Impact of a Soil Conditioner Integrated into Fertilization Scheme on Orange and Lemon Seedling Physiological Performances
Abstract
1. Introduction
2. Results
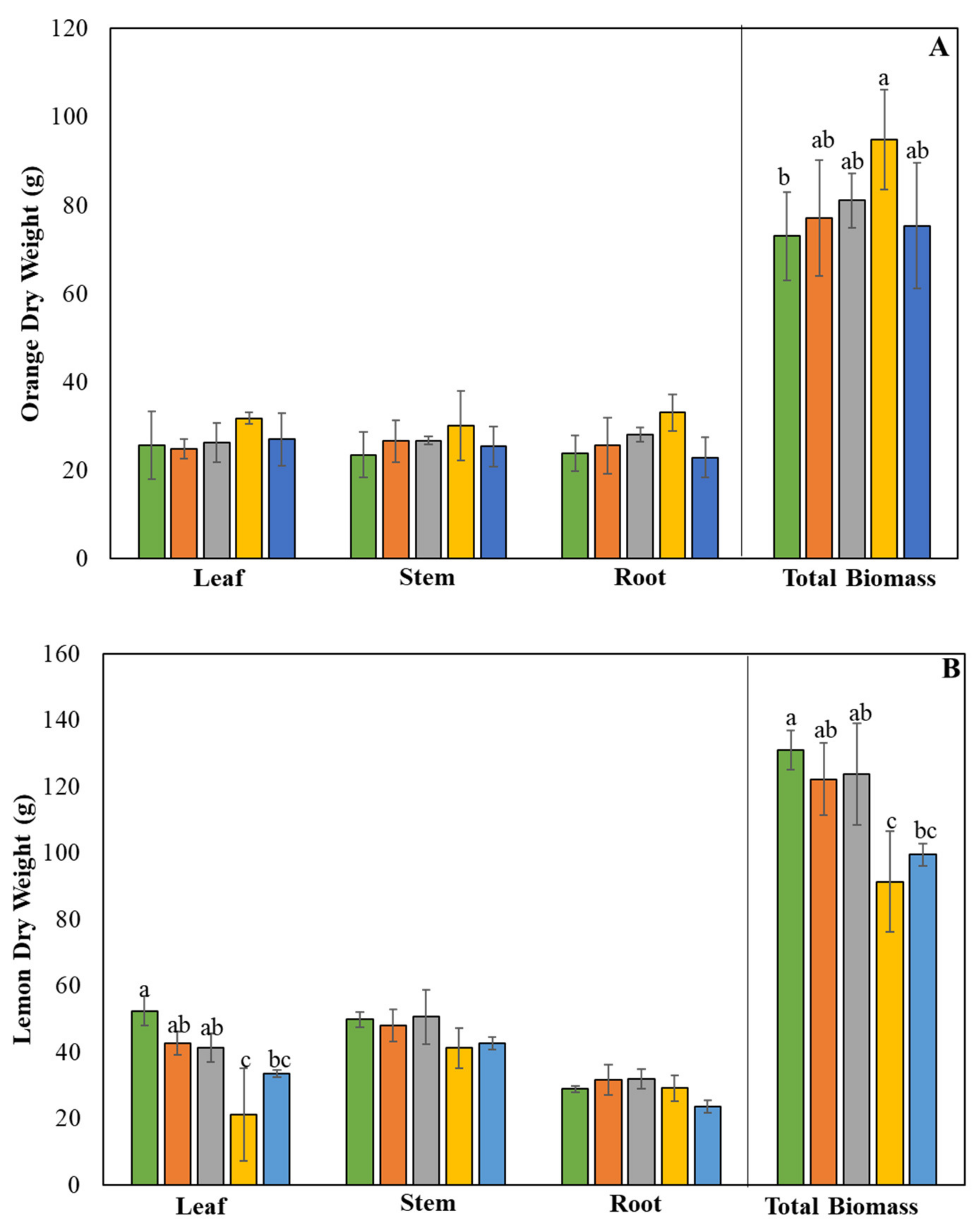
2.1. Plant Biomass (Dry Weight)
2.2. Plant Growth and Development (Height and Stem Diameter)
2.3. Chlorophyll Contents
2.4. Stomatal Conductance
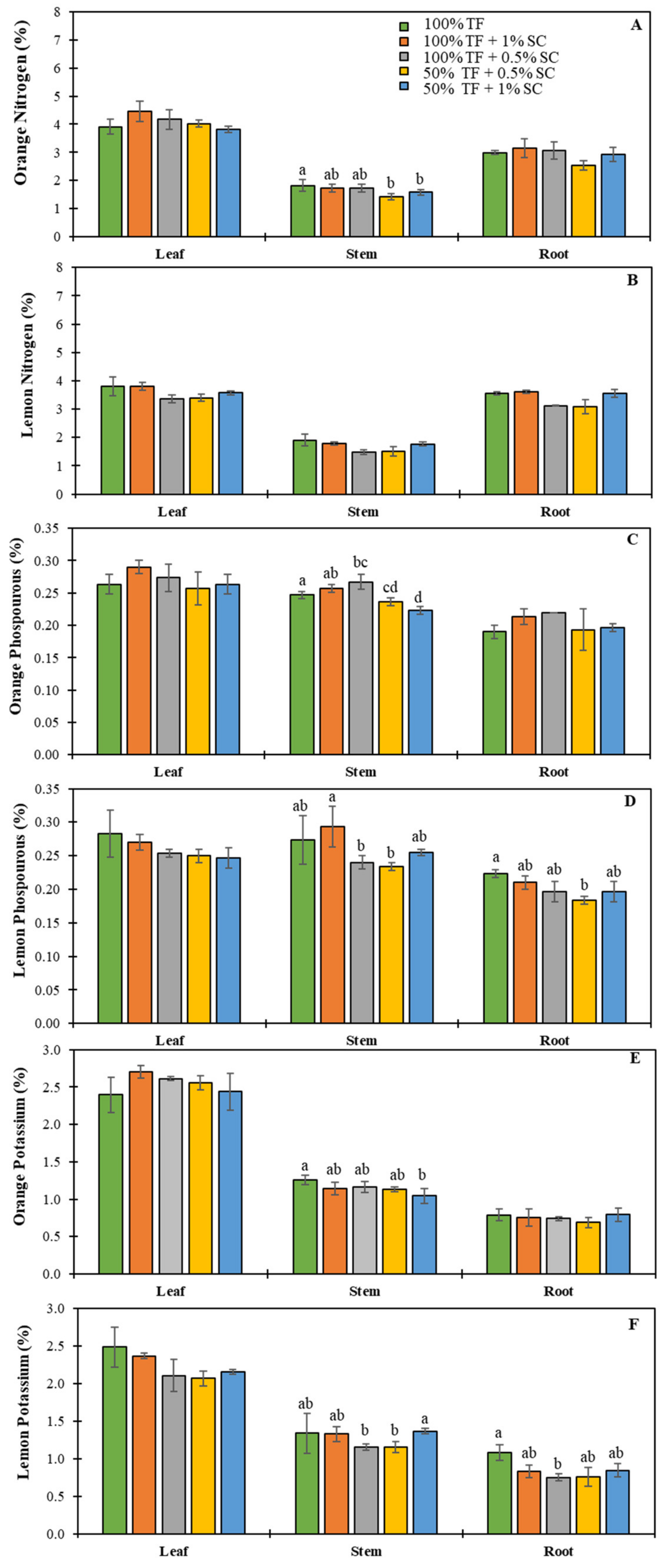
2.5. Nutrient Concentrations
3. Discussion
4. Materials and Methods
4.1. Plant Material and Treatments
4.2. Treatments and Cultivation
4.3. Vegetative Measurements
4.4. Mineral Analysis
4.5. Chlorophyll Contents
4.6. Experimental Design and Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO. Citrus Fruit, Fresh and Processed. Available online: http://www.fao.org/economic/est/est-commodities/citrus-fruit/en/ (accessed on 28 June 2020).
- Ackerman, E.A. Influences of climate on the cultivation of citrus fruits. Geogr. Rev. 1938, 28, 289–302. [Google Scholar] [CrossRef]
- USDA. Available online: https://www.nass.usda.gov/Statistics_by_State/Florida/Publications/Citrus/Citrus_Forecast/2017-18/cit0518.pdf (accessed on 28 June 2020).
- White, P.J.; Brown, P.H. Plant nutrition for sustainable development and global health. Ann. Bot. 2010, 105, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Talon, M.; Caruso, M.; Gmitter, F.G., Jr. The Genus Citrus; Woodhead Publishing: Cambridge, UK, 2020. [Google Scholar]
- Kadyampakeni, D.M.; Morgan, K.T.; Nkedi-Kizza, P.; Kasozi, G.N. Nutrient Management Options for Florida Citrus: A Review of NPK Application and Analytical Methods. J. Plant Nutr. 2015, 38, 568–583. [Google Scholar] [CrossRef]
- Quaggio, J.A.; Souza, T.R.; Zambrosi, F.C.B.; Boaretto, R.M.; Mattos, D., Jr. Nitrogen-fertilizer forms affect the nitrogen-use efficiency in fertigated citrus groves. J. Plant Nutr. Soil Sci. 2014, 177, 404–411. [Google Scholar] [CrossRef]
- Quaggio, J.A.; Cantarella, H.; van Raij, B. Phosphorus and potassium soil test and nitrogen leaf analysis as a base for citrus fertilization. Nutr. Cycl. Agroecosyst. 1998, 52, 67–74. [Google Scholar] [CrossRef]
- Lea-Cox, J.D.; Syvertsen, J.P.; Graetz, D.A. Springtime 15Nitrogen Uptake, Partitioning, and Leaching Losses from Young Bearing Citrus Trees of Differing Nitrogen Status. J. Amer. Soc. Hort. Sci. 2001, 126, 242. [Google Scholar] [CrossRef]
- Schachtman, D.P.; Reid, R.J.; Ayling, S.M. Phosphorus Uptake by Plants: From Soil to Cell. Plant Physiol. 1998, 116, 447–453. [Google Scholar] [CrossRef]
- Harris, W.G.; Chrysostome, M.; Obreza, T.A.; Nair, V.D. Soil Properties Pertinent to Horticulture in Florida. HortTechnology 2010, 20, 10. [Google Scholar] [CrossRef]
- Alva, A. Micronutrients status of Florida soils under citrus production. Commun. Soil Sci. Plant Anal. 1992, 23, 2493–2510. [Google Scholar] [CrossRef]
- Hendricks, G.S.; Shukla, S. Water and nitrogen management effects on water and nitrogen fluxes in Florida Flatwoods. J. Environ. Qual. 2011, 40, 1844–1856. [Google Scholar] [CrossRef]
- Boman, B.J. Evapotranspiration by young Florida flatwoods citrus trees. J. Irrig. Drain. Eng. 1994, 120, 80–88. [Google Scholar] [CrossRef]
- Davies, F.S.; Jackson, L.K. Citrus Growing in Florida, 5th ed.; University Press of Florida: Gainesville, FL, USA, 2009; 310p. [Google Scholar]
- Bauer, M.G.; Castle, W.S.; Boman, B.J.; Obreza, T.A.; Stover, E.W. Root systems of healthy and declining citrus trees on Swingle citrumelo rootstock growing in the southern Florida flatwoods. Proc. Florida State Hortic. Soc. 2004, 117, 103–109. [Google Scholar]
- Obreza, T.; Admire, K. Shallow Water Table Fluctuations in Response to Rainfall, Irrigation and Evapotranspiration in Flatwoods Citrus. Proc. Florida State Hortic. Soc. 1985, 98, 32–37. [Google Scholar]
- Mann, K.K.; Schumann, A.W.; Obreza, T.A.; Harris, W.G.; Shukla, S. Spatial variability of soil physical properties affecting Florida citrus production. Soil Sci. 2010, 175, 487–499. [Google Scholar] [CrossRef]
- Johnson, E.G.; Wu, J.; Bright, D.B.; Graham, J.H. Association of ‘Candidatus Liberibacter asiaticus’ root infection, but not phloem plugging with root loss on huanglongbing-affected trees prior to appearance of foliar symptoms. Plant Pathol. 2014, 63, 290–298. [Google Scholar] [CrossRef]
- Roberts, P.D.; Rouse, R.E.; Teems, S.S.; System, R.E.; Shobert, Z. The effect of nutritional spray programs applied to mitigate symptoms of Huanglongbing on fruit drop caused by HLB and citrus canker on ‘Hamlin’ orange trees. J. Citrus Pathl. 2014, 1, 204. [Google Scholar]
- Ramirez Acosta, D.F. Mitigation of Huanglongbing Effects on Grapefruit Trees Using Enhanced Nutritional Programs. Master’s Thesis, University of Florida, Gainesville, FL, USA, 2016. [Google Scholar]
- Xu, M.; Liang, M.; Chen, J.; Xia, Y.; Zheng, Z.; Zhu, Q.; Deng, X. Preliminary research on soil conditioner mediated citrus Huanglongbing mitigation in the field in Guangdong, China. Eur. J. Plant Pathol. 2013, 137, 283–293. [Google Scholar] [CrossRef]
- Rhizolizer®. Locus Agricultural Solutions. Available online: https://locusag.com/rhizolizer/ (accessed on 28 June 2020).
- Tiger-Sul. Global Agriculture Firm Tiger-Sul Products Launches New Citrus Greening Product. Available online: https://www.tigersul.com/global-agriculture-firm-tigersul-launches-new-citrus-greening-product/ (accessed on 28 June 2020).
- oGrowing, LCC; Farming. Available online: https://www.apollo.io/companies/oGrowing--LLC/5e57aba6f6d38f0001ebc78d?chart=count.oGrowing (accessed on 28 June 2020).
- Zekri, M.; Obreza, T.A.; Koo, R. Irrigation, Nutrition, and Citrus Fruit Quality; SL-207; Institute of Food and Agricultural Sciences (IFAS), University of Florida: Gainesville, FL, USA, 2009. [Google Scholar]
- Alvarez, S.; Rohrig, E.; Solís, D.; Thomas, M.H. Citrus Greening Disease (Huanglongbing) in Florida: Economic Impact, Management and the Potential for Biological Control. Agric. Res. 2016, 5, 109–118. [Google Scholar] [CrossRef]
- Tréguer, P.; Nelson, D.M.; Van Bennekom, A.J.; DeMaster, D.J.; Leynaert, A.; Quéguiner, B. The Silica Balance in the World Ocean: A Reestimate. Science 1995, 268, 375–379. [Google Scholar] [CrossRef]
- Epstein, E. Silicon: Its manifold roles in plants. Ann. Appl. Biol. 2009, 155, 155–160. [Google Scholar] [CrossRef]
- Epstein, E. SILICON. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 641–664. [Google Scholar] [CrossRef]
- Kostopoulou, Z.; Therios, I. Growth and inorganic composition of ‘Nova’ mandarin plants grafted on two commercial rootstocks in response to salinity and silicon. Acta Physiol. Plant. 2014, 36, 1363–1372. [Google Scholar] [CrossRef]
- Hamed, N.; Abdel-Aziz, R.A.; H. Abou-Baker, N. Effect of Some Applications on the Performance of Mandarin Trees under Soil Salinity Conditions. Egypt. J. Hortic. 2017, 44, 141–153. [Google Scholar] [CrossRef]
- Sun, D.; Hussain, H.I.; Yi, Z.; Siegele, R.; Cresswell, T.; Kong, L.; Cahill, D.M. Uptake and cellular distribution, in four plant species, of fluorescently labeled mesoporous silica nanoparticles. Plant Cell Rep. 2014, 33, 1389–1402. [Google Scholar] [CrossRef] [PubMed]
- Boaretto, R.; Mattos, D., Jr.; Quaggio, J.; Cantarella, H.; Trivelin, P. Nitrogen-15 uptake and distribution in two citrus species. In Proceedings of the 2010 19th World Congress of Soil Science, Soil Solutions for a Changing World, Brisbane, Australia, 1–6 August 2010. [Google Scholar]
- Graham, J.H.; Duncan, L.W.; Eissenstat, D.M. Carbohydrate allocation patterns in citrus genotypes as affected by phosphorus nutrition, mycorrhizal colonization and mycorrhizal dependency. New Phytol. 1997, 135, 335–343. [Google Scholar] [CrossRef][Green Version]
- Syvertsen, J.P.; Graham, J.H. Phosphorus supply and arbuscular mycorrhizas increase growth and net gas exchange responses of two Citrus spp. grown at elevated [CO2]. Plant Soil 1999, 208, 209. [Google Scholar] [CrossRef]
- Yang, L.-T.; Jiang, H.-X.; Qi, Y.-P.; Chen, L.-S. Differential expression of genes involved in alternative glycolytic pathways, phosphorus scavenging and recycling in response to aluminum and phosphorus interactions in Citrus roots. Mol. Biol. Rep. 2012, 39, 6353–6366. [Google Scholar] [CrossRef] [PubMed]
- Obreza, T.A.; Rouse, R.E.; Morgan, K.T. Managing Phosphorus for Citrus Yield and Fruit Quality in Developing Orchards. HortScience 2008, 43, 2162–2166. [Google Scholar] [CrossRef]
- Yu, S.; He, Z.L.; Stoffella, P.J.; Calvert, D.V.; Yang, X.E.; Banks, D.J.; Baligar, V.C. Surface runoff phosphorus (P) loss in relation to phosphatase activity and soil P fractions in Florida sandy soils under citrus production. Soil Biol. Biochem. 2006, 38, 619–628. [Google Scholar] [CrossRef]
- Zekri, M.; Obreza, T.A. Phosphorus (P) for Citrus Trees; SL379 EDIS UF/IFAS Extension; Institute of Food and Agricultural Sciences (IFAS), University of Florida: Gainesville, FL, USA, 2019. [Google Scholar]
- Antunes, V.; Cardoso, E.J.B.N. Growth and nutrient status of citrus plants as influenced by mycorrhiza and phosphorus application. Plant Soil 1991, 131, 11–19. [Google Scholar] [CrossRef]
- Bondada, B.R.; Syvertsen, J.P. Leaf chlorophyll, net gas exchange and chloroplast ultrastructure in citrus leaves of different nitrogen status. Tree Physiol. 2003, 23, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Bleda, F.J.; Madrid, R.; García-Torres, A.L.; García-Lidón, Á.; Porras, I. chlorophyll fluorescence and mineral nutrition in citrus leaves under salinity stress. J. Plant Nutr. 2011, 34, 1579–1592. [Google Scholar] [CrossRef]
- Melgar, J.C.; Syvertsen, J.P.; Martínez, V.; García-Sánchez, F. Leaf gas exchange, water relations, nutrient content and growth in citrus and olive seedlings under salinity. Biol. Plant. 2008, 52, 385–390. [Google Scholar] [CrossRef]
- Nemec, S.; Vu, J.C.V. Effects of soil phosphorus and Glomus intraradices on growth, nonstructural carbohydrates, and photosynthetic activity of Citrus aurantium. Plant Soil 1990, 128, 257–263. [Google Scholar] [CrossRef]
- Bloomfield, K.J.; Farquhar, G.D.; Lloyd, J. Photosynthesis–nitrogen relationships in tropical forest tree species as affected by soil phosphorus availability: A controlled environment study. Funct. Plant Biol. 2014, 41, 820–832. [Google Scholar] [CrossRef] [PubMed]
- Obreza, T.A.; Rouse, R.E.; Sherrod, J.B. Economics of controlled-release fertilizer use on young citrus trees. J. Prod. Agric. 1999, 12, 69–73. [Google Scholar] [CrossRef]
- Zekri, M.; Obreza, T. Potassium (K) for Citrus Trees; #SL381 EDIS; Institute of Food and Agricultural Sciences (IFAS), University of Florida: Gainesville, FL, USA, 2013. [Google Scholar]
- Anderson, D.; Henderson, L. Comparing sealed chamber digestion with other digestion methods used for plant-tissue analysis. Agron. J. 1988, 80, 549–552. [Google Scholar] [CrossRef]
- Munter, R.; Halverson, T.; Anderson, R. Quality assurance for plant tissue analysis by ICP-AES. Commun. Soil Sci. Plant Anal. 1984, 15, 1285–1322. [Google Scholar] [CrossRef]
- Inskeep, W.P.; Bloom, P.R. Extinction Coefficients of Chlorophyll a and b in N,n-Dimethylformamide and 80% Acetone. Plant Physiol. 1985, 77, 483–485. [Google Scholar] [CrossRef] [PubMed]
- Moran, R. Formulae for Determination of Chlorophyllous Pigments Extracted with N,n-Dimethylformamide. Plant Physiol. 1982, 69, 1376–1381. [Google Scholar] [CrossRef]


| Products | Traditional Fertilizer (TF; Osmocote® Plus) | Soil Conditioner (SC; oGrowing™) |
|---|---|---|
| Total Nitrogen (N) % Ammoniacal Nitrogen % Nitrate Nitrogen | 15.00 8.40 6.60 | 0.19 0.07 0.12 |
| Available Phosphorus P2O5 | 9.00 | n/a |
| Soluble Potassium K2O | 12.00 | 5.21 |
| Element | Content (mg/Kg of Dry Soil) |
|---|---|
| Nitrogen (N) | 3.35 |
| Phosphorus (P) | 13.5 |
| Potassium (K) | 30.33 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossi, L.; Hallman, L.M.; Adams, S.N.; Ac-Pangan, W.O. Impact of a Soil Conditioner Integrated into Fertilization Scheme on Orange and Lemon Seedling Physiological Performances. Plants 2020, 9, 812. https://doi.org/10.3390/plants9070812
Rossi L, Hallman LM, Adams SN, Ac-Pangan WO. Impact of a Soil Conditioner Integrated into Fertilization Scheme on Orange and Lemon Seedling Physiological Performances. Plants. 2020; 9(7):812. https://doi.org/10.3390/plants9070812
Chicago/Turabian StyleRossi, Lorenzo, Lukas M. Hallman, Sawyer N. Adams, and Walter O. Ac-Pangan. 2020. "Impact of a Soil Conditioner Integrated into Fertilization Scheme on Orange and Lemon Seedling Physiological Performances" Plants 9, no. 7: 812. https://doi.org/10.3390/plants9070812
APA StyleRossi, L., Hallman, L. M., Adams, S. N., & Ac-Pangan, W. O. (2020). Impact of a Soil Conditioner Integrated into Fertilization Scheme on Orange and Lemon Seedling Physiological Performances. Plants, 9(7), 812. https://doi.org/10.3390/plants9070812






