Comparative Metabolomics Profiling of Polyphenols, Nutrients and Antioxidant Activities of Two Red Onion (Allium cepa L.) Cultivars
Abstract
:1. Introduction
2. Results and Discussion
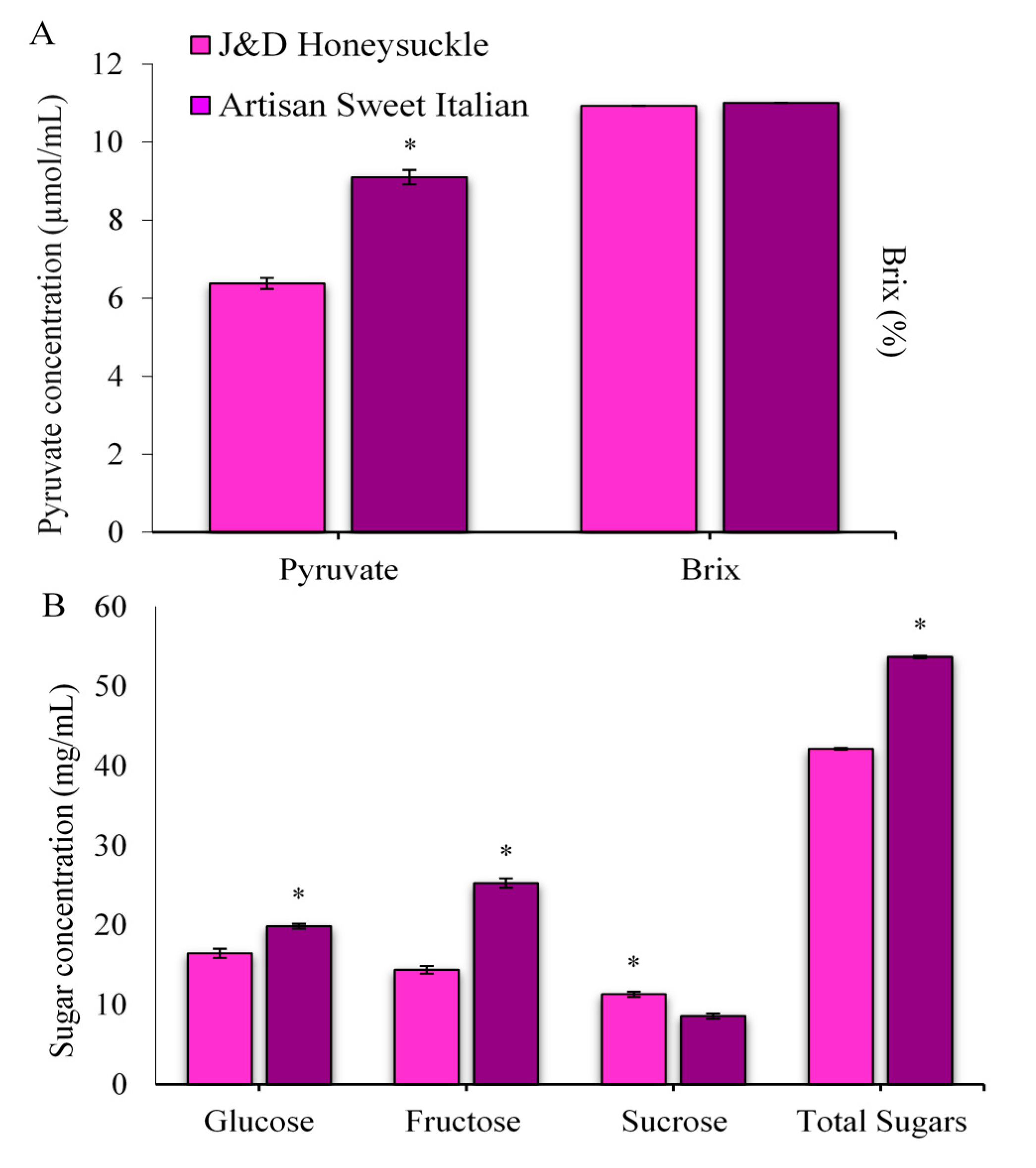
2.1. Pungency, Total Soluble Solids, and Sugars
2.2. Evaluation of Protein and Minerals
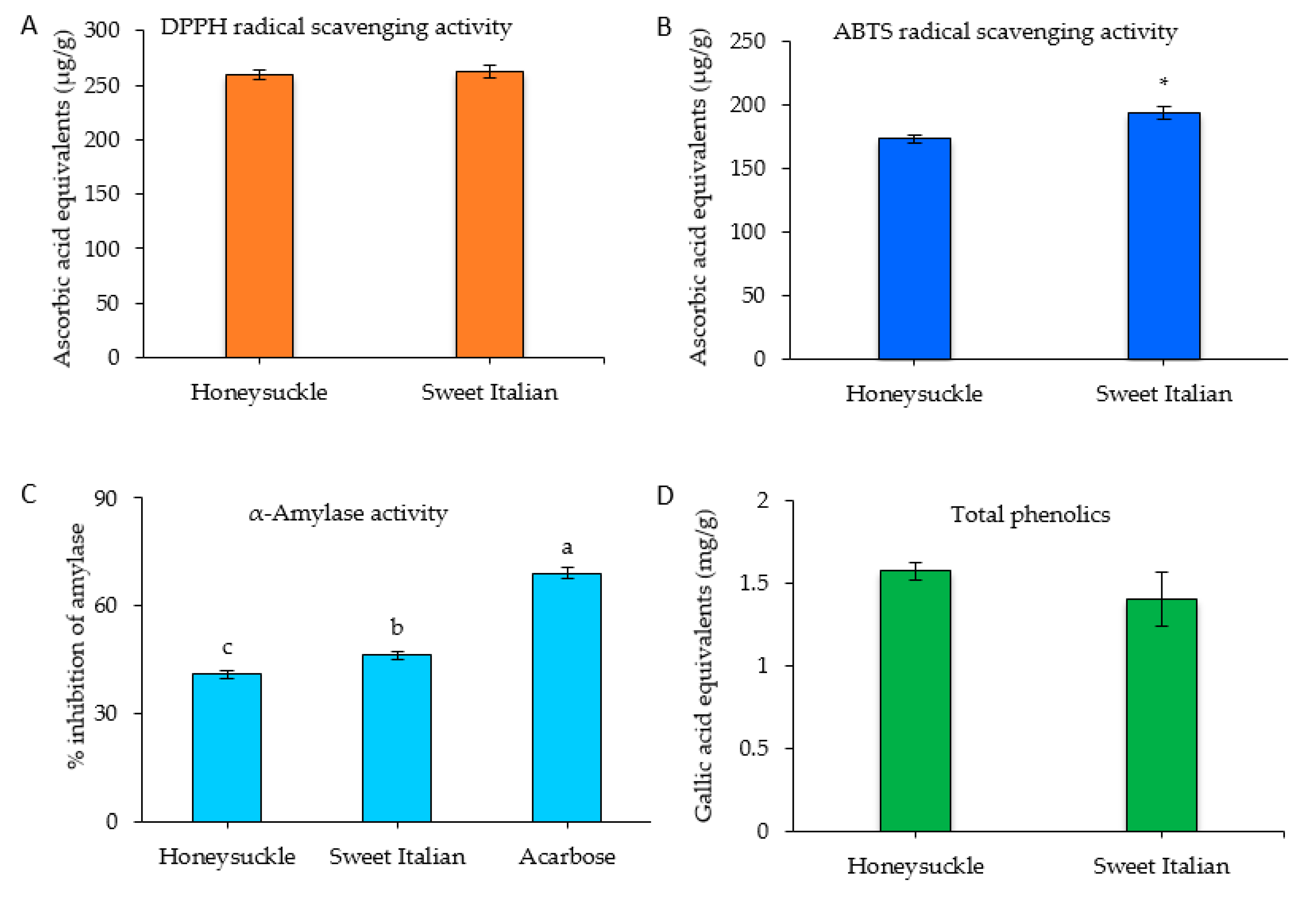
2.3. Antioxidant Activity, Total Phenolic Content, and α-Amylase Inhibitory Activity
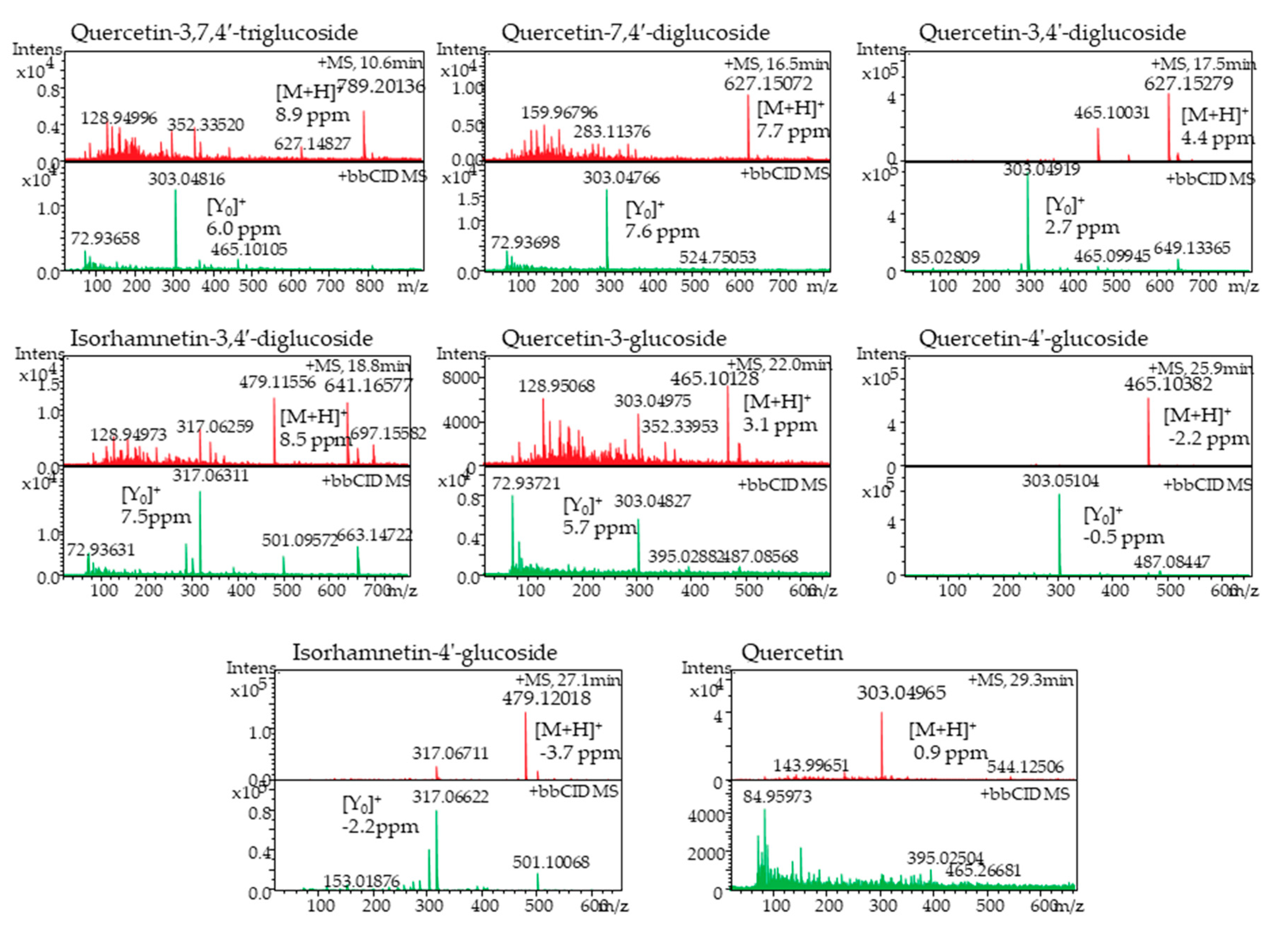
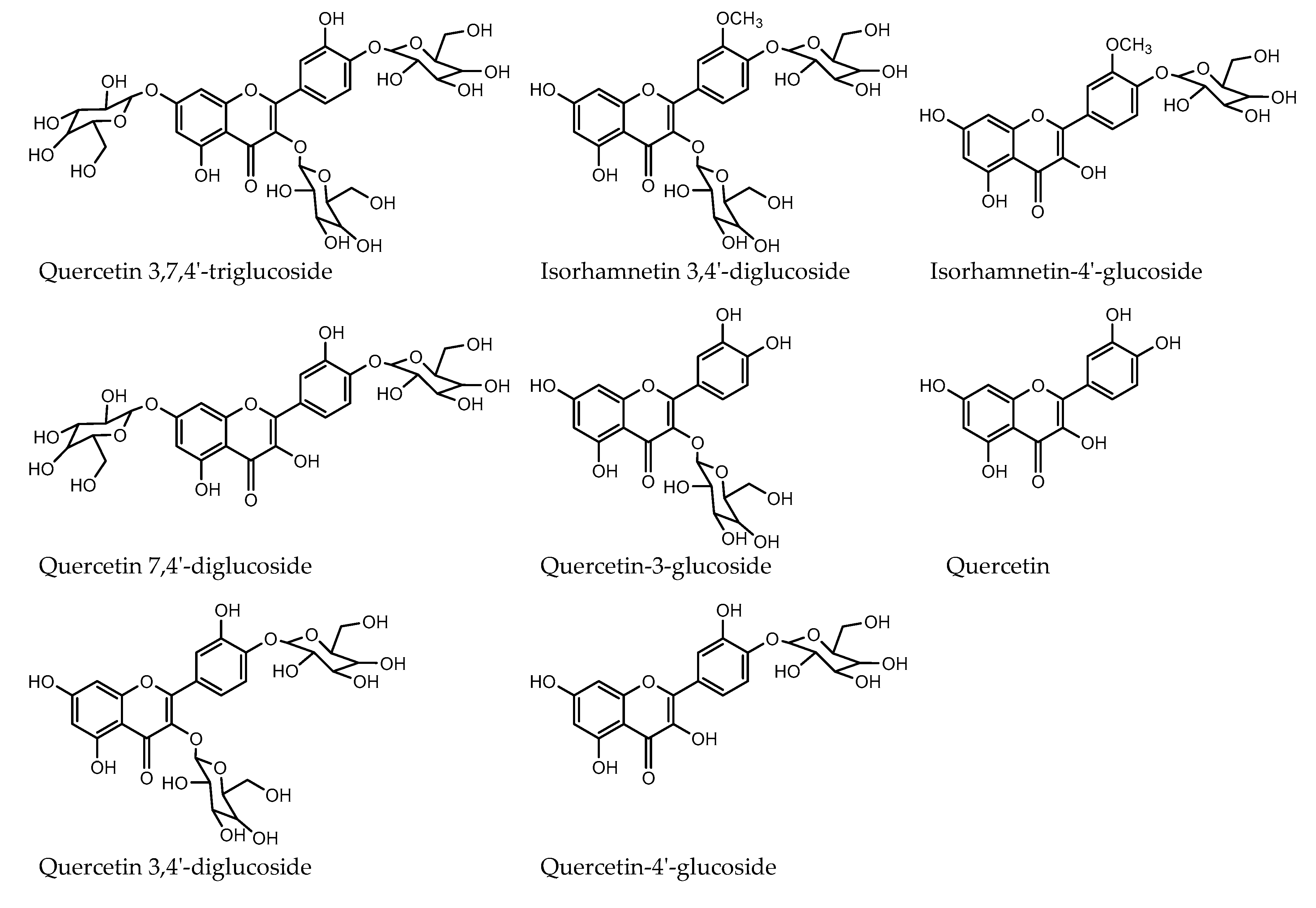
2.4. Quantification of Red Onion Flavonoids
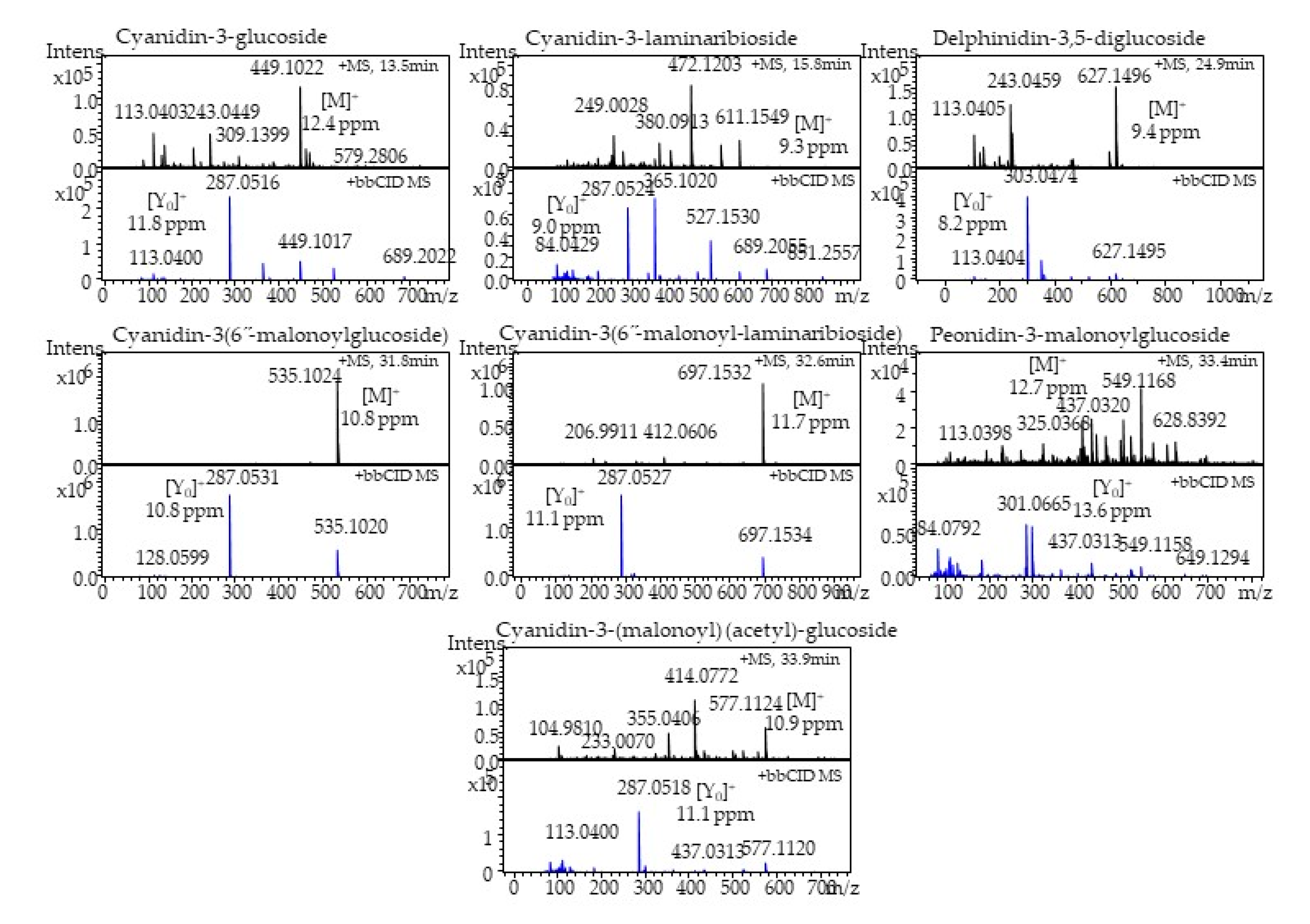
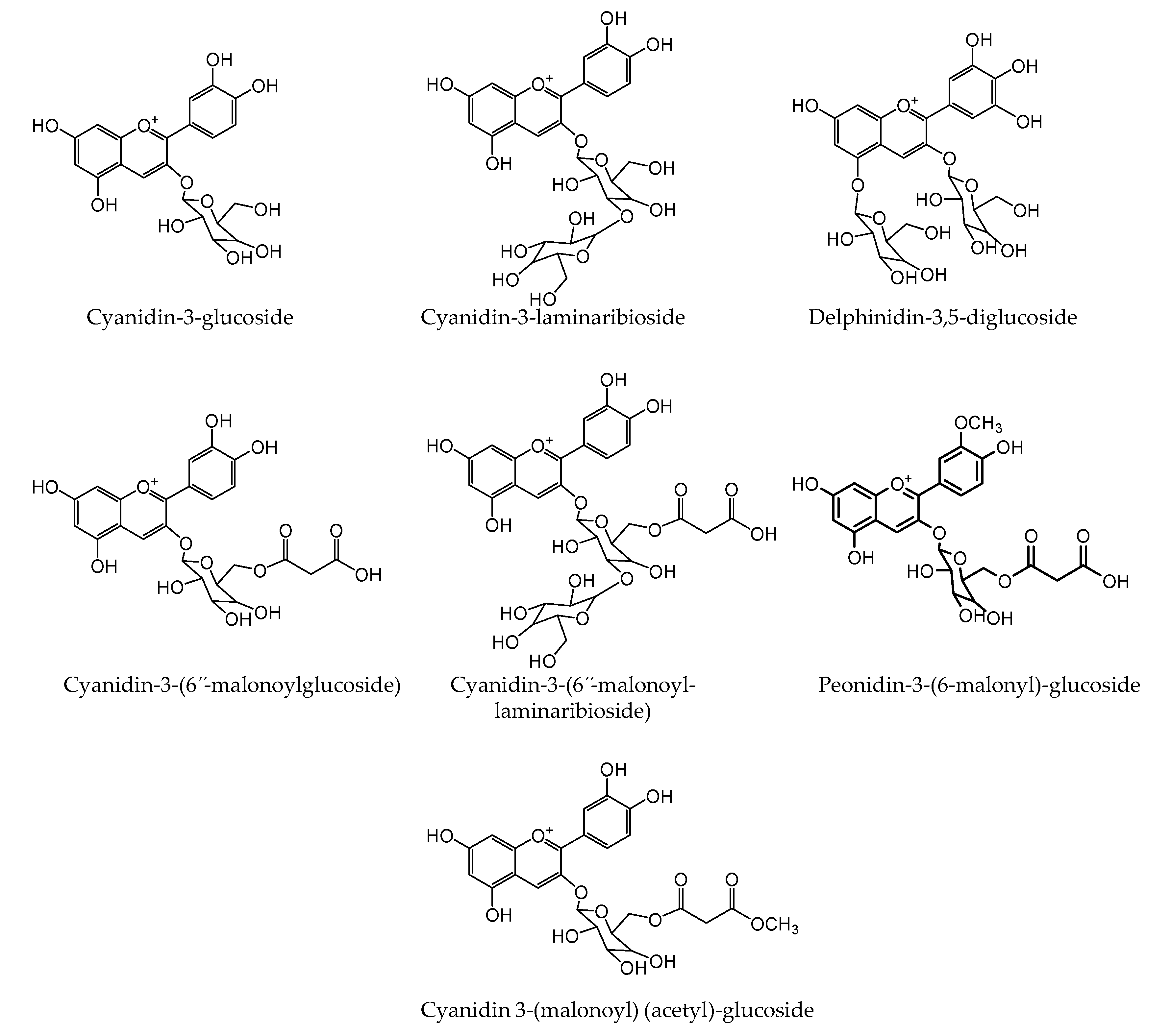
2.5. Identification and Quantitation of Anthocyanins
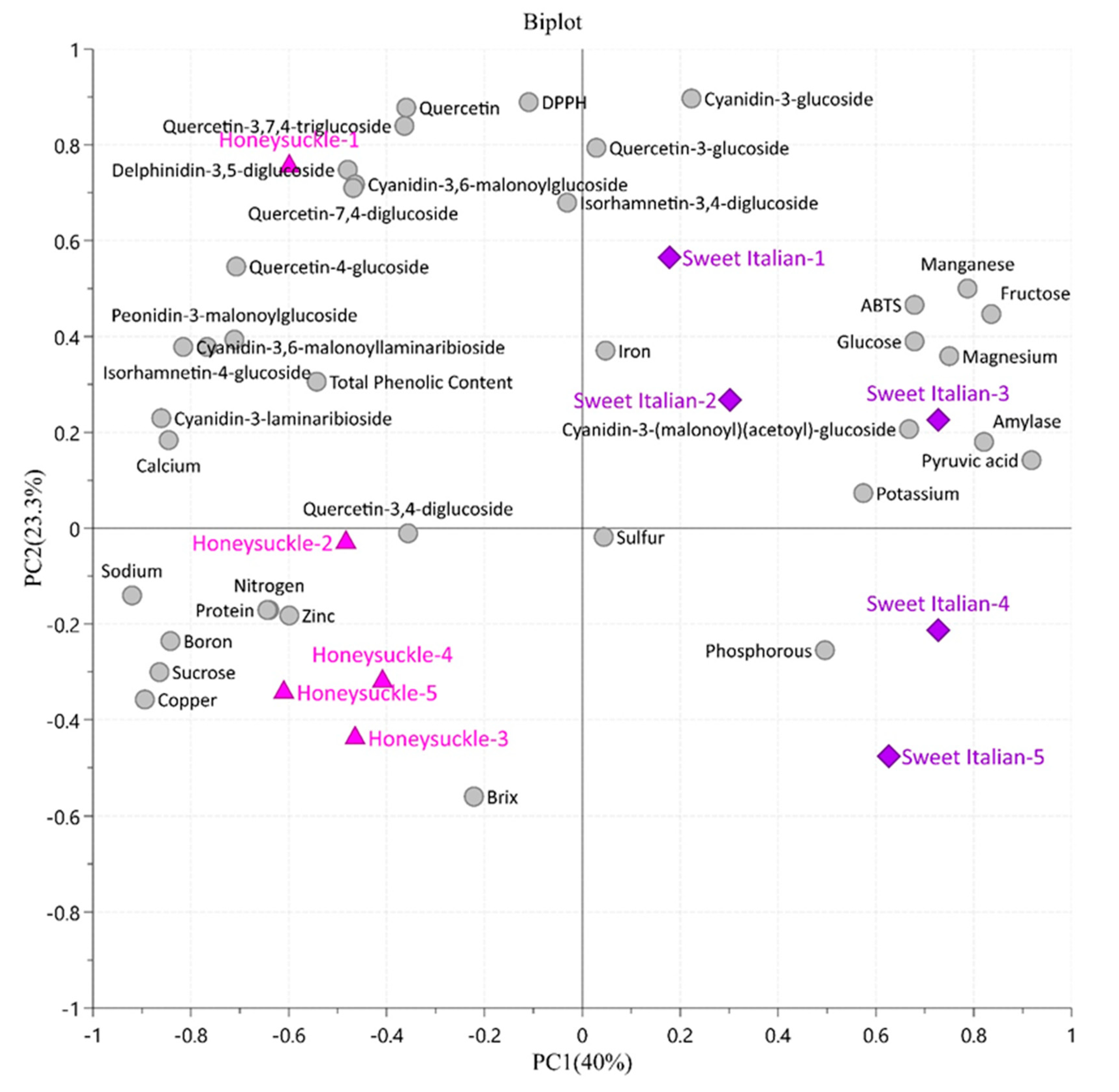
2.6. Principal Component Analysis and Pearson’s Correlation Coefficients
3. Material and Methods
3.1. Plant Materials and Chemicals
3.2. Pyruvic Acid Content
3.3. Sugar Analysis
3.4. Protein and Mineral Analysis
3.5. Analysis of Total Phenolic Content, Antioxidant Capacity, and α-Amylase Assay
3.6. Analysis of Flavonoids
3.7. LC-HR-ESI-QTOF-MS Identification of Flavonoids
3.8. Analysis of Anthocyanins
3.9. LC-HR-ESI-QTOF-MS Identification of Onion Anthocyanins
3.10. Statistical Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sellappan, S.; Akoh, C.C. Flavonoids and antioxidant capacity of Georgia-grown Vidalia onions. J. Agric. Food Chem. 2002, 50, 5338–5342. [Google Scholar] [CrossRef]
- Lombard, K.; Peffley, E.; Geoffriau, E.; Thompson, L.; Herring, A. Quercetin in onion (Allium cepa L.) after heat-treatment simulating home preparation. J. Food Compos. Anal. 2005, 18, 571–581. [Google Scholar] [CrossRef]
- US Department of Agriculture, & Economic Research Service. (April 12, 2020). Per Capita Consumption of Fresh Onions in the United States from 2000 to 2019 (in Pounds). Statista. Available online: https://www.statista.com/statistics/257306/per-capita-consumption-of-fresh-onions-in-the-us/ (accessed on 12 August 2020).
- Yoo, K.S.; Lee, E.J.; Patil, B.S. Changes in flavor precursor, pungency, and sugar contents in shortday onion bulbs during five month storage at various temperatures and controlled-atmosphere. J. Food Sci. 2012, 77, C216–C221. [Google Scholar] [CrossRef]
- Hossain, M.B.; Lebelle, J.; Birsan, R.; Rai, D.K. Enrichment and Assessment of the Contributions of the Major Polyphenols to the Total Antioxidant Activity of Onion Extracts: A Fractionation by Flash Chromatography Approach. Antioxidants 2018, 7, 175. [Google Scholar] [CrossRef] [Green Version]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [Green Version]
- Slimestad, R.; Fossen, T.; Vågen, I.M. Onions: A source of unique dietary flavonoids. J. Agric. Food Chem. 2007, 55, 10067–10080. [Google Scholar] [CrossRef]
- Sytar, O. Phenolic acids in the inflorescences of different varieties of buckwheat and their antioxidant activity. J. King Saud Univ. Sci. 2015, 27, 136–142. [Google Scholar] [CrossRef] [Green Version]
- Kaushik, P.; Andújar, I.; Vilanova, S.; Plazas, M.; Gramazio, P.; Herraiz, F.J.; Brar, N.S.; Prohens, J. Breeding vegetables with increased content in bioactive phenolic acids. Molecules 2015, 20, 18464–18481. [Google Scholar] [CrossRef] [Green Version]
- Kaur, C.; Joshi, S.; Kapoor, H. Antioxidants in onion (Allium Cepa L.) cultivars grown in India. J. Food Biochem. 2009, 33, 184–200. [Google Scholar] [CrossRef]
- Søltoft, M.; Christensen, J.H.; Nielsen, J.; Knuthsen, P. Pressurised liquid extraction of flavonoids in onions. Method development and validation. Talanta 2009, 80, 269–278. [Google Scholar] [CrossRef]
- Rajeswari, G.; Susanna, S.; Prabhasankar, P.; Venkateswara Rao, G. Influence of onion powder and its hydrocolloid blends on pasta dough, pasting, microstructure, cooking and sensory characteristics. Food Biosci. 2013, 4, 13–20. [Google Scholar] [CrossRef]
- Nishimura, M.; Muro, T.; Kobori, M.; Nishihira, J. Effect of Daily Ingestion of Quercetin-Rich Onion Powder for 12 Weeks on Visceral Fat: A Randomised, Double-Blind, Placebo-Controlled, Parallel-Group Study. Nutrients 2020, 12, 91. [Google Scholar] [CrossRef] [Green Version]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]
- Nile, S.H.; Park, S.W. Total phenolics, antioxidant and xanthine oxidase inhibitory activity of three colored onions (Allium cepa L.). Front. Life Sci. 2013, 7, 224–228. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Wang, Y.; Mai, Y.; Li, H.; Wang, Z.; Xu, J.; He, X. Health Benefits of the Flavonoids from Onion: Constituents and Their Pronounced Antioxidant and Anti-neuroinflammatory Capacities. J. Agric. Food Chem. 2020, 68, 799–807. [Google Scholar] [CrossRef]
- Lee, J.; Mitchell, A.E. Quercetin and isorhamnetin glycosides in onion (Allium cepa L.): Varietal comparison, physical distribution, coproduct evaluation, and long-term storage stability. J. Agric. Food Chem. 2011, 59, 857–863. [Google Scholar] [CrossRef]
- Yoo, K.S.; Lee, E.J.; Patil, B.S. Quantification of quercetin glycosides in 6 onion cultivars and comparisons of hydrolysis-HPLC and spectrophotometric methods in measuring total quercetin concentrations. J. Food Sci. 2010, 75, C160–C165. [Google Scholar] [CrossRef]
- Bonaccorsi, P.; Caristi, C.; Gargiulli, C.; Leuzzi, U. Flavonol glucoside profile of southern Italian red onion (Allium cepa L.). J. Agric. Food Chem. 2005, 53, 2733–2740. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [Green Version]
- Saha, S.; Singh, J.; Paul, A.; Sarkar, R.; Khan, Z.; Banerjee, K. Anthocyanin profiling using uv-vis spectroscopy and liquid chromatography mass spectrometry. J. AOAC Int. 2020, 103, 23–39. [Google Scholar] [CrossRef]
- Winter, A.N.; Ross, E.K.; Khatter, S.; Miller, K.; Linseman, D.A. Chemical basis for the disparate neuroprotective effects of the anthocyanins, callistephin and kuromanin, against nitrosative stress. Free Radic. Biol. Med. 2017, 103, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Venancio, V.P.; Cipriano, P.A.; Kim, H.; Antunes, L.M.; Talcott, S.T.; Mertens-Talcott, S.U. Cocoplum (Chrysobalanus icaco L.) anthocyanins exert anti-inflammatory activity in human colon cancer and non-malignant colon cells. Food Funct. 2017, 8, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Jalao, I.; Sánchez-Moreno, C.; de Ancos, B. Influence of food matrix and high-pressure processing on onion flavonols and antioxidant activity during gastrointestinal digestion. J. Food Eng. 2017, 213, 60–68. [Google Scholar] [CrossRef]
- Duangjit, J.; Welsh, K.; Wise, M.L.; Bohanec, B.; Havey, M.J. Genetic analyses of anthocyanin concentrations and intensity of red bulb color among segregating haploid progenies of onion. Mol. Breed. 2014, 34, 75–85. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Azizi, F. Dietary polyphenols as potential nutraceuticals in management of diabetes: A review. J. Diabetes Metab. Disord. 2013, 12, 43. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Gonzalez, A.I.; Díaz-Sánchez, Á.; de La Rosa, L.; Bustos-Jaimes, I.; Alvarez-Parrilla, E. Inhibition of α-amylase by flavonoids: Structure activity relationship (SAR). Spectrochim. Acta Part A 2019, 206, 437–447. [Google Scholar] [CrossRef]
- Gois Ruivo da Silva, M.; Skrt, M.; Komes, D.; Poklar Ulrih, N.; Pogačnik, L. Enhanced Yield of Bioactivities from Onion (Allium cepa L.) Skin and Their Antioxidant and Anti-α-Amylase Activities. Int. J. Mol. Sci. 2020, 21, 2909. [Google Scholar] [CrossRef]
- Anthon, G.E.; Barrett, D.M. Modified method for the determination of pyruvic acid with dinitrophenylhydrazine in the assessment of onion pungency. J. Sci. Food Agric. 2003, 83, 1210–1213. [Google Scholar] [CrossRef]
- Yoo, K.S.; Lee, E.J.; Hamilton, B.K.; Patil, B.S. A comparison of juice extraction methods in the pungency measurement of onion bulbs. J. Sci. Food Agric. 2016, 96, 735–741. [Google Scholar] [CrossRef]
- Liguori, L.; Califano, R.; Albanese, D.; Raimo, F.; Crescitelli, A.; Di Matteo, M. Chemical Composition and Antioxidant Properties of Five White Onion (Allium cepa L.) Landraces. J. Food Qual. 2017, 2017, 9. [Google Scholar] [CrossRef] [Green Version]
- Takeda, E.; Yamamoto, H.; Yamanaka-Okumura, H.; Taketani, Y. Dietary phosphorus in bone health and quality of life. Nutr. Rev. 2012, 70, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Ahmed, M.J.; Shah, S.Z.A. Comparative analysis of mineral content and proximate composition from chilli pepper (Capsicum annuum L.) germplasm. Pure Appl. Biol. 2019, 8, 1338–1347. [Google Scholar] [CrossRef]
- Glasdam, S.-M.; Glasdam, S.; Peters, G.H. The Importance of Magnesium in the Human Body: A Systematic Literature Review. Adv. Clin. Chem. 2016, 73, 169–193. [Google Scholar]
- Sardesai, V. Introduction to Clinical Nutrition; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Maret, W.; Sandstead, H.H. Zinc requirements and the risks and benefits of zinc supplementation. J. Trace Elem. Med. Biol. 2006, 20, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, F.H.; Meacham, S.L. Growing evidence for human health benefits of boron. J. Evid. Based Complement. Altern. Med. 2011, 16, 169–180. [Google Scholar] [CrossRef]
- Mithen, R. Sulphur-Containing Compounds. In Plant Secondary Metabolites: Occurrence, Structure and Role in the Human Diet; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2006; pp. 25–46. [Google Scholar]
- Schaich, K.M.; Tian, X.; Xie, J. Hurdles and pitfalls in measuring antioxidant efficacy: A critical evaluation of ABTS, DPPH, and ORAC assays. J. Funct. Foods 2015, 14, 111–125. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Girennavar, B.; Patil, B.S. Antioxidant capacity of pummelo and navel oranges: Extraction efficiency of solvents in sequence. Lwt-Food Sci. Technol. 2008, 41, 376–384. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S.; Gonzalez-Aguilar, G.A. Quantification of flavonoids, total phenols and antioxidant properties of onion skin: A comparative study of fifteen Indian cultivars. J. Food Sci. Technol. 2020, 57, 2423–2432. [Google Scholar] [CrossRef]
- Wu, H.; Xu, B. Inhibitory Effects of Onion Against α-Glucosidase Activity and its Correlation with Phenolic Antioxidants. Int. J. Food Prop. 2014, 17, 599–609. [Google Scholar] [CrossRef]
- Lu, X.; Wang, J.; Al-Qadiri, H.M.; Ross, C.F.; Powers, J.R.; Tang, J.; Rasco, B.A. Determination of total phenolic content and antioxidant capacity of onion (Allium cepa) and shallot (Allium oschaninii) using infrared spectroscopy. Food Chem. 2011, 129, 637–644. [Google Scholar] [CrossRef]
- Suleria, H.A.R.; Butt, M.S.; Anjum, F.M.; Saeed, F.; Khalid, N. Onion: Nature Protection Against Physiological Threats. Crit. Rev. Food Sci. Nutr. 2015, 55, 50–66. [Google Scholar] [CrossRef] [PubMed]
- Prakash, D.; Singh, B.N.; Upadhyay, G. Antioxidant and free radical scavenging activities of phenols from onion (Allium cepa). Food Chem. 2007, 102, 1389–1393. [Google Scholar] [CrossRef]
- Yang, J.; Meyers, K.J.; van der Heide, J.; Liu, R.H. Varietal Differences in Phenolic Content and Antioxidant and Antiproliferative Activities of Onions. J. Agric. Food Chem. 2004, 52, 6787–6793. [Google Scholar] [CrossRef]
- Lee, S.U.; Lee, J.H.; Choi, S.H.; Lee, J.S.; Ohnisi-Kameyama, M.; Kozukue, N.; Levin, C.E.; Friedman, M. Flavonoid content in fresh, home-processed, and light-exposed onions and in dehydrated commercial onion products. J. Agric. Food Chem. 2008, 56, 8541–8548. [Google Scholar] [CrossRef] [PubMed]
- Serban, M.C.; Sahebkar, A.; Zanchetti, A.; Mikhailidis, D.P.; Howard, G.; Antal, D.; Andrica, F.; Ahmed, A.; Aronow, W.S.; Muntner, P.; et al. Effects of Quercetin on Blood Pressure: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2016, 5, e002713. [Google Scholar] [CrossRef] [Green Version]
- Kozlowska, A.; Szostak-Wegierek, D. Flavonoids--food sources and health benefits. Rocz. Panstw. Zakl. Hig. 2014, 65, 79–85. [Google Scholar]
- Böttcher, C.; Krähmer, A.; Stürtz, M.; Widder, S.; Schulz, H. Effect of cultivar and cultivation year on the metabolite profile of onion bulbs (Allium cepa L.). J. Agric. Food Chem. 2018, 66, 3229–3238. [Google Scholar] [CrossRef]
- Zhang, S.-l.; Peng, D.; Xu, Y.-C.; Lü, S.-W.; Wang, J.-J. Quantification and analysis of anthocyanin and flavonoids compositions, and antioxidant activities in onions with three different colors. J. Integr. Agric. 2016, 15, 2175–2181. [Google Scholar] [CrossRef] [Green Version]
- Fossen, T.; Andersen, Ø.M. Anthocyanins from red onion, Allium cepa, with novel aglycone. Phytochemistry 2003, 62, 1217–1220. [Google Scholar] [CrossRef]
- Pérez-Gregorio, R.M.; García-Falcón, M.S.; Simal-Gándara, J.; Rodrigues, A.S.; Almeida, D.P.F. Identification and quantification of flavonoids in traditional cultivars of red and white onions at harvest. J. Food Compos. Anal. 2010, 23, 592–598. [Google Scholar] [CrossRef]
- Ferreres, F.; Gil, M.I.; Tomás-Barberán, F.A. Anthocyanins and flavonoids from shredded red onion and changes during storage in perforated films. Food Res. Int. 1996, 29, 389–395. [Google Scholar] [CrossRef]
- Gennaro, L.; Leonardi, C.; Esposito, F.; Salucci, M.; Maiani, G.; Quaglia, G.; Fogliano, V. Flavonoid and Carbohydrate Contents in Tropea Red Onions: Effects of Homelike Peeling and Storage. J. Agric. Food Chem. 2002, 50, 1904–1910. [Google Scholar] [CrossRef] [PubMed]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Chemistry, Pharmacology and Health Benefits of Anthocyanins. Phytother. Res. 2016, 30, 1265–1286. [Google Scholar] [CrossRef] [PubMed]
- Amić, D.; Davidović-Amić, D.; Bešlo, D.; Trinajstić, N. Structure-radical scavenging activity relationships of flavonoids. Croat. Chem. Acta 2003, 76, 55–61. [Google Scholar]
- Murota, K.; Mitsukuni, Y.; Ichikawa, M.; Tsushida, T.; Miyamoto, S.; Terao, J. Quercetin-4‘-glucoside Is More Potent than Quercetin-3-glucoside in Protection of Rat Intestinal Mucosa Homogenates against Iron Ion-Induced Lipid Peroxidation. J. Agric. Food Chem. 2004, 52, 1907–1912. [Google Scholar] [CrossRef]
- Olivas-Aguirre, F.J.; Rodrigo-García, J.; Martínez-Ruiz, N.D.R.; Cárdenas-Robles, A.I.; Mendoza-Díaz, S.O.; Álvarez-Parrilla, E.; González-Aguilar, G.A.; de la Rosa, L.A.; Ramos-Jiménez, A.; Wall-Medrano, A. Cyanidin-3-O-glucoside: Physical-Chemistry, Foodomics and Health Effects. Molecules 2016, 21, 1264. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Chen, S.; Zhou, G.; Li, H.; Zhong, L.; Liu, S. Potential role of cyanidin 3-glucoside (C3G) in diabetic cardiomyopathy in diabetic rats: An in vivo approach. Saudi J. Biol. Sci. 2018, 25, 500–506. [Google Scholar] [CrossRef] [Green Version]
- Akkarachiyasit, S.; Charoenlertkul, P.; Yibchok-Anun, S.; Adisakwattana, S. Inhibitory activities of cyanidin and its glycosides and synergistic effect with acarbose against intestinal α-glucosidase and pancreatic α-amylase. Int. J. Mol. Sci. 2010, 11, 3387–3396. [Google Scholar] [CrossRef] [Green Version]
- Metrani, R.; Jayaprakasha, G.K.; Patil, B.S. Optimized method for the quantification of pyruvic acid in onions by microplate reader and confirmation by high resolution mass spectra. Food Chem. 2018, 242, 451–458. [Google Scholar] [CrossRef]
- Acharya, P.; Jayaprakasha, G.K.; Crosby, K.M.; Jifon, J.L.; Patil, B.S. Green-Synthesized Nanoparticles Enhanced Seedling Growth, Yield, and Quality of Onion (Allium cepa L.). Acs Sustain. Chem. Eng. 2019, 7, 14580–14590. [Google Scholar] [CrossRef]
- Chaudhary, P.; Jayaprakasha, G.; Porat, R.; Patil, B.S. Degreening and postharvest storage influences ‘Star Ruby’grapefruit (Citrus paradisi Macf.) bioactive compounds. Food Chem. 2012, 135, 1667–1675. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jayaprakasha, G.K.; Patil, B.S. Chemometric characterization of 30 commercial thermal and cold processed juices using UPLC-QTOF-HR-MS fingerprints. Lwt-Food Sci. Technol. 2019, 114, 108322. [Google Scholar] [CrossRef]
- Barkat, N.; Singh, J.; Jayaprakasha, G.K.; Patil, B.S. Effect of harvest time on the levels of phytochemicals, free radical-scavenging activity, α-amylase inhibition and bile acid-binding capacity of spinach (Spinacia oleracea). J. Sci. Food Agric. 2018, 98, 3468–3477. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Jayaprakasha, G.K.; Patil, B.S. Rapid ultra-high-performance liquid chromatography/quadrupole time-of-flight tandem mass spectrometry and selected reaction monitoring strategy for the identification and quantification of minor spinacetin derivatives in spinach. Rapid Commun. Mass Spectrom. 2017, 31, 1803–1812. [Google Scholar] [CrossRef]
- Gomez, M.K.; Singh, J.; Acharya, P.; Jayaprakasha, G.; Patil, B.S. Identification and Quantification of Phytochemicals, Antioxidant Activity, and Bile Acid-Binding Capacity of Garnet Stem Dandelion (Taraxacum officinale). J. Food Sci. 2018, 83, 1569–1578. [Google Scholar] [CrossRef]
- Singh, J.; Jayaprakasha, G.; Patil, B.S. An optimized solvent extraction and characterization of unidentified flavonoid glucuronide derivatives from spinach by UHPLC-HR-QTOF-MS. Talanta 2018, 188, 763–771. [Google Scholar] [CrossRef]


| Nutritional Component | Honeysuckle | Sweet Italian |
|---|---|---|
| μg/g | ||
| Phosphorus | 2525.63 | 2677.64 |
| Potassium | 12,720.67 | 13,550.1 |
| Calcium | 3183.54 | 2506.5 |
| Magnesium | 980.43 | 1100.62 |
| Sodium | 1001.34 | 314.13 |
| Zinc | 14.43 | 12.23 |
| Iron | 23.16 | 27.69 |
| Copper | 7.38 | 3.19 |
| Manganese | 6.03 | 9.22 |
| Sulfur | 3421.58 | 3415.67 |
| Boron | 18.34 | 15.34 |
| % | ||
| Nitrogen | 1.47 | 1.31 |
| Protein | 9.21 | 8.2 |
| Phytochemical | Compound | Experimental MS (m/z) | Theoretical MS (m/z) | Mass Error (ppm) | Aglycone Ion (m/z) |
|---|---|---|---|---|---|
| Flavonoids | Quercetin-3,7,4′-triglucoside | 789.20136 | 789.208399 | 8.9 | 303.04816 |
| Quercetin-7,4′-diglucoside | 627.15072 | 627.155576 | 7.7 | 303.04766 | |
| Quercetin-3,4′-diglucoside | 627.15279 | 627.155576 | 4.4 | 303.04919 | |
| Isorhamnetin-3,4′-diglucoside | 641.16577 | 641.171226 | 8.5 | 317.06311 | |
| Quercetin-3- glucoside | 465.10128 | 465.102753 | 3.1 | 303.04827 | |
| Quercetin-4′-glucoside | 465.10382 | 465.102753 | −2.2 | 303.05104 | |
| Isorhamnetin-4′-glucoside | 479.12018 | 479.118403 | −3.7 | 317.06622 | |
| Quercetin | 303.04965 | 303.049929 | 0.9 | - | |
| Anthocyanins | Cyanidin-3-glucoside | 449.1022 | 449.1078 | 12.4 | 287.0516 |
| Cyanidin-3-laminaribioside | 611.1549 | 611.1606 | 9.3 | 287.0524 | |
| Delphinidin-3,5-diglucoside | 627.1496 | 627.1555 | 9.4 | 303.0474 | |
| Cyanidin-3-(6”-malonoylglucoside) | 535.1024 | 535.1082 | 10.8 | 287.0531 | |
| Cyanidin-3-(6”-malonoyl-laminaribioside) | 697.1532 | 697.161 | 11.1 | 287.0527 | |
| Peonidin-3-malonoylglucoside | 549.1168 | 549.1238 | 12.7 | 301.0665 | |
| Cyanidin-3(malonoyl)-(acetyl)-glucoside | 577.1124 | 577.1187 | 10.9 | 287.0518 |
| Phytochemical | Compound | Honeysuckle (μg/g FW) | Sweet Italian (μg/g FW) |
|---|---|---|---|
| Flavonoids | Quercetin-3,7,4′-triglucoside | 8.44 ± 0.95 | 6.95 ± 0.47 |
| Quercetin-7,4′-diglucoside | 35.34 ± 1.80 | 28.73 ± 1.16 | |
| Quercetin-3,4′-diglucoside | 168.91 ± 8.71 | 154.79 ± 3.91 | |
| Isorhamnetin-3,4′-diglucoside | 13.18 ± 1.56 | 13.42 ± 0.53 | |
| Quercetin-3-glucoside | 5.88 ± 0.34 | 6.47 ± 0.27 | |
| Quercetin-4′-glucoside | 184.60 ± 7.11 * | 115.14 ± 3.35 | |
| Isorhamnetin-4′-glucoside | 25.58 ± 1.19 * | 13.52 ± 0.64 | |
| Quercetin | 7.44 ± 0.59 | 6.31 ± 0.35 | |
| Total Flavonoids | 449.37 ± 75.21 * | 345.34 ± 58.09 | |
| Anthocyanins | Cyanidin-3-glucoside | 6.42 ± 0.94 | 9.30 ± 1.77 |
| Cyanidin-3-laminaribioside | 5.34 ± 0.49 * | 3.03 ± 0.67 | |
| Delphinidin-3,5-diglucoside | 1.25 ± 0.08 | 1.07 ± 0.19 | |
| Cyanidin-3-(6”-malonoylglucoside) | 58.88 ± 3.76 | 50.90 ± 6.79 | |
| Cyanidin-3-(6”-malonoyl-laminaribioside) | 30.18 ± 2.14 * | 20.44 ± 2.84 | |
| Peonidin-3-malonoylglucoside | 1.01 ± 0.08 * | 0.66 ± 0.12 | |
| Cyanidin-3(malonoyl)-(acetyl)-glucoside | 0.33 ± 0.09 | 1.05 ± 0.35* | |
| Total Anthocyanins | 103.40 ± 22.06 | 86.47 ± 18.43 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Metrani, R.; Singh, J.; Acharya, P.; K. Jayaprakasha, G.; S. Patil, B. Comparative Metabolomics Profiling of Polyphenols, Nutrients and Antioxidant Activities of Two Red Onion (Allium cepa L.) Cultivars. Plants 2020, 9, 1077. https://doi.org/10.3390/plants9091077
Metrani R, Singh J, Acharya P, K. Jayaprakasha G, S. Patil B. Comparative Metabolomics Profiling of Polyphenols, Nutrients and Antioxidant Activities of Two Red Onion (Allium cepa L.) Cultivars. Plants. 2020; 9(9):1077. https://doi.org/10.3390/plants9091077
Chicago/Turabian StyleMetrani, Rita, Jashbir Singh, Pratibha Acharya, Guddadarangavvanahally K. Jayaprakasha, and Bhimanagouda S. Patil. 2020. "Comparative Metabolomics Profiling of Polyphenols, Nutrients and Antioxidant Activities of Two Red Onion (Allium cepa L.) Cultivars" Plants 9, no. 9: 1077. https://doi.org/10.3390/plants9091077





