Genomic Characterization and Expression Analysis of Basic Helix-Loop-Helix (bHLH) Family Genes in Traditional Chinese Herb Dendrobium officinale
Abstract
1. Introduction
2. Results
2.1. Genome-Wide Identification of DobHLH Members in Dendrobium officinale
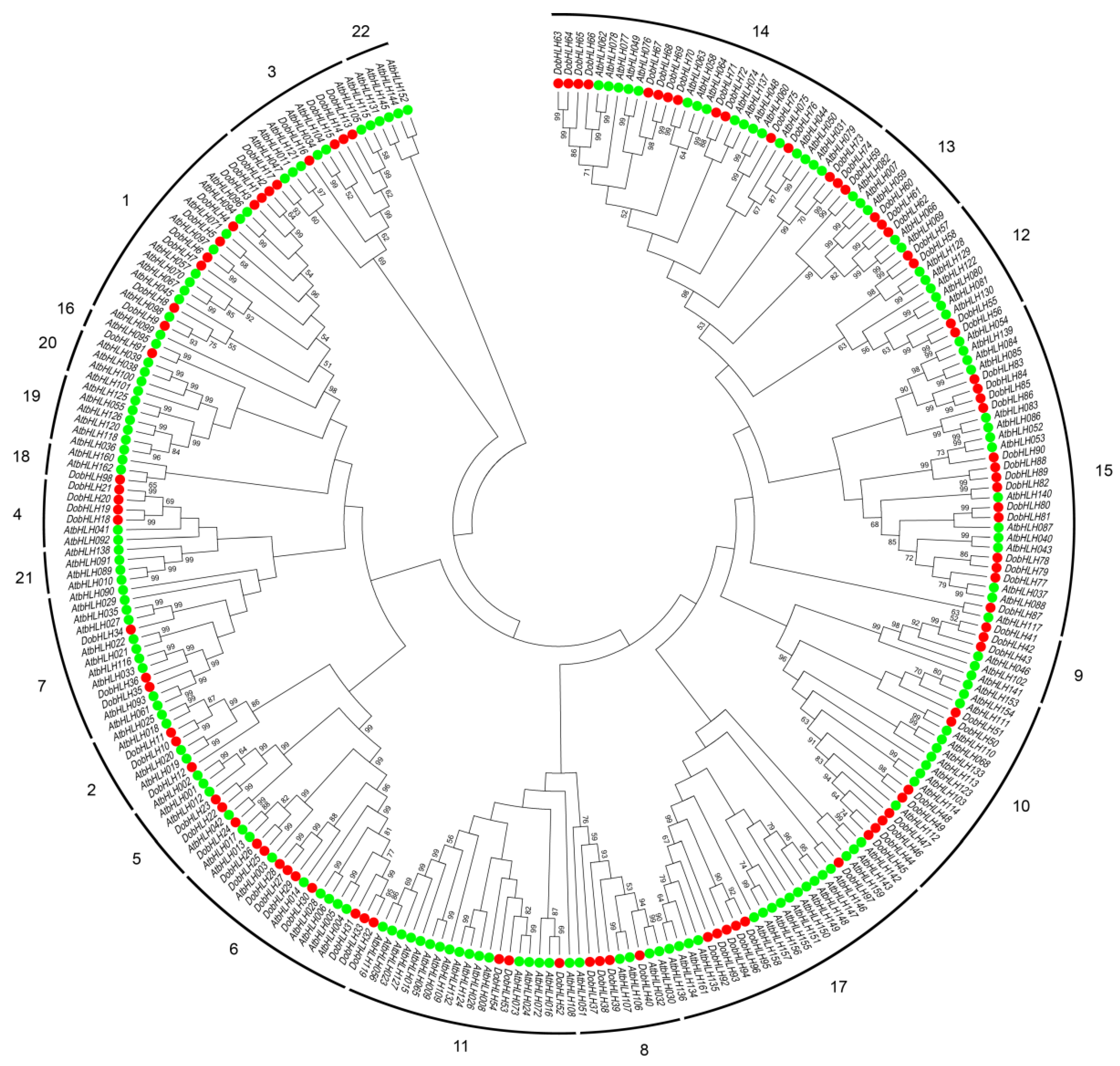
2.2. Phylogenetic Relationship of DobHLH Proteins
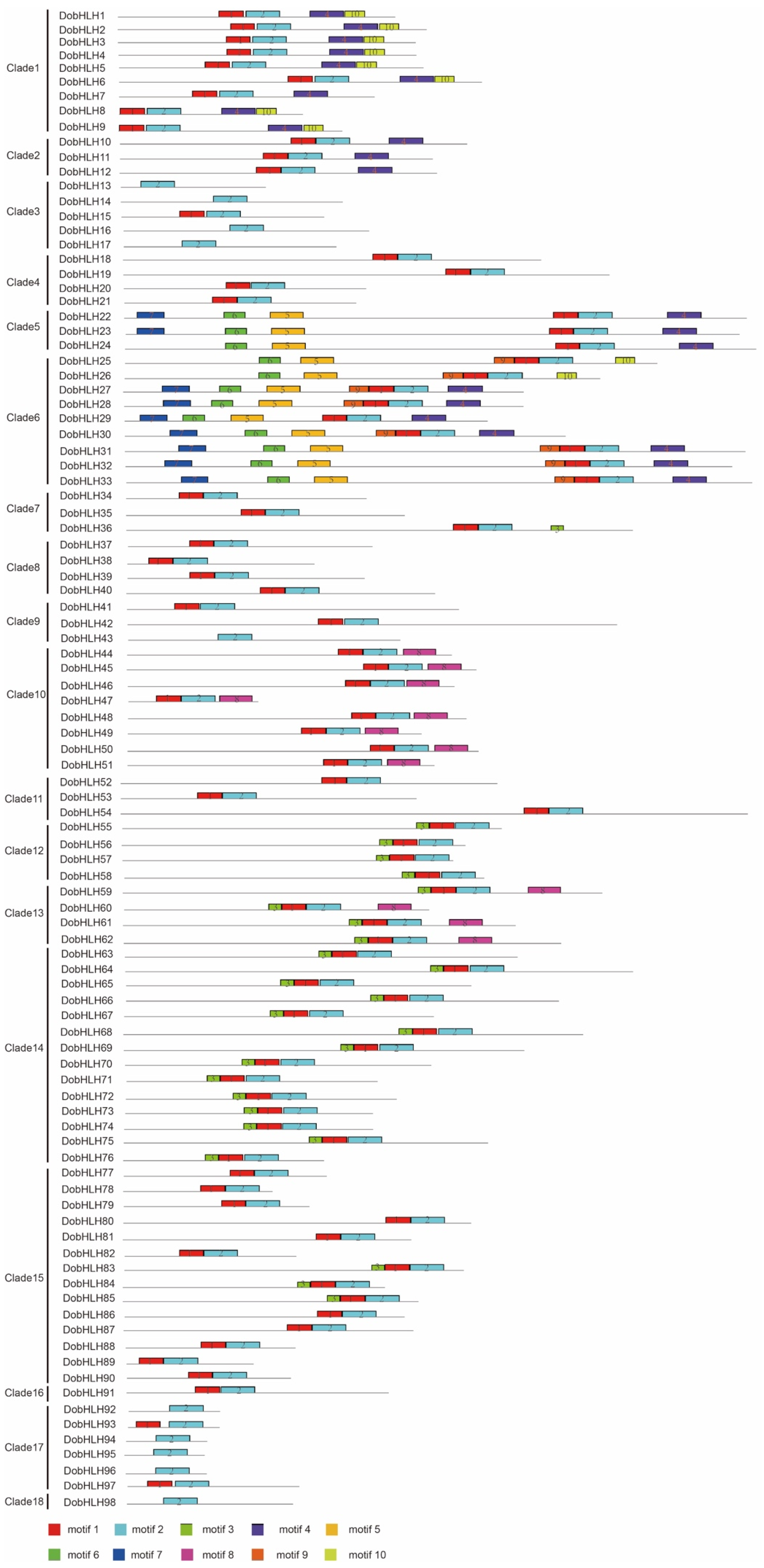
2.3. Gene Structures and Conserved Motifs of DobHLH Genes
2.4. Conserved Amino Acids in the DobHLH Domains and DNA-Binding Ability
2.5. Cis-Elements in the Promoter Regions of DobHLH Genes
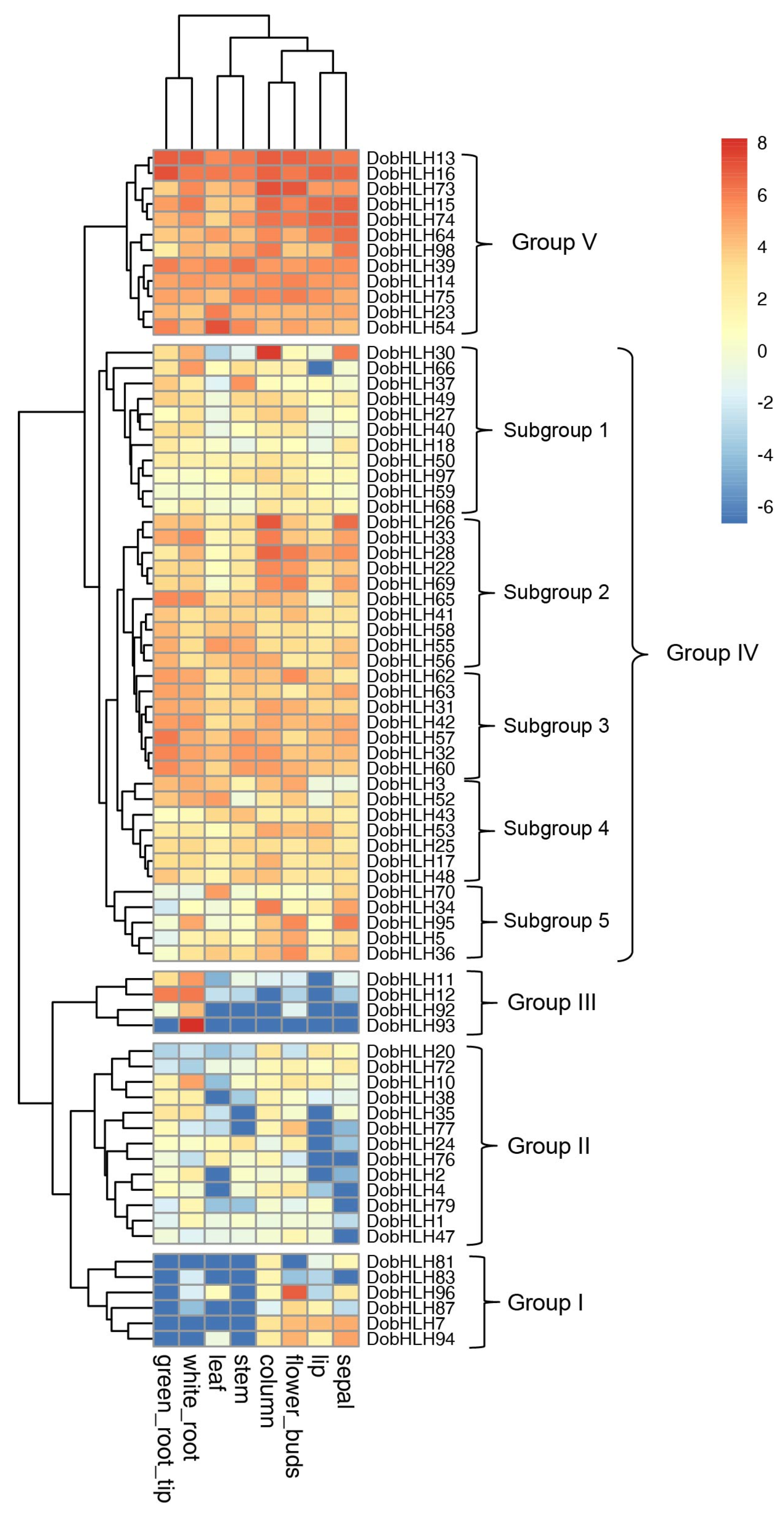
2.6. Expression Patterns of DobHLH Genes in Eight Tissues
2.7. Expressional Changes of DobHLH Genes Concerned with ABA and JA Signals under MeJA and ABA Treatments
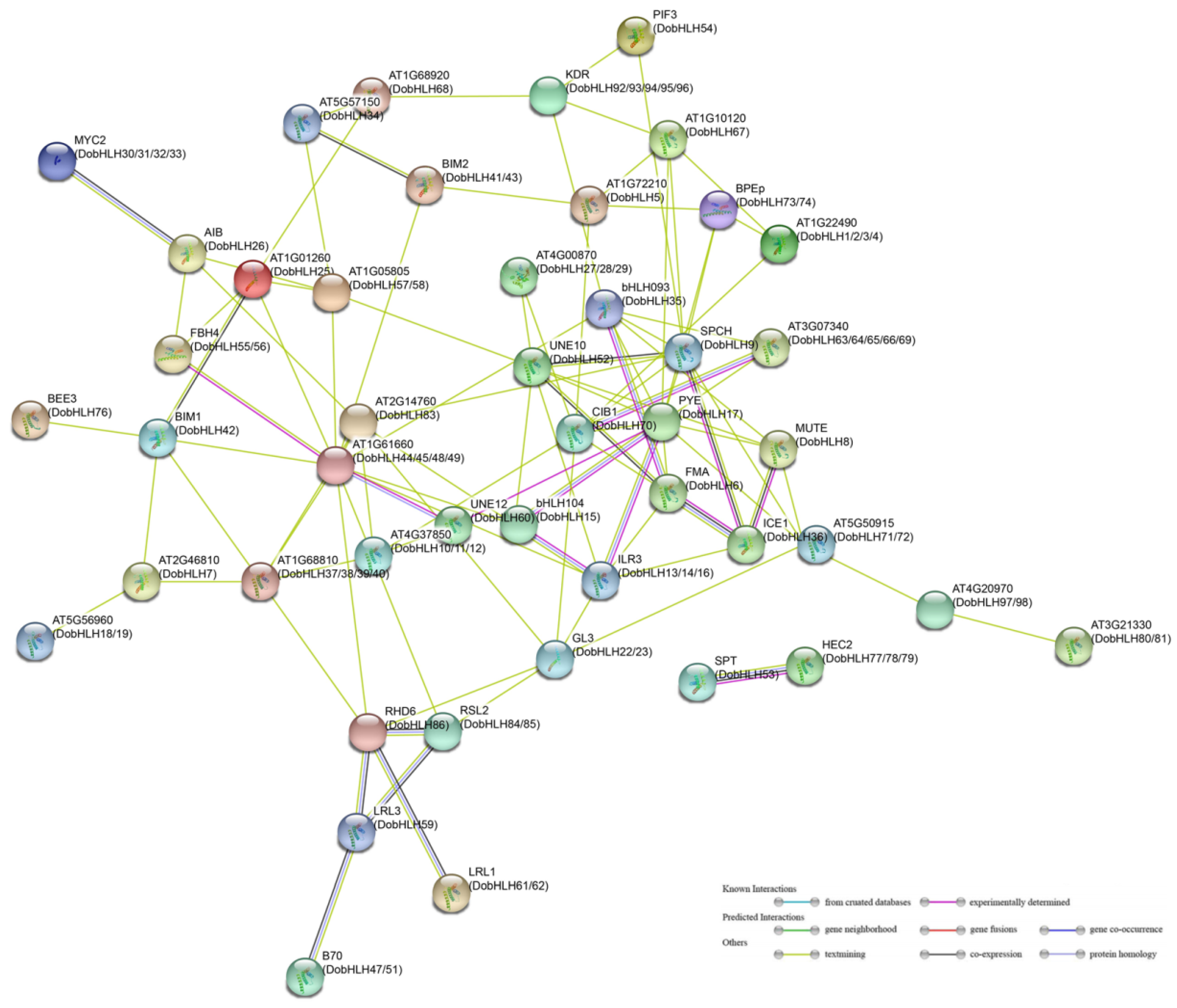
2.8. Protein–Protein Interaction Networks
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Identification of the bHLH TF Family in Dendrobium officinale
4.3. Phylogenetic Analysis of DobHLH Proteins
4.4. Gene Structure and Conserved Motif Analysis of DobHLHs
4.5. Expression Patterns of DobHLH Genes in Different Tissues
4.6. Predicted Protein–Protein Interaction of DobHLH Members
4.7. Cis-Element Analysis in Promoter Regions of DobHLH Genes
4.8. RNA Extraction and cDNA Preparation
4.9. Yeast Two Hybrid Assays
4.10. qRT-PCR Assays
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ng, T.B.; Liu, J.; Wong, J.H.; Ye, X.; Sze, S.C.W.; Tong, Y.; Zhang, K.Y. Review of research on Dendrobium, a prized folk medicine. Appl. Microbiol. Biotechnol. 2012, 93, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Wang, X.; Liu, H.; Tian, Y.; Lian, J.; Yang, R.; Hao, S.; Wang, X.; Yang, S.; Li, Q.; et al. The Genome of Dendrobium officinale Illuminates the Biology of the Important Traditional Chinese Orchid Herb. Mol. Plant 2015, 8, 922–934. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Zhao, T.; Sheng, Y.; Zheng, T.; Fu, L.; Zhang, Y. Dendrobium officinale Kimura et Migo: A Review on Its Ethnopharmacology, Phytochemistry, Pharmacology, and Industrialization. Evid. Based Complement. Alternat. Med. 2017, 2017, 7436259. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Guo, H.; Chen, H.; Shi, Y.; Meng, Y.; Lu, J.; Feng, S.; Wang, H. Identification and analysis of genes associated with the synthesis of bioactive constituents in Dendrobium officinale using RNA-Seq. Sci. Rep. 2017, 7, 187. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.; Zhang, X.; Ke, H.; Lin, J.; Huang, Y.; Wei, G. Physicochemical properties of polysaccharides from Dendrobium officinale by fractional precipitation and their preliminary antioxidant and anti-HepG2 cells activities in vitro. Chem. Cent. J. 2018, 12, 100. [Google Scholar] [CrossRef]
- Liang, J.; Wu, Y.; Yuan, H.; Yang, Y.; Xiong, Q.; Liang, C.; Li, Z.; Li, C.; Zhang, G.; Lai, X.; et al. Dendrobium officinale polysaccharides attenuate learning and memory disabilities via anti-oxidant and anti-inflammatory actions. Int. J. Biol. Macromol. 2019, 126, 414–426. [Google Scholar] [CrossRef]
- Tao, S.C.; Lei, Z.X.; Huang, K.W.; Li, Y.R.; Ren, Z.Y.; Zhang, X.F.; Wei, G.; Chen, H.M. Structural characterization and immunomodulatory activity of two novel polysaccharides derived from the stem of Dendrobium officinale Kimura et Migo. J. Funct. Foods. 2019, 57, 121–134. [Google Scholar] [CrossRef]
- Kuang, M.T.; Li, J.Y.; Yang, X.B.; Yang, L.; Xu, J.Y.; Yan, S.; Lv, Y.F.; Ren, F.C.; Hu, J.M.; Zhou, J. Structural characterization and hypoglycemic effect via stimulating glucagon-like peptide-1 secretion of two polysaccharides from Dendrobium officinale. Carbohydr. Polym. 2020, 241, 116326. [Google Scholar] [CrossRef]
- Pan, L.H.; Li, X.F.; Wang, M.N.; Zha, X.Q.; Yang, X.F.; Liu, Z.J.; Luo, Y.B.; Luo, J.P. Comparison of hypoglycemic and antioxidative effects of polysaccharides from four different Dendrobium species. Int. J. Biol. Macromol. 2014, 64, 420–427. [Google Scholar] [CrossRef]
- Zeng, X.; Ling, H.; Chen, X.; Guo, S. Genome-wide identification, phylogeny and function analysis of GRAS gene family in Dendrobium catenatum (Orchidaceae). Gene 2019, 705, 5–15. [Google Scholar] [CrossRef]
- Huang, K.; Li, Y.; Tao, S.; Wei, G.; Huang, Y.; Chen, D.; Wu, C. Purification, Characterization and Biological Activity of Polysaccharides from Dendrobium officinale. Molecules 2016, 21, 701. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Liao, Y.; da Silva, J.A.T.; Yang, Z.; Duan, J. Differential Accumulation of Anthocyanins in Dendrobium officinale Stems with Red and Green Peels. Int. J. Mol. Sci. 2018, 19, 2857. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhang, J.; Liu, X.; Meng, M.; Wang, J.; Lin, J. Tissue-specific transcriptome for Dendrobium officinale reveals genes involved in flavonoid biosynthesis. Genomics 2020, 112, 1781–1794. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Zhou, C.; Ji, X.; Wei, G.; Huang, Y.; Yu, W.; Luo, Y.; Qiu, Y. Transcriptome Analysis Reveals genes involved in flavonoid biosynthesis and accumulation in Dendrobium catenatum From Different Locations. Sci. Rep. 2018, 8, 6373. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, Y.; Li, C.; Luo, H.; Wang, L.; Qian, J.; Luo, X.; Xiang, L.; Song, J.; Sun, C.; et al. Analysis of the Dendrobium officinale transcriptome reveals putative alkaloid biosynthetic genes and genetic markers. Gene 2013, 527, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, Y.; Sui, N. Transcriptional regulation of bHLH during plant response to stress. Biochem. Biophys. Res. Commun. 2018, 503, 397–401. [Google Scholar] [CrossRef]
- Jones, S. An overview of the basic helix-loop-helix proteins. Genome Biol. 2004, 5, 226. [Google Scholar] [CrossRef]
- Pires, N.; Dolan, L. Origin and diversification of basic-helix-loop-helix proteins in plants. Mol. Biol. Evol. 2010, 27, 862–874. [Google Scholar] [CrossRef]
- Moore, A.W.; Barbel, S.; Jan, L.Y.; Jan, Y.N. A genomewide survey of basic helix-loop-helix factors in Drosophila. Proc. Natl. Acad. Sci. USA 2000, 97, 10436–10441. [Google Scholar] [CrossRef]
- Riechmann, J.L.; Heard, J.; Martin, G.; Reuber, L.; Jiang, C.; Keddie, J.; Adam, L.; Pineda, O.; Ratcliffe, O.J.; Samaha, R.R.; et al. Arabidopsis transcription factors: Genome-wide comparative analysis among eukaryotes. Science 2000, 290, 2105–2110. [Google Scholar] [CrossRef]
- Murre, C.; McCaw, P.S.; Baltimore, D. A new DNA binding and dimerization motif in immunoglobulin enhancer binding, daughterless, MyoD, and myc proteins. Cell 1989, 56, 777–783. [Google Scholar] [CrossRef]
- Toledo-Ortiz, G.; Huq, E.; Quail, P.H. The Arabidopsis basic/helix-loop-helix transcription factor family. Plant Cell 2003, 15, 1749–1770. [Google Scholar] [CrossRef] [PubMed]
- Atchley, W.R.; Terhalle, W.; Dress, A. Positional dependence, cliques, and predictive motifs in the bHLH protein domain. J. Mol. Evol. 1999, 48, 501–516. [Google Scholar] [CrossRef] [PubMed]
- Massari, M.E.; Murre, C. Helix-loop-helix proteins: Regulators of transcription in eucaryotic organisms. Mol. Cell Biol. 2000, 20, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Gao, W.; Xue, C.; Zhang, Y.; Liu, Z.; Zhang, Y.; Meng, X.; Liu, M.; Zhao, J. Genome-wide analysis of the bHLH gene family in Chinese jujube (Ziziphus jujuba Mill.) and wild jujube. BMC Genom. 2019, 20, 568. [Google Scholar] [CrossRef]
- Bailey, P.C.; Martin, C.; Toledo-Ortiz, G.; Quail, P.H.; Huq, E.; Heim, M.A.; Jakoby, M.; Werber, M.; Weisshaar, B. Update on the basic helix-loop-helix transcription factor gene family in Arabidopsis thaliana. Plant Cell 2003, 15, 2497–2502. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Fan, H.J.; Ling, H.Q. Genome-wide identification and characterization of the bHLH gene family in tomato. BMC Genom. 2015, 16, 9. [Google Scholar] [CrossRef]
- Zhang, T.; Lv, W.; Zhang, H.; Ma, L.; Li, P.; Ge, L.; Li, G. Genome-wide analysis of the basic Helix-Loop-Helix (bHLH) transcription factor family in maize. BMC Plant Biol. 2018, 18, 235. [Google Scholar] [CrossRef]
- Wang, P.; Su, L.; Gao, H.; Jiang, X.; Wu, X.; Li, Y.; Zhang, Q.; Wang, Y.; Ren, F. Genome-Wide Characterization of bHLH Genes in Grape and Analysis of their Potential Relevance to Abiotic Stress Tolerance and Secondary Metabolite Biosynthesis. Front. Plant Sci. 2018, 9, 64. [Google Scholar] [CrossRef]
- Lu, R.; Zhang, J.; Liu, D.; Wei, Y.L.; Wang, Y.; Li, X.B. Characterization of bHLH/HLH genes that are involved in brassinosteroid (BR) signaling in fiber development of cotton (Gossypium hirsutum). BMC Plant Biol. 2018, 18, 304. [Google Scholar] [CrossRef]
- Niu, X.; Guan, Y.; Chen, S.; Li, H. Genome-wide analysis of basic helix-loop-helix (bHLH) transcription factors in Brachypodium distachyon. BMC Genom. 2017, 18, 619. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, S.R.; Wessler, S.R. Maize R Gene Family: Tissue-Specific Helix-Loop-Helix Proteins. Cell 1990, 62, 849–851. [Google Scholar] [CrossRef]
- Xu, W.; Dubos, C.; Lepiniec, L. Transcriptional control of flavonoid biosynthesis by MYB-bHLH-WDR complexes. Trends Plant Sci. 2015, 20, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Van Moerkercke, A.; Steensma, P.; Schweizer, F.; Pollier, J.; Gariboldi, I.; Payne, R.; van den Bossche, R.; Miettinen, K.; Espoz, J.; Purnama, P.C.; et al. The bHLH transcription factor BIS1 controls the iridoid branch of the monoterpenoid indole alkaloid pathway in Catharanthus roseus. Proc. Natl. Acad. Sci. USA 2015, 112, 8130–8135. [Google Scholar] [CrossRef]
- Zhang, X.; Luo, H.; Xu, Z.; Zhu, Y.; Ji, A.; Song, J.; Chen, S. Genome-wide characterisation and analysis of bHLH transcription factors related to tanshinone biosynthesis in Salvia miltiorrhiza. Sci. Rep. 2015, 5, 11244. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Qiu, J.; Ding, L.; Huang, M.; Huang, S.; Yang, G.; Yin, J. Anthocyanin biosynthesis regulation of DhMYB2 and DhbHLH1 in Dendrobium hybrids petals. Plant Physiol. Biochem. 2017, 112, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.; Yamaguchi, S.; Kamiya, Y.; Bae, G.; Chung, W.I.; Choi, G. Light activates the degradation of PIL5 protein to promote seed germination through gibberellin in Arabidopsis. Plant J. 2006, 47, 124–139. [Google Scholar] [CrossRef]
- Chinnusamy, V.; Ohta, M.; Kanrar, S.; Lee, B.H.; Hong, X.; Agarwal, M.; Zhu, J.K. ICE1: A regulator of cold-induced transcriptome and freezing tolerance in Arabidopsis. Genes Dev. 2003, 17, 1043–1054. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Xu, Q.; Bian, C.; Tsai, W.C.; Yeh, C.M.; Liu, K.W.; Yoshida, K.; Zhang, L.S.; Chang, S.B.; Chen, F.; et al. The Dendrobium catenatum Lindl. genome sequence provides insights into polysaccharide synthase, floral development and adaptive evolution. Sci. Rep. 2016, 6, 19029. [Google Scholar] [CrossRef]
- Li, X.; Duan, X.; Jiang, H.; Sun, Y.; Tang, Y.; Yuan, Z.; Guo, J.; Liang, W.; Chen, L.; Yin, J.; et al. Genome-wide analysis of basic/helix-loop-helix transcription factor family in rice and Arabidopsis. Plant Physiol. 2006, 141, 1167–1184. [Google Scholar] [CrossRef]
- Zhao, K.; Li, S.; Yao, W.; Zhou, B.; Li, R.; Jiang, T. Characterization of the basic helix-loop-helix gene family and its tissue-differential expression in response to salt stress in poplar. PeerJ 2018, 6, e4502. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.J.; Wang, J.R. Global identification, structural analysis and expression characterization of bHLH transcription factors in wheat. BMC Plant Biol. 2017, 17, 90. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Cui, X.; Li, H.; Teng, R.-M.; Wang, Y.-X.; Liu, H.; Zhuang, J. Genome-wide identification and analyses of bHLH family genes in Brassica napus. Can. J. Plant Sci. 2019, 99, 589–598. [Google Scholar] [CrossRef]
- Zhang, C.; Feng, R.; Ma, R.; Shen, Z.; Cai, Z.; Song, Z.; Peng, B.; Yu, M. Genome-wide analysis of basic helix-loop-helix superfamily members in peach. PLoS ONE 2018, 13, e0195974. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, Y.; Lyu, P.; Chen, L.; Shen, C.; Sun, C. Comparative transcriptomic analysis reveal the regulation mechanism underlying MeJA-induced accumulation of alkaloids in Dendrobium officinale. J. Plant Res. 2019, 132, 419–429. [Google Scholar] [CrossRef] [PubMed]
- He, C.; da Silva, J.A.T.; Wang, H.; Si, C.; Zhang, M.; Zhang, X.; Li, M.; Tan, J.; Duan, J. Mining MYB transcription factors from the genomes of orchids (Phalaenopsis and Dendrobium) and characterization of an orchid R2R3-MYB gene involved in water-soluble polysaccharide biosynthesis. Sci. Rep. 2019, 9, 1381. [Google Scholar] [CrossRef]
- Chen, Y.; Shen, Q.; Lyu, P.; Lin, R.; Sun, C. Identification and expression profiling of selected MADS-box family genes in Dendrobium officinale. Genetica 2019, 147, 303–313. [Google Scholar] [CrossRef]
- Hong, Y.; Naveed, A.; Tian, Y.; Liu, J.; Wang, L.; Wang, G.; Liu, X.; Dong, Y.; Wang, F.; Liu, W.; et al. Genome-Wide Identification, Expression Analysis, and Subcellular Localization of Carthamus tinctorius bHLH Transcription Factors. Int. J. Mol. Sci. 2019, 20, 3044. [Google Scholar] [CrossRef]
- Wang, L.; Xiang, L.; Hong, J.; Xie, Z.; Li, B. Genome-wide Analysis of bHLH Transcription Factor Family Reveals Their Involvement in Biotic and Abiotic Stress Responses in Wheat (Triticum aestivum L.). 3 Biotech 2019, 9, 236. [Google Scholar] [CrossRef]
- Chu, Y.; Xiao, S.; Su, H.; Liao, B.; Zhang, J.; Xu, J.; Chen, S. Genome-wide characterization and analysis of bHLH transcription factors in Panax ginseng. Acta Pharm. Sin. B 2018, 8, 666–677. [Google Scholar] [CrossRef]
- Yang, J.; Gao, M.; Huang, L.; Wang, Y.; van Nocker, S.; Wan, R.; Guo, C.; Wang, X.; Gao, H. Identification and expression analysis of the apple (Malus × domestica) basic helix-loop-helix transcription factor family. Sci. Rep. 2017, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Mao, T.Y.; Liu, Y.Y.; Zhu, H.H.; Zhang, J.; Yang, J.X.; Fu, Q.; Wang, N.; Wang, Z. Genome-wide analyses of the bHLH gene family reveals structural and functional characteristics in the aquatic plant Nelumbo nucifera. PeerJ 2019, 7, e7153. [Google Scholar] [CrossRef] [PubMed]
- Carretero-Paulet, L.; Galstyan, A.; Roig-Villanova, I.; Martínez-García, J.F.; Bilbao-Castro, J.R.; Robertson, D.L. Genome-wide classification and evolutionary analysis of the bHLH family of transcription factors in Arabidopsis, poplar, rice, moss, and algae. Plant Physiol. 2010, 153, 1398–1412. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhao, P.; Kong, N.; Lu, R.; Pei, Y.; Huang, C.; Ma, H.; Chen, Q. Genome-Wide Identification and Characterization of the Potato bHLH Transcription Factor Family. Genes 2018, 9, 54. [Google Scholar] [CrossRef] [PubMed]
- Varaud, E.; Brioudes, F.; Szécsi, J.; Leroux, J.; Brown, S.; Perrot-Rechenmann, C.; Bendahmane, M. AUXIN RESPONSE FACTOR8 regulates Arabidopsis petal growth by interacting with the bHLH transcription factor BIGPETALp. Plant Cell 2011, 23, 973–983. [Google Scholar] [CrossRef]
- Zhu, Y.; Meng, C.; Zhu, L.; Li, D.; Jin, Q.; Song, C.; Cai, Y.; Fan, H.; Lin, Y. Cloning and characterization of DoMYC2 from Dendrobium officinale. Plant Cell Tiss. Organ. Cult. 2017, 129, 533–541. [Google Scholar] [CrossRef]
- Braun, P.; Carvunis, A.-R.; Charloteaux, B.; Dreze, M.; Ecker, R.J.; Hill, E.D.; Roth, P.F.; Vidal, M.; Galli, M.; Balumuri, P. Evidence for network evolution in an Arabidopsis interactome map. Science 2011, 333, 601–607. [Google Scholar]
- Finn, R.D.; Clements, J.; Arndt, W.; Miller, B.L.; Wheeler, T.J.; Schreiber, F.; Bateman, A.; Eddy, S.R. HMMER web server: 2015 update. Nucleic Acids Res. 2015, 43, W30–W38. [Google Scholar] [CrossRef]
- Wilkins, M.R.; Gasteiger, E.; Bairoch, A.; Sanchez, J.C.; Williams, K.L.; Appel, R.D.; Hochstrasser, D.F. Protein identification and analysis tools in the ExPASy server. Methods Mol. Biol. 1999, 112, 531–552. [Google Scholar] [CrossRef]
- McWilliam, H.; Li, W.; Uludag, M.; Squizzato, S.; Park, Y.M.; Buso, N.; Cowley, A.P.; Lopez, R. Analysis Tool Web Services from the EMBL-EBI. Nucleic Acids Res. 2013, 41, W597–W600. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Jin, J.; Guo, A.Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef] [PubMed]
- Apweiler, R.; Attwood, T.K.; Bairoch, A.; Bateman, A.; Birney, E.; Biswas, M.; Bucher, P.; Cerutti, L.; Corpet, F.; Croning, M.D.; et al. The InterPro database, an integrated documentation resource for protein families, domains and functional sites. Nucleic Acids Res. 2001, 29, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.Q.; Liu, K.W.; Li, Z.; Lohaus, R.; Hsiao, Y.Y.; Niu, S.C.; Wang, J.Y.; Lin, Y.C.; Xu, Q.; Chen, L.J.; et al. The Apostasia genome and the evolution of orchids. Nature 2017, 549, 379–383. [Google Scholar] [CrossRef]
- Pertea, M.; Kim, D.; Pertea, G.M.; Leek, J.T.; Salzberg, S.L. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016, 11, 1650–1667. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, W.; Chen, Z.; Han, B.; Haque, M.E.; Liu, A. Gene structure, expression pattern and interaction of Nuclear Factor-Y family in castor bean (Ricinus communis). Planta 2018, 247, 559–572. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]




| Gene Name | Gene ID | NCBI References | Clade | Gene Name | Gene ID | NCBI References | Clade |
|---|---|---|---|---|---|---|---|
| DobHLH1 | LOC110116721 | XP_020706080.1 | 1 | DobHLH50 | LOC110116300 | XP_020705489.1 | 10 |
| DobHLH2 | LOC110115756 | XP_020704772.1 | 1 | DobHLH51 | LOC114579352 | XP_028549432.1 | 10 |
| DobHLH3 | LOC110107102 | XP_020692919.1 | 1 | DobHLH52 | LOC110095032 | XP_020676060.1 | 11 |
| DobHLH4 | LOC110096422 | XP_020678032.1 | 1 | DobHLH53 | LOC110113329 | XP_020701532.1 | 11 |
| DobHLH5 | LOC110116474 | XP_020705700.1 | 1 | DobHLH54 | LOC110113204 | XP_020701328.1 | 11 |
| DobHLH6 | LOC110098508 | XP_020681021.1 | 1 | DobHLH55 | LOC110114625 | XP_020703217.1 | 12 |
| DobHLH7 | LOC110111619 | XP_020699225.1 | 1 | DobHLH56 | LOC110106166 | XP_020691604.1 | 12 |
| DobHLH8 | LOC110112576 | XP_020700508.1 | 1 | DobHLH57 | LOC110112285 | XP_020700113.1 | 12 |
| DobHLH9 | LOC110108487 | XP_020694816.2 | 1 | DobHLH58 | LOC110108563 | XP_020694908.1 | 12 |
| DobHLH10 | LOC110110298 | XP_020697356.1 | 2 | DobHLH59 | LOC110101829 | XP_020685553.1 | 13 |
| DobHLH11 | LOC110093619 | XP_020674221.1 | 2 | DobHLH60 | LOC110095526 | XP_020676768.1 | 13 |
| DobHLH12 | LOC110115484 | XP_020704390.1 | 2 | DobHLH61 | LOC110111433 | XP_028555769.1 | 13 |
| DobHLH13 | LOC110093741 | XP_020674407.1 | 3 | DobHLH62 | LOC110093428 | XP_020673967.1 | 13 |
| DobHLH14 | LOC110102219 | XP_020686105.1 | 3 | DobHLH63 | LOC110104725 | XP_020689612.1 | 14 |
| DobHLH15 | LOC110098909 | XP_020681520.1 | 3 | DobHLH64 | LOC110102342 | XP_028552167.1 | 14 |
| DobHLH16 | LOC110107046 | XP_028551992.1 | 3 | DobHLH65 | LOC110107722 | XP_020693735.1 | 14 |
| DobHLH17 | LOC110096269 | XP_020677790.1 | 3 | DobHLH66 | LOC110100649 | XP_020683910.1 | 14 |
| DobHLH18 | LOC110116147 | XP_020705280.1 | 4 | DobHLH67 | LOC110114258 | XP_028550306.1 | 14 |
| DobHLH19 | LOC110094813 | XP_028551573.1 | 4 | DobHLH68 | LOC110114447 | XP_028556766.1 | 14 |
| DobHLH20 | LOC110107826 | XP_020693884.2 | 4 | DobHLH69 | LOC110109277 | XP_028547820.1 | 14 |
| DobHLH21 | LOC110094726 | XP_020675682.1 | 4 | DobHLH70 | LOC110096494 | XP_020678137.1 | 14 |
| DobHLH22 | LOC110114654 | XP_020703261.1 | 5 | DobHLH71 | LOC110111863 | XP_028551171.1 | 14 |
| DobHLH23 | LOC110111891 | XP_020699606.1 | 5 | DobHLH72 | LOC110107433 | XP_028552737.1 | 14 |
| DobHLH24 | LOC110097687 | XP_020679864.1 | 5 | DobHLH73 | LOC110114740 | XP_020703378.2 | 14 |
| DobHLH25 | LOC110103241 | XP_020687528.1 | 6 | DobHLH74 | LOC110095482 | XP_020676694.1 | 14 |
| DobHLH26 | LOC110094094 | XP_020674906.1 | 6 | DobHLH75 | LOC110099461 | XP_020682274.1 | 14 |
| DobHLH27 | LOC110116682 | XP_020706025.1 | 6 | DobHLH76 | LOC110099214 | XP_028548138.1 | 14 |
| DobHLH28 | LOC110099101 | XP_020681804.1 | 6 | DobHLH77 | LOC110098199 | XP_020680601.1 | 15 |
| DobHLH29 | LOC110113808 | XP_020702166.2 | 6 | DobHLH78 | LOC110098270 | XP_020680696.1 | 15 |
| DobHLH30 | LOC110094435 | XP_020675326.1 | 6 | DobHLH79 | LOC110094287 | XP_020675140.1 | 15 |
| DobHLH31 | LOC110116479 | XP_020705705.1 | 6 | DobHLH80 | LOC110113891 | XP_020702260.1 | 15 |
| DobHLH32 | LOC110114462 | XP_020703009.1 | 6 | DobHLH81 | LOC110112441 | XP_020700324.1 | 15 |
| DobHLH33 | LOC110092865 | XP_020673218.1 | 6 | DobHLH82 | LOC110101026 | XP_020684454.1 | 15 |
| DobHLH34 | LOC110109085 | XP_020695652.1 | 7 | DobHLH83 | LOC110111081 | XP_020698439.1 | 15 |
| DobHLH35 | LOC110096203 | XP_020677673.1 | 7 | DobHLH84 | LOC110104845 | XP_020689772.1 | 15 |
| DobHLH36 | LOC110112097 | XP_020699850.1 | 7 | DobHLH85 | LOC110095998 | XP_020677402.1 | 15 |
| DobHLH37 | LOC110107930 | XP_020694038.1 | 8 | DobHLH86 | LOC110110399 | XP_020697513.2 | 15 |
| DobHLH38 | LOC110115493 | XP_020704404.1 | 8 | DobHLH87 | LOC110109507 | XP_028547425.1 | 15 |
| DobHLH39 | LOC110094861 | XP_020675853.1 | 8 | DobHLH88 | LOC114578594 | XP_028547764.1 | 15 |
| DobHLH40 | LOC110112072 | XP_020699818.1 | 8 | DobHLH89 | LOC110105526 | XP_020690722.2 | 15 |
| DobHLH41 | LOC110114469 | XP_028556744.1 | 9 | DobHLH90 | LOC110100961 | XP_020684346.1 | 15 |
| DobHLH42 | LOC110107963 | XP_020694091.1 | 9 | DobHLH91 | LOC110112336 | XP_020700191.1 | 16 |
| DobHLH43 | LOC110106259 | XP_028550041.1 | 9 | DobHLH92 | LOC110110400 | XP_020697514.1 | 17 |
| DobHLH44 | LOC110107318 | XP_028547365.1 | 10 | DobHLH93 | LOC110112399 | XP_028555091.1 | 17 |
| DobHLH45 | LOC110103817 | XP_028551326.1 | 10 | DobHLH94 | LOC110108630 | XP_020695011.1 | 17 |
| DobHLH46 | LOC110114754 | XP_020703396.1 | 10 | DobHLH95 | LOC110110529 | XP_020697710.1 | 17 |
| DobHLH47 | LOC110107832 | XP_020693899.1 | 10 | DobHLH96 | LOC110108826 | XP_020695307.1 | 17 |
| DobHLH48 | LOC110107031 | XP_020692824.1 | 10 | DobHLH97 | LOC110105593 | XP_020690812.1 | 17 |
| DobHLH49 | LOC110098511 | XP_020681024.1 | 10 | DobHLH98 | LOC110100228 | XP_020683310.1 | 18 |
| Predicted DNA-Binding Types Based on the bHLH Domain | Number of DobHLHs | The Ratio |
|---|---|---|
| DNA binding | ||
| E-box | 65 | 62.25% |
| G-box | 61 | 62.24% |
| Non-G-box | 4 | 4.08% |
| Non-E-box | 11 | 11.22% |
| Total | 76 | 77.55% |
| Non-DNA binding | 22 | 22.45% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Liu, A. Genomic Characterization and Expression Analysis of Basic Helix-Loop-Helix (bHLH) Family Genes in Traditional Chinese Herb Dendrobium officinale. Plants 2020, 9, 1044. https://doi.org/10.3390/plants9081044
Wang Y, Liu A. Genomic Characterization and Expression Analysis of Basic Helix-Loop-Helix (bHLH) Family Genes in Traditional Chinese Herb Dendrobium officinale. Plants. 2020; 9(8):1044. https://doi.org/10.3390/plants9081044
Chicago/Turabian StyleWang, Yue, and Aizhong Liu. 2020. "Genomic Characterization and Expression Analysis of Basic Helix-Loop-Helix (bHLH) Family Genes in Traditional Chinese Herb Dendrobium officinale" Plants 9, no. 8: 1044. https://doi.org/10.3390/plants9081044
APA StyleWang, Y., & Liu, A. (2020). Genomic Characterization and Expression Analysis of Basic Helix-Loop-Helix (bHLH) Family Genes in Traditional Chinese Herb Dendrobium officinale. Plants, 9(8), 1044. https://doi.org/10.3390/plants9081044





