020, 154, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Smil, V. Detonator of the population explosion. Nature 1999, 400, 415. [Google Scholar] [CrossRef]
- Pingali, P.L. Green revolution: Impacts, limits, and the path ahead. Proc. Natl. Acad. Sci. USA 2012, 109, 12302–12308. [Google Scholar] [CrossRef] [PubMed]
- Pretty, J. Intensification for redesigned and sustainable agricultural systems. Science 2018, 362. [Google Scholar] [CrossRef]
- Chen, J.G.; Crooks, R.M.; Seefeldt, L.C.; Bren, K.L.; Bullock, R.M.; Darensbourg, M.Y.; Holland, P.L.; Hoffman, B.M.; Janik, M.J.; Jones, A.K.; et al. Beyond fossil fuel–driven nitrogen transformations. Science 2018, 360, eaar6611. [Google Scholar] [CrossRef]
- Withers, P.J.; Neal, C.; Jarvie, H.P.; Doody, D.G. Agriculture and eutrophication: Where do we go from here? Sustainability 2014, 6, 5853–5875. [Google Scholar] [CrossRef]
- Jansa, J.; Forczek, S.T.; Rozmoš, M.; Püschel, D.; Bukovská, P.; Hršelová, H. Arbuscular mycorrhiza and soil organic nitrogen: Network of players and interactions. Chem. Biol. Technol. Agric. 2019, 6, 10. [Google Scholar] [CrossRef]
- Cassman, K.G. Ecological intensification of cereal production systems: Yield potential, soil quality, and precision agriculture. Proc. Natl. Acad. Sci. USA 1999, 96, 5952–5959. [Google Scholar] [CrossRef] [PubMed]
- Dellagi, A.; Quillere, I.; Hirel, B. Beneficial soil-borne bacteria and fungi: A promising way to improve plant nitrogen acquisition. J. Exp. Bot. 2020, 71, 4469–4479. [Google Scholar] [CrossRef] [PubMed]
- Coskun, D.; Britto, D.T.; Shi, W.; Kronzucker, H.J. How plant root exudates shape the nitrogen cycle. Trends Plant Sci. 2017, 22, 661–673. [Google Scholar] [CrossRef]
- Jacoby, R.; Peukert, M.; Succurro, A.; Koprivova, A.; Kopriva, S. The role of soil microorganisms in plant mineral nutrition—Current knowledge and future directions. Front. Plant Sci. 2017, 8, 1617. [Google Scholar] [CrossRef]
- Liu, H.; Carvalhais, L.C.; Crawford, M.; Singh, E.; Dennis, P.G.; Pieterse, C.M.; Schenk, P.M. Inner plant values: Diversity, colonization and benefits from endophytic bacteria. Front. Microbiol. 2017, 8, 2552. [Google Scholar] [CrossRef]
- Cordovez, V.; Dini-Andreote, F.; Carrión, V.J.; Raaijmakers, J.M. Ecology and evolution of plant microbiomes. Annu. Rev. Microbiol. 2019, 73, 69–88. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Cheng, Y.-H.; Chen, K.-E.; Tsay, Y.-F. Nitrate transport, signaling, and use efficiency. Annu. Rev. Plant Biol. 2018, 69, 85–122. [Google Scholar] [CrossRef] [PubMed]
- Hirel, B.; Krapp, A. Nitrogen Utilization in Plants I Biological and Agronomic Importance. In Reference Module in Life Sciences; Elsevier BV: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Courty, P.E.; Smith, P.; Koegel, S.; Redecker, D.; Wipf, D. Inorganic nitrogen uptake and transport in beneficial plant root-microbe interactions. Crit. Rev. Plant Sci. 2015, 34, 4–16. [Google Scholar] [CrossRef]
- Valkov, V.T.; Sol, S.; Rogato, A.; Chiurazzi, M. The functional characterization of LjNRT2. 4 indicates a novel, positive role of nitrate for an efficient nodule N2-fixation activity. New Phytol. 2020. [Google Scholar] [CrossRef]
- Hirel, B.; Le Gouis, J.; Ney, B.; Gallais, A. The challenge of improving nitrogen use efficiency in crop plants: Towards a more central role for genetic variability and quantitative genetics within integrated approaches. J. Exp. Bot. 2007, 58, 2369–2387. [Google Scholar] [CrossRef]
- Spiertz, J.; De Vos, N. Agronomical and physiological aspects of the role of nitrogen in yield formation of cereals. Plant Soil 1983, 75, 379–391. [Google Scholar] [CrossRef]
- Bellaloui, N.; Pilbeam, D. Reduction of nitrate in leaves of tomato during vegetative growth. J. Plant Nutr. 1990, 13, 39–55. [Google Scholar] [CrossRef]
- Gloser, V.; Zwieniecki, M.A.; Orians, C.M.; Holbrook, N.M. Dynamic changes in root hydraulic properties in response to nitrate availability. J. Exp. Bot. 2007, 58, 2409–2415. [Google Scholar] [CrossRef]
- Clarkson, D.T.; Carvajal, M.; Henzler, T.; Waterhouse, R.N.; Smyth, A.J.; Cooke, D.T.; Steudle, E. Root hydraulic conductance: Diurnal aquaporin expression and the effects of nutrient stress. J. Exp. Bot. 2000, 51, 61–70. [Google Scholar] [CrossRef]
- Field, C.; Mooney, H. Leaf age and seasonal effects on light, water, and nitrogen use efficiency in a California shrub. Oecologia 1983, 56, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Pilbeam, D.J. The utilization of nitrogen by plants: A whole plant perspective. Ann. Plant Rev. Online 2018, 305–351. [Google Scholar] [CrossRef]
- Evans, J.R.; Clarke, V.C. The nitrogen cost of photosynthesis. J. Exp. Bot. 2019, 70, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Rogers, A.; Medlyn, B.E.; Dukes, J.S.; Bonan, G.; Von Caemmerer, S.; Dietze, M.C.; Kattge, J.; Leakey, A.D.; Mercado, L.M.; Niinemets, Ü. A roadmap for improving the representation of photosynthesis in Earth system models. New Phytol. 2017, 213, 22–42. [Google Scholar] [CrossRef] [PubMed]
- Tao, K.; Kelly, S.; Radutoiu, S. Microbial associations enabling nitrogen acquisition in plants. Curr. Opin. Microbiol. 2019, 49, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Lehnert, N.; Dong, H.T.; Harland, J.B.; Hunt, A.P.; White, C.J. Reversing nitrogen fixation. Nat. Rev. Chem. 2018, 2, 278–289. [Google Scholar] [CrossRef]
- Veresoglou, S.D.; Chen, B.; Rillig, M.C. Arbuscular mycorrhiza and soil nitrogen cycling. Soil Biol. Biochem. 2012, 46, 53–62. [Google Scholar] [CrossRef]
- Marschner, P.; Baumann, K. Changes in bacterial community structure induced by mycorrhizal colonisation in split-root maize. Plant Soil 2003, 251, 279–289. [Google Scholar] [CrossRef]
- Vázquez, M.M.; César, S.; Azcón, R.; Barea, J.M. Interactions between arbuscular mycorrhizal fungi and other microbial inoculants (Azospirillum, Pseudomonas, Trichoderma) and their effects on microbial population and enzyme activities in the rhizosphere of maize plants. Appl. Soil Ecol. 2000, 15, 261–272. [Google Scholar] [CrossRef]
- Chen, A.; Gu, M.; Wang, S.; Chen, J.; Xu, G. Transport Properties and Regulatory Roles of Nitrogen in Arbuscular Mycorrhizal Symbiosis; Elsevier: Amsterdam, The Netherlands, 2018; Volume 74, pp. 80–88. [Google Scholar]
- Hodge, A. Arbuscular mycorrhizal fungi influence decomposition of, but not plant nutrient capture from, glycine patches in soil. New Phytol. 2001, 151, 725–734. [Google Scholar] [CrossRef]
- Hodge, A.; Campbell, C.D.; Fitter, A.H. An arbuscular mycorrhizal fungus accelerates decomposition and acquires nitrogen directly from organic material. Nature 2001, 413, 297–299. [Google Scholar] [CrossRef]
- Whiteside, M.D.; Garcia, M.O.; Treseder, K.K. Amino acid uptake in arbuscular mycorrhizal plants. PLoS ONE 2012, 7, e47643. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Pfeffer, P.; Douds, D.; Piotrowski, E.; Lammers, P.; Shachar-Hill, Y. The uptake, metabolism, transport and transfer of nitrogen in an arbuscular mycorrhizal symbiosis. New Phytol. 2005, 168, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Belmondo, S.; Fiorilli, V.; Pérez-Tienda, J.; Ferrol, N.; Marmeisse, R.; Lanfranco, L. A dipeptide transporter from the arbuscular mycorrhizal fungus Rhizophagus irregularis is upregulated in the intraradical phase. Front. Plant Sci. 2014, 5, 436. [Google Scholar] [CrossRef] [PubMed]
- Johansen, A.; Finlay, R.D.; OLSSON, P.A. Nitrogen metabolism of external hyphae of the arbuscular mycorrhizal fungus Glomus intraradices. New Phytol. 1996, 133, 705–712. [Google Scholar] [CrossRef]
- Govindarajulu, M.; Pfeffer, P.E.; Jin, H.; Abubaker, J.; Douds, D.D.; Allen, J.W.; Bücking, H.; Lammers, P.J.; Shachar-Hill, Y. Nitrogen transfer in the arbuscular mycorrhizal symbiosis. Nature 2005, 435, 819–823. [Google Scholar] [CrossRef]
- Breuninger, M.; Trujillo, C.G.; Serrano, E.; Fischer, R.; Requena, N. Different nitrogen sources modulate activity but not expression of glutamine synthetase in arbuscular mycorrhizal fungi. Fungal Genet. Biol. 2004, 41, 542–552. [Google Scholar] [CrossRef]
- Kaldorf, M.; Schmelzer, E.; Bothe, H. Expression of maize and fungal nitrate reductase genes in arbuscular mycorrhiza. Mol. Plant Microbe Interact. 1998, 11, 439–448. [Google Scholar] [CrossRef]
- Tharanathan, R.N.; Kittur, F.S. Chitin—The undisputed biomolecule of great potential. Crit. Rev. Food Sci. Nutr. 2003, 43, 61–87. [Google Scholar] [CrossRef]
- Bukovská, P.; Bonkowski, M.; Konvalinková, T.; Beskid, O.; Hujslová, M.; Püschel, D.; Řezáčová, V.; Gutiérrez-Núñez, M.S.; Gryndler, M.; Jansa, J. Utilization of organic nitrogen by arbuscular mycorrhizal fungi—Is there a specific role for protists and ammonia oxidizers? Mycorrhiza 2018, 28, 269–283. [Google Scholar] [CrossRef]
- Kobae, Y.; Kawachi, M.; Saito, K.; Kikuchi, Y.; Ezawa, T.; Maeshima, M.; Hata, S.; Fujiwara, T. Up-regulation of genes involved in N-acetylglucosamine uptake and metabolism suggests a recycling mode of chitin in intraradical mycelium of arbuscular mycorrhizal fungi. Mycorrhiza 2015, 25, 411–417. [Google Scholar] [CrossRef]
- Smith, S.E.; Smith, F.A. Roles of arbuscular mycorrhizas in plant nutrition and growth: New paradigms from cellular to ecosystem scales. Annu. Rev. Plant Biol. 2011, 62, 227–250. [Google Scholar] [CrossRef] [PubMed]
- Guether, M.; Neuhäuser, B.; Balestrini, R.; Dynowski, M.; Ludewig, U.; Bonfante, P. A mycorrhizal-specific ammonium transporter from Lotus japonicus acquires nitrogen released by arbuscular mycorrhizal fungi. Plant Physiol. 2009, 150, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Uehlein, N.; Fileschi, K.; Eckert, M.; Bienert, G.P.; Bertl, A.; Kaldenhoff, R. Arbuscular mycorrhizal symbiosis and plant aquaporin expression. Phytochemistry 2007, 68, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Hogekamp, C.; Arndt, D.; Pereira, P.A.; Becker, J.D.; Hohnjec, N.; Küster, H. Laser microdissection unravels cell-type-specific transcription in arbuscular mycorrhizal roots, including CAAT-box transcription factor gene expression correlating with fungal contact and spread. Plant Physiol. 2011, 157, 2023–2043. [Google Scholar] [CrossRef]
- Xu, G.; Fan, X.; Miller, A.J. Plant nitrogen assimilation and use efficiency. Annu. Rev. Plant Biol. 2012, 63, 153–182. [Google Scholar] [CrossRef]
- Hildebrandt, U.; Schmelzer, E.; Bothe, H. Expression of nitrate transporter genes in tomato colonized by an arbuscular mycorrhizal fungus. Physiol. Plant. 2002, 115, 125–136. [Google Scholar] [CrossRef]
- Hohnjec, N.; Vieweg, M.F.; Pühler, A.; Becker, A.; Küster, H. Overlaps in the transcriptional profiles of Medicago truncatula roots inoculated with two different Glomus fungi provide insights into the genetic program activated during arbuscular mycorrhiza. Plant Physiol. 2005, 137, 1283–1301. [Google Scholar] [CrossRef]
- Guether, M.; Balestrini, R.; Hannah, M.; He, J.; Udvardi, M.K.; Bonfante, P. Genome-wide reprogramming of regulatory networks, transport, cell wall and membrane biogenesis during arbuscular mycorrhizal symbiosis in Lotus japonicus. New Phytol. 2009, 182, 200–212. [Google Scholar] [CrossRef]
- Guether, M.; Volpe, V.; Balestrini, R.; Requena, N.; Wipf, D.; Bonfante, P. LjLHT1.2—A mycorrhiza-inducible plant amino acid transporter from Lotus japonicus. Biol. Fertil. Soils 2011, 47, 925. [Google Scholar] [CrossRef]
- Cliquet, J.B.; Murray, P.J.; Boucaud, J. Effect of the arbuscular mycorrhizal fungus Glomus fasciculatum on the uptake of amino nitrogen by Lolium perenne. New Phytol. 1997, 137, 345–349. [Google Scholar] [CrossRef]
- Nouri, E.; Breuillin-Sessoms, F.; Feller, U.; Reinhardt, D. Phosphorus and nitrogen regulate arbuscular mycorrhizal symbiosis in Petunia hybrida. PLoS ONE 2014, 9, e90841. [Google Scholar] [CrossRef] [PubMed]
- Nanjareddy, K.; Blanco, L.; Arthikala, M.; Affantrange, X.A.; Sánchez, F.; Lara, M. Nitrate regulates rhizobial and mycorrhizal symbiosis in common bean (Phaseolus vulgaris L.). J. Integr. Plant Biol. 2014, 56, 281–298. [Google Scholar] [CrossRef] [PubMed]
- Püschel, D.; Janoušková, M.; Hujslová, M.; Slavíková, R.; Gryndlerová, H.; Jansa, J. Plant–fungus competition for nitrogen erases mycorrhizal growth benefits of Andropogon gerardii under limited nitrogen supply. Ecol. Evol. 2016, 6, 4332–4346. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, L.; Palla, M.; Agnolucci, M.; Avio, L.; Sbrana, C.; Turrini, A.; Giovannetti, M. Arbuscular mycorrhizal fungi and associated microbiota as plant biostimulants: Research strategies for the selection of the best performing inocula. Agronomy 2020, 10, 106. [Google Scholar] [CrossRef]
- Jansa, J.; Bukovská, P.; Gryndler, M. Mycorrhizal hyphae as ecological niche for highly specialized hypersymbionts–or just soil free-riders? Front. Plant Sci. 2013, 4, 134. [Google Scholar] [CrossRef]
- Irankhah, S.; Chitarra, W.; Nerva, L.; Antoniou, C.; Lumini, E.; Volpe, V.; Ganjeali, A.; Cheniany, M.; Mashreghi, M.; Fotopoulos, V. Impact of an arbuscular mycorrhizal fungal inoculum and exogenous MeJA on fenugreek secondary metabolite production under water deficit. Environ. Exp. Bot. 2020, 176, 104096. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, M.; Liu, Y.; Zhang, F.; Hodge, A.; Feng, G. Carbon and phosphorus exchange may enable cooperation between an arbuscular mycorrhizal fungus and a phosphate-solubilizing bacterium. New Phytol. 2016, 210, 1022–1032. [Google Scholar] [CrossRef]
- Hestrin, R.; Hammer, E.C.; Mueller, C.W.; Lehmann, J. Synergies between mycorrhizal fungi and soil microbial communities increase plant nitrogen acquisition. Commun. Biol. 2019, 2, 1–9. [Google Scholar] [CrossRef]
- Torres-Cortés, G.; Ghignone, S.; Bonfante, P.; Schüßler, A. Mosaic genome of endobacteria in arbuscular mycorrhizal fungi: Transkingdom gene transfer in an ancient mycoplasma-fungus association. Proc. Natl. Acad. Sci. USA 2015, 112, 7785–7790. [Google Scholar] [CrossRef]
- Paul, K.; Saha, C.; Nag, M.; Mandal, D.; Naiya, H.; Sen, D.; Mitra, S.; Kumar, M.; Bose, D.; Mukherjee, G. A tripartite interaction among the basidiomycete Rhodotorula mucilaginosa, N2-fixing endobacteria, and rice improves plant nitrogen nutrition. Plant Cell 2020, 32, 486–507. [Google Scholar] [CrossRef]
- De Novais, C.B.; Sbrana, C.; da Conceição Jesus, E.; Rouws, L.F.M.; Giovannetti, M.; Avio, L.; Siqueira, J.O.; Júnior, O.J.S.; da Silva, E.M.R.; de Faria, S.M. Mycorrhizal networks facilitate the colonization of legume roots by a symbiotic nitrogen-fixing bacterium. Mycorrhiza 2020, 30, 389–396. [Google Scholar] [CrossRef]
- Simard, S.W. Mycorrhizal networks facilitate tree communication, learning, and memory. In Memory and Learning in Plants; Springer: Berlin, Germany, 2018; pp. 191–213. [Google Scholar]
- He, Y.; Cornelissen, J.H.; Wang, P.; Dong, M.; Ou, J. Nitrogen transfer from one plant to another depends on plant biomass production between conspecific and heterospecific species via a common arbuscular mycorrhizal network. Environ. Sci. Pollut. Res. 2019, 26, 8828–8837. [Google Scholar] [CrossRef]
- Sylvia, D.; Hammond, L.; Bennett, J.; Haas, J.; Linda, S. Field response of maize to a VAM fungus and water management. Agron. J. 1993, 85, 193–198. [Google Scholar] [CrossRef]
- Ruiz-Lozano, J.; Azcón, R.; Gomez, M. Effects of arbuscular-mycorrhizal glomus species on drought tolerance: Physiological and nutritional plant responses. Appl. Environ. Microbiol. 1995, 61, 456–460. [Google Scholar] [CrossRef] [PubMed]
- Augé, R.M. Arbuscular mycorrhizae and soil/plant water relations. Can. J. Soil Sci. 2004, 84, 373–381. [Google Scholar] [CrossRef]
- Kaya, C.; Higgs, D.; Kirnak, H.; Tas, I. Mycorrhizal colonisation improves fruit yield and water use efficiency in watermelon (Citrullus lanatus Thunb.) grown under well-watered and water-stressed conditions. Plant Soil 2003, 253, 287–292. [Google Scholar] [CrossRef]
- Boomsma, C.R.; Vyn, T.J. Maize drought tolerance: Potential improvements through arbuscular mycorrhizal symbiosis? Field Crop. Res. 2008, 108, 14–31. [Google Scholar] [CrossRef]
- Basu, S.; Rabara, R.C.; Negi, S. AMF: The future prospect for sustainable agriculture. Physiol. Mol. Plant Pathol. 2018, 102, 36–45. [Google Scholar] [CrossRef]
- Ruiz-Lozano, J.; Azcón, R. Hyphal contribution to water uptake in mycorrhizal plants as affected by the fungal species and water status. Physiol. Plant. 1995, 95, 472–478. [Google Scholar] [CrossRef]
- Augé, R.M.; Toler, H.D.; Sams, C.E.; Nasim, G. Hydraulic conductance and water potential gradients in squash leaves showing mycorrhiza-induced increases in stomatal conductance. Mycorrhiza 2008, 18, 115–121. [Google Scholar] [CrossRef]
- Ruiz-Lozano, J.M. Arbuscular mycorrhizal symbiosis and alleviation of osmotic stress. New perspectives for molecular studies. Mycorrhiza 2003, 13, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Poca, M.; Coomans, O.; Urcelay, C.; Zeballos, S.R.; Bodé, S.; Boeckx, P. Isotope fractionation during root water uptake by Acacia caven is enhanced by arbuscular mycorrhizas. Plant Soil 2019, 441, 485–497. [Google Scholar] [CrossRef]
- Bitterlich, M.; Franken, P.; Graefe, J. Arbuscular mycorrhiza improves substrate hydraulic conductivity in the plant available moisture range under root growth exclusion. Front. Plant Sci. 2018, 9, 301. [Google Scholar] [CrossRef]
- Zhu, Y.-G.; Smith, A.F.; Smith, S.E. Phosphorus efficiencies and responses of barley (Hordeum vulgare L.) to arbuscular mycorrhizal fungi grown in highly calcareous soil. Mycorrhiza 2003, 13, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Hoeksema, J.D.; Chaudhary, V.B.; Gehring, C.A.; Johnson, N.C.; Karst, J.; Koide, R.T.; Pringle, A.; Zabinski, C.; Bever, J.D.; Moore, J.C. A meta-analysis of context-dependency in plant response to inoculation with mycorrhizal fungi. Ecol. Lett. 2010, 13, 394–407. [Google Scholar] [CrossRef]
- Quiroga, G.; Erice, G.; Aroca, R.; Delgado-Huertas, A.; Ruiz-Lozano, J.M. Elucidating the Possible Involvement of Maize Aquaporins and Arbuscular Mycorrhizal Symbiosis in the Plant Ammonium and Urea Transport under Drought Stress Conditions. Plants 2020, 9, 148. [Google Scholar] [CrossRef]
- Wulf, A.; Manthey, K.; Doll, J.; Perlick, A.M.; Linke, B.; Bekel, T.; Meyer, F.; Franken, P.; Küster, H.; Krajinski, F. Transcriptional changes in response to arbuscular mycorrhiza development in the model plant Medicago truncatula. Mol. Plant Microbe Interact. 2003, 16, 306–314. [Google Scholar] [CrossRef]
- Krajinski, F.; Biela, A.; Schubert, D.; Gianinazzi-Pearson, V.; Kaldenhoff, R.; Franken, P. Arbuscular mycorrhiza development regulates the mRNA abundance of Mtaqp1 encoding a mercury-insensitive aquaporin of Medicago truncatula. Planta 2000, 211, 85–90. [Google Scholar] [CrossRef]
- Groppa, M.D.; Benavides, M.P.; Zawoznik, M.S. Root hydraulic conductance, aquaporins and plant growth promoting microorganisms: A revision. Appl. Soil Ecol. 2012, 61, 247–254. [Google Scholar] [CrossRef]
- Jia-Dong, H.; Tao, D.; Hui-Hui, W.; Ying-Ning, Z.; Qiang-Sheng, W.; Kamil, K. Mycorrhizas induce diverse responses of root TIP aquaporin gene expression to drought stress in trifoliate orange. Sci. Hortic. 2019, 243, 64–69. [Google Scholar] [CrossRef]
- Bienert, G.P.; Bienert, M.D.; Jahn, T.P.; Boutry, M.; Chaumont, F. Solanaceae XIPs are plasma membrane aquaporins that facilitate the transport of many uncharged substrates. Plant J. 2011, 66, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Maurel, C.; Verdoucq, L.; Rodrigues, O. Aquaporins and plant transpiration. Plant Cell Environ. 2016, 39, 2580–2587. [Google Scholar] [CrossRef] [PubMed]
- Laloux, T.; Junqueira, B.; Maistriaux, L.C.; Ahmed, J.; Jurkiewicz, A.; Chaumont, F. Plant and mammal aquaporins: Same but different. Int. J. Mol. Sci. 2018, 19, 521. [Google Scholar] [CrossRef] [PubMed]
- Bárzana, G.; Aroca, R.; Bienert, G.P.; Chaumont, F.; Ruiz-Lozano, J.M. New insights into the regulation of aquaporins by the arbuscular mycorrhizal symbiosis in maize plants under drought stress and possible implications for plant performance. Mol. Plant Microbe Interact. 2014, 27, 349–363. [Google Scholar] [CrossRef]
- Dynowski, M.; Schaaf, G.; Loque, D.; Moran, O.; Ludewig, U. Plant plasma membrane water channels conduct the signalling molecule H2O2. Biochem. J. 2008, 414, 53–61. [Google Scholar] [CrossRef]
- Watts-Williams, S.J.; Cavagnaro, T.R.; Tyerman, S.D. Variable effects of arbuscular mycorrhizal fungal inoculation on physiological and molecular measures of root and stomatal conductance of diverse Medicago truncatula accessions. Plant Cell Environ. 2019, 42, 285–294. [Google Scholar] [CrossRef]
- Bárzana, G.; Aroca, R.; Paz, J.A.; Chaumont, F.; Martinez-Ballesta, M.C.; Carvajal, M.; Ruiz-Lozano, J.M. Arbuscular mycorrhizal symbiosis increases relative apoplastic water flow in roots of the host plant under both well-watered and drought stress conditions. Ann. Bot. 2012, 109, 1009–1017. [Google Scholar] [CrossRef]
- Levy, Y.; Syvertsen, J.; Nemec, S. Effect of drought stress and vesicular–arbuscular mycorrhiza on citrus transpiration and hydraulic conductivity of roots. New Phytol. 1983, 93, 61–66. [Google Scholar] [CrossRef]
- Graham, J.; Syvertsen, J. Influence of vesicular–arbuscular mycorrhiza on the hydraulic conductivity of roots of two citrus rootstocks. New Phytol. 1984, 97, 277–284. [Google Scholar] [CrossRef]
- Duan, X.; Neuman, D.S.; Reiber, J.M.; Green, C.D.; Saxton, A.M.; Augé, R.M. Mycorrhizal influence on hydraulic and hormonal factors implicated in the control of stomatal conductance during drought. J. Exp. Bot. 1996, 47, 1541–1550. [Google Scholar] [CrossRef]
- Dell’Amico, J.; Torrecillas, A.; Rodriguez, P.; Morte, A.; Sánchez-Blanco, M.J. Responses of tomato plants associated with the arbuscular mycorrhizal fungus Glomus clarum during drought and recovery. J. Agric. Sci. 2002, 138, 387. [Google Scholar] [CrossRef]
- Sánchez-Blanco, M.J.; Ferrández, T.; Morales, M.A.; Morte, A.; Alarcón, J.J. Variations in water status, gas exchange, and growth in Rosmarinus officinalis plants infected with Glomus deserticola under drought conditions. J. Plant Physiol. 2004, 161, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Augé, R.M.; Toler, H.D.; Saxton, A.M. Arbuscular mycorrhizal symbiosis alters stomatal conductance of host plants more under drought than under amply watered conditions: A meta-analysis. Mycorrhiza 2015, 25, 13–24. [Google Scholar] [CrossRef]
- Chitarra, W.; Pagliarani, C.; Maserti, B.; Lumini, E.; Siciliano, I.; Cascone, P.; Schubert, A.; Gambino, G.; Balestrini, R.; Guerrieri, E. Insights on the impact of arbuscular mycorrhizal symbiosis on tomato tolerance to water stress. Plant Physiol. 2016, 171, 1009–1023. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Facelli, E.; Pope, S.; Smith, F.A. Plant performance in stressful environments: Interpreting new and established knowledge of the roles of arbuscular mycorrhizas. Plant Soil 2010, 326, 3–20. [Google Scholar] [CrossRef]
- Subramanian, K.; Charest, C.; Dwyer, L.; Hamilton, R. Effects of arbuscular mycorrhizae on leaf water potential, sugar content, and P content during drought and recovery of maize. Can. J. Bot. 1997, 75, 1582–1591. [Google Scholar] [CrossRef]
- Kubikova, E.; Jennifer, L.M.; Bonnie, H.O.; Michael, D.M.; Augé, M.R. Mycorrhizal impact on osmotic adjustment in Ocimum basilicum during a lethal drying episode. J. Plant Physiol. 2001, 158, 1227–1230. [Google Scholar] [CrossRef]
- Porcel, R.; Ruiz-Lozano, J.M. Arbuscular mycorrhizal influence on leaf water potential, solute accumulation, and oxidative stress in soybean plants subjected to drought stress. J. Exp. Bot. 2004, 55, 1743–1750. [Google Scholar] [CrossRef]
- Wu, Q.-S.; Xia, R.-X. Arbuscular mycorrhizal fungi influence growth, osmotic adjustment and photosynthesis of citrus under well-watered and water stress conditions. J. Plant Physiol. 2006, 163, 417–425. [Google Scholar] [CrossRef]
- Yooyongwech, S.; Samphumphuang, T.; Tisarum, R.; Theerawitaya, C.; Cha-um, S. Arbuscular mycorrhizal fungi (AMF) improved water deficit tolerance in two different sweet potato genotypes involves osmotic adjustments via soluble sugar and free proline. Sci. Hortic. 2016, 198, 107–117. [Google Scholar] [CrossRef]
- Mo, Y.; Wang, Y.; Yang, R.; Zheng, J.; Liu, C.; Li, H.; Ma, J.; Zhang, Y.; Wei, C.; Zhang, X. Regulation of plant growth, photosynthesis, antioxidation and osmosis by an arbuscular mycorrhizal fungus in watermelon seedlings under well-watered and drought conditions. Front. Plant Sci. 2016, 7, 644. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Bie, Z.; Liu, Z.; Zhen, A.; Jiao, X. Improving cucumber photosynthetic capacity under NaCl stress by grafting onto two salt-tolerant pumpkin rootstocks. Biol. Plant. 2011, 55, 285–290. [Google Scholar] [CrossRef]
- Porcel, R.; Redondo-Gómez, S.; Mateos-Naranjo, E.; Aroca, R.; Garcia, R.; Ruiz-Lozano, J.M. Arbuscular mycorrhizal symbiosis ameliorates the optimum quantum yield of photosystem II and reduces non-photochemical quenching in rice plants subjected to salt stress. J. Plant Physiol. 2015, 185, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.-Q.; Zhu, H.-H.; Zhao, H.-Q.; Yao, Q. Arbuscular mycorrhizal fungal inoculation increases phenolic synthesis in clover roots via hydrogen peroxide, salicylic acid and nitric oxide signaling pathways. J. Plant Physiol. 2013, 170, 74–79. [Google Scholar] [CrossRef]
- Joshi, J.; Mueller-Cajar, O.; Tsai, Y.-C.C.; Hartl, F.U.; Hayer-Hartl, M. Role of small subunit in mediating assembly of red-type form I Rubisco. J. Biol. Chem. 2015, 290, 1066–1074. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, H.; Zou, C.; Li, Y.; Chen, Y.; Wang, Z.; Jiang, Y.; Liu, A.; Zhao, P.; Wang, M. Combined inoculation with multiple arbuscular mycorrhizal fungi improves growth, nutrient uptake and photosynthesis in cucumber seedlings. Front. Microbiol. 2017, 8, 2516. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, S.A.L.; Domingues Jr, A.P.; Mazzafera, P. Photosynthesis is induced in rice plants that associate with arbuscular mycorrhizal fungi and are grown under arsenate and arsenite stress. Chemosphere 2015, 134, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.Q.; Tang, M.; Zhang, H. Arbuscular mycorrhizal fungi enhanced the growth, photosynthesis, and calorific value of black locust under salt stress. Photosynthetica 2017, 55, 378–385. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, H.; Zhang, X.; Tang, M. Arbuscular mycorrhizal symbiosis alleviates salt stress in black locust through improved photosynthesis, water status, and K+/Na+ homeostasis. Front. Plant Sci. 2017, 8, 1739. [Google Scholar] [CrossRef]
- Li, J.; Meng, B.; Chai, H.; Yang, X.; Song, W.; Li, S.; Lu, A.; Zhang, T.; Sun, W. Arbuscular mycorrhizal fungi alleviate drought stress in C3 (Leymus chinensis) and C4 (Hemarthria altissima) grasses via altering antioxidant enzyme activities and photosynthesis. Front. Plant Sci. 2019, 10, 499. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, H.; Zhang, X.; Tang, M. Arbuscular mycorrhizal symbiosis mitigates oxidative injury in black locust under salt stress through modulating antioxidant defence of the plant. Environ. Exp. Bot. 2020, 175, 104034. [Google Scholar] [CrossRef]
- Baslam, M.; Goicoechea, N. Water deficit improved the capacity of arbuscular mycorrhizal fungi (AMF) for inducing the accumulation of antioxidant compounds in lettuce leaves. Mycorrhiza 2012, 22, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Begum, N.; Ahanger, M.A.; Su, Y.; Lei, Y.; Mustafa, N.S.A.; Ahmad, P.; Zhang, L. Improved Drought Tolerance by AMF Inoculation in Maize (Zea mays) Involves Physiological and Biochemical Implications. Plants 2019, 8, 579. [Google Scholar] [CrossRef] [PubMed]
- Begum, N.; Ahanger, M.A.; Zhang, L. AMF inoculation and phosphorus supplementation alleviates drought induced growth and photosynthetic decline in Nicotiana tabacum by up-regulating antioxidant metabolism and osmolyte accumulation. Environ. Exp. Bot. 2020, 176, 104088. [Google Scholar] [CrossRef]
- Wu, Q.S.; Zou, Y.N.; Liu, W.; Ye, X.; Zai, H.; Zhao, L. Alleviation of salt stress in citrus seedlings inoculated with mycorrhiza: Changes in leaf antioxidant defense systems. Plant Soil Environ. 2010, 56, 470–475. [Google Scholar] [CrossRef]
- Ren, C.-G.; Kong, C.-C.; Yan, K.; Xie, Z.-H. Transcriptome analysis reveals the impact of arbuscular mycorrhizal symbiosis on Sesbania cannabina expose to high salinity. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Mathur, S.; Tomar, R.S.; Jajoo, A. Arbuscular mycorrhizal fungi (AMF) protects photosynthetic apparatus of wheat under drought stress. Photosynth. Res. 2019, 139, 227–238. [Google Scholar] [CrossRef]
- Hazzoumi, Z.; Moustakime, Y.; Joutei, K.A. Effect of arbuscular mycorrhizal fungi (AMF) and water stress on growth, phenolic compounds, glandular hairs, and yield of essential oil in basil (Ocimum gratissimum L.). Chem. Biol. Technol. Agric. 2015, 2, 10. [Google Scholar] [CrossRef]
- Krishna, H.; Singh, S.; Sharma, R.; Khawale, R.; Grover, M.; Patel, V. Biochemical changes in micropropagated grape (Vitis vinifera L.) plantlets due to arbuscular-mycorrhizal fungi (AMF) inoculation during ex vitro acclimatization. Sci. Hortic. 2005, 106, 554–567. [Google Scholar] [CrossRef]
- Larose, G.; Chênevert, R.; Moutoglis, P.; Gagné, S.; Piché, Y.; Vierheilig, H. Flavonoid levels in roots ofMedicago sativa are modulated by the developmental stage of the symbiosis and the root colonizing arbuscular mycorrhizal fungus. J. Plant Physiol. 2002, 159, 1329–1339. [Google Scholar] [CrossRef]
- Devi, M.C.; Reddy, M. Phenolic acid metabolism of groundnut (Arachis hypogaea L.) plants inoculated with VAM fungus and Rhizobium. Plant Growth Regul. 2002, 37, 151–156. [Google Scholar] [CrossRef]
- Ceccarelli, N.; Curadi, M.; Martelloni, L.; Sbrana, C.; Picciarelli, P.; Giovannetti, M. Mycorrhizal colonization impacts on phenolic content and antioxidant properties of artichoke leaves and flower heads two years after field transplant. Plant Soil 2010, 335, 311–323. [Google Scholar] [CrossRef]
- Ding, L.; Chaumont, F. Are aquaporins expressed in stomatal complexes promising targets to enhance stomatal dynamics? Front. Plant Sci. 2020, 11, 458. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balestrini, R.; Brunetti, C.; Chitarra, W.; Nerva, L. Photosynthetic Traits and Nitrogen Uptake in Crops: Which Is the Role of Arbuscular Mycorrhizal Fungi? Plants 2020, 9, 1105. https://doi.org/10.3390/plants9091105
Balestrini R, Brunetti C, Chitarra W, Nerva L. Photosynthetic Traits and Nitrogen Uptake in Crops: Which Is the Role of Arbuscular Mycorrhizal Fungi? Plants. 2020; 9(9):1105. https://doi.org/10.3390/plants9091105
Chicago/Turabian StyleBalestrini, Raffaella, Cecilia Brunetti, Walter Chitarra, and Luca Nerva. 2020. "Photosynthetic Traits and Nitrogen Uptake in Crops: Which Is the Role of Arbuscular Mycorrhizal Fungi?" Plants 9, no. 9: 1105. https://doi.org/10.3390/plants9091105
APA StyleBalestrini, R., Brunetti, C., Chitarra, W., & Nerva, L. (2020). Photosynthetic Traits and Nitrogen Uptake in Crops: Which Is the Role of Arbuscular Mycorrhizal Fungi? Plants, 9(9), 1105. https://doi.org/10.3390/plants9091105







