Nutritional Content and Antioxidant Capacity of the Seed and the Epicarp in Different Ecotypes of Pistacia atlantica Desf. Subsp. atlantica
Abstract
:1. Introduction
2. Results
2.1. Biochemical Analysis
2.1.1. Protein and Soluble Sugar Content of Seeds
2.1.2. Mineral Analysis
2.1.3. Total Phenolic Content
2.1.4. Phenolic Compound Identification
2.2. Total Antioxidant Activity (TAA)
3. Discussion
4. Materials and Methods
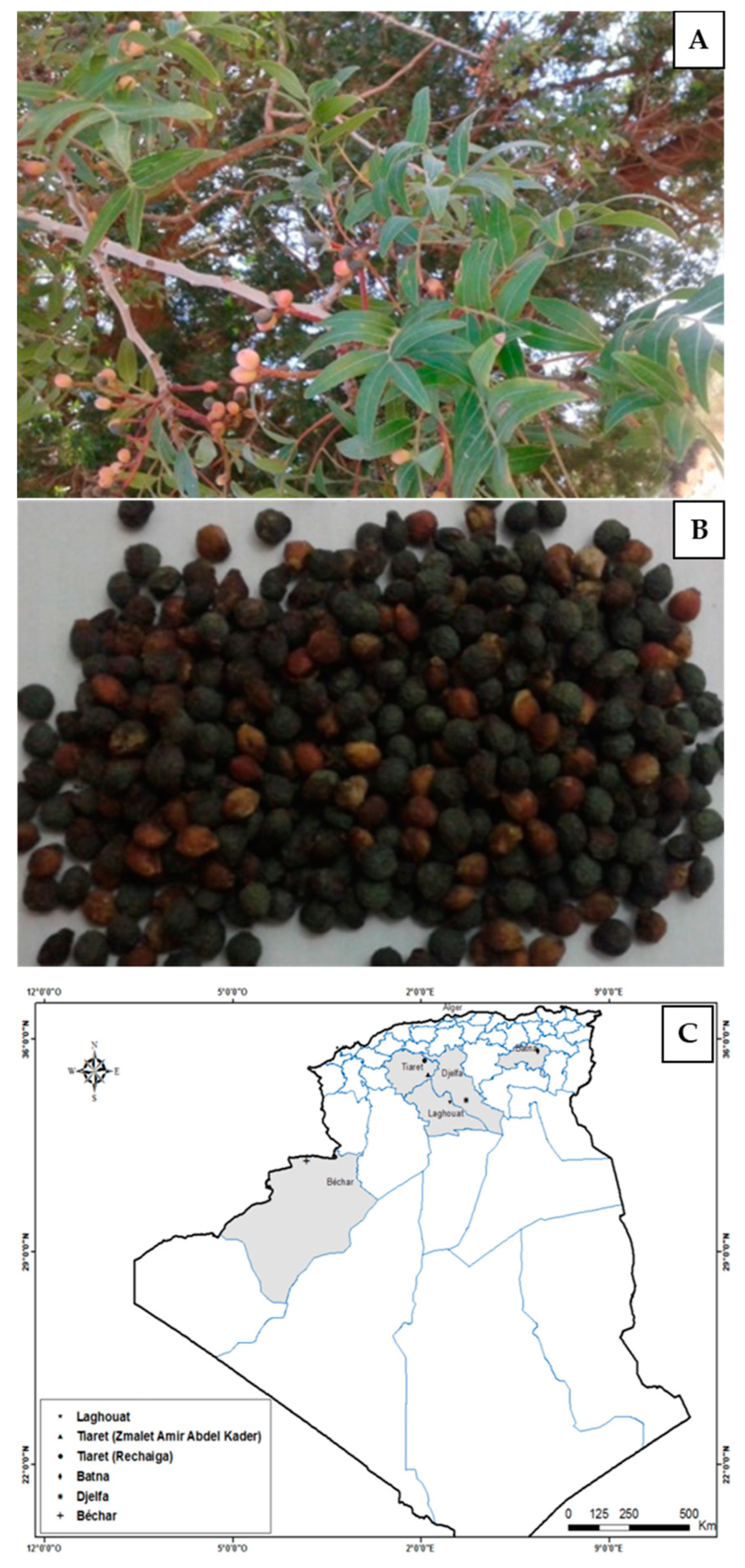
4.1. Geographical Origin of Seeds
4.2. Chemicals and Reagents
4.3. Biochemical Analysis
4.3.1. Nitrogen, Protein Contents and Soluble Sugars Content
4.3.2. Mineral Analysis
4.3.3. Total Phenolic Content
Plant Sampling and Preparation for Extract
4.3.4. Chromatographic Separation of Phenolic Compounds by HPLC
4.4. Total Antioxidant Activity (TAA)
4.5. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zohary, M. A monographical study of the genus Pistacia. Palest. J. Bot. 1952, 5, 128–187. [Google Scholar]
- Ifticene-Habani, N.; Messaoudene, M. Croissance radiale et sensibilité au climat du pistachier de l’atlas, pistacia atlantica desf., en algérie. Bois For. Des Trop. 2017, 329, 3. [Google Scholar] [CrossRef] [Green Version]
- Mahjoub, F.; Rezayat, K.A.; Yousefi, M.; Mohebbi, M.; Salari, R. Pistacia atlantica Desf. A review of its traditional uses, phytochemicals and pharmacology. J. Med. Life 2018, 11, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Labdelli, A.; Zemour, K.; Simon, V.; Cerny, M.; Adda, A.; Merah, O. Pistacia Atlantica Desf., a Source of Healthy Vegetable Oil. Appl. Sci. 2019, 9, 2552. [Google Scholar] [CrossRef] [Green Version]
- Chelghoum, M.; Guenane, H.; Harrat, M.; Yousfi, M. Total Tocopherols, Carotenoids, and Fatty Acids Content Variation of Pistacia atlantica from Different Organs’ Crude Oils and Their Antioxidant Activity during Development Stages. Chem. Biodivers. 2020, 1–16. [Google Scholar] [CrossRef]
- Gourine, N.; Yousfi, M.; Bombarda, I.; Nadjemi, B.; Stocker, P.; Gaydou, E.M. Antioxidant activities and chemical composition of essential oil of Pistacia atlantica from Algeria. Ind. Crop. Prod. 2010, 31, 203–208. [Google Scholar] [CrossRef]
- Bozorgi, M.; Memariani, Z.; Mobli, M.; Surmaghi, M.H.S.; Shams-Ardekani, M.R.; Rahimi, R. Five Pistacia species (P. vera, P. atlantica, P. terebinthus, P. khinjuk, and P. lentiscus): A Review of Their Traditional Uses, Phytochemistry, and Pharmacology. Sci. World J. 2013, 2013, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Peksel, A.; Arisan, I.; Yanardag, R. Radical scavenging and anti-acetylcholinesterase activities of aqueous extract of wild pistachio (Pistacia atlantica Desf.) leaves. Food Sci. Biotechnol. 2013, 22, 515–522. [Google Scholar] [CrossRef]
- Sayed-Ahmad, B.; Talou, T.; Saad, Z.; Hijazi, A.; Merah, O. The Apiaceae: Ethnomedicinal family as source for industrial uses. Ind. Crop. Prod. 2017, 109, 661–671. [Google Scholar] [CrossRef] [Green Version]
- Auddy, B.; Ferreira, M.; Blasina, F.; Lafon, L.; Arredondo, F.; Dajas, F.; Tripathi, P.C.; Seal, T.; Mukherjee, B. Screening of antioxidant activity of three Indian medicinal plants, traditionally used for the management of neurodegenerative diseases. J. Ethnopharmacol. 2003, 84, 131–138. [Google Scholar] [CrossRef]
- Isabelle, M.; Lee, B.L.; Lim, M.T.; Koh, W.-P.; Huang, D.; Ong, C.N. Antioxidant activity and profiles of common fruits in Singapore. Food Chem. 2010, 123, 77–84. [Google Scholar] [CrossRef]
- Engin, A.B.; Bukan, N.; Kurukahvecioglu, O.; Memis, L.; Engin, A. Effect of butylated hydroxytoluene (E321) pretreatment versus l-arginine on liver injury after sub-lethal dose of endotoxin administration. Environ. Toxicol. Pharmacol. 2011, 32, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Randhawa, S.; Bahna, S.L. Hypersensitivity reactions to food additives. Curr. Opin. Allergy Clin. Immunol. 2009, 9, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Kahl, R.; Kappus, H. Toxicology of the synthetic antioxidants BHA and BHT in comparison with the natural antioxidant vitamin E. Z. Lebensm. Unters. Forsch 1993, 196, 329–338. [Google Scholar] [CrossRef]
- Oksana, S. Plant phenolic compounds for food, pharmaceutical and cosmetics production. J. Med. Plants Res. 2012, 6, 2526–2539. [Google Scholar] [CrossRef]
- Aqil, F.; Ahmad, I.; Mehmood, Z. Antioxidant and Free Radical Scavenging Properties of Twelve Traditionally Used Indian Medicinal Plants. Turk. J. Biol. 2006, 30, 177–183. [Google Scholar]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant Capacity of Tea and Common Vegetables. J. Agric. Food Chem. 1996, 44, 3426–3431. [Google Scholar] [CrossRef]
- Kaur, C.; Kapoor, H.C. Anti-oxidant activity and total phenolic content of some Asian vegetables. Int. J. Food Sci. Technol. 2002, 37, 153–161. [Google Scholar] [CrossRef]
- Mohammadi, B.; Maboud, H.E.; Seyedi, S.M. Nutritional value and antioxidant properties of hull and kernel in Pistacia atlantica and Pistacia khinjuk fruits. J. Food Sci. Technol. 2019, 56, 3571–3578. [Google Scholar] [CrossRef]
- Klepacka, J.; Najda, A.; Klimek, K. Effect of Buckwheat Groats Processing on the Content and Bioaccessibility of Selected Minerals. Foods 2020, 9, 832. [Google Scholar] [CrossRef]
- Black, M.M. Micronutrient Deficiencies and Cognitive Functioning. J. Nutr. 2003, 133, 3927S–3931S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adjepong, M.; Jain, R.; Pickens, C.A.; Appaw, W.; Fenton, J.I. Quantification of fatty acid and mineral levels of selected seeds, nuts, and oils in Northern Ghana. J. Food Sci. Technol. 2018, 55, 4615–4622. [Google Scholar] [CrossRef] [PubMed]
- Belyagoubi, L.; Belyagoubi-Benhammou, N.; Atik-Bekkara, F.; Coustard, J.M. Effects of extraction solvents on phenolic content and antioxidant properties of Pistacia atlantica Desf. fruits from Algeria. Int. Food Res. J. 2016, 23, 948–953. [Google Scholar]
- Hatamnia, A.A.; Abbaspour, N.; Darvishzadeh, R. Antioxidant activity and phenolic profile of different parts of Bene (Pistacia atlantica subsp. kurdica) fruits. Food Chem. 2014, 145, 306–311. [Google Scholar] [CrossRef]
- Rezaie, M.; Farhoosh, R.; Iranshahi, M.; Sharif, A.; Golmohamadzadeh, S. Ultrasonic-assisted extraction of antioxidative compounds from Bene (Pistacia atlantica subsp. mutica) hull using various solvents of different physicochemical properties. Food Chem. 2015, 173, 577–583. [Google Scholar] [CrossRef]
- Saffarzadeh, A.; Vincze, L.; CsapÓ, J. Determination of chemical composition of acorn (Quercus branti), Pistacia atlantica and Pistacia khinjuk seeds as non-conventional feedstuffs. Acta Agraria Kaposváriensis 1999, 3, 59–69. [Google Scholar]
- Benhassaini, H.; Bendahmane, M.; Benchalgo, N. The chemical composition of fruits of Pistacia atlantica Desf. subsp. atlantica from Algeria. Chem. Nat. Compd. 2007, 43, 121–124. [Google Scholar] [CrossRef]
- Tan-Wilson, A.L.; Wilson, K.A. Mobilization of seed protein reserves. Physiol. Plant. 2011, 145, 140–153. [Google Scholar] [CrossRef]
- Sugimoto, M.; Goto, H.; Otomo, K.; Ito, M.; Onuma, H.; Suzuki, A.; Sugawara, M.; Abe, S.; Tomita, M.; Soga, T. Metabolomic Profiles and sensory attributes of edamame under various storage duration and temperature conditions. J. Agric. Food Chem. 2010, 58, 8418–8425. [Google Scholar] [CrossRef]
- Young, G.; Mebrahtu, T.; Johnson, J. Acceptability of green soybeans as a vegetable entity. Plant Foods Hum. Nutr. 2000, 55, 323–333. [Google Scholar] [CrossRef]
- Shanmugasundaram, S.; Yan, M.R.; Yang, R.Y. Association between protein, oil and sugar in vegetable soybean. In Proceedings of the 2nd International Vegetable Soybean Conference, Tacoma, WA, USA, 10–12 August 2001. [Google Scholar]
- Kumar, V.; Rani, A.; Goyal, L.; Pratap, D.; Billore, S.; Chauhan, G. Evaluation of vegetable-type soybean for sucrose, taste-related amino acids, and isoflavones contents. Int. J. Food Prop. 2011, 14, 1142–1151. [Google Scholar] [CrossRef]
- Özcan, M.M. Characteristics of fruit and oil of terebinth(Pistacia terebinthus L) growing wild in Turkey. J. Sci. Food Agric. 2004, 84, 517–520. [Google Scholar] [CrossRef]
- Martinec, N.; Balbino, S.; Dobša, J.; Šimunić-Mežnarić, V.; Legen, S. Macro- and microelements in pumpkin seed oils: Effect of processing, crop season, and country of origin. Food Sci. Nutr. 2019, 7, 1634–1644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roche, J.; Mouloungui, Z.; Cerny, M.; Merah, O. Effect of sowing date on fatty acid and phytosterols patterns of Carthamus tinctoria L. Appl. Sci. 2019, 9, 2839. [Google Scholar] [CrossRef] [Green Version]
- Zemour, K.; Labdelli, A.; Adda, A.; Dellal, A.; Talou, T.; Merah, O. Phenol Content and Antioxidant and Antiaging Activity of Safflower Seed Oil (Carthamus tinctorius L.). Cosmetics 2019, 6, 55. [Google Scholar] [CrossRef] [Green Version]
- Alphan, E.; Pala, M.; Açkurt, F.; Yilmaz, T. Nutritional composition of hazelnuts and its effects on glucose and lipid metabolism. Acta Hortic. 1997, 445, 305–310. [Google Scholar] [CrossRef]
- Tomlinson, D.J.; Mülling, C.; Fakler, T. Invited Review: Formation of Keratins in the Bovine Claw: Roles of Hormones, Minerals, and Vitamins in Functional Claw Integrity. J. Dairy Sci. 2004, 87, 797–809. [Google Scholar] [CrossRef] [Green Version]
- Khomri, Z.-E.; Chabaca, M.N. Contribution to the determination of potential areas for recharge of the El Madher plain water table by a cartographic approach (Batna, Algeria). Ponte Int. Sci. Res. J. 2019, 75, 12. [Google Scholar] [CrossRef]
- Adamou-Djerbaouiz, M.; Djelaila, Y.; Adamou, M.S.; Baziz, B.; Nicolas, V.; Denys, C. Préférence édaphique et pullulation chez Meriones shawii (Mammalia, Rodentia) dans la région de Tiaret (Algérie). Rev. Écol. 2010, 65, 63–72. [Google Scholar]
- Penfield, S.; MacGregor, D.R. Effects of environmental variation during seed production on seed dormancy and germination. J. Exp. Bot. 2016, 68, 819–825. [Google Scholar] [CrossRef] [Green Version]
- Bouabdelli, Z.; Belhadj, S.; Smail-Saadoun, N.; Mévy, J.P.; Notonnier, R.; Tonetto, A.; Ortas, I.; Gauquelin, T. Influence de l’aridité sur la variation de la colonisation mycorhizienne arbusculaire chez cinq populations naturelles algériennes du pistachier de l’atlas (Pistacia atlantica Desf.). Rev. Écol. 2018, 73, 330–344. [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine. Dietary Reference Intakes for Sodium and Potassium; The National Academies Press: Washington, DC, USA, 2019; p. 26. [Google Scholar]
- Sanchez-Castillo, C.P.; Dewey, P.J.; Aguirre, A.; Lara, J.J.; Vaca, R.; De La Barra, P.L.; Ortiz, M.; Escamilla, I.; James, W.T. The Mineral Content of Mexican Fruits and Vegetables. J. Food Compos. Anal. 1998, 11, 340–356. [Google Scholar] [CrossRef]
- Özcan, M.M.; Akbulut, M. Estimation of minerals, nitrate and nitrite contents of medicinal and aromatic plants used as spices, condiments and herbal tea. Food Chem. 2008, 106, 852–858. [Google Scholar] [CrossRef]
- Brody, T. Nutritional Biochemistry; Academic Press: San Diego, CA, USA, 1999. [Google Scholar]
- López-Amorós, M.; Hernández, T.; Estrella, I. Effect of germination on legume phenolic compounds and their antioxidant activity. J. Food Compos. Anal. 2006, 19, 277–283. [Google Scholar] [CrossRef]
- Özbek, H.N.; Halahlih, F.; Göğüş, F.; Yanık, D.K.; Azaizeh, H. Pistachio (Pistacia vera L.) Hull as a Potential Source of Phenolic Compounds: Evaluation of Ethanol–Water Binary Solvent Extraction on Antioxidant Activity and Phenolic Content of Pistachio Hull Extracts. Waste Biomass Valorization 2018, 11, 2101–2110. [Google Scholar] [CrossRef]
- Benamar, H.; Marouf, A.; Bennaceur, M. Phytochemical composition, antioxidant and acetylcholinesterase inhibitory activities of aqueous extract and fractions of Pistacia atlantica subsp. atlantica from Algeria. J. Herbs, Spices Med. Plants 2018, 24, 229–244. [Google Scholar] [CrossRef]
- Labdelli, A. Study of seed dormancy origins in three atlas pistachio ecotypes (Pistacia atlantica Desf.). Appl. Ecol. Environ. Res. 2019, 17, 13555–13565. [Google Scholar] [CrossRef]
- Xu, J.G.; Tian, C.R.; Hu, Q.P.; Luo, J.Y.; Wang, X.D.; Tian, X.D. Dynamic Changes in Phenolic Compounds and Antioxidant Activity in Oats (Avena nuda L.) during Steeping and Germination. J. Agric. Food Chem. 2009, 57, 10392–10398. [Google Scholar] [CrossRef]
- Ti, H.; Zhang, R.; Zhang, M.; Li, Q.; Wei, Z.; Zhang, Y.; Tang, X.; Deng, Y.; Liu, L.; Ma, Y. Dynamic changes in the free and bound phenolic compounds and antioxidant activity of brown rice at different germination stages. Food Chem. 2014, 161, 337–344. [Google Scholar] [CrossRef]
- Velioglu, Y.S.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Gan, R.-Y.; Li, S.; Zhou, Y.; Li, A.-N.; Xu, D.-P.; Li, H.-B. Antioxidant Phytochemicals for the Prevention and Treatment of Chronic Diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef] [PubMed]
- Huyut, Z.; Beydemir, S.; Gulcin, I. Antioxidant and Antiradical Properties of Selected Flavonoids and Phenolic Compounds. Biochem. Res. Int. 2017, 2017, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.-Z.; Yang, S.; Wu, G. Free radicals, antioxidants, and nutrition. Nutrition 2002, 18, 872–879. [Google Scholar] [CrossRef]
- Shahidi, F.; Naczk, M. Antioxidant Properties of Food Phenolics. In Phenolics in Food and Nutraceuticals; Shahidi, F., Naczk, M., Eds.; CRC Press: Boca Raton, FL, USA, 2003; pp. 1–403. [Google Scholar]
- Xu, B.; Chang, S.K. A Comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. J. Food Sci. 2007, 72, S159–S166. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H. Health-Promoting components of fruits and vegetables in the diet12. Adv. Nutr. 2013, 4, 384S–392S. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality—A systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef]
- Waddell, K.L. Sampling coarse woody debris for multiple attributes in extensive resource inventories. Ecol. Indic. 2002, 1, 139–153. [Google Scholar] [CrossRef]
- Kirk, P.L. Kjeldahl Method for Total Nitrogen. Anal. Chem. 1950, 22, 354–358. [Google Scholar] [CrossRef]
- Gomez, L.; Rubio, E.; Auge, M. A new procedure for extraction and measurement of soluble sugars in ligneous plants. J. Sci. Food Agric. 2002, 82, 360–369. [Google Scholar] [CrossRef]
- Galedar, M.N.; Tabatabaeefar, A.; Jafari, A.; Sharifi, A.; Mohtasebi, S.S.; Fadaei, H. Moisture Dependent Geometric and Mechanical Properties of Wild Pistachio (Pistacia vera L.) Nut and Kernel. Int. J. Food Prop. 2010, 13, 1323–1338. [Google Scholar] [CrossRef] [Green Version]
- Guéguen, L. Étude de la composition minérale de quelques espèces fourragères. influence du stade de développement et du cycle de végétation. Anim. Res. 1959, 8, 245–268. [Google Scholar] [CrossRef] [Green Version]
- Joret, G.; Hébert, J. Contribution à la détermination du besoin des sols en acide phosphorique. Ann. Agron. 1955, VI 2, 233–299. [Google Scholar]
- Kjeldahl, J. Neue Methode zur Bestimmung des Stickstoffs in organischen Körpern. Anal. Bioanal. Chem. 1883, 22, 366–382. [Google Scholar] [CrossRef] [Green Version]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.-M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef] [PubMed]

| Parameters | A | B | D | L | T-R | T-Z | Test F |
|---|---|---|---|---|---|---|---|
| Proteins (%) | 7.73 ± 0.13 b | 7.69 ± 0.55 b | 8.45 ± 0.20 a | 8.82 ± 0.35 a | 9.56 ± 0.32 | 8.46 ± 0.42 a | 11.57 *** |
| Soluble sugars (mg/gFW) | 128.06 ± 8.39 b | 55.24 ± 5.02 c | 125.29 ± 9.55 b | 94.96 ± 4.59 a | 77.17 ± 7.85 d | 93.16 ± 5.57 a | 46.54 *** |
| Parameters | A | B | D | L | T-R | T-Z | F Value |
|---|---|---|---|---|---|---|---|
| Moisture (%) | 3.2 | 4.03 | 3.72 | 4.38 | 4.5 | 4.1 | - |
| Ash (%) | 5.03 ± 0.84 b | 5.12 ± 1.10 b | 3.29 ± 0.01 a | 3.66 ± 0.74 a | 3.99 ± 0.38 a | 3.42 ± 0.10 a | 2.85 ns |
| N (%) | 1.24 ± 0.02 b | 1.23 ± 0.09 b | 1.35 ± 0.03 a | 1.41 ± 0.06 a | 1.53 ± 0.05 c | 1.35 ± 0.07 a | 11.57 *** |
| P | 2.42 ± 0.03 e | 1.60 ± 0.02 a | 2.00 ± 0.003 c | 2.32 ± 0.01 d | 1.80 ± 0.02 b | 2.44 ± 0.01 f | 3235.1 *** |
| Ca | 2.57 ± 0.06 d | 3.77 ± 0.23 a | 2.35 ± 0.06 c | 1.40 ± 0.00 b | 3.76 ± 0.10 a | 2.99 ± 0.10 e | 191.86 *** |
| Na | 0.29 ± 0.05 c,d | 0.23 ± 0.05 b,c | 0.34 ± 0.05 d | 0.19 ± 0.05 a,b | 0.11 ± 0.0 a | 0.19 ± 0.05 a,b | 8.87 ** |
| K | 12.68 ± 0.25 b | 12.11 ± 0.24 a | 15.83 ± 0.25 e | 10.18 ± 0.25 c | 12.29 ± 0.25 a,b | 14.06 ± 0.14 d | 200.09 *** |
| Mg | 0.37 ± 0.01 d | 0.24 ± 0.01 | 0.18 ± 0.01 a | 0.13 ± 0.01 b | 0.18 ± 0.06 a | 0.19 ± 0.01 a | 34.48 *** |
| Se | 0.43 | 0.82 | 0.22 | 0.15 | 0.47 | 0.17 | - |
| Fe | 0.051 | 0.01 | 0.05 | 0.019 | 0.056 | 0.026 | - |
| Mn | 0.005 | 0.002 | 0.008 | 0.001 | 0.002 | 0.002 | - |
| Zn | 0.002 | 0.003 | 0.002 | 0.001 | 0.001 | 0.001 | - |
| Cu | 0.005 | 0.003 | 0.004 | 0.005 | 0.005 | 0.005 | - |
| Pb | 0.002 | 0.006 | 0.012 | 0.014 | 0.001 | 0.017 | - |
| Polyphenols (mg/gFW) | Ecotype | Phenolic Acids | Flavonoids | Phenolic Aldehyde | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Caffeic Acid | Chlorogenic Acid | p-Coumaric Acid | Gallic Acid | Vanillic Acid | Total Phenolic Acids | Quercetin | Rutin | Naringin | Total Flavonoids | Vanillin | ||
| Epicarp | A | ND | 0.92 | ND | 1.62 | ND | 2.54 | 23.39 | 0.21 | ND | 23.6 | ND |
| B | 0.02 | 0.13 | ND | 0.04 | 0.02 | 0.21 | 104.13 | 0.25 | 0.09 | 104.48 | 0.09 | |
| D | 0.39 | 43.86 | 0.28 | 48.8 | 0.82 | 94.15 | 452.89 | 5.46 | 1.51 | 459.86 | 0.07 | |
| L | 0.15 | 16.28 | 0.11 | 9.65 | 0.06 | 26.26 | 158.57 | 1.16 | 0.23 | 159.96 | 0.03 | |
| T-R | ND | 2.79 | 0.02 | 3.26 | ND | 6.07 | 46.76 | 0.19 | 0.22 | 47.17 | 0.02 | |
| T-Z | ND | 10.79 | 0.03 | 5.91 | 0.05 | 16.77 | 25.74 | 1.48 | 0.07 | 27.29 | 0.04 | |
| Seed | A | 0.03 | 9.7 | 0.15 | 6.44 | 0.07 | 16.39 | 41.8 | 1.13 | 0.3 | 43.23 | ND |
| B | 0.01 | 3.48 | 0.03 | 1.46 | ND | 4.99 | 42.84 | 0.13 | 0.21 | 43.18 | ND | |
| D | ND | 9.83 | 0.16 | 6.28 | 0.09 | 16.36 | 43.99 | 1.02 | 0.07 | 45.08 | 0.11 | |
| L | ND | 8.01 | 0.15 | 4.51 | 0.03 | 12.7 | 32.26 | 0.72 | 0.16 | 33.15 | 0.02 | |
| T-R | 0.02 | 6.09 | 0.07 | 2.51 | ND | 8.69 | 39.46 | 0.4 | 0.13 | 39.98 | ND | |
| T-Z | ND | 4.31 | 0.04 | 1.27 | 0.03 | 5.64 | 13.35 | 0.39 | 0.08 | 13.82 | ND | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Labdelli, A.; Rebiai, A.; Tahirine, M.; Adda, A.; Merah, O. Nutritional Content and Antioxidant Capacity of the Seed and the Epicarp in Different Ecotypes of Pistacia atlantica Desf. Subsp. atlantica. Plants 2020, 9, 1065. https://doi.org/10.3390/plants9091065
Labdelli A, Rebiai A, Tahirine M, Adda A, Merah O. Nutritional Content and Antioxidant Capacity of the Seed and the Epicarp in Different Ecotypes of Pistacia atlantica Desf. Subsp. atlantica. Plants. 2020; 9(9):1065. https://doi.org/10.3390/plants9091065
Chicago/Turabian StyleLabdelli, Amina, Abdelkrim Rebiai, Mohammed Tahirine, Ahmed Adda, and Othmane Merah. 2020. "Nutritional Content and Antioxidant Capacity of the Seed and the Epicarp in Different Ecotypes of Pistacia atlantica Desf. Subsp. atlantica" Plants 9, no. 9: 1065. https://doi.org/10.3390/plants9091065







