Polyphenols of Frangula alnus and Peganum harmala Leaves and Associated Biological Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling of Plant Material
2.2. Phenolic Compounds
2.3. Antiproliferative Effects
- AB is absorbance
- and are the absorbances of the control and sample, respectively.
2.4. Cytotoxic Effects
2.5. Antioxidant Activity
2.6. ROS Intercellular Accumulation
2.7. Caspase Activity by Colorimetric Assay
2.8. Statistical Analyses
3. Results
3.1. F. alnus and P. harmala Polyphenol Profiling of Leaf Extracts
3.2. Antioxidant Effects
3.3. MTT Assay
3.4. Flow Cytometry
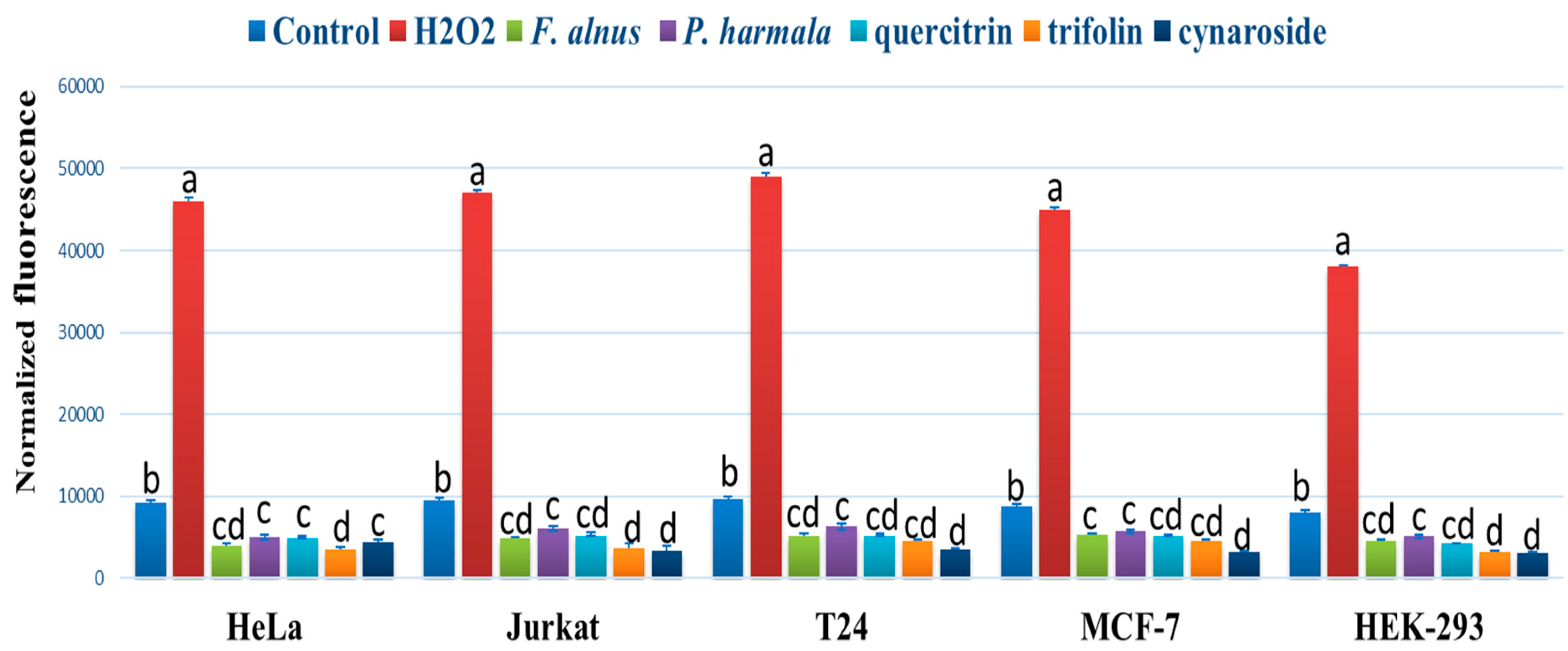
3.5. ROS Accumulation Assay
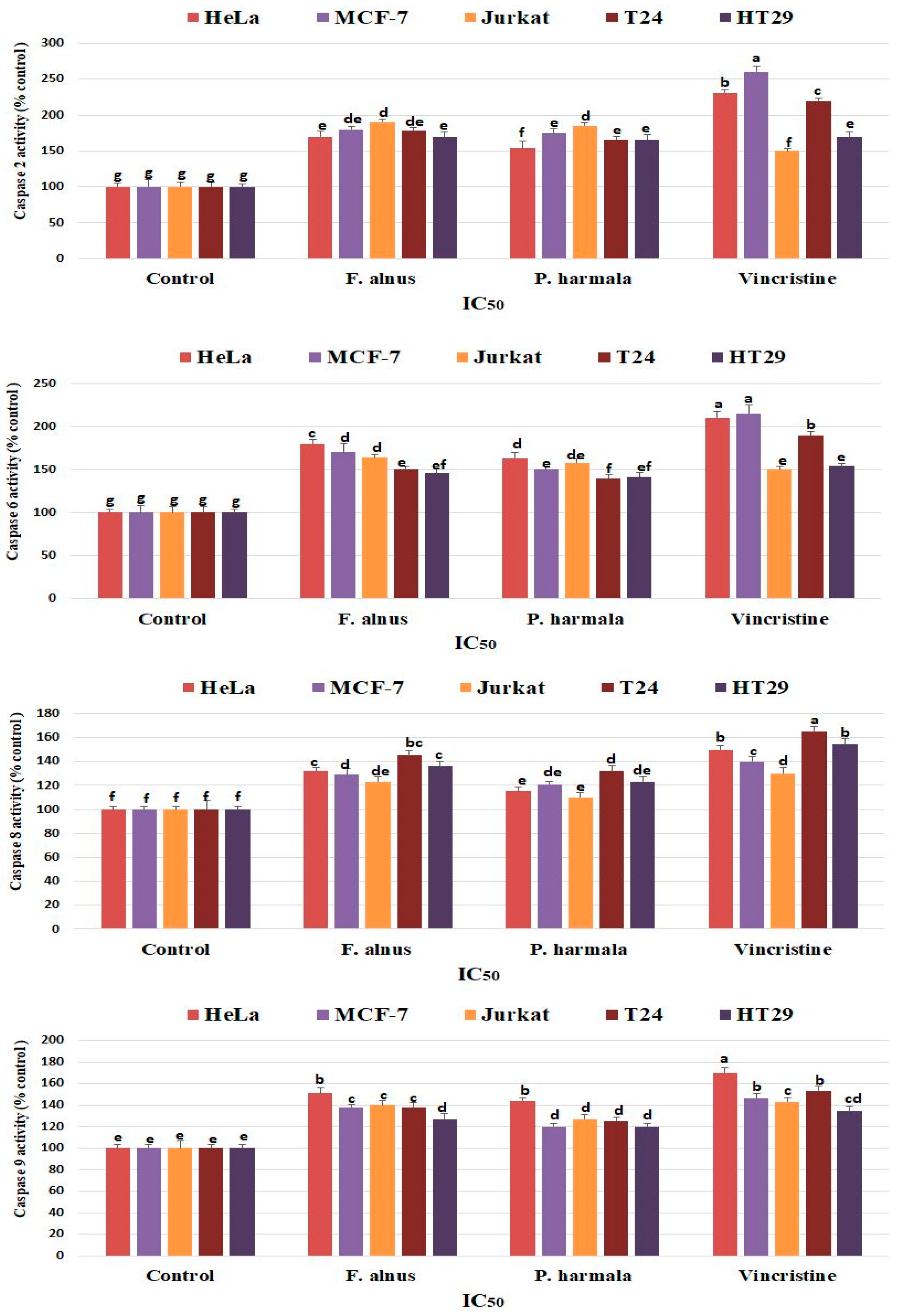
3.6. Detection of Caspase Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Traditional Medicine Strategy: 2014–2023; WHO Press: Geneva, Switzerland, 2013; Available online: https://apps.who.int/iris/bitstream/handle/10665/92455/9786167697581-tha.pdf (accessed on 21 August 2020).
- Queen, B.; Tollefsbol, T. Polyphenols and Aging. Curr. Aging Sci. 2010, 3, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Luo, Q.; Nie, R.; Yang, X.; Tang, Z.; Chen, H. Potential implications of polyphenols on aging considering oxidative stress, inflammation, autophagy and gut microbiota. Crit. Rev. Food Sci. Nutr. 2020, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Alvarado-Sansininea, J.J.; Sánchez-Sánchez, L.; López-Muñoz, H.; Escobar, M.L.; Flores-Guzmán, F.; Tavera-Hernández, R.; Jiménez-Estrada, M. Quercetagetin and patuletin: Antiproliferative, necrotic and apoptotic activity in tumor cell lines. Molecules 2018, 23, 2579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sezer, D.E.; Oktay, L.M.; Karadadaş, E.; Memmedov, H.; Selvi Gunel, N.; Sözmen, E. Assessing anticancer potential of blueberry flavonoids, quercetin, kaempferol and gentisic acid, through oxidative stress and apoptosis parameters on HCT-116 cells. J. Med. Food 2019, 22, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- Elansary, H.O.; Szopa, A.; Kubica, P.; Al-Mana, F.A.; Mahmoud, E.A.; El-Abedin, T.K.A.Z.; Mattar, M.A.; Ekiert, H. Phenolic compounds of Catalpa speciosa, Taxus cuspidata and Magnolia acuminata have antioxidant and anticancer activity. Molecules 2019, 24, 412. [Google Scholar] [CrossRef] [Green Version]
- Elansary, O.H.; Szopa, A.; Kubica, P.; Ekiert, H.; Mattar, M.A.; Al-Yafrasi, A.M.; El-Ansary, O.D.; Zin El-Abedin, K.T.; Yessoufou, K. Polyphenol profile and pharmaceutical potential of Quercus spp. bark extracts. Plants 2019, 8, 486. [Google Scholar] [CrossRef] [Green Version]
- Saleh, A.; El Fayoumi, H.M.; Youns, M.; Barakat, W. Rutin and orlistat produce antitumor effects via antioxidant and apoptotic actions. Naunyn-Schmiede Arch. Pharmacol. 2019, 392, 165–175. [Google Scholar] [CrossRef]
- Abdal Dayem, A.; Choi, H.Y.; Yang, G.-M.; Kim, K.; Saha, S.K.; Cho, S.-G. The anti-cancer effect of polyphenols against breast cancer and cancer stem cells: Molecular mechanisms. Nutrients 2016, 8, 581. [Google Scholar] [CrossRef]
- Frappier, B.; Lee, T.; Olson, K.; Eckert, R. Small-scale invasion pattern, spread rate and lag-phase behavior of Rhamnus frangula L. For. Ecol. Manag. 2003, 186, 1–6. [Google Scholar] [CrossRef]
- Males, Z.; Kremer, D.; Randić, Z.; Randić, M.; Hazler Pilepic, K.; Bojić, M. Quantitative analysis of glucofrangulins and phenolic compounds in Croatian Rhamnus and Frangula species. Acta Biol. Crac. Ser. Bot. 2010, 52. [Google Scholar] [CrossRef]
- Brkanac, S.R.; Gerić, M.; Gajski, G.; Vujčić, V.; Garaj-Vrhovac, V.; Kremer, D.; Domijan, A.-M. Toxicity and antioxidant capacity of Frangula alnus Mill. bark and its active component emodin. Regul. Toxicol. Pharmacol. 2015, 73, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Sadowska, B.; Paszkiewicz, M.; Anna, P.; Redzynia, M.; Rozalska, B. Vaccinium myrtillus leaves and Frangula alnus bark derived extracts as potential antistaphylococcal agents. Acta Biochim. Pol. 2014, 61. [Google Scholar] [CrossRef] [Green Version]
- Berg, J.A.; Meyer, G.A.; Young, E.B. Propagule pressure and environmental conditions interact to determine establishment success of an invasive plant species, glossy buckthorn (Frangula alnus), across five different wetland habitat types. Biol. Invasions 2016, 18, 1363–1373. [Google Scholar] [CrossRef]
- Ncube, B.; Finnie, J.F.; Van Staden, J. Quality from the field: The impact of environmental factors as quality determinants in medicinal plants. S. Afr. J. Bot. 2012, 82, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Passos, I.D.; Mironidou-Tzouveleki, M. Chapter 71—Hallucinogenic Plants in the Mediterranean Countries. In Neuropathology of Drug Addictions and Substance Misuse; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 761–772. [Google Scholar]
- Abolhasani, L.; Salehi, E.; Kenari, R. Study of antioxidant capacity and stability of phenolic compounds from the seeds of Peganum harmala. J. Appl. Environ. Biol. Sci. 2015, 4, 218–222. [Google Scholar]
- Edziri, H.; Mastouri, M.; Mahjoub, M.A.; Patrich, G.; Matieu, M.; Ammar, S.; Ali, S.M.; Laurent, G.; Zine, M.; Aouni, M. Antibacterial, antiviral and antioxidant activities of aerial part extracts of Peganum harmala L. grown in Tunisia. Toxicol. Environ. Chem. 2010, 92, 1283–1292. [Google Scholar] [CrossRef]
- Fatma, B.; Fatiha, M.; Elattafia, B.; Noureddine, D. Phytochemical and antimicrobial study of the seeds and leaves of Peganum harmala L. against urinary tract infection pathogens. Asian Pac. J. Trop. Dis. 2016, 6, 822–826. [Google Scholar] [CrossRef]
- Lamchouri, F.; Settaf, A.; Jossang, A.; Zemzami, M.; Atif, N.; Cherrah, Y.; Hassar, M. Antiproliferative activity of vasicinone, harmine, peganine and harmalacidine extacted from Peganum harmala seeds. In Proceedings of the Vème Ecole Internationale d’endocrinologie et VIème Congrès d’endocrinologie, Settat, Morocco, 2000; Available online: https://hal.archives-ouvertes.fr/hal-00087883/ (accessed on 21 August 2020).
- Lamchouri, F.; Zemzami, M.; Jossang, A.; Abdellatif, A.; Israili, Z.H.; Lyoussi, B. Cytotoxicity of alkaloids isolated from Peganum harmala seeds. Pak. J. Pharm. Sci. 2013, 26, 699–706. [Google Scholar]
- Li, Y.; He, Q.; Du, S.; Guo, S.; Geng, Z.; Deng, Z. Study of methanol extracts from different parts of Peganum harmala L. Using 1H-NMR plant metabolomics. J. Anal. Methods Chem. 2018, 2018, 6532789. [Google Scholar] [CrossRef] [Green Version]
- Ababou, A.; Chouieb, M.; Bouthiba, A.; Saidi, D.; Bouzina, M.; Mederbal, K. Spatial pattern analysis of Peganum harmala on the salted lower Chelif plain, Algeria. Turk. J. Bot. 2013, 37, 111–121. [Google Scholar]
- Elansary, H.O.; Szopa, A.; Kubica, P.; El-Ansary, D.O.; Ekiert, H.; Al-Mana, F.A. Malus baccata var. gracilis and Malus toringoides bark polyphenol studies and antioxidant, antimicrobial and anticancer activities. Processes 2020, 8, 283. [Google Scholar] [CrossRef] [Green Version]
- Ellnain-Wojtaszek, M.; Zgórka, G. High-performance liquid chromatography and thin-layer chromatography of phenolic acids from Ginkgo biloba L. leaves collected within vegetative period. J. Liq. Chromatogr. Relat. Technol. 1999, 22, 1457–1471. [Google Scholar] [CrossRef]
- Sułkowska-Ziaja, K.; Maślanka, A.; Szewczyk, A.; Muszyńska, B. Physiologically active compounds in four species of Phellinus. Nat. Prod. Commun. 2017, 12, 363–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yessoufou, K.; Elansary, H.O.; Mahmoud, E.A.; Skalicka-Woźniak, K. Antifungal, antibacterial and anticancer activities of Ficus drupacea L. stem bark extract and biologically active isolated compounds. Ind. Crop. Prod. 2015, 74, 752–758. [Google Scholar] [CrossRef]
- Elansary, H.O.; Szopa, A.; Kubica, P.; Ekiert, H.; Al-Mana, F.A.; Al-Yafrasi, M.A. Antioxidant and biological activities of Acacia saligna and Lawsonia inermis natural populations. Plants 2019, 9, 908. [Google Scholar] [CrossRef]
- Elansary, H.O.; Abdelgaleil, S.A.M.; Mahmoud, E.A.; Yessoufou, K.; Elhindi, K.; El-Hendawy, S. Effective antioxidant, antimicrobial and anticancer activities of essential oils of horticultural aromatic crops in northern Egypt. BMC Complement. Altern. Med. 2018, 18. [Google Scholar] [CrossRef]
- Elansary, H.O.; Yessoufou, K.; Abdel-Hamid, A.M.E.; El-Esawi, M.A.; Ali, H.M.; Elshikh, M.S. Seaweed extracts enhance salam turfgrass performance during prolonged irrigation intervals and saline shock. Front. Plant Sci. 2017, 8, 830. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, J.P.A.; Miranda, I.; Sousa, V.B.; Pereira, H. Chemical composition of barks from Quercus faginea trees and characterization of their lipophilic and polar extracts. PLoS ONE 2018, 13, e0197135. [Google Scholar] [CrossRef]
- El-Esawi, A.M.; Elkelish, A.; Soliman, M.; Elansary, O.H.; Zaid, A.; Shabir, W.H. Serratia marcescens BM1 enhances cadmium stress tolerance and phytoremediation potential of soybean through modulation of osmolytes, leaf gas exchange, antioxidant machinery and stress-responsive genes expression. Antioxidants 2020, 9, 43. [Google Scholar] [CrossRef] [Green Version]
- Elansary, H.O.; Szopa, A.; Klimek-Szczykutowicz, M.; Ekiert, H.; Barakat, A.A.; Al-Mana, F.A. Antiproliferative, antimicrobial and antifungal activities of polyphenol extracts from Ferocactus species. Processes 2020, 8, 138. [Google Scholar] [CrossRef] [Green Version]
- Elansary, H.O.; Mahmoud, E.A. Basil cultivar identification using chemotyping still favored over genotyping using core barcodes and possible resources of antioxidants. J. Essent. Oil Res. 2015, 27, 82–87. [Google Scholar] [CrossRef]
- Nizioł-Łukaszewska, Z.; Furman-Toczek, D.; Zagórska-Dziok, M. Antioxidant activity and cytotoxicity of Jerusalem artichoke tubers and leaves extract on HaCaT and BJ fibroblast cells. Lipids Health Dis. 2018, 17, 280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nejabatdoust, A.; Daemi, H.; Salehzadeh, A.; Azimi, S.; Darafkan, S.; Digsara, F.; Pourebrahim, M.; Seighalani, R. Comparing of effects of hydro-alcoholic, ethanolic and methanolic extracts of the Frangula alnus: chemical composition, antimicrobial and synergism. J. Genet. Resour. 2020, 6, 20–33. [Google Scholar]
- Hemmateenejad, B.; Abbaspour, A.; Maghami, H.; Miri, R.; Panjehshahin, M.R. Partial least squares-based multivariate spectral calibration method for simultaneous determination of beta-carboline derivatives in Peganum harmala seed extracts. Anal. Chim. Acta 2006, 575, 290–299. [Google Scholar] [CrossRef]
- Sodaeizadeh, H.; Rafieiolhossaini, M.; Havlik, J.; Van Damme, P. Allelopathic activity of different plant parts of Peganum harmala L. and identification of their growth inhibitors substances. Plant Growth Reg. 2009, 59, 227–236. [Google Scholar] [CrossRef]
- Sharaf, M.; El-Ansari, M.A.; Matlin, S.A.; Saleh, N.A.M. Four flavonoid glycosides from Peganum harmala. Phytochemistry 1997, 44, 533–536. [Google Scholar] [CrossRef]
- Li, Y.; He, Q.; Geng, Z.; Du, S.; Deng, Z.; Hasi, E. NMR-based metabolomic profiling of Peganum harmala L. reveals dynamic variations between different growth stages. R. Soc. Open Sci. 2018, 5, 171722. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Luo, Z.; Wang, D.; He, F.; Li, D. Phytochemical profiles and antioxidant and antimicrobial activities of the leaves of Zanthoxylum bungeanum. Sci. World J. 2014, 2014, 181072. [Google Scholar]
- Kilic, I.; Yesiloglu, Y. Spectroscopic studies on the antioxidant activity of p-coumaric acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 115, 719–724. [Google Scholar] [CrossRef]
- Buravlev, E.V.; Dvornikova, I.A.; Schevchenko, O.G.; Kutchin, A.V. Synthesis and antioxidant ability of novel derivatives based on para-coumaric acid containing isobornyl groups. Chem. Biodivers. 2019, 16, e1900362. [Google Scholar] [CrossRef]
- Elansary, H.O.; Szopa, A.; Kubica, P.; Ekiert, H.; El-Ansary, D.O.; Al-Mana, F.A.; Mahmoud, E.A. Polyphenol content and biological activities of Ruta graveolens L. and Artemisia abrotanum L. in northern Saudi Arabia. Processes 2020, 8, 531. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, M.; Zhang, T.; Wu, J.; Wang, J.; Liu, K.; Zhan, N. Antioxidant and anti-inflammatory activities of cynaroside from Elsholtiza bodinieri. Nat. Prod. Commun. 2018, 13, 1501–1504. [Google Scholar] [CrossRef] [Green Version]
- Cenic-Milosevic, D.; Tambur, Z.; Ivančajić, S.; Bokonjić, D.; Cukovic, A.; Stanojkovic, T.; Grozdanić, N.; Kulišić, Z.; Juranic, Z. Antiproliferative effects of Camellia sinensis, Frangula alnus and Rosmarinus officinalis. Arch. Biol. Sci. 2013, 65, 885–891. [Google Scholar] [CrossRef]
- Murakami, Y.; Kawata, A.; Ito, S.; Katayama, T.; Fujisawa, S. Radical-scavenging and anti-inflammatory activity of quercetin and related compounds and their combinations against RAW264.7 cells stimulated with porphyromonas gingivalis fimbriae. relationships between anti-inflammatory activity and quantum chemical parameters. In Vivo 2015, 29, 701–710. [Google Scholar] [PubMed]
- Zhao, M.H.; Yuan, L.; Meng, L.Y.; Qiu, J.L.; Wang, C.B. Quercetin-loaded mixed micelles exhibit enhanced cytotoxic efficacy in non-small cell lung cancer in vitro. Exp. Ther. Med. 2017, 14, 5503–5508. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.-J.; Kwon, S.-B.; Kim, M.-S.; Jin, S.; Ryu, H.W.; Oh, S.-R.; Yoon, D.-Y. Trifolin induces apoptosis via extrinsic and intrinsic pathways in the NCI-H460 human non-small cell lung-cancer cell line. Phytomedicine 2016, 23, 998–1004. [Google Scholar] [CrossRef] [PubMed]
- Gecibesler, I.H.; Demirtas, I.; Koldas, S.; Behcet, L.; Gul, F.; Altun, M. Bioactivity-guided isolation of compounds with antiproliferative activity from Teucrium chamaedrys L. subsp. sinuatum (Celak.) Rech. F. Progr. Nutr. 2019, 21, 458–470. [Google Scholar]
- Semaming, Y.; Pannengpetch, P.; Chattipakorn, S.C.; Chattipakorn, N. Pharmacological properties of protocatechuic acid and its potential roles as complementary medicine. Evid-Based Compl. Alt. Med. 2015, 593902. [Google Scholar] [CrossRef] [Green Version]
- Deepak, K.G.K.; Challa, S.; Suhasin, G.; Reddy, N.N.R.; Elansary, H.O.; El-Ansary, D.O. Antidiabetic and antilipidemic activity of root extracts of Salacia oblonga against streptozotocin-induced diabetes in Wistar rats. Processes 2020, 8, 301. [Google Scholar] [CrossRef] [Green Version]
- Elansary, H.O.; Szopa, A.; Kupica, P.; Ekiert, H.; El-Ansary, D.O.; Al-Mana, F.A.; Mahmoud, E.A. Saudi Rosmarinus officinalis and Ocimum basilicum L. polyphenols and biological activities. Processes 2020, 8, 446. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Yuan, J. Caspases in apoptosis and beyond. Oncogene 2008, 27, 6194–6206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srinivasula, S.M.; Ahmad, M.; Fernandes-Alnemri, T.; Litwack, G.; Alnemri, E.S. Molecular ordering of the Fas-apoptotic pathway: The Fas/APO-1 protease Mch5 is a CrmA-inhibitable protease that activates multiple Ced-3/ICE-like cysteine proteases. Biochemistry 1996, 93, 14486–14491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwan, Y.P.; Saito, T.; Ibrahim, D.; Al-Hassan, F.M.S.; Ein Oon, C.; Chen, Y.; Jothy, S.L.; Kanwar, J.R.; Sasidharan, S. Evaluation of the cytotoxicity, cell-cycle arrest and apoptotic induction by Euphorbia hirta in MCF-7 breast cancer cells. Pharm. Biol. 2016, 54, 1223–1236. [Google Scholar] [PubMed] [Green Version]
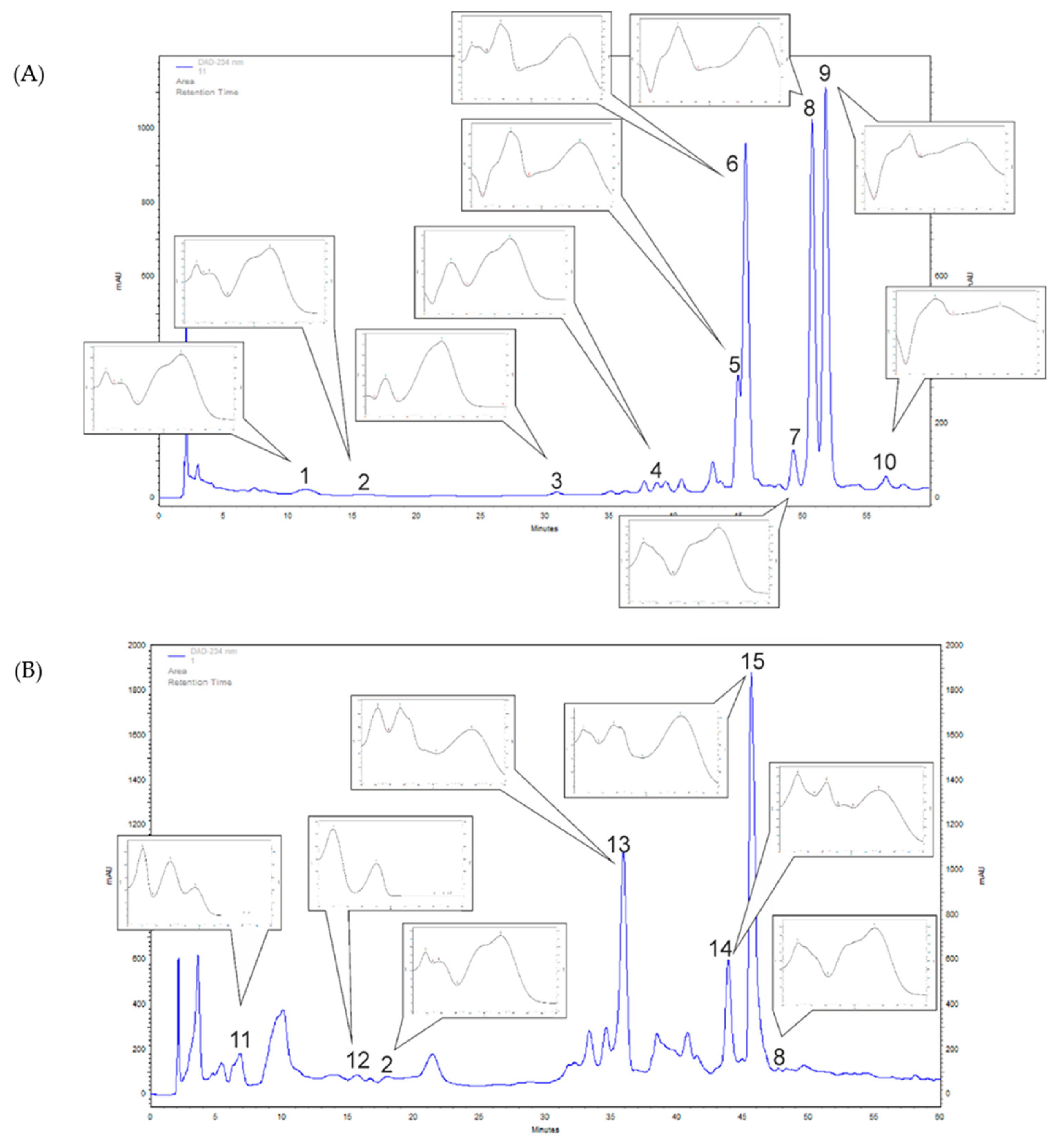
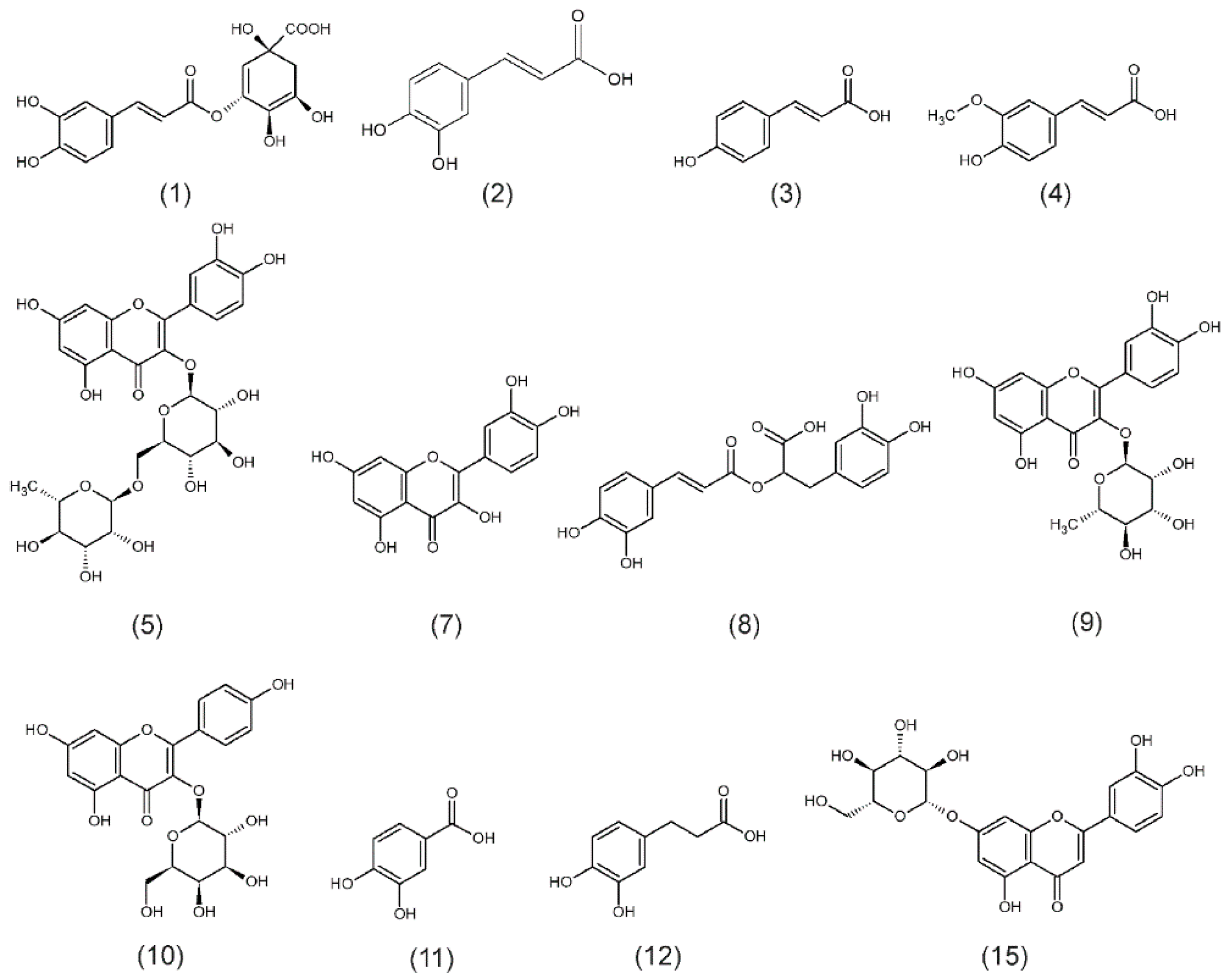

| Phenolic Compounds | (mg/100 g DW ± SD) | |
|---|---|---|
| Frangula alnus | Chlorogenic acid (1) | 126.8 ± 2.5 |
| Caffeic acid (2) | 33.8 ± 2.1 | |
| p-Coumaric acid (3) | 41.4 ± 2.7 | |
| Ferulic acid (4) | 44.0 ± 3.4 | |
| Rutoside (Quercetin 3-rutinoside) (5) | 283.4 ± 23.9 | |
| Unknown compound (6) | 1072.4 ± 94.7 | |
| Quercetin (7) | 115.1 ± 16.4 | |
| Rosmarinic acid (8) | 269.5 ± 14.7 | |
| Quercitrin (9) | 1132.3 ± 76.2 | |
| Trifolin (Kaempferol 3-galactoside) (10) | 914.3 ± 20.7 | |
| Peganum harmala | Caffeic acid (2) | 20.6 ± 1.8 |
| Rosmarinic acid (8) | 18.9 ± 0.3 | |
| Protocatechuic acid (11) | 51.6 ± 6.8 | |
| Hydrocaffeic acid (12) | 199.4 ± 12.9 | |
| Unknown compound (13) | 503.8 ± 47.3 | |
| Unknown compound (14) | 254.0 ± 28.1 | |
| Cynaroside (Luteolin 7-glucoside) (15) | 713.5 ± 88.0 |
| β-Carotene-Bleaching Assay (IC50, µg/mL) | DPPH (IC50, µg/mL) | FRAP (IC50, mM TEAC/g extract) | |
|---|---|---|---|
| F.alnus | 18.3 ± 2.3 b | 14.1 ± 1.2 b | 23.1 ± 1.3 b |
| P. harmala | 26.8 ± 2.9 a | 21.5± 2.3 a | 32.4 ± 3.5 a |
| Quercitrin | 26.0 ± 1.9 a | 21.5 ± 1.5 a | 32.6 ± 3.1 a |
| Trifolin | 6.9 ± 0.3 d | 5.6 ± 0.2 d | 8.6 ± 0.3 e |
| p-Coumaric acid | 4.0 ± 0.3 e | 3.8 ± 0.1 e | 4.3 ± 0.1 g |
| Cynaroside | 6.6 ± 0.3 de | 5.8 ± 0.3 d | 8.3 ± 0.3 e |
| Rutoside | 17.5 ± 0.7 b | 14.3 ± 0.9 b | 19.3 ± 1.5 c |
| Rosmarinic acid | 3.0 ± 0.2 e | 2.5 ± 0.3 f | 3.4 ± 0.3 g |
| Quercetin | 6.3 ± 0.2 e | 5.6 ± 0.3 d | 8.0 ± 0.3 e |
| Chlorogenic acid | 5.0 ± 0.5 ef | 4.1 ± 0.2 e | 6.7 ± 0.5 f |
| Ferulic acid | 9.8 ± 0.3 c | 8.0 ± 0.3 c | 11.7± 1.1 d |
| Hydrocaffeic acid | 4.5 ± 0.1 f | 4.1 ± 0.1 e | 5.3 ± 0.1 f |
| Protocatechuic acid | 9.2 ± 0.1 c | 7.3 ± 0.1 c | 11.2 ± 0.1 d |
| BHT | 3.3 ± 0.1 g | 2.9 ± 0.1 f | - |
| Trolox | - | - | 3.2 ± 0.1g |
| HT-29 * | HeLa | MCF-7 | Jurkat | HEK-293 | |
|---|---|---|---|---|---|
| F. alnus | 31.53 ± 2.1 c | 28.45 ± 1.3 d | 39.64 ± 3.3 b | 43.75 ± 3.8 d | ˃400 |
| P. harmala | 49.05 ± 3.2 b | 43.86 ± 2.7 b | 58.64 ± 4.2 a | 59.53 ± 4.2 b | ˃400 |
| Rutoside | 19.1 ± 0.5 fe | 4.1 ± 02 f | 5.74± 0.6 fg | 4.7 ± 0.2 e | ˃400 |
| Cynaroside | 7.9 ± 0.6 f | 4.8 ± 0.5 f | 25.97 ± 1.7 e | 42.32 ± 2.1 d | ˃400 |
| Chlorogenic acid | 14.11 ± 2.6 e | 4.1 ± 0.5 d | 37.25 ± 3.9 c | 40.53 ± 2.1 d | ˃400 |
| Ferulic acid | 21.32 ± 7.1 d | 50.35 ± 3.9 a | 41.32 ± 3.5 b | 38.53 ± 3.8 d | ˃400 |
| Quercetin | 7.50 ± 0.8 f | 4.9 ± 0.7 f | 22.53 ± 1.5 e | 39.63 ± 2.3 d | ˃400 |
| Quercitrin | 20.32 ± 1.8 d | 17.3 ± 1.2 e | 45.23 ± 4.1 b | 68.42 ± 3.8 a | ˃400 |
| Trifolin | 18.9 ± 0.9 d | 16.1 ± 1.7 e | 28.21 ± 1.6 d | 45.31 ± 2.1 c | ˃400 |
| p-Coumaric acid | 8.2 ± 0.3 f | 6.3 ± 0.6 f | 18.5 ± 2.1 f | 33.5 ± 2.7 d | ˃400 |
| Protocatechuic acid | 97.42 ± 4.1 a | 38.19 ± 3.5 c | 19.31 ± 2.9 f | 48.31 ± 3.1 c | ˃400 |
| Hydrocaffeic acid | 19.3 ± 1.9 d | 15.42 ± 1.3 e | 23.1 ± 2.3 e | 39.12 ± 2.5 d | ˃400 |
| Vinblastine sulfate | 15.3 ± 0.2 e | 2.2 ± 0.01 f | ‒ | 0.14 ± 0.02 f | 43.5 ± 2.5 |
| Taxol | ‒ | ‒ | 0.06 ± 0.005 h | ‒ | ‒ |
| Vincristine | 8.4 ± 0.4 | 4.64 ± 1.5 | 0.4 ± 0.07 | 90.1 ± 3.2 | 46.5 ± 0.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elansary, H.O.; Szopa, A.; Kubica, P.; Ekiert, H.; Al-Mana, F.A.; El-Shafei, A.A. Polyphenols of Frangula alnus and Peganum harmala Leaves and Associated Biological Activities. Plants 2020, 9, 1086. https://doi.org/10.3390/plants9091086
Elansary HO, Szopa A, Kubica P, Ekiert H, Al-Mana FA, El-Shafei AA. Polyphenols of Frangula alnus and Peganum harmala Leaves and Associated Biological Activities. Plants. 2020; 9(9):1086. https://doi.org/10.3390/plants9091086
Chicago/Turabian StyleElansary, Hosam O., Agnieszka Szopa, Paweł Kubica, Halina Ekiert, Fahed A. Al-Mana, and Ahmed A. El-Shafei. 2020. "Polyphenols of Frangula alnus and Peganum harmala Leaves and Associated Biological Activities" Plants 9, no. 9: 1086. https://doi.org/10.3390/plants9091086
APA StyleElansary, H. O., Szopa, A., Kubica, P., Ekiert, H., Al-Mana, F. A., & El-Shafei, A. A. (2020). Polyphenols of Frangula alnus and Peganum harmala Leaves and Associated Biological Activities. Plants, 9(9), 1086. https://doi.org/10.3390/plants9091086








