Phytoremediation of a Highly Arsenic Polluted Site, Using Pteris vittata L. and Arbuscular Mycorrhizal Fungi
Abstract
:1. Introduction
2. Results
2.1. P. vittata Growth in Greenhouse: Experiment in Controlled Conditions
2.1.1. Arsenic Bioaccumulation in P. vittata
2.1.2. Mycorrhizal Colonization
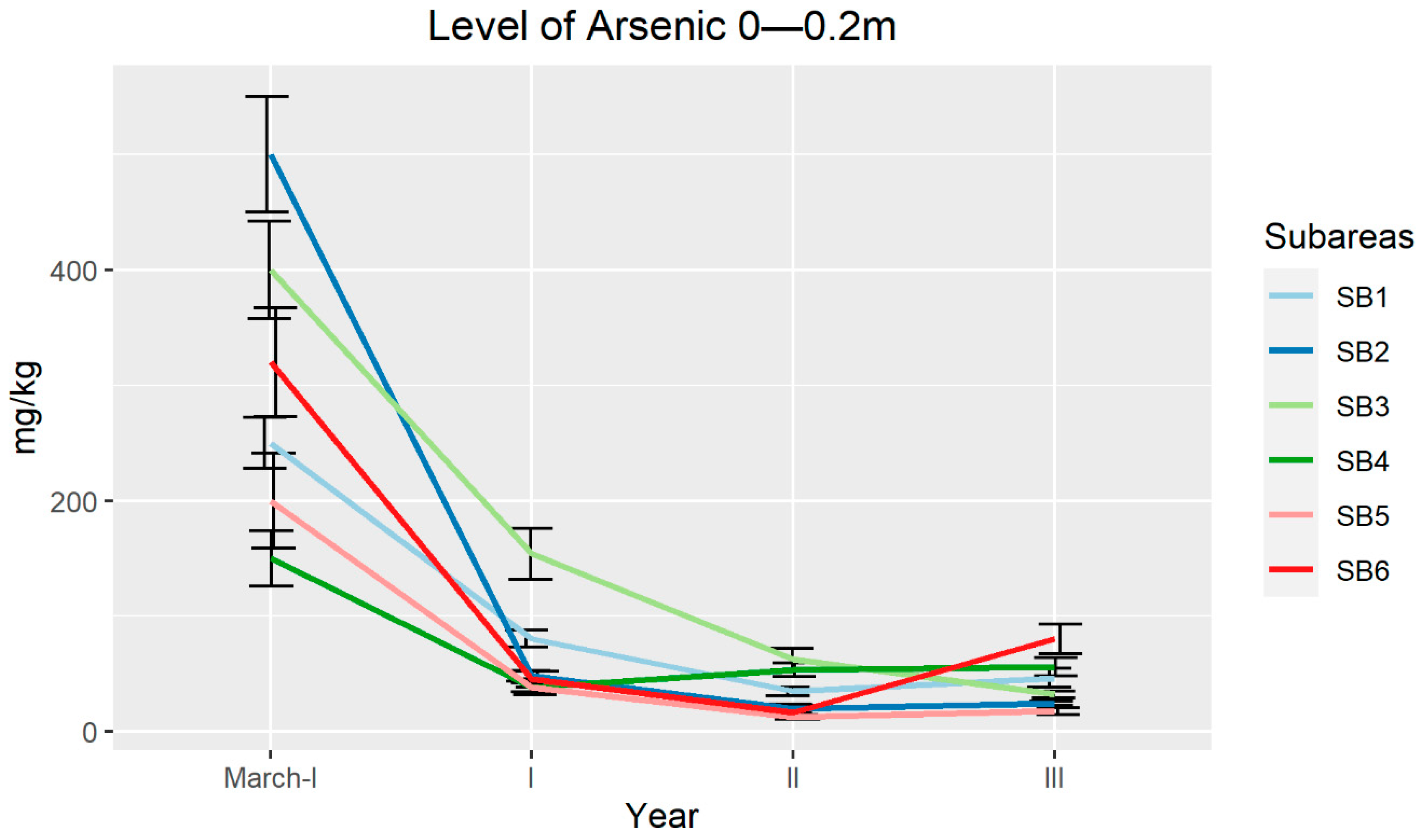
2.2. In Field Experiment
3. Discussion
4. Materials and Methods
4.1. Fern Propagation and Growth
4.2. Experiment in Controlled Condition
Mycorrhizal Colonization
4.3. Field Experiment: Study Site and Phytoremediation Design
4.4. Soil Sampling and Determination of Arsenic and Heavy Metals in Soil
4.5. Arsenic Determination in P. vittata
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Angle, J.S. Plants that hyperaccumulate heavy metals: Their role in phytoremediation, microbiology, archaeology, mineral exploration and phytomining. J. Environ. Qual. 1999, 28, 1045. [Google Scholar] [CrossRef]
- NRC. Arsenic in Drinking Water: 2001 Update; National Academy Press: Washington, DC, USA, 2001. [Google Scholar]
- Azcue, J.M.; Nriagu, J.O. Arsenic: Historical perspectives. In Arsenic in the Environment, Part 1: Cycling and Characterization, 1st ed.; Wiley and Sons: New York, NY, USA, 1994; pp. 1–16. [Google Scholar]
- Bettiol, C.; Minello, F.; Gobbo, L.; Rigo, C.; Bedini, S.; Bona, E.; Berta, G.; Argese, E. Phytoremediation potential of the arsenic hyperaccumulator Pteris vittata: Preliminary results from a field study. Sci. Ca’ Foscari 2012, 1, 25–31. [Google Scholar] [CrossRef]
- Reimann, C.; de Caritat, P. Chemical Elements in the Environment; Springer: Berlin/Heidelberg, Germany, 1998. [Google Scholar]
- Chen, W.Q.; Shi, Y.L.; Wu, S.L.; Zhu, Y.G. Anthropogenic arsenic cycles: A research framework and features. J. Clean. Prod. 2016, 139, 328–336. [Google Scholar] [CrossRef]
- Williams, P.N.; Villada, A.; Deacon, C.; Raab, A.; Figuerola, J.; Green, A.J.; Feldmann, J.; Meharg, A.A. Greatly enhanced arsenic shoot assimilation in rice leads to elevated grain levels compared to wheat and barley. Environ. Sci. Technol. 2007, 41, 6854–6859. [Google Scholar] [CrossRef]
- Kuramata, M.; Abe, T.; Kawasaki, A.; Ebana, K.; Shibaya, T.; Yano, M.; Ishikawa, S. Genetic diversity of arsenic accumulation in rice and QTL analysis of methylated arsenic in rice grains. Rice 2013, 6, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hua, B.; Yan, W.; Wang, J.; Deng, B.; Yang, J. Arsenic accumulation in rice grains: Effects of cultivars and water management practices. Environ. Eng. Sci. 2011, 28, 591–596. [Google Scholar] [CrossRef]
- Meharg, A.A.; Williams, P.N.; Adomako, E.; Lawgali, Y.Y.; Deacon, C.; Villada, A.; Cambell, R.C.J.; Sun, G.; Zhu, Y.G.; Feldmann, J.; et al. Geographical variation in total and inorganic arsenic content of polished (white) rice. Environ. Sci. Technol. 2009, 43, 1612–1617. [Google Scholar] [CrossRef]
- Wan, X.; Lei, M.; Chen, T. Cost–benefit calculation of phytoremediation technology for heavy-metal-contaminated soil. Sci. Total Environ. 2016, 563–564, 796–802. [Google Scholar] [CrossRef]
- Naila, A.; Meerdink, G.; Jayasena, V.; Sulaiman, A.Z.; Ajit, A.B.; Berta, G. A review on global metal accumulators—Mechanism, enhancement, commercial application, and research trend. Environ. Sci. Pollut. Res. 2019, 26, 26449–26471. [Google Scholar] [CrossRef]
- Ronzan, M.; Zanella, L.; Fattorini, L.; Della Rovere, F.; Urgast, D.; Cantamessa, S.; Nigro, A.; Barbieri, M.; Sanità di Toppi, L.; Berta, G.; et al. The morphogenic responses and phytochelatin complexes induced by arsenic in Pteris vittata change in the presence of cadmium. Environ. Exp. Bot. 2017, 133, 176–187. [Google Scholar] [CrossRef]
- Cantamessa, S.; D’agostino, G.; Berta, G. Hydathode structure and localization in Pteris vittata fronds and evidence for their involvement in arsenic leaching. Plant Biosyst. 2016, 150, 1208–1215. [Google Scholar] [CrossRef]
- Trotta, A.; Falaschi, P.; Cornara, L.; Minganti, V.; Fusconi, A.; Drava, G.; Berta, G. Arbuscular mycorrhizae increase the arsenic translocation factor in the As hyperaccumulating fern Pteris vittata L. Chemosphere 2006, 65, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Ma, L.Q. Arsenic speciation, and arsenic and phosphate distribution in arsenic hyperaccumulator Pteris vittata L. and non-hyperaccumulator Pteris ensiformis L. Environ. Pollut. 2006, 141, 238–246. [Google Scholar] [CrossRef]
- Ma, L.Q.; Komar, K.M.; Tu, C.; Zhang, W.; Cai, Y.; Kennelley, E.D. A fern that hyperaccumulates arsenic. Nature 2001, 409, 579. [Google Scholar] [CrossRef]
- Bona, E.; Cattaneo, C.; Cesaro, P.; Marsano, F.; Lingua, G.; Cavaletto, M.; Berta, G. Proteomic analysis of Pteris vittata fronds: Two arbuscular mycorrhizal fungi differentially modulate protein expression under arsenic contamination. Proteomics 2010, 10, 3811–3834. [Google Scholar] [CrossRef]
- Silva Gonzaga, M.I.; Gonzaga Santos, J.A.; Ma, L.Q. Arsenic phytoextraction and hyperaccumulation by fern species. Sci. Agric. 2006, 63, 90–101. [Google Scholar] [CrossRef]
- Spatafora, J.W.; Chang, Y.; Benny, G.L.; Lazarus, K.; Smith, M.E.; Berbee, M.L.; Bonito, G.; Corradi, N.; Grigoriev, I.; Gryganskyi, A.; et al. A phylum-level phylogenetic classification of zygomycete fungi based on genome-scale data. Mycologia 2016, 108, 1028–1046. [Google Scholar] [CrossRef] [Green Version]
- Dhalaria, R.; Kumar, D.; Kumar, H.; Nepovimova, E.; Kuča, K.; Torequl Islam, M.; Verma, R. Arbuscular Mycorrhizal Fungi as Potential Agents in Ameliorating Heavy Metal Stress in Plants. Agronomy 2020, 10, 815. [Google Scholar] [CrossRef]
- Harrison, M.J. Signaling in the arbuscular mycorrhizal symbiosis. Annu. Rev. Microbiol. 2005, 59, 19–42. [Google Scholar] [CrossRef] [PubMed]
- Bagyaraj, D.J.; Sharma, M.P.; Maiti, D. Phosphorus nutrition of crops through arbuscular mycorrhizal fungi. Curr. Sci. 2015, 108, 1288–1293. [Google Scholar]
- Atkinson, D.; Berta, G.; Hooker, J.E. Impact of mycorrhizal colonisation on root architecture, root longevity and the formation of growth regulators. In Impact of Arbuscular Mycorrhizas on Sustainable Agriculture and Natural Ecosystems; Birkhäuser: Basel, Switzerland, 1994; pp. 89–99. [Google Scholar]
- Lingua, G.; D’Agostino, G.; Massa, N.; Antosiano, M.; Berta, G. Mycorrhiza-induced differential response to a yellows disease in tomato. Mycorrhiza 2002, 12, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Sampò, S.; Massa, N.; Cantamessa, S.; D’Agostino, U.; Bosco, D.; Marzachì, C.; Berta, G. Effects of two AM fungi on phytoplasma infection in the model plant Chrysanthemum carinatum. Agric. Food Sci. 2012, 21, 39–51. [Google Scholar] [CrossRef]
- Jung, S.C.; Martinez-Medina, A.; Lopez-Raez, J.A.; Pozo, M.J. Mycorrhiza-Induced Resistance and Priming of Plant Defenses. J. Chem. Ecol. 2012, 38, 651–664. [Google Scholar] [CrossRef] [PubMed]
- Mathur, S.; Tomar, R.S.; Jajoo, A. Arbuscular Mycorrhizal fungi (AMF) protects photosynthetic apparatus of wheat under drought stress. Photosynth. Res. 2019, 139, 227–238. [Google Scholar] [CrossRef]
- Kumar, A.; Dames, J.F.; Gupta, A.; Sharma, S.; Gilbert, J.A.; Ahmad, P. Current developments in arbuscular mycorrhizal fungi research and its role in salinity stress alleviation: A biotechnological perspective. Crit. Rev. Biotechnol. 2015, 35, 461–474. [Google Scholar] [CrossRef]
- Neidhardt, H. Arbuscular mycorrhizal fungi alleviate negative effects of arsenic-induced stress on crop plants: A meta-analysis. Plants People Planet 2020. [Google Scholar] [CrossRef]
- de Souza Moreira, F.M.; Ferreira, P.A.A.; Vilela, L.A.F.; Carneiro, M.A.C. Symbioses of Plants with Rhizobia and Mycorrhizal Fungi in Heavy Metal-Contaminated Tropical Soils; Springer: Cham, Switzerland, 2015; pp. 215–243. [Google Scholar]
- Bai, J.; Lin, X.; Yin, R.; Zhang, H.; Junhua, W.; Xueming, C.; Yongming, L. The influence of arbuscular mycorrhizal fungi on As and P uptake by maize (Zea mays L.) from As-contaminated soils. Appl. Soil Ecol. 2008, 38, 137–145. [Google Scholar] [CrossRef]
- Orłowska, E.; Godzik, B.; Turnau, K. Effect of different arbuscular mycorrhizal fungal isolates on growth and arsenic accumulation in Plantago lanceolata L. Environ. Pollut. 2012, 168, 121–130. [Google Scholar] [CrossRef]
- Gonzalez-Chavez, C.; Harris, P.J.; Dodd, J.; Meharg, A.A. Arbuscular mycorrhizal fungi confer enhanced arsenate resistance on Holcus lanatus. New Phytol. 2002, 155, 163–171. [Google Scholar] [CrossRef]
- Zhang, X.; Ren, B.H.; Wu, S.L.; Sun, Y.Q.; Lin, G.; Chen, B.D. Arbuscular mycorrhizal symbiosis influences arsenic accumulation and speciation in Medicago truncatula L. in arsenic-contaminated soil. Chemosphere 2015, 119, 224–230. [Google Scholar] [CrossRef]
- Ultra, V.U.Y.; Tanaka, S.; Sakurai, K.; Iwasaki, K. Arbuscular mycorrhizal fungus (Glomus aggregatum) influences biotransformation of arsenic in the rhizosphere of sunflower (Helianthus annuus L.). Soil Sci. Plant Nutr. 2007, 53, 499–508. [Google Scholar] [CrossRef]
- Ahmed, F.R.S.; Alexander, I.J.; Mwinyihija, M.; Killham, K. Effect of superphosphate and arbuscular mycorrhizal fungus Glomus mosseae on phosphorus and arsenic uptake in lentil (Lens culinaris L.). Water. Air. Soil Pollut. 2011, 221, 169–182. [Google Scholar] [CrossRef]
- Berta, G.; Bona, E.; Cattaneo, C.; Marsano, F.; D’Agostino, G.; Lingua, G.; Fusconi, A.; Cesaro, P.; Bonelli, G.; Cavaletto, M. AM symbiosis improved As tolerance in the As-hyperaccumulating brake fern Pteris vittata: Cytological and molecular study. In Proceedings of the FESPB 2008: XVI Congress of the Federation of European Societies of Plant Biology (FESPB), Tampere, Finland, 18–22 August 2008; Volume 133, p. P13-011. [Google Scholar]
- Bona, E.; Marsano, F.; Massa, N.; Cattaneo, C.; Cesaro, P.; Argese, E.; di Toppi, L.S.; Cavaletto, M.; Berta, G. Proteomic analysis as a tool for investigating arsenic stress in Pteris vittata roots colonized or not by arbuscular mycorrhizal symbiosis. J. Proteomics 2011, 74, 1338–1350. [Google Scholar] [CrossRef] [PubMed]
- Decreto Legislativo 3 Aprile 2006, n. 152. Norme in Materia Ambientale. (GU n. 88 del 14.04.2006—Suppl. Ordinario n. 96). Available online: http://www.camera.it/parlam/leggi/deleghe/06152dl.htm (accessed on 26 September 2012).
- Lim, K.T.; Shukor, M.Y.; Wasoh, H. Physical, chemical, and biological methods for the removal of arsenic compounds. Biomed Res. Int. 2014. [Google Scholar] [CrossRef] [Green Version]
- Beans, C. Phytoremediation advances in the lab but lags in the field. Proc. Natl. Acad. Sci. USA 2017, 114, 7475–7477. [Google Scholar] [CrossRef] [Green Version]
- Kertulis-Tartar, G.M.; Ma, L.Q.; Tu, C.; Chirenje, T. Phytoremediation of an arsenic-contaminated site using Pteris vittata L.: A two-year study. Int. J. Phytoremediation 2006, 8, 311–322. [Google Scholar] [CrossRef]
- Eze, V.C.; Harvey, A.P. Extractive recovery and valorisation of arsenic from contaminated soil through phytoremediation using Pteris cretica. Chemosphere 2018, 208, 484–492. [Google Scholar] [CrossRef] [Green Version]
- da Silva, E.B.; Lessl, J.T.; Wilkie, A.C.; Liu, X.; Liu, Y.; Ma, L.Q. Arsenic removal by As-hyperaccumulator Pteris vittata from two contaminated soils: A 5-year study. Chemosphere 2018, 206, 736–741. [Google Scholar] [CrossRef]
- Ma, J.; Lei, E.; Lei, M.; Liu, Y.; Chen, T. Remediation of Arsenic contaminated soil using malposed intercropping of Pteris vittata L. and maize. Chemosphere 2018, 194, 737–744. [Google Scholar] [CrossRef]
- Wan, X.; Lei, M. Intercropping efficiency of four arsenic hyperaccumulator Pteris vittata populations as intercrops with Morus alba. Environ. Sci. Pollut. Res. 2018, 25, 12600–12611. [Google Scholar] [CrossRef]
- Chen, B.D.; Zhu, Y.G.; Smith, F.A. Effects of arbuscular mycorrhizal inoculation on uranium and arsenic accumulation by Chinese brake fern (Pteris vittata L.) from a uranium mining-impacted soil. Chemosphere 2006, 62, 1464–1473. [Google Scholar] [CrossRef] [PubMed]
- Yizhu, L.; Imtiaz, M.; Ditta, A.; Rizwan, M.S.; Ashraf, M.; Mehmood, S.; Aziz, O.; Mubeen, F.; Ali, M.; Elahi, N.N.; et al. Response of growth, antioxidant enzymes and root exudates production towards As stress in Pteris vittata and in Astragalus sinicus colonized by arbuscular mycorrhizal fungi. Environ. Sci. Pollut. Res. 2020, 27, 2340–2352. [Google Scholar] [CrossRef] [PubMed]
- Danh, L.T.; Truong, P.; Mammucari, R.; Foster, N. A Critical Review of the Arsenic Uptake Mechanisms and Phytoremediation Potential of Pteris vittata. Int. J. Phytoremediation 2014, 16, 429–453. [Google Scholar] [CrossRef]
- Li, X.; Christie, P. Changes in soil solution Zn and pH and uptake of Zn by arbuscular mycorrhizal red clover in Zn-contaminated soil. Chemosphere 2001, 42, 201–207. [Google Scholar] [CrossRef]
- Turnau, K.; Orlowska, E.; Ryszka, P.; Zubek, S.; Anielska, T.; Gawronski, S.; Jurkiewicz, A. Role of mycorrhizal fungi in phytoremediation and toxicity monitoring of heavy metal rich industrial wastes in southern poland. In Soil and Water Pollution Monitoring, Protection and Remediation; Springer: Dordrecht, The Netherlands, 2007; pp. 533–551. [Google Scholar]
- Leung, H.M.; Ye, Z.H.; Wong, M.H. Survival strategies of plants associated with arbuscular mycorrhizal fungi on toxic mine tailings. Chemosphere 2007, 66, 905–915. [Google Scholar] [CrossRef]
- Baghaie, A.H.; Aghili, F.; Jafarinia, R. Soil-indigenous arbuscular mycorrhizal fungi and zeolite addition to soil synergistically increase grain yield and reduce cadmium uptake of bread wheat (through improved nitrogen and phosphorus nutrition and immobilization of Cd in roots). Environ. Sci. Pollut. Res. 2019, 26, 30794–30807. [Google Scholar] [CrossRef]
- Tu, S.; Ma, L.; Luongo, T. Root exudates and arsenic accumulation in arsenic hyperaccumulating Pteris vittata and non-hyperaccumulating Nephrolepis exaltata. Plant Soil 2004, 258, 9–19. [Google Scholar] [CrossRef]
- Balestri, M.; Ceccarini, A.; Forino, L.M.C.; Zelko, I.; Martinka, M.; Lux, A.; Ruffini Castiglione, M. Cadmium uptake, localization and stress-induced morphogenic response in the fern Pteris vittata. Planta 2014, 239, 1055–1064. [Google Scholar] [CrossRef]
- Hewitt, E.J.; Eden, A. Sand and water culture methods used in the study of plant nutrition. Analyst 1953, 78, 329–330. [Google Scholar]
- Trouvelot, A.; Kough, J.L.; Gianinazzi-Pearson, V. Estimation of VA mycorrhizal infection levels. Research for methods having a functional significance. In Physiological and Genetical Aspects of Mycorrhizae. Aspects Physiologiques et Genetiques des Mycorhizes; INRA: Dijon, France, 1986. [Google Scholar]






| Mean ± SE | Limits by Italian Law for Industrial Areas | |
|---|---|---|
| As | 170 ± 31 | 50 |
| Cd | 2 ± 0.7 | 15 |
| Cr | 401 ± 37 | 800 |
| Cu | 221 ± 14 | 600 |
| Fe | 82,726 ± 11,258 | - |
| Mn | 301 ± 71 | - |
| Ni | 82 ± 6 | 500 |
| Sb | 33 ± 5.2 | 30 |
| Se | 29 ± 7.2 | 15 |
| Sn | 15 ± 3 | 300 |
| Zn | 428 ± 69 | 1500 |
| Soil Depth 0–0.2 m [a] | Soil Depth 0.2–0.4 m [a] | Limits by Italian Law for Commercial Area | |
|---|---|---|---|
| As | 230 ± 80 | 270 ± 72 | 50 |
| Cd | 5 ± 1 | 7 ± 0,1 | 15 |
| Cr | 492 ± 30 | 553 ± 70 | 800 |
| Cu | 248 ± 34 | 167 ± 39 | 600 |
| Fe | 84,667 ± 12,831 | 56,000 ± 6398 | - |
| Mn | 389 ± 11 | 743 ± 32 | - |
| Ni | 104 ± 6 | 105 ± 3 | 500 |
| Sb | 37 ± 8 | 15 ± 3 | 30 |
| Se | 21 ± 7 | 7 ± 2 | 15 |
| Sn | 15 ± 3 | 10 ± 2 | 300 |
| Zn | 572 ± 95 | 303 ± 49 | 1500 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cantamessa, S.; Massa, N.; Gamalero, E.; Berta, G. Phytoremediation of a Highly Arsenic Polluted Site, Using Pteris vittata L. and Arbuscular Mycorrhizal Fungi. Plants 2020, 9, 1211. https://doi.org/10.3390/plants9091211
Cantamessa S, Massa N, Gamalero E, Berta G. Phytoremediation of a Highly Arsenic Polluted Site, Using Pteris vittata L. and Arbuscular Mycorrhizal Fungi. Plants. 2020; 9(9):1211. https://doi.org/10.3390/plants9091211
Chicago/Turabian StyleCantamessa, Simone, Nadia Massa, Elisa Gamalero, and Graziella Berta. 2020. "Phytoremediation of a Highly Arsenic Polluted Site, Using Pteris vittata L. and Arbuscular Mycorrhizal Fungi" Plants 9, no. 9: 1211. https://doi.org/10.3390/plants9091211
APA StyleCantamessa, S., Massa, N., Gamalero, E., & Berta, G. (2020). Phytoremediation of a Highly Arsenic Polluted Site, Using Pteris vittata L. and Arbuscular Mycorrhizal Fungi. Plants, 9(9), 1211. https://doi.org/10.3390/plants9091211









