A Review on Applications and Uses of Thymus in the Food Industry
Abstract
1. Introduction
2. Thyme
3. Composition of Thyme (from Plants, Plant Extracts or Essential Oils)
4. Antioxidant Activity of Thyme
5. Antimicrobial Activity of Thyme
6. Thyme as Functional Food
Potential Health Benefits of Thyme
7. Food Applications
7.1. Sensory Implications
7.2. Incorporation of Thyme in Meat
7.3. Incorporation of Thyme in Fish and Seafood
7.4. Incorporation of Thyme in Milk
8. Public Health and Dietary Implications Concerning the Use of Thyme in Foods
9. Conclusions
Funding
Conflicts of Interest
References
- Carocho, M.; Barreiro, M.F.; Morales, P.; Ferreira, I.C.F.R.; Morales, P. Adding Molecules to Food, Pros and Cons: A Review on Synthetic and Natural Food Additives. Compr. Rev. Food Sci. Food Saf. 2014, 13, 377–399. [Google Scholar] [CrossRef]
- Gould, G.W. Industry Perspectives on the Use of Natural Antimicrobials and Inhibitors for Food Applications. J. Food Prot. 1996, 59, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Farag, R.S.; Badei, A.Z.M.A.; Hewei, F.M.; El-Baroty, G.S.A. Antioxidant activity of some spice essential oils on linoleic acis oxidation in aqueous media. J. Am. Oil Chem. Soc. 1989, 66, 792–799. [Google Scholar] [CrossRef]
- Chipault, J.H.; Mizuno, G.R.; Hawkins, J.M.; Lundberg, W.O. the antioxidant properties of natural spices. J. Food Sci. 1952, 17, 46–55. [Google Scholar] [CrossRef]
- Chipault, J.R.; Mizuno, G.R.; Lundberg, W.O. Antioxidant properties of spices in oil-in-water emulsion. J. Food Sci. 1955, 20, 443–448. [Google Scholar] [CrossRef]
- Hagerman, A.E.; Riedl, K.M.; Jones, G.A.; Sovik, K.N.; Ritchard, N.T.; Hartzfeld, P.W.; Riechel, T.L. High Molecular Weight Plant Polyphenolics (Tannins) as Biological Antioxidants. J. Agric. Food Chem. 1998, 46, 1887–1892. [Google Scholar] [CrossRef]
- Martínez, L.; Bastida, P.; Castillo, J.; Ros, G.; Nieto, G. Green Alternatives to Synthetic Antioxidants, Antimicrobials, Nitrates, and Nitrites in Clean Label Spanish Chorizo. Antioxidants 2019, 8, 184. [Google Scholar] [CrossRef]
- Chen, C.; Pearson, A.; Gray, J. Effects of synthetic antioxidants (BHA, BHT and PG) on the mutagenicity of IQ-like compounds. Food Chem. 1992, 43, 177–183. [Google Scholar] [CrossRef]
- Imaida, K.; Fukishima, S.; Shirai, T.; Ohtami, M.; Nakamishi, K.; Ito, N. Promoting activities of butylated hydroxyanisole and butylated hydroxytoluene on 2-stage urinary bladder carcinogenesis and inhibition of gamma-glutamyl trans-peptide-positive for development in the liver of rats. Carcinogenesis 1983, 4, 895–899. [Google Scholar] [CrossRef]
- Botsoglou, N.; Christaki, E.; Fletouris, D.; Florou-Paneri, P.; Spais, A. The effect of dietary oregano essential oil on lipid oxidation in raw and cooked chicken during refrigerated storage. Meat Sci. 2002, 62, 259–265. [Google Scholar] [CrossRef]
- Wanasundara, U.N.; Shahidi, F. Stabilization of seal blubber and menhaden oils with green tea catechins. J. Am. Oil Chem. Soc. 1996, 73, 1183–1190. [Google Scholar] [CrossRef]
- Jung, M.Y.; Yoon, S.H.; Min, D.B. Effects of processing steps on the contents of minor compounds and oxidation of soybean oil. J. Am. Oil Chem. Soc. 1989, 66, 118–120. [Google Scholar] [CrossRef]
- Yen, G.C.; Chen, H.-Y. Antioxidant Activity of Various Tea Extracts in Relation to Their Antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Fachini-Queiroz, F.C.; Kummer, R.; Estevão-Silva, C.F.; Carvalho, M.D.D.B.; Da Cunha, J.M.; Grespan, R.; Bersani-Amado, C.A.; Cuman, R.K.N. Effects of Thymol and Carvacrol, Constituents of Thymus vulgaris L. Essential Oil, on the Inflammatory Response. Evid. Based Complement. Altern. Med. 2012, 2012, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rota, C.; Herrera, A.; Martinez, R.M.; Sotomayor, J.A.; Jordán, M.J. Antimicrobial activity and chemical composition of Thymus vulgaris, Thymus zygis and Thymus hyemalis essential oils. Food Control 2008, 19, 681–687. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Tepe, B.; Sökmen, M.; Akpulat, H.A.; Daferera, D.; Polissiou, M.; Sokmen, A. Antioxidative activity of the essential oils of Thymus sipyleus subsp. sipyleus var. sipyleus and Thymus sipyleus subsp. sipyleus var. rosulans. J. Food Eng. 2005, 66, 447–454. [Google Scholar] [CrossRef]
- Roby, M.H.H.; Sarhan, M.A.; Selim, K.A.H.; Khalel, K.I. Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme (Thymus vulgaris L.), sage (Salvia officina- lis L.), and marjoram (Origanum majorana L.) extracts. Ind. Crop. Prod. 2013, 48, 43–48. [Google Scholar] [CrossRef]
- Hossain, A.; Al-Raqmi, K.A.S.; Al-Mijizy, Z.H.; Weli, A.M.; Al-Riyami, Q.; Rahman, S.M.M. Study of total phenol, flavonoids contents and phytochemical screening of various leaves crude extracts of locally grown Thymus vulgaris. Asian Pac. J. Trop. Biomed. 2013, 3, 705–710. [Google Scholar] [CrossRef]
- Sienkiewicz, M.; Łysakowska, M.E.; Denys, P.; Kowalczyk, E. The Antimicrobial Activity of Thyme Essential Oil Against Multidrug Resistant Clinical Bacterial Strains. Microb. Drug Resist. 2012, 18, 137–148. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S. Essential Oils: Extraction, Bioactivities, and Their Uses for Food Preservation. J. Food Sci. 2014, 79, R1231–R1249. [Google Scholar] [CrossRef]
- McGimpsey, J.A.; Douglas, M.H.; Van Klink, J.W.; Beauregard, D.A.; Perry, N.B. Seasonal variation in essential oil yield and composition from naturalized Thymus vulgaris L. in New Zealand. Flavour Fragr. J. 1994, 9, 347–352. [Google Scholar] [CrossRef]
- Airoldi, C.; Sironi, E.; Dias, C.; Marcelo, F.; Martins, A.; Rauter, A.P.; Nicotra, F.; Jiménez-Barbero, J. Natural Compounds against Alzheimer’s Disease: Molecular Recognition of Aβ1-42 Peptide bySalvia sclareoidesExtract and its Major Component, Rosmarinic Acid, as Investigated by NMR. Chem.—Asian J. 2013, 8, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Furtado, M.A.; De Almeida, L.C.F.; Furtado, R.A.; Cunha, W.R.; Tavares, D.C. Antimutagenicity of rosmarinic acid in Swiss mice evaluated by the micronucleus assay. Mutat. Res. Toxicol. Environ. Mutagen. 2008, 657, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.; Figueira, M.E.; Barateiro, A.; Fernandes, A.; Brites, D.; Bronze, R.; Duarte, C.M.M.; Serra, A.T.; Pinto, R.M.; Freitas, M.; et al. Anti-inflammatory Effect of Rosmarinic Acid and an Extract ofRosmarinus officinalisin Rat Models of Local and Systemic Inflammation. Basic Clin. Pharmacol. Toxicol. 2014, 116, 398–413. [Google Scholar] [CrossRef] [PubMed]
- Ladopoulou, E.M.; Matralis, A.N.; Nikitakis, A.; Kourounakis, A.P. Antihyperlipidemic morpholine derivatives with antioxidant activity: An investigation of the aromatic substitution. Bioorg. Med. Chem. 2015, 23, 7015–7023. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, K.; Kawazoe, K.; Takaishi, Y. Human platelet aggregation inhibitors from thyme (Thymus vulgaris L.). Phytother. Res. 2002, 16, 398–399. [Google Scholar] [CrossRef]
- Vallverdú-Queralt, A.; Regueiro, J.; Martínez-Huélamo, M.; De Alvarenga, J.F.R.; Leal, L.N.; Lamuela-Raventós, R.M. A comprehensive study on the phenolic profile of widely used culinary herbs and spices: Rosemary, thyme, oregano, cinnamon, cumin and bay. Food Chem. 2014, 154, 299–307. [Google Scholar] [CrossRef]
- Burdock, G.A. Fenaroli’s Handbook of Flavor Ingredients; Informa UK Limited: Boca Raton, FL, USA, 2016. [Google Scholar]
- Merola, N.; Castillo, J.; Benavente-García, O.; Ros, G.; Nieto, G. The Effect of Consumption of Citrus Fruit and Olive Leaf Extract on Lipid Metabolism. Nutrients 2017, 9, 1062. [Google Scholar] [CrossRef]
- Gavliakova, S.; Biringerova, Z.; Buday, T.; Brozmanova, M.; Calkovsky, V.; Poliacek, I.; Plevkova, J. Antitussive effects of nasal thymol challenges in healthy volunteers. Respir. Physiol. Neurobiol. 2013, 187, 104–107. [Google Scholar] [CrossRef]
- Höferl, M.; Buchbauer, G.; Jirovetz, L.; Schmidt, E.; Stoyanova, A.; Denkova, Z.; Slavchev, A.; Geissler, M. Correlation of Antimicrobial Activities of Various Essential Oils and Their Main Aromatic Volatile Constituents. J. Essent. Oil Res. 2009, 21, 459–463. [Google Scholar] [CrossRef]
- Quiroga, P.R.; Asensio, C.M.; Nepote, V. Antioxidant effects of the monoterpenes carvacrol, thymol and sabinene hydrate on chemical and sensory stability of roasted sunflower seeds. J. Sci. Food Agric. 2014, 95, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.M.; Khaneghah, A.M.; Gavahian, M.; Marszałek, K.; Es, I.; Munekata, P.; Ferreira, I.C.F.R.; Barba, F.J. Understanding the potential benefits of thyme and its derived products for food industry and consumer health: From extraction of value-added compounds to the evaluation of bioaccessibility, bioavailability, anti-inflammatory, and antimicrobial activities. Crit. Rev. Food Sci. Nutr. 2018, 59, 2879–2895. [Google Scholar] [CrossRef]
- Yanishlieva, N.V.; Marinova, E.M.; Gordon, M.H.; Raneva, V.G. Antioxidant activity and mechanism of action of thymol and carvacrol in two lipid systems. Food Chem. 1999, 64, 59–66. [Google Scholar] [CrossRef]
- Frankel, E.N.; Huang, S.-W.; Aeschbach, R.; Prior, E. Antioxidant Activity of a Rosemary Extract and Its Constituents, Carnosic Acid, Carnosol, and Rosmarinic Acid, in Bulk Oil and Oil-in-Water Emulsion. J. Agric. Food Chem. 1996, 44, 131–135. [Google Scholar] [CrossRef]
- Moure, A.; Cruz, J.M.; Franco, D.; Domínguez, J.; Sineiro, J.; Domínguez, H.; Núñez, M.J.; Parajó, J.C. Natural antioxidants from residual sources. Food Chem. 2001, 72, 145–171. [Google Scholar] [CrossRef]
- Economou, K.D.; Oreopoulou, V.; Thomopoulos, C.D. Antioxidant properties of some plant extracts of the Labiatae family. J. Amer. Oil Chem. Soc. 1991, 68, 109–113. [Google Scholar] [CrossRef]
- Chang, S.S.; Hsieh, O.A.L.; Huang, C.-L.; Ostric-Matijasevic, B. Natural antioxidants from rosemary and sage. J. Food Sci. 1977, 42, 1102–1106. [Google Scholar] [CrossRef]
- Dorman, H.; Peltoketo, A.; Hiltunen, R.; Tikkanen, M. Characterisation of the antioxidant properties of de-odourised aqueous extracts from selected Lamiaceae herbs. Food Chem. 2003, 83, 255–262. [Google Scholar] [CrossRef]
- Soare, J.R.; Dinis, T.C.P.; Cunha, A.P.; Almeida, L.M. Antioxidant Activities of Some Extracts ofThymus zygis. Free. Radic. Res. 1997, 26, 469–478. [Google Scholar] [CrossRef]
- Yu, Y.-M.; Chao, T.-Y.; Chang, W.-C.; Chang, M.J.; Lee, M.-F. Thymol reduces oxidative stress, aortic intimal thickening, and inflammation-related gene expression in hyperlipidemic rabbits. J. Food Drug Anal. 2016, 24, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Aouam, I.; El Atki, Y.; Taroq, A.; El Kamari, F.; Abdellaoui, A. Chemical composition, antimicrobial and antioxidant activities of two Moroccan Thymus essential oils. Asian J. Pharm. Clin. Res. 2019, 12, 447–451. [Google Scholar] [CrossRef]
- Golkar, P.; Mosavat, N.; Jalali, S.A.H. Essential oils, chemical constituents, antioxidant, antibacterial and in vitro cytotoxic activity of different Thymus species and Zataria multiflora collected from Iran. S. Afr. J. Bot. 2020, 130, 250–258. [Google Scholar] [CrossRef]
- Lee, K.-G.; Shibamoto, T. Determination of Antioxidant Potential of Volatile Extracts Isolated from Various Herbs and Spices. J. Agric. Food Chem. 2002, 50, 4947–4952. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, K.; Ernst, W. Evaluation of antioxidative constituents from thyme. J. Sci. Food Agric. 1996, 70, 217. [Google Scholar] [CrossRef]
- Youdim, K.A.; Deans, S.G.; Finlayson, H.J. The Antioxidant Properties of Thyme (Thymus zygis L.) Essential Oil: An Inhibitor of Lipid Peroxidation and a Free Radical Scavenger. J. Essent. Oil Res. 2002, 14, 210–215. [Google Scholar] [CrossRef]
- Haraguchi, H.; Saito, T.; Ishikawa, H.; Date, H.; Kataoka, S.; Tamura, Y.; Mizutani, K. Antiperoxidative Components in Thymus vulgaris. Planta Medica 1996, 62, 217–221. [Google Scholar] [CrossRef]
- Miura, K.; Kikuzaki, H.; Nakatani, N. Antioxidant activity of chemical components fron sage (Salvia officinalis L.) and Thyme (Thymus vulgaris L.) measured by the oil satabilittty index method. J. Agric. Food Chem. 2002, 50, 1845–1851. [Google Scholar] [CrossRef]
- Venskutonis, P.; Gruzdienè, D.; Tirzite, D.; Tirzitis, G. Assessment of antioxidant activity of plant extracts by different methods. Acta Hortic. 2005, 677, 99–107. [Google Scholar] [CrossRef]
- Gavaric, N.; Možina, S.S.; Kladar, N.; Bozin, B. Chemical Profile, Antioxidant and Antibacterial Activity of Thyme and Oregano Essential Oils, Thymol and Carvacrol and Their Possible Synergism. J. Essent. Oil Bear. Plants 2015, 18, 1013–1021. [Google Scholar] [CrossRef]
- Meeran, M.F.N.; Javed, H.; Al Taee, H.; Azimullah, S.; Ojha, S. Pharmacological Properties and Molecular Mechanisms of Thymol: Prospects for Its Therapeutic Potential and Pharmaceutical Development. Front. Pharmacol. 2017, 8, 380. [Google Scholar] [CrossRef] [PubMed]
- Salgado-Garciglia, R.; Torres-Martínez, R.; Hernández-García, A.; Saavedra- Molina, A.; López-Meza, J.E.; Ochoa-Zarzosa, A. In vitro evaluation of cytotoxic and antioxidant activity of essential oil and terpenes of Satureja macrostema. FASEB J. 2016, 30, 1090.8. [Google Scholar]
- Llana-Ruiz-Cabello, M.; Gutiérrez-Praena, D.; Puerto, M.; Pichardo, S.; Jos, Á.; Cameán, A.M. In vitro pro-oxidant/antioxidant role of carvacrol, thymol and their mixture in the intestinal Caco-2 cell line. Toxicol. In Vitro 2015, 29, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Youdim, K.A.; Deans, S.G. Dietary supplementation of thyme (Thymus vulgaris L.) essential oil during the lifetime of the rat: Its effects on the antioxidant status in liver, kidney and heart tissues. Mech. Ageing Dev. 1999, 109, 163–175. [Google Scholar] [CrossRef]
- Youdim, K.A.; Deans, S.G. Beneficial effects of thyme oil on age-related changes in the phospholipid C20 and C22 polyunsaturated fatty acid composition of various rat tissues. Biochim. Biophys. Acta (BBA)—Mol. Cell Boil. Lipids 1999, 1438, 140–146. [Google Scholar] [CrossRef]
- Vardar-Ünlü, G.; Candan, F.; Sökmen, A.; Daferera, D.; Polissiou, M.; Sökmen, M.; Dönmez, E.; Tepe, B. Antimicrobial and antioxidant activity of the essential oil and methanol extracts of Thymus pectinatus Fisch. Et Mey. Var. pectinatus (Lamiaceae). J. Agric. Food Chem. 2003, 51, 63–67. [Google Scholar] [CrossRef]
- Deans, S.; Simpson, E.; Noble, R.; MacPherson, A.; Pénzes, L. Natural antioxidants from thymus vulgaris (thyme) volatile oil: The beneficial effects upon mammalian lipid metabolism. Acta Hortic. 1993, 332, 177–182. [Google Scholar] [CrossRef]
- Meeran, M.F.N.; Prince, P.S.M. Protective effects of thymol on altered plasma lipid peroxidation and nonenzymic antioxidants in isoproterenol-induced myocardial infarcted rats. J. Biochem. Mol. Toxicol. 2012, 26, 368–373. [Google Scholar] [CrossRef]
- Kalemba, D.; Kunicka-Styczyńska, A. Antibacterial and antifungal properties of essential oils. Curr. Med. Chem. 2003, 10, 813–829. [Google Scholar] [CrossRef]
- Juven, B.; Kanner, J.; Schved, F.; Weisslowicz, H. Factors that interact with the antibacterial action of thyme essential oil and its active constituents. J. Appl. Bacteriol. 1994, 76, 626–631. [Google Scholar] [CrossRef]
- Singh, N.; Singh, R.; Bhunia, A.K. Sequential disinfection of Escherichia coli O157:H7 inoculated alfalfa seeds before and during sprouting using aqueous chlorine dioxide, ozonated water, and thyme essential oil. LWT 2003, 36, 235–243. [Google Scholar] [CrossRef]
- Shapiro, S.; Guggenheim, B. The action of thymol on oral bacteria. Oral Microbiol. Immunol. 1995, 10, 241–246. [Google Scholar] [CrossRef]
- Evans, J.D.; Martin, S.A. Effects of thymol on ruminal microorganisms. Curr. Microbiol. 2000, 41, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Borugă, O.; Jianu, C.; Mişcă, C.; Goleţ, I.; Gruia, A.T.; Horhat, F.G. Thymus vulgaris essential oil: Chemical composition and antimicrobial activity. J. Med. Life 2014, 7, 56–60. [Google Scholar] [PubMed]
- Manconi, M.; Petretto, G.L.; D’Hallewin, G.; Escribano, E.; Milia, E.; Pinna, R.; Palmieri, A.; Firoznezhad, M.; Peris, J.E.; Usach, I.; et al. Thymus essential oil extraction, characterization and incorporation in phospholipid vesicles for the antioxidant/antibacterial treatment of oral cavity diseases. Colloids Surf. B: Biointerfaces 2018, 171, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Ultee, A.; Bennik, M.H.J.; Moezelaar, R. The Phenolic Hydroxyl Group of Carvacrol Is Essential for Action against the Food-Borne Pathogen Bacillus cereus. Appl. Environ. Microbiol. 2002, 68, 1561–1568. [Google Scholar] [CrossRef]
- Di Pasqua, R.; Hoskins, N.; Betts, G.; Mauriello, G. Changes in Membrane Fatty Acids Composition of Microbial Cells Induced by Addiction of Thymol, Carvacrol, Limonene, Cinnamaldehyde, and Eugenol in the Growing Media. J. Agric. Food Chem. 2006, 54, 2745–2749. [Google Scholar] [CrossRef]
- Lambert, R.; Skandamis, P.; Coote, P.; Nychas, G.-J. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef]
- Burt, S.A.; Reinders, R. Antibacterial activity of selected plant essential oils against Escherichia coli O157:H7. Lett. Appl. Microbiol. 2003, 36, 162–167. [Google Scholar] [CrossRef]
- Ultee, A.; Slump, R.A.; Steging, G.; Smid, E.J. Antimicrobial Activity of Carvacrol toward Bacillus cereus on Rice. J. Food Prot. 2000, 63, 620–624. [Google Scholar] [CrossRef]
- Vasala, A.; Isomäki, R.; Myllykosky, L.; Alatossava, T. Thymol-triggered lysis of Escherichia coli expressing Lactobacillus phage LL-H muramidasa. J. Ind. Microbiol. Biotechnol. 1999, 22, 39–43. [Google Scholar] [CrossRef]
- Dorman, H.J.D.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef]
- Domingo, D.; López-Brea, M. Revisión: Plantas con acción antimicrobiana. Revis. Esp. Quimioter. 2003, 16, 385–393. [Google Scholar]
- Faleiro, L.; Miguel, G.; Guerrero, C.; Brito, J. Antimicrobial activity of essential oils of rosmarinus officinalis l., thymus mastichina (l) l. ssp mastichina and thymus albicans hofmanns link. Acta Hortic. 1999, 501, 45–48. [Google Scholar] [CrossRef]
- Rosa, M.D.S.S.; Mendonça-Filho, R.R.; Bizzo, H.R.; Rodrigues, I.D.A.; Soares, R.M.A.; Souto-Padroón, T.; Alviano, C.S.; Lopes, A.H.C.S. Antileishmanial Activity of a Linalool-Rich Essential Oil from Croton cajucara. Antimicrob. Agents Chemother. 2003, 47, 1895–1901. [Google Scholar] [CrossRef] [PubMed]
- Rota, M.C.; Carramiñana, J.J.; Burillo, J.; Herrera, A. In Vitro Antimicrobial Activity of Essential Oils from Aromatic Plants against Selected Foodborne Pathogens. J. Food Prot. 2004, 67, 1252–1256. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Shiota, S.; Kuroda, T.; Hatano, T.; Yoshida, T.; Mizushima, T.; Tsuchiya, T. Remarkable synergies between baicalein and tetracycline, and baicalein and beta-lactams against methicillin-resistant Staphylococcus aureus. Microbiol. Immunol. 2005, 49, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Sagdic, O.; Yasar, S.; Kisioglu, A.N. Antibacterial effects of single or combined plant extracts. Annals Microbiol. 2005, 55, 67–71. [Google Scholar]
- Tabak, M.; Armon, R.; Potasman, I.; Neeman, I. In vitro inhibition of Helicobacter pylori by extracts of thyme. J. Appl. Bacteriol. 1996, 80, 667–672. [Google Scholar] [CrossRef]
- Alzoreky, N.S.; Nakahara, K. Antibacterial activity of extracts from some edible plants commonly consumed in Asia. Int. J. Food Microbiol. 2003, 80, 223–230. [Google Scholar] [CrossRef]
- Sokmen, A.; Gulluce, M.; Akpulat, H.A.; Daferera, D.; Tepe, B.; Polissiou, M.; Sokmen, M.; Şahin, F. The in vitro antimicrobial and antioxidant activities of the essential oils and methanol extracts of endemic Thymus spathulifolius. Food Control 2004, 15, 627–634. [Google Scholar] [CrossRef]
- Martínez, L.; Castillo, J.; Ros, G.; Nieto, G. Antioxidant and Antimicrobial Activity of Rosemary, Pomegranate and Olive Extracts in Fish Patties. Antioxidants 2019, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Asadbegi, M.; Yaghmaei, P.; Salehi, I.; Komaki, A.; Ebrahim-Habibi, A. Investigation of thymol effect on learning and memory impairment induced by intrahippocampal injection of amyloid beta peptide in high fat diet- fed rats. Metab. Brain Dis. 2017, 32, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Serrano, A.; González-Sarrías, A.; Tomás-Barberán, F.A.; Avellaneda, A.; Gironés-Vilaplana, A.; Nieto, G.; Ros-Berruezo, G. Anti-Inflammatory and Antioxidant Effects of Regular Consumption of Cooked Ham Enriched with Dietary Phenolics in Diet-Induced Obese Mice. Antioxidants 2020, 9, 639. [Google Scholar] [CrossRef]
- Kensara, O.A.; Elsawy, N.A.; El-shemi, A.G.; Header, E.A. Thymus vulgaris supplementation attenuates blood pressure and aorta damage in hypertensive rats. J. Medic. Plants Res. 2013, 7, 669–676. [Google Scholar]
- Haque, M.R.; Ansari, S.H.; Najmi, A.K.; Ahmad, M.A. Monoterpene phenolic compound thymol prevents high fat diet induced obesity in murine model. Toxicol. Mech. Methods 2013, 24, 116–123. [Google Scholar] [CrossRef]
- Deb, D.D.; Parimala, G.; Devi, S.S.; Chakraborty, T. Effect of thymol on peripheral blood mononuclear cell PBMC and acute promyelotic cancer cell line HL-60. Chem. Interact. 2011, 193, 97–106. [Google Scholar] [CrossRef]
- Souilah, N.; Ullah, Z.; Bendif, H.; Medjroubi, K.; Hazmoune, T.; Hamel, T.; Öztürk, M.; Nieto, G.; Akkal, S. Phenolic Compounds from An Algerian Endemic Species of Hypochaeris laevigata var. hipponensis and Investigation of Antioxidant Activities. Plants 2020, 9, 514. [Google Scholar] [CrossRef]
- Rhee, K.; Anderson, L.; Sams, A. Lipid Oxidation Potential of Beef, Chicken, and Pork. J. Food Sci. 1996, 61, 8–12. [Google Scholar] [CrossRef]
- Opara, E.I.; Chohan, M. Culinary Herbs and Spices: Their Bioactive Properties, the Contribution of Polyphenols and the Challenges in Deducing Their True Health Benefits. Int. J. Mol. Sci. 2014, 15, 19183–19202. [Google Scholar] [CrossRef]
- Braga, P.C.; Sasso, M.D.; Culici, M.; Bianchi, T.; Bordoni, L.; Marabini, L. Anti-Inflammatory Activity of Thymol: Inhibitory Effect on the Release of Human Neutrophil Elastase. Pharmacology 2006, 77, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Li, F.; Fu, Y.; Cao, Y.; Song, X.; Wang, T.; Wang, W.; Guo, M.; Zhou, E.; Li, D.; et al. Thymol Inhibits LPS-Stimulated Inflammatory Response via Down-Regulation of NF-κB and MAPK Signaling Pathways in Mouse Mammary Epithelial Cells. Inflammation 2013, 37, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Ocaña, A.; Reglero, G. Effects of Thyme Extract Oils (from Thymus vulgaris, Thymus zygis, and Thymus hyemalis) on Cytokine Production and Gene Expression of oxLDL-Stimulated THP-1-Macrophages. J. Obes. 2012, 2012, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Karami-Osboo, R.; Khodaverdi, M.; Ali-Akbari, F. Antibacterial effect of effective com- pounds of Satureja hortensis and Thymus vulgaris essential oils against Erwinia amylovora. J. Agric. Sci. Techn. 2010, 12, 35–45. [Google Scholar]
- Bosquez-Molina, E.; Jesús, E.R.-D.; Bautista-Baños, S.; Verde-Calvo, J.; Morales-López, J. Inhibitory effect of essential oils against Colletotrichum gloeosporioides and Rhizopus stolonifer in stored papaya fruit and their possible application in coatings. Postharvest Boil. Technol. 2010, 57, 132–137. [Google Scholar] [CrossRef]
- Arras, G.; Usai, M. Fungitoxic activity of 12 essential oils against four post-harvest cit- rus pathogens, chemical analysis of Thymus capitatus oil and its effect in sub-atmo- spheric pressure conditions. J. Food Prot. 2001, 64, 1025–1029. [Google Scholar] [CrossRef]
- Satyal, P.; Murray, B.L.; McFeeters, R.L.; Setzer, W.N. Essential Oil Characterization of Thymus vulgaris from Various Geographical Locations. Foods 2016, 5, 70. [Google Scholar] [CrossRef]
- Soković, M.; Glamočlija, J.; Marin, P.D.; Brkic, D.; Van Griensven, L.J.L.D. Antibacterial Effects of the Essential Oils of Commonly Consumed Medicinal Herbs Using an In Vitro Model. Molecules 2010, 15, 7532–7546. [Google Scholar] [CrossRef]
- Rezaei, A.; Fathi, M.; Jafari, S.M. Nanoencapsulation of hydrophobic and low-soluble food bioactive compounds within different nanocarriers. Food Hydrocoll. 2019, 88, 146–162. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, M.; Bhandari, B.; Xu, J.; Yang, C. Effects of nanoemulsion-based active coatings with composite mixture of star anise essential oil, polylysine, and nisin on the quality and shelf life of ready-to-eat Yao meat products. Food Control 2020, 107, 106771. [Google Scholar] [CrossRef]
- Donsi, F.; Annunziata, M.; Seesa, M.; Ferrari, G. Nanoencapsulation of essential oils to enhance their antimicrbial activity in foods. Food Sci. Technol. 2010, 44, 1908–1914. [Google Scholar]
- Nuguefack, J.; Tamgue, O.; Dongmo, J.B.L.; Dakole, C.D.; Leth, V.; Vismer, H.F.; Amvam Zollo, P.H.; Nkengfack, A.E. Synergistic action between facrtions of essential oils from Cymbopogon citratus, ocimum gratissimum and thymis vulgaris againts Penicilium expansum. Food Control 2012, 23, 377–383. [Google Scholar] [CrossRef]
- Lv, F.; Liang, H.; Yuan, Q.; Li, C. IN vitro natimicrobial effect and mechanism of action of selected plant essential oil combinations against four food-related microorganisms. Food Res. Int. 2011, 44, 3057–3064. [Google Scholar] [CrossRef]
- Cava-Roda, R.M.; Taboada-Rodríguez, A.; Valverde-Franco, M.T.; Marín-Iniesta, F. Antimicrobial Activity of Vanillin and Mixtures with Cinnamon and Clove Essential Oils in Controlling Listeria monocytogenes and Escherichia coli O157:H7 in Milk. Food Bioprocess Technol. 2010, 5, 2120–2131. [Google Scholar] [CrossRef]
- Rattanachaikunsopon, P.; Phumkhachorn, P. Assessment of factors influencing antimicrobial activity of carvacrol and cymene against Vibrio cholerae in food. J. Biosci. Bioeng. 2010, 110, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.; Barry-Ryan, C.; Bourke, P. Antimicrobial activity of plant essential oils using food model media: Efficacy, synergistic potential and interactions with food components. Food Microbiol. 2009, 26, 142–150. [Google Scholar] [CrossRef]
- Cerrutti, P.; Alzamora, S.M. Inhibitory effects of vanillin on some food spoilage yeasts in laboratory media and fruit purées. Int. J. Food Microbiol. 1996, 29, 379–386. [Google Scholar] [CrossRef]
- Somolinos, M.; García, D.; Condón, S.; Mackey, B.; Pagán, R. Inactivation ofEscherichia coliby citral. J. Appl. Microbiol. 2009, 108, 1928–1939. [Google Scholar] [CrossRef]
- Harpaz, S.; Glatman, L.; Drabkin, V.; Gelman, A. Effects of Herbal Essential Oils Used To Extend the Shelf Life of Freshwater-Reared Asian Sea Bass Fish (Lates calcarifer). J. Food Prot. 2003, 66, 410–417. [Google Scholar] [CrossRef]
- Tanabe, H.; Yoshida, M.; Tomita, N. Comparison of the antioxidant activities of 22 commonly used culinary herbs and spices on the lipid oxidation of pork meat. Anim. Sci. J. 2002, 73, 389–393. [Google Scholar] [CrossRef]
- Medina, R.; Miralles, S. Aceite Esencial de Tomillo Mendocino (Acantholippia Seriphiioides): Efecto Antioxidante en Medallones de Carne Vacuna, 2000.
- Kykkidou, S.; Giatrakou, V.; Papavergou, A.; Kontominas, M.G.; Savvaidis, I.N. Effect of thyme essential oil and packaging treatments on fresh Mediterranean swordfish fillets during storage at 4 °C. Food Chem. 2009, 115, 169–175. [Google Scholar] [CrossRef]
- Skandamis, P.N.; Tsigarida, E.; Nychas, G.J.E. The effect of oregano essential oil on survival/death of Salmonella typhimurium in meat stored at 5ºC under aerobic, VP/MAP conditions. Food Microbiol. 2002, 19, 97–103. [Google Scholar] [CrossRef]
- Ismail, A.A.; Pierson, M.D. Effect of sodium nitrite and origenum oil on growth and toxin production of Clostridium botulinum in TYG broth and ground pork. J. Food Prot. 1990 53, 958–960.
- Moñino, M.I.; Martínez, C.; Sotomayor, J.A.; Lafuente, A.; Jordán, M.J. Polyphenolic transmission to segureño lamb meat from ewes dietary supplemented with the distillate from rosemary (Rosmarinus officinalis) leaves. J. Agric. Food Chem. 2008, 56, 3363–3367. [Google Scholar] [CrossRef]
- Mytle, N.; Anderson, G.; Doyle, M.; Smith, M. Antimicrobial activity of clove (Syzgium aromaticum) oil in inhibiting Listeria monocytogenes on chicken frankfurters. Food Control 2006, 17, 102–107. [Google Scholar] [CrossRef]
- Nissen, L.R.; Byrne, D.V.; Bertelsen, G.; Skibsted, L.H. The antioxidative activity of plant extracts in cooked pork patties as evaluated by descriptive sensory profiling and chemical analysis. Meat Sci. 2004, 68, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Grün, I.U.; Mustapha, A. Effects of plant extracts on microbial growth, color change, and lipid oxidation in cooked beef. Food Microbiol. 2007, 24, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Solomakos, N.; Govaris, A.; Koidis, P.; Botsoglou, N. The antimicrobial effect of thyme essential oil, nisin, and their combination against Listeria monocytogenes in minced beef during refrigerated storage. Food Microbiol. 2008, 25, 120–127. [Google Scholar] [CrossRef]
- Solomakos, N.; Govaris, A.; Koidis, P.; Botsoglou, N. The antimicrobial effect of thyme essential oil, nisin and their combination against Escherichia coli O157:H7 in minced beef during refrigerated storage. Meat Sci. 2008, 80, 159–166. [Google Scholar] [CrossRef]
- Botsoglou, N.A.; Yannakopoulos, A.L.; Fletouris, D.J.; Tserveni-Goussi, A.S.; Fortomaris, P.D. Effect of Dietary Thyme on the Oxidative Stability of Egg Yolk. J. Agric. Food Chem. 1997, 45, 3711–3716. [Google Scholar] [CrossRef]
- Ouattara, B.; Sabato, S.F.; Lacroix, M. Combinated effect of antimicrobial coating and gamma irradiation on shelf life extension of pre-cooked shrimp (Penaus spp.). Int. J. Food Microbiol. 2001, 68, 1–9. [Google Scholar] [CrossRef]
- Holley, R.A.; Patel, D. Improvement in shelf-life and safety of perishable foods by plant essential oils and smoke antimicrobials. Food Microbiol. 2005, 22, 273–292. [Google Scholar] [CrossRef]
- Hao, Y.Y.; Brackett, R.E.; Doyle, M.P. Efficacy of plant extracts in inhibiting Aeromonas hydrophila and Listeria monocytogenes in refrigerated cooked poultry. Food Microbiol. 1998, 15, 367–378. [Google Scholar] [CrossRef]
- Nieto, G.; Díaz, P.; Bañon, S.; Garrido, M.D.; Nieto, G. Effect on lamb meat quality of including thyme (Thymus zygis ssp. gracilis) leaves in ewes’ diet. Meat Sci. 2010, 85, 82–88. [Google Scholar] [CrossRef]
- Nieto, G.; Bañón, S.; Garrido, M.D.; Nieto, G. Effect of supplementing ewes’ diet with thyme (Thymus zygis ssp. gracilis) leaves on the lipid oxidation of cooked lamb meat. Food Chem. 2011, 125, 1147–1152. [Google Scholar] [CrossRef]
- Martínez, L.; Ros, G.; Nieto, G. Effect of natural extracts obtained from food industry by-products on nutritional quality and shelf life of chicken nuggets enriched with organic Zn and Se provided in broiler diet. Poult. Sci. 2020, 99, 1491–1501. [Google Scholar] [CrossRef]
- Nieto, G.; Banon, S.; Garrido, M.D.; Nieto, G. Administration of distillate thyme leaves into the diet of Segureña ewes: Effect on lamb meat quality. Animal 2012, 6, 2048–2056. [Google Scholar] [CrossRef]
- Nieto, G. Incorporation of by-products of rosemary and thyme in the diet of ewes: Effect on the fatty acid profile of lamb. Eur. Food Res. Technol. 2013, 236, 379–389. [Google Scholar] [CrossRef]
- Nieto, G.; Jongberg, S.; Andersen, M.L.; Skibsted, L.H.; Nieto, G. Thiol oxidation and protein cross-link formation during chill storage of pork patties added essential oil of oregano, rosemary, or garlic. Meat Sci. 2013, 95, 177–184. [Google Scholar] [CrossRef]
- Nieto, G.; Martínez-Zamora, L.; Sánchez, J.C.; Ros, G.; Nieto, G. Hydroxytyrosol extracts, olive oil and walnuts as functional components in chicken sausages. J. Sci. Food Agric. 2017, 97, 3761–3771. [Google Scholar] [CrossRef]
- Martinez, J.; Nieto, G.; Castillo, J.; Ros, G. Influence of in vitro gastrointestinal digestion and/or grape seed extract addition on antioxidant capacity of meat emulsions. LWT-Food Sci. Technol. 2014, 59, 834–840. [Google Scholar] [CrossRef]
- Nieto, G.; Ros, G.; Nieto, G. Hydroxytyrosol: Health Benefits and Use as Functional Ingredient in Meat. Medicine 2018, 5, 13. [Google Scholar] [CrossRef]
- Nieto, G.; Ros-Berruezo, G.; Nieto, G. Fe, Zn and Se Bioavailability in Chicken Meat Emulsions Enriched with Minerals, Hydroxytyrosol and Extra Virgin Olive Oil as Measured by Caco-2 Cell Model. Nutrients 2018, 10, 969. [Google Scholar] [CrossRef]
- Martínez-Zamora, L.; Jongberg, S.; Ros, G.; Skibsted, L.H.; Nieto, G. Plant derived ingredients rich in nitrates or phenolics for protection of pork against protein oxidation. Food Res. Int. 2020, 129, 108789. [Google Scholar] [CrossRef]
- Singh, A.; Singh, R.; Bhunia, A.K.; Singh, N. Efficacy of plant essential oils as antimicrobial agents against Listeria monocytogenes in hotdogs. LWT 2003, 36, 787–794. [Google Scholar] [CrossRef]
- Özkan, G.; Sagdic, O.; Özcan, M. Note: Inhibition of Pathogenic Bacteria by Essential Oils at Different Concentrations. Food Sci. Technol. Int. 2003, 9, 85–88. [Google Scholar] [CrossRef]
- Sagdic, O.; Kuşçu, A.; Özcan, M.; Özçelik, S. Effects of Turkish spice extracts at various concentrations on the growth of Escherichia coli O157:H7. Food Microbiol. 2002, 19, 473–480. [Google Scholar] [CrossRef]
- Gaysinsky, S.; Davidson, P.M.; Bruce, B.D.; Weiss, J. Growth Inhibition of Escherichia coli O157:H7 and Listeria monocytogenes by Carvacrol and Eugenol Encapsulated in Surfactant Micelles. J. Food Prot. 2005, 68, 2559–2566. [Google Scholar] [CrossRef]
- Gill, A.; Holley, R.A. Disruption of Escherichia coli, Listeria monocytogenes and Lactobacillus sakei cellular membranes by plant oil aromatics. Int. J. Food Microbiol. 2006, 108, 1–9. [Google Scholar] [CrossRef]
- Aureli, P.; Costantini, A.; Zolea, S. Antimicrobial Activity of Some Plant Essential Oils Against Listeria monocytogenes1. J. Food Prot. 1992, 55, 344–348. [Google Scholar] [CrossRef]
- Deans, S.; Ritchie, G. Antibacterial properties of plant essential oils. Int. J. Food Microbiol. 1987, 5, 165–180. [Google Scholar] [CrossRef]
- Conner, D.E.; Beuchat, L.R. Sensity of heat-stressed yeasts to essential oil of plant. Appl. Environ. Microbiol. 1984, 47, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Karaman, S.; Digrak, M.; Ravid, U.; Ilcim, A. Antibacterial and antifungal activity of the essential oils of Thymus revolutus Celak from Turkey. J. Ethnopharmacol. 2001, 76, 183–186. [Google Scholar] [CrossRef]
- Rasooli, I.; Mirmostafa, S.A. Bacterial Susceptibility to and Chemical Composition of Essential Oils from Thymus kotschyanus and Thymus persicus. J. Agric. Food Chem. 2003, 51, 2200–2205. [Google Scholar] [CrossRef]
- Montes-Belmont, R.; Convajal, M. Control of Aspergellus flavus in Maize with plant essential oils and their components. J. Food Prot. 1998, 61, 616–619. [Google Scholar] [CrossRef]
- Kumar, A.; Shukla, R.; Singh, P.; Prasad, C.S.; Dubey, N.K. Assessment of Thymus vulgaris L. essential oil as a safe botanical preservative against post harvest fungal infestation of food commodities. Innov. Food Sci. Emerg. Technol. 2008, 9, 575–580. [Google Scholar] [CrossRef]
- Bagamboula, C.; Uyttendaele, M.; Debevere, J. Inhibitory effect of thyme and basil essential oils, carvacrol, thymol, estragol, linalool and p-cymene towards Shigella sonnei and S. flexneri. Food Microbiol. 2004, 21, 33–42. [Google Scholar] [CrossRef]
- Burt, S.A.; Vlielander, R.; Haagsman, H.P.; Veldhuizen, E.J.A. Increase in Activity of Essential Oil Components Carvacrol and Thymol against Escherichia coli O157:H7 by Addition of Food Stabilizers. J. Food Prot. 2005, 68, 919–926. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, F.; Ji, B.P.; Pei, R.S.; Xu, N. The antibacterial mechanism of carvacrol and thymol against Escherichia coli. Lett. Appl. Microbiol. 2008, 47, 174–179. [Google Scholar] [CrossRef]
- Uhart, M.; Maks, N.; Ravishankar, S. Effect of spices on growth and survival of Salmonella typhimurium DT 104 in ground beef stored at 4 and 8 °C. J. Food Safe 2006, 26, 115–125. [Google Scholar] [CrossRef]
- Firouzi, R.; Shekarforoush, S.S.; Nazer, A.H.K.; Borumand, Z.; Jooyandeh, A.R. Effects of Essential Oils of Oregano and Nutmeg on Growth and Survival of Yersinia enterocolitica and Listeria monocytogenes in Barbecued Chicken. J. Food Prot. 2007, 70, 2626–2630. [Google Scholar] [CrossRef]
- Tassou, C.; Drosinos, E.H.; Nychas, G.-J.E. Inhibition of Resident Microbial Flora and Pathogen Inocula on Cold Fresh Fish Fillets in Olive Oil, Oregano, and Lemon Juice under Modified Atmosphere or Air. J. Food Prot. 1996, 59, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Koutsoumanis, K.; Lambropoulou, K.; Nychas, G.-J.E. A predictive model for the non-thermal inactivation of Salmonella enteritidis in a food model system supplemented with a natural antimicrobial. Int. J. Food Microbiol. 1999, 49, 63–74. [Google Scholar] [CrossRef]
- Tsigarida, T.; Skandamis, P.; Nychas, G.J.E. Behaviour of Listeria monocytogenes and autochtonus flora on meat stored under aerobic, vacuum and modified atmosphere packaging conditions with or without the presence of oregano essential oil at 5 °C. J. Appl. Microbiol. 2000, 89, 901–909. [Google Scholar] [CrossRef]
- Skandamis, P.; Koutsoumanis, K.; Fasseas, K.; Nychas, G.J.E. Inhibition of oregano essential oil and EDTA on Escherichia coli O157:H7. Ital. J. Food Sci. 2001, 13, 65–75. [Google Scholar]
- Smith-Palmer, A.; Stewart, J.; Fyfe, L. The potential application of plant essential oils as natural food preservatives in soft cheese. Food Microbiol. 2001, 18, 463–470. [Google Scholar] [CrossRef]
- Gill, A.O.; Delaquis, P.; Russo, P.; Holley, R.A. Evaluation of antilisterial action of cilantro oil on vaccum packed ham. Int. J. Food Microbiol. 2002, 73, 83–92. [Google Scholar] [CrossRef]
- Ceylan, E.; Fung, D. Antimicrobial activity of spices. J. Rapid Methods Autom. Microbiol. 2004, 12, 1–55. [Google Scholar] [CrossRef]
- Naidu, A.; Davidson, P. Phyto-phenols. In Natural Food Antimicrobial Systems; CRC Press: Boca Raton, FL, USA, 2000; pp. 265–294. [Google Scholar]
- Raccach, M. The antimicrobial activity of phenolic antioxidants in foods: A review. J. Food Saf. 1984, 6, 141–170. [Google Scholar] [CrossRef]
- Magi, G.; Marini, E.; Facinelli, B. Antimicrobial activity of essential oils and carvacrol, and synergy of carvacrol and erythromycin, against clinical, erythromycin-resistant Group A Streptococci. Front. Microbiol. 2015, 6, 165. [Google Scholar] [CrossRef]
- Knowles, J.R.; Roller, S.; Murray, D.B.; Naidu, A.S. Antimicrobial Action of Carvacrol at Different Stages of Dual-Species Biofilm Development by Staphylococcus aureus and Salmonella enterica Serovar Typhimurium. Appl. Environ. Microbiol. 2005, 71, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Carson, C.; Mee, B.J.; Riley, T.V. Mechanism of Action of Melaleuca alternifolia (Tea Tree) Oil on Staphylococcus aureus Determined by Time-Kill, Lysis, Leakage, and Salt Tolerance Assays and Electron Microscopy. Antimicrob. Agents Chemother. 2002, 46, 1914–1920. [Google Scholar] [CrossRef] [PubMed]
- Sikkema, J.; de Bont, J.A.; Poolman, B. Interactions of cyclic hydrocarbons with biological membranes. J. Boil. Chem. 1994, 269, 8022–8028. [Google Scholar]
- Denyer, S.P.; Hugo, W.B. Biocide-induced damage to the bacterial cytoplasmic membrane. In Mechanisms of Action of Chemical Biocides; Oxford The society for Applied Bacteriology, Technical Series No 27; Denyer, S.P., Hugo, W.B., Eds.; Blackwell Scientific Publication: Oxford, UK, 1991; pp. 171–188. [Google Scholar]
- Mejlholm, O.; Dalgaard, P. Antimicrobial effect of essential oils on the seafood spoilage micro-organism Photobacterium phosphoreum in liquid media and fish products. Lett. Appl. Microbiol. 2002, 34, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Goulas, A.E.; Kontominas, M.G. Combined effect of light salting, modified atmosphere packaging and oregano essential oil on the shelf-life of sea bream (Sparus aurata): Biochemical and sensory attributes. Food Chem. 2007, 100, 287–296. [Google Scholar] [CrossRef]
- Nychas, G.-J. Natural antimicrobials from plants. In New Methods of Food Preservation; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 1995; pp. 58–89. [Google Scholar]
- Román, S.; Sanchez-Siles, L.M.; Siegrist, M. The importance of food naturalness for consumers: Results of a systematic review. Trends Food Sci. Technol. 2017, 67, 44–57. [Google Scholar] [CrossRef]
- Jouki, M.; Yazdi, F.T.; Mortazavi, S.A.; Koocheki, A.; Khazaei, N. Effect of quince seed mucilage edible films incorporated with oregano or thyme essential oil on shelf life extension of refrigerated rainbow trout fillets. Int. J. Food Microbiol. 2014, 174, 88–97. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; De Lacey, A.L.; López-Caballero, M.; Gómez-Guillén, M.C.; Montero, P. Biodegradable gelatin–chitosan films incorporated with essential oils as antimicrobial agents for fish preservation. Food Microbiol. 2010, 27, 889–896. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, X.; Jia, S.; Zhang, L.; Luo, Y. The effect of essential oils on microbial composition and quality of grass carp (Ctenopharyngodon idellus) fillets during chilled storage. Int. J. Food Microbiol. 2018, 266, 52–59. [Google Scholar] [CrossRef]
- Yano, Y.; Satomi, M.; Oikawa, H. Antimicrobial effect of spices and herbs on Vibrio parahaemolyticus. Int. J. Food Microbiol. 2006, 111, 6–11. [Google Scholar] [CrossRef]
- Navarro-Segura, L.; Ros-Chumillas, M.; López-Cánovas, A.E.; García-Ayala, A.; López-Gómez, A. Nanoencapsulated essential oils embedded in ice improve the quality and shelf life of fresh whole seabream stored on ice. Heliyon 2019, 5, e01804. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, B.S.; Yamazaki, K.; Miyashita, K.; Il-Shik, S.; Dong-Suk, C.; Suzuki, T. Bacterial microflora of carp (Cyprinus carpio) and its shelf-life extension by essential oil compounds. Food Microbiol. 2004, 21, 657–666. [Google Scholar] [CrossRef]
- Kraszewski, J.; Strzetelski, J.A.; Niwińska, B. Effects of dietary herb supplements for cows on milk yield and technological quality of milk. In Proceedings of the 55th Annual Meeting of the European Association for Animal Production, Bled, Slovenia, 5–9 September 2004. [Google Scholar]
- Sharma, R.; Gupta, M.; Ogra, J. Factors affecting yield and chemical quality of goat milk chhana. Small Rumin. Res. 1998, 27, 257–262. [Google Scholar] [CrossRef]
- Boutoial, K.; Garcia, V.; Rovira, S.; Ferrandini, E.; Abdelkhalek, O.; López, M.B. Effect of feeding goats with distilled and non-distilled thyme leaves (Thymus zygis subp. gracilis) on milk and cheese properties. J. Dairy Res. 2013, 80, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Güran, H.Ş.; Öksüztepe, G.; Coban, O.E.; Incili, G.K. Influence of different essential oils on refrigerated fish patties produced from bonito fish (Sarda sarda Bloch, 1793). Czech J. Food Sci. 2016, 33, 37–44. [Google Scholar] [CrossRef]
- El Adab, S.; Hassouna, M. Proteolysis, Lipolysis and Sensory Characteristics of a Tunisian Dry Fermented Poultry Meat Sausage with Oregano and Thyme Essential Oils. J. Food Saf. 2015, 36, 19–32. [Google Scholar] [CrossRef]
- Krisch, J.; Pardi, Z.; Tserennadmid, R.; Papp, T.; Vágvölgyi, C. Antimicrobial effects of commercial herbs, spices and essential oils in minced pork. Acta Biol. Szeged. 2010, 54, 131–134. [Google Scholar]
- Sharma, H.; Mendiratta, S.K.; Agarwal, R.K.; Kumar, S.; Soni, A. Evaluation of anti-oxidant and anti-microbial activity of various essential oils in fresh chicken sausages. J. Food Sci. Technol. 2017, 54, 279–292. [Google Scholar] [CrossRef]
- Erkan, N.; Tosun, Ş.Y.; Ulusoy, Ş.; Üretener, G. The use of thyme and laurel essential oil treatments to extend the shelf life of bluefish (Pomatomus saltatrix) during storage in ice. J. Consum. Prot. Food Saf. 2010, 6, 39–48. [Google Scholar] [CrossRef]

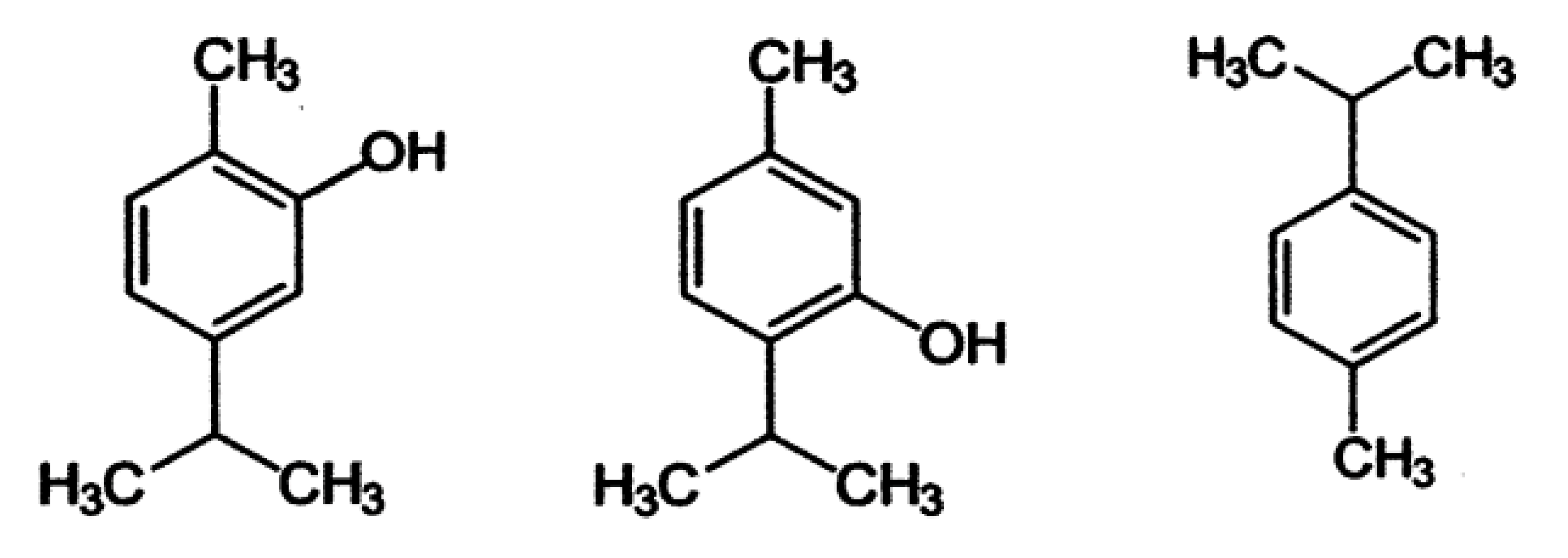
| Compounds | Quantity (%) | |
|---|---|---|
| Thymus zygis, subsp. gracilis | Thymus vulgaris L. | |
| α-Pinene | 1.10–3.50 | 1.9 |
| Camphene | 0.40–1.50 | 1.2 |
| Sabinene | 0.10–0.20 | - |
| Myrecene | 1.10–2.90 | 1.1 |
| α-Terpineol | 0.50–1.30 | 0.3 |
| 1.8-Cineole | 1.70–3.10 | 2.1 |
| γ-Terpinene | 2.90–9.70 | 5.2 |
| p-Cymene | 24.70–40.90 | 29.1 |
| Linalool | 3.50–4.20 | 3.7 |
| Terpinen-4-ol | 1.10–3.50 | 1.3 |
| α-Terpineol | 1.00–3.10 | 0.3 |
| Caryophyllene Oxide | 0.40–1.00 | 0.5 |
| Thymol | 22.30–43.30 | 38.1 |
| Carvacrol | 1.50–2.70 | 2.3 |
| β-Caryophyllene | - | 3.1 |
| Phytochemical Composition of Thyme | Main Components | Functional Properties | Ref. |
|---|---|---|---|
| Phenolics acids | Quercetin | Anti-oxidative polyphenol health benefits: preventive effect against Alzheimer’s disease, anti-infl- ammatory and anti-mutagenic properties. | [22,23,24] |
| Ferulic acid | |||
| Syringic acid | |||
| Caffeic acid | |||
| Rosmarinic acid | |||
| p-cumaric acid | |||
| Biphenyl compounds | 4,4′-dihydroxy-5,5′-disopropyl-2,2′-dimethylbiphenyl-3,6-dione | Antioxidant activity, deodorant effect | [25,26] |
| 5,5′-diisopropyl-2,2′- -dimethylbiphenyl-3,4,3′,4/-tetraone | |||
| 4′-hydroxy-5,5′-diisopropyl-2,2′-dimethylbiphenyl-3,4-dione | |||
| Flavonoids | Flavonols, Flavones | Antioxidant activity, anti-inflammatory | [25,27] |
| Flavonols, Flavone glycosides | |||
| Methyl flavones, Flavonols | |||
| Apigenin, Luteolin, Hesperitin | |||
| Rutin, Quercetin, Hesperidin | |||
| Kaempferol, Kaempferol-3-O-rutinoside | |||
| Essential oils | Limonene | Antioxidant, antimicrobial, antitussive, expectorant, antispasmodic, antibacterial effects | [28,29,30,31,32] |
| Linalool | |||
| γ- Terpinene | |||
| p- Cymene | |||
| Carvacrol | |||
| Thymol |
| Material and Food | Amount | Model | Results | Ref. |
|---|---|---|---|---|
| Thyme (essential oil) Bonito fish | 880 μL/kg | Application: Mixture in fish patty and stored at 4 °C for 14 days. | During the storage period, peroxide values, total volatile basic nitrogen (TVB-N), and thiobarbituric acid index (TBA-i), were significantly lower in thyme group compared to control group. | [181] |
| Distilled thyme leaves (Thymus zygis, subsp. gracilis) Lamb meat | Replacing 10% and 20% of the basal diet of pregnant sheep, with pellets elaborated from 50% barley and 50% distilled thyme leaves. | Application: In vivo. Inclusion of distilled thyme leaves in the diet of pregnant sheep and study their effect on the final meat quality of lamb, which was studied during the storage of meat in a MA (modified atmosphere). A total of 36 sheep were randomly divided into 3 homogeneous groups. One group was fed a basal diet. The diet of the other two groups was modified by distilled thyme leaves. | In general, the diet supplemented with distilled thyme leaves inhibits lipid oxidation and reduced the content of psychotrophs. In contrast, the a* values (redness) was significantly greater in lamb meat treated with thyme, (compared with control meat) at 7 and 14 days of storage. | [126] |
| Fermented poultry sausage Thyme essential oil | 0.25% | Thyme essential oil (Thymus vulgaris) incorporated into fermented poultry sausages for 28 days of ripening. | TBA values were significantly affected by the addition of thyme EO. Decrease on total coliform counts, Enterobacteriaceae counts, and Staphylococcus aureus counts. | [182] |
| Thyme essential oil Grass carp (Ctenopharyngodon idellus) | 0.1% | Application by immersion and storage at 4 °C. | Thyme essential oils treatment was found to be effective in delaying lipid oxidation, inhibiting microbial growth, and retarding the increase of K-value, putrescine, TVB-N and hypoxanthine. | [174] |
| Minced pork | 1% | Fresh and dried thyme with and without 1% salt, were mixed in 1% concentration to minced pork (100 g). The meat was stored at 5 °C. | Decreased E. coli cell numbers in minced pork with 1 log cfu after 24 h storage at 5 °C. | [183] |
| Chicken sausages | 0.125% | Thyme essential oil incorporated into fresh chicken sausages for 20 days at refrigeration temperature (4 ± 1 °C). | Storage studies revealed that thyme oil (0.125%) incorporated aerobically in packaged and refrigerated fresh chicken sausages had approx. 2–3 days longer shelf life than control. Microbial count of thyme essential oil incorporated products were significantly lower than control and remained well below the permissible limit of fresh meat products (log107 cfu/g). Decrease on TBARS values, total viable, psychrophilic bacteria and yeast and mold counts. | [184] |
| Thyme or laurel essential oil (1%/each) Bluefish (Pomatomus saltatrix) | 0.1% | Stored in ice inoculation thyme on the surface of fish. | Shelf life of treated bluefish with thyme was extended 2 days, compared with control samples. Trimethylamine values and total volatile base nitrogen gave acceptable results for up to 9 days for the control samples and 13 days for samples with thyme. Peroxide values, free fatty acid and thiobarbituric acid were lower for treated samples with thyme than the control. Microbial growth in control samples was significantly higher than treated samples with thyme. | [185] |
| Distilled thyme leaves (Thymus zygis, subsp. gracilis) Feeding goats with distilled and non-distilled thyme leaves (Thymus zygis subsp. gracilis) | One group of goats was fed the basal diet (control), the second and third groups were fed with different levels of distilled (10 and 20%) or non-distilled (3.75 and 7.5%) thyme leaves. | Application: In vivo. Inclusion in goats diet with distilled and non-distilled thyme leaves (Thymus zygis subsp. gracilis) on the physicochemical composition and technological properties of pasteurized goat milk, and on the physicochemical composition, phenolic content, oxidative stability, microbiology, sensory and texture profile of goat cheese. | Incorporation of T. zygis leaves to goats provided cheeses and milks with added bromatological values, which increased the oxidative stability of a typical cheese with wine. | [180] |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nieto, G. A Review on Applications and Uses of Thymus in the Food Industry. Plants 2020, 9, 961. https://doi.org/10.3390/plants9080961
Nieto G. A Review on Applications and Uses of Thymus in the Food Industry. Plants. 2020; 9(8):961. https://doi.org/10.3390/plants9080961
Chicago/Turabian StyleNieto, Gema. 2020. "A Review on Applications and Uses of Thymus in the Food Industry" Plants 9, no. 8: 961. https://doi.org/10.3390/plants9080961
APA StyleNieto, G. (2020). A Review on Applications and Uses of Thymus in the Food Industry. Plants, 9(8), 961. https://doi.org/10.3390/plants9080961





