Micropropagation and Production of Health Promoting Lignans in Linum usitatissimum
Abstract
:1. Introduction
2. Results and Discussion
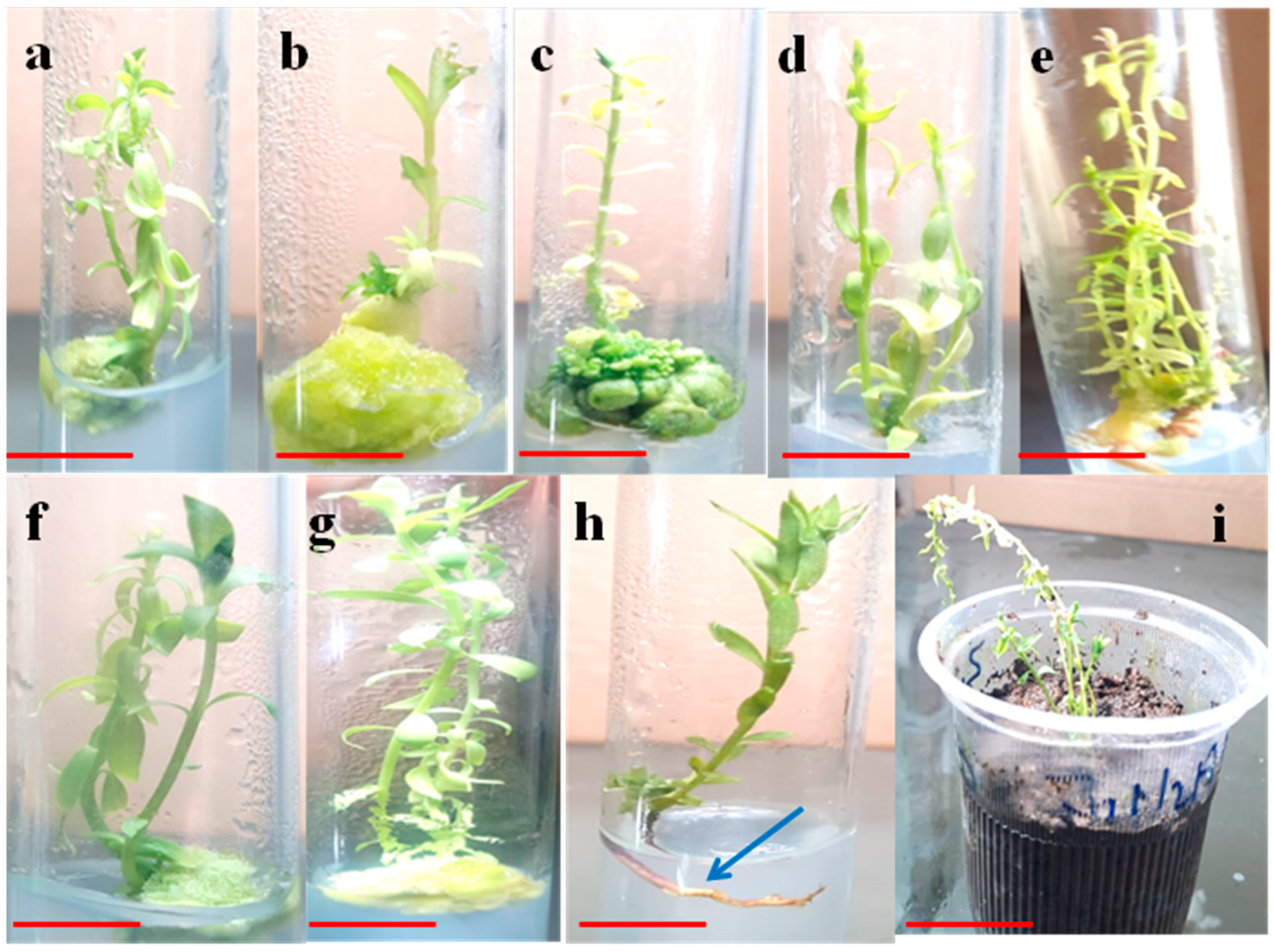
2.1. Optimization of the Suitable Explant and Plant Growth Regulators (PGRs) for In Vitro Shoot Induction
2.2. TDZ Mediated Micropropagation and Growth Parameters
2.3. Roots Formation and Acclimatization
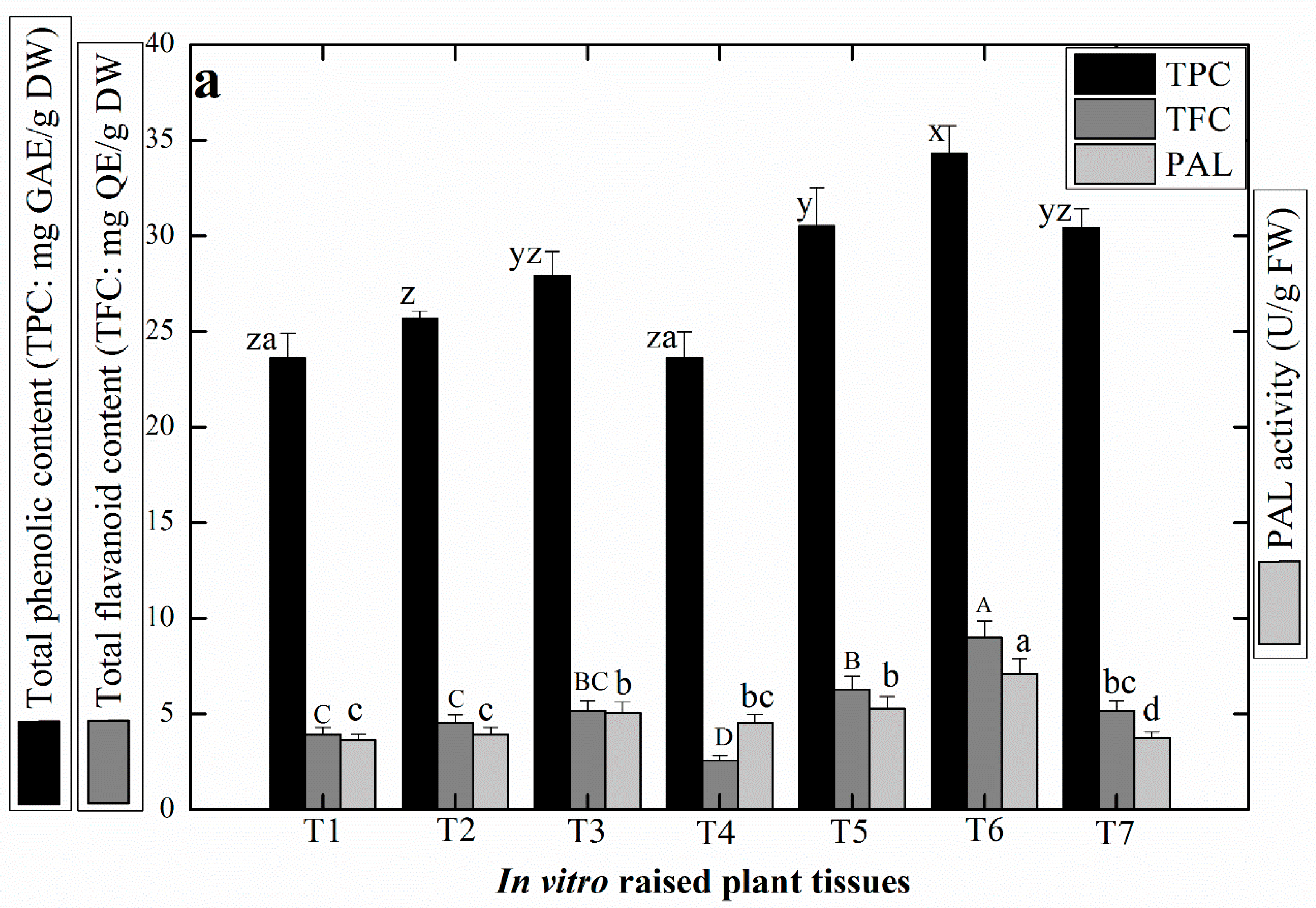
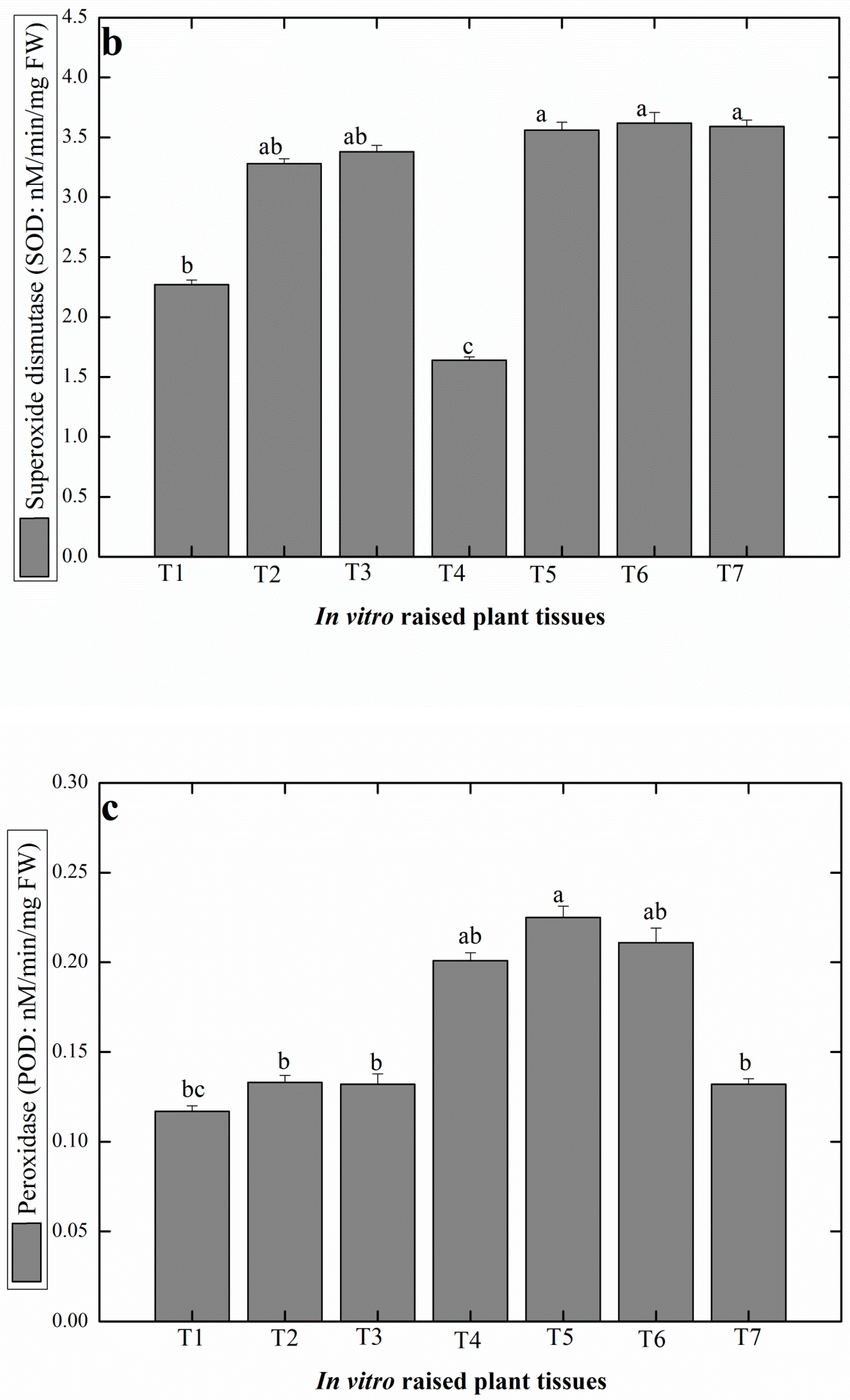
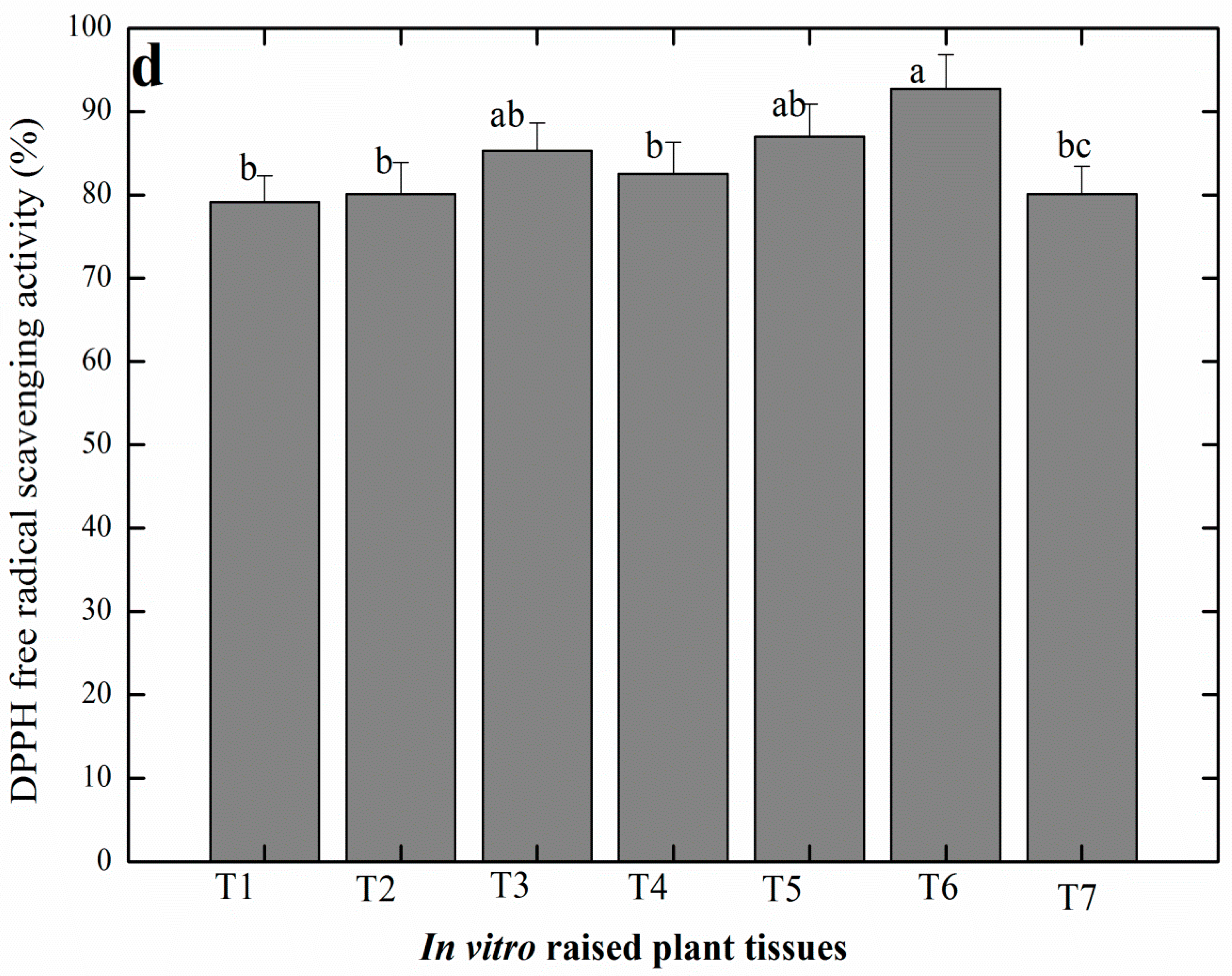
2.4. Production of Antioxidant Secondary Metabolites in Linseed
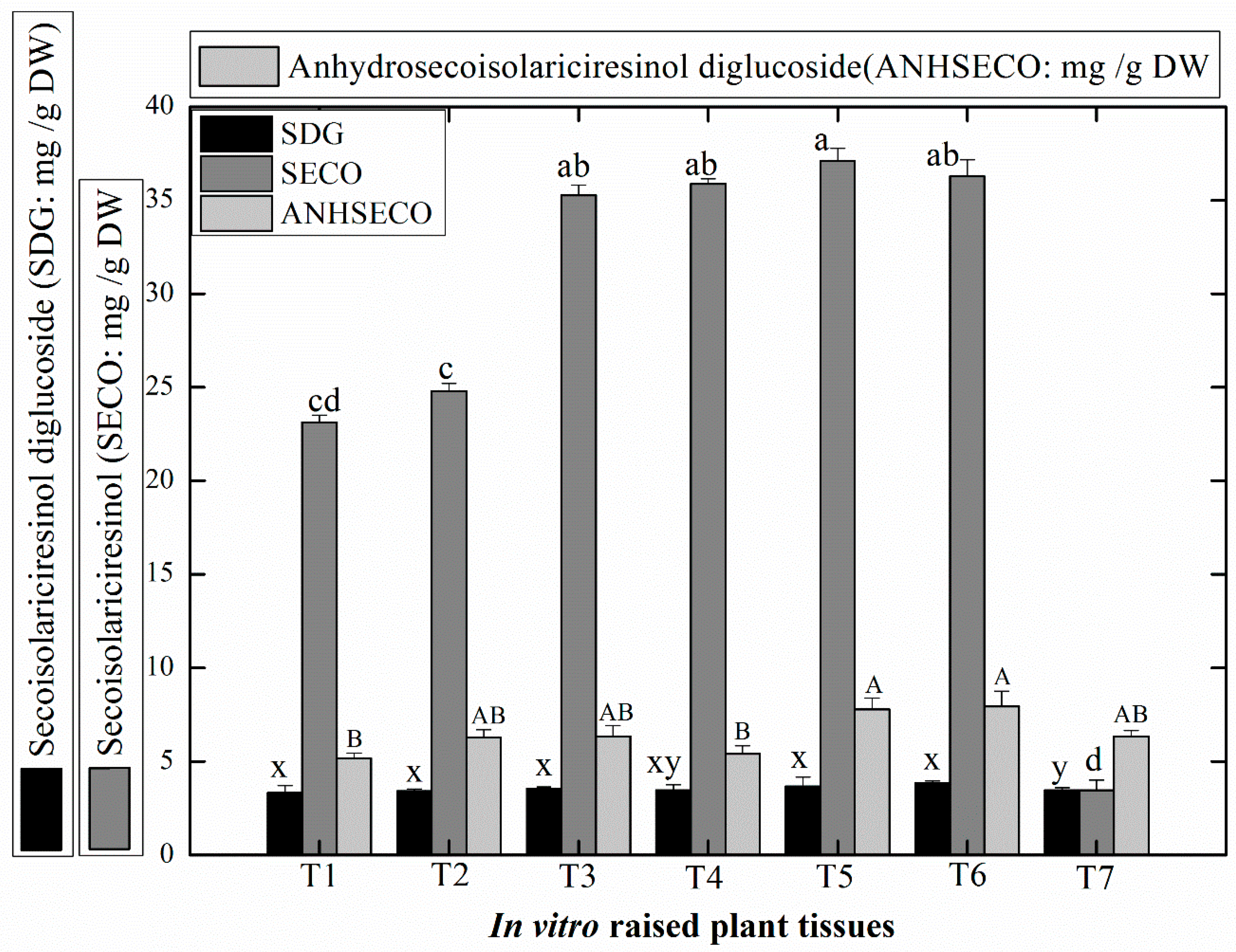
2.5. Production of Medicinally Potent Lignans in Linseed
3. Materials and Methods
3.1. Plant Material, Sterilization and In Vitro Germination
3.2. Optimization of In Vitro Conditions for Micropropagation and Plantlet Development
3.3. Phytochemical Analysis
3.3.1. Total Phenolic Content
3.3.2. Total Flavonoid Content
3.3.3. Analysis of Antioxidant Enzyme Activities
3.3.4. Phenylalanine Ammonia-Lyase (PAL) Activity
3.3.5. Peroxidase (POD) Activity
3.3.6. Determination of Free Radicle Scavenging Activity
3.4. Analysis of Lignans in the Micro Propagated Shoots of L. Usitatissimum
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barozai, M.Y.K. Insilico identification of microRNAs and their targets in fiber and oil producing plant Flax (Linum usitatissimum L.). Pak. J. Bot. 2012, 44, 1357–1362. [Google Scholar]
- Jhala, A.J.; Hall, L.M. Flax (Linum usitatissimum L.): Current uses and future applications. Aust. J. Basic Appl. Sci. 2010, 4, 4304–4312. [Google Scholar]
- Akhtar, K.; Dickinson, M.; Shah, T.; Sarwar, N. Natural occurrence, identification and transmission of the phytoplasma associated with flax phyllody and stem fasciation in Pakistan. Phytoparasitica 2013, 41, 383–389. [Google Scholar] [CrossRef]
- Halligudi, N. Pharmacological properties of flax seeds: A Review. Hygeia J. Drugs Med. 2012, 4, 70–77. [Google Scholar]
- Commission, C.G. Nutritional profile of No. 1 Canada Western flaxseed and of yellow flaxseed samples. Can. Grain Commun. Winn. Manit. 2001, 3, 63–67. [Google Scholar]
- Clarence-Smith, W.G. Cocoa and Chocolate, 1765–1914. Routledge: Abingdon-on-Thames, England, UK, 2003. [Google Scholar]
- Das, U.N. Essential fatty acids—A review. Curr. Pharm. Biotechnol. 2006, 7, 467–482. [Google Scholar] [CrossRef]
- Katare, C.; Saxena, S.; Agrawal, S.; Prasad, G.; Bisen, P. Flax seed: A potential medicinal food. J. Nutr. Food Sci. 2012, 2, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Kasote, D. Flaxseed phenolics as natural antioxidants. Int. Food Res. J. 2013, 20, 27. [Google Scholar]
- Perryman, S.; Gladders, P.; Barrow, A.; Simons, B.; Fitt, B.D. Diseases of Winter Linseed: Occurrence, Effects and Importance. Available online: uhra.herts.ac.uk (accessed on 13 January 2020).
- Hano, C.; Addi, M.; Bensaddek, L.; Crônier, D.; Baltora-Rosset, S.; Doussot, J.; Maury, S.; Mesnard, F.; Chabbert, B.; Hawkins, S. Differential accumulation of monolignol-derived compounds in elicited flax (Linum usitatissimum) cell suspension cultures. Planta 2006, 223, 975–989. [Google Scholar] [CrossRef]
- Szewczyk, M.; Abarzua, S.; Schlichting, A.; Nebe, B.; Piechulla, B.; Briese, V.; Richter, D.U. Effects of extracts from Linum usitatissimum on cell vitality, proliferation and cytotoxicity in human breast cancer cell lines. J. Med. Plants Res. 2014, 8, 237–245. [Google Scholar]
- Sen, C.K.; Khanna, S.; Roy, S. Tocotrienols: Vitamin E beyond tocopherols. Life Sci. 2006, 78, 2088–2098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, M.C.; Evans, D.A.; Tangney, C.C.; Bienias, J.L.; Wilson, R.S.; Aggarwal, N.T.; Scherr, P.A. Relation of the tocopherol forms to incident Alzheimer disease and to cognitive change. Am. J. Clin. Nutr. 2005, 81, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Gutte, K.B.; Sahoo, A.; Ranveer, R.C. Bioactive components of flaxseed and its health benefits. Int. J. Pharma. Sci. Rev. Res 2015, 31, 42–51. [Google Scholar]
- Yildiz, M. The prerequisite of the success in plant tissue culture: High frequency shoot regeneration. In Recent Advances in Plant in vitro Culture; IntechOpen: London, UK, 2012. [Google Scholar]
- Ling, A.P.K.; Tan, K.P.; Hussein, S. Comparative effects of plant growth regulators on leaf and stem explants of Labisia pumila var. alata. J. Zhejiang Univ. Sci. B 2013, 14, 621–631. [Google Scholar] [CrossRef] [Green Version]
- Wannakrairoj, S.; Tefera, W. Thidiazuron and other plant Bioregulators for Axenic culture of siam Cardamom (Amomum krervanh Pierre ex Gangnep). Kasetsart J. 2012, 46, 335–345. [Google Scholar]
- Matand, K.; Prakash, C. Evaluation of peanut genotypes for in vitro plant regeneration using thidiazuron. J. Biotechnol. 2007, 130, 202–207. [Google Scholar] [CrossRef]
- Khan, M.A.; Abbasi, B.H.; Shinwari, Z.K. Thidiazuron enhanced regeneration and silymarin content in Silybum marianum L. Pak. J. Bot. 2014, 46, 185–190. [Google Scholar]
- Khan, T.; Abbasi, B.H.; Khan, M.A.; Shinwari, Z.K. Differential effects of thidiazuron on production of anticancer phenolic compounds in callus cultures of Fagonia indica. Appl. Biochem. Biotechnol. 2016, 179, 46–58. [Google Scholar] [CrossRef]
- Torres, G.R.C.; Houllou, L.M. Control of contaminants during introduction and establishment of Bambusa vulgaris in vitro. Res. Biotechnol. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Luciani, G.F.; Mary, A.K.; Pellegrini, C.; Curvetto, N. Effects of explants and growth regulators in garlic callus formation and plant regeneration. Plant Cell Tissue Organ Cult. 2006, 87, 139–143. [Google Scholar] [CrossRef]
- Takashina, T.; Suzuki, T.; Egashira, H.; Imanishi, S. New molecular markers linked with the high shoot regeneration capacity of the wild tomato species Lycopersicon chilense. Jpn. J. Breed. 1998, 48, 109–113. [Google Scholar] [CrossRef] [Green Version]
- Janowicz, J.; Niemann, J.; Wojciechowski, A. The effect of growth regulators on the regeneration ability of flax (Linum usitatissimum L.) hypocotyl explants in in vitro culture. Biotechnol. J. Biotechnol. Comput. Biol. Bionanotechnol. 2012, 93, 135–138. [Google Scholar] [CrossRef] [Green Version]
- Burbulis, N.; Blinstrubiene, A.; Kupriene, R. Regeneration of adventitious shoots of linseed (Linum usitatissimum L.) from hypocotyls explants. Zemdirbyste-Agricult 2009, 96, 168–175. [Google Scholar]
- Naggar, A.; Kadi, D.; Mcrughen, A. In vitro direct regeneration of egyptian and canadian flax (Linum usitatissimum L.). PJB 2009, 2, 412–415. [Google Scholar]
- Akter, F.; Parvez, M.; Islam, M.; Mondol, P.; Alam, M. Callus culture and plant regeneration in linseed (Linum usitatissimum L.). Plant Environ. Dev. 2008, 2, 101. [Google Scholar]
- Hutchinson, M.J.; Saxena, P.K. Acetylsalicylic acid enhances and synchronizes thidiazuron-induced somatic embryogenesis in geranium (Pelargonium x hortorum Bailey) tissue cultures. Plant Cell Rep. 1996, 15, 512–515. [Google Scholar] [CrossRef]
- Victor, J.; Murthy, B.; Murch, S.; KrishnaRaj, S.; Saxena, P. Role of endogenous purine metabolism in thidiazuron-induced somatic embryogenesis of peanut (Arachis hypogaea L.). Plant Growth Regul. 1999, 28, 41–47. [Google Scholar] [CrossRef]
- Nikolić, R.; Mitić, N.; Miletić, R.; Nešković, M. Effects of cytokinins on in vitro seed germination and early seedling morphogenesis in Lotus corniculatus L. J. Plant Growth Regul. 2006, 25, 187. [Google Scholar] [CrossRef]
- Ali, H.; Khan, M.A.; Kayani, W.K.; Khan, T.; Khan, R.S. Thidiazuron regulated growth, secondary metabolism and essential oil profiles in shoot cultures of Ajuga bracteosa. Ind. Crop. Prod. 2018, 121, 418–427. [Google Scholar] [CrossRef]
- Thomas, T.D.; Puthur, J.T. Thidiazuron induced high frequency shoot organogenesis in callus from Kigelia pinnata L. Bot. Bull. Acad. Sin. 2004, 45, 307–313. [Google Scholar]
- Gairi, A.; Rashid, A. Direct differentiation of somatic embryos on different regions of intact seedlings of Azadirachta in response to thidiazuron. J. Plant Physiol. 2004, 161, 1073–1077. [Google Scholar] [CrossRef] [PubMed]
- Burbulis, N.; Blinstrubiene, A.; Kupriene, R.; Sliesaravicius, A.; Venskutoniene, E. Optimization of linseed flax (Linum usitatissimum L.) in vitro cultures. Zemdirbyste-Agricult 2007, 94, 120–128. [Google Scholar]
- Chen, J.; Wang, L.; Thompson, L.U. Flaxseed and its components reduce metastasis after surgical excision of solid human breast tumor in nude mice. Cancer Lett. 2006, 234, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Lincy, A.; Sasikumar, B. Enhanced adventitious shoot regeneration from aerial stem explants of ginger using TDZ and its histological studies. Turk. J. Bot. 2010, 34, 21–29. [Google Scholar]
- Yucesan, B.; Turker, A.U.; Gurel, E. TDZ-induced high frequency plant regeneration through multiple shoot formation in witloof chicory (Cichorium intybus L.). Plant Cell Tissue Organ Cult. 2007, 91, 243–250. [Google Scholar] [CrossRef]
- Malik, K.A.; Saxena, P.K. Regeneration in Phaseolus vulgaris L.: High-frequency induction of direct shoot formation in intact seedlings by N 6-benzylaminopurine and thidiazuron. Planta 1992, 186, 384–389. [Google Scholar] [CrossRef]
- Thomas, J.C.; Katterman, F.R. Cytokinin activity induced by thidiazuron. Plant Physiol. 1986, 81, 681–683. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.R.; Anis, M. Role of TDZ in the quick regeneration of multiple shoots from nodal explant of Vitex trifolia L.—An important medicinal plant. Appl. Biochem. Biotechnol. 2012, 168, 957–966. [Google Scholar] [CrossRef]
- Murthy, B.; Murch, S.; Saxena, P.K. Thidiazuron: A potent regulator ofin vitro plant morphogenesis. Vitr. Cell. Dev. Biol. Plant 1998, 34, 267. [Google Scholar] [CrossRef]
- Murthy, B.; Victor, J.; Singh, R.P.; Fletcher, R.; Saxena, P.K. In vitro regeneration of chickpea (Cicer arietinum L.): Stimulation of direct organogenesis and somatic embryogenesis by thidiazuron. Plant Growth Regul. 1996, 19, 233–240. [Google Scholar] [CrossRef]
- Guo, B.; Abbasi, B.H.; Zeb, A.; Xu, L.; Wei, Y. Thidiazuron: A multi-dimensional plant growth regulator. Afr. J. Biotechnol. 2011, 10, 8984–9000. [Google Scholar]
- Aytekin Polat, A.; Caliskan, O. Effect of indolebutyric acid (IBA) on rooting of cutting in various pomegranate genotypes. In Proceedings of the I International Symposium on Pomegranate and Minor Mediterranean Fruits 818, Adana, Turkey, 3–6 May 2016; pp. 187–192. [Google Scholar]
- Ling, A.P.K.; Kok, K.M.; Hussein, S.; Ong, S.L. Effects of plant growth regulators on adventitious roots induction from different explants of Orthosiphon stamineus. Am. Eurasian J. Sustain. Agric. 2009, 3, 493–501. [Google Scholar]
- Sun, W.Q.; Bassuk, N.L. Auxin-induced Ethylene Synthesis during Rooting and Inhibition of Budbreak ofRoyalty’Rose Cuttings. J. Am. Soc. Hortic. Sci. 1993, 118, 638–643. [Google Scholar] [CrossRef] [Green Version]
- Arena, C.; Tsonev, T.; Doneva, D.; De Micco, V.; Michelozzi, M.; Brunetti, C.; Centritto, M.; Fineschi, S.; Velikova, V.; Loreto, F. The effect of light quality on growth, photosynthesis, leaf anatomy and volatile isoprenoids of a monoterpene-emitting herbaceous species (Solanum lycopersicum L.) and an isoprene-emitting tree (Platanus orientalis L.). Environ. Exp. Bot. 2016, 130, 122–132. [Google Scholar] [CrossRef]
- Khan, M.A.; Abbasi, B.H.; Ahmed, N.; Ali, H. Effects of light regimes on in vitro seed germination and silymarin content in Silybum marianum. Ind. Crop. Prod. 2013, 46, 105–110. [Google Scholar] [CrossRef]
- Bakar, M.; Karim, F.A.; Perisamy, E. Comparison of phytochemicals and antioxidant properties of different fruit parts of selected Artocarpus species from Sabah, Malaysia. Sains Malays. 2015, 44, 355–363. [Google Scholar] [CrossRef]
- Anjum, S.; Abbasi, B.H. Thidiazuron-enhanced biosynthesis and antimicrobial efficacy of silver nanoparticles via improving phytochemical reducing potential in callus culture of Linum usitatissimum L. Int. J. Nanomed. 2016, 11, 715. [Google Scholar]
- KÖksal, E.; Gülçin, İ.; Beyza, S.; Sarikaya, Ö.; Bursal, E. In vitro antioxidant activity of silymarin. J. Enzym. Inhib. Med. Chem. 2009, 24, 395–405. [Google Scholar] [CrossRef] [Green Version]
- Hayyan, M.; Hashim, M.A.; AlNashef, I.M. Superoxide ion: Generation and chemical implications. Chem. Rev. 2016, 116, 3029–3085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vatankhah, E.; Niknam, V.; Ebrahimzadeh, H. Activity of antioxidant enzyme during in vitro organogenesis in Crocus sativus. Biol. Plant. 2010, 54, 509–514. [Google Scholar] [CrossRef]
- Mathur, M.; Ramawat, K. Guggulsterone production in cell suspension cultures of the guggul tree, Commiphora wightii, grown in shake-flasks and bioreactors. Biotechnol. Lett. 2007, 29, 979–982. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Newton, R.J. Peroxidase and catalase activities are involved in direct adventitious shoot formation induced by thidiazuron in eastern white pine (Pinus strobus L.) zygotic embryos. Plant Physiol. Biochem. 2005, 43, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.; Abbasi, B.H.; Hano, C. Trends in accumulation of pharmacologically important antioxidant-secondary metabolites in callus cultures of Linum usitatissimum L. Plant Cell Tissue Organ Cult. 2017, 129, 73–87. [Google Scholar] [CrossRef]
- Danya, U.; Udhayasankar, M.; Punitha, D.; Arumugasamy, K.; Suresh, S. In vitro regeneration of Tecomella undulata (Sm.) Seem-an endangered medicinal plant. Int. J. Plant Anim. Environ. Sci. 2012, 2, 44–49. [Google Scholar]
- Fazal, H.; Abbasi, B.H.; Ahmad, N. Optimization of adventitious root culture for production of biomass and secondary metabolites in Prunella vulgaris L. Appl. Biochem. Biotechnol. 2014, 174, 2086–2095. [Google Scholar] [CrossRef]
- Shibli, R.A.; Smith, M.; Kushad, M. Headspace ethylene accumulation effects on secondary metabolite production in Vaccinium pahalae cell culture. Plant Growth Regul. 1997, 23, 201–205. [Google Scholar] [CrossRef]
- Ford, J.D.; Huang, K.S.; Wang, H.B.; Davin, L.B.; Lewis, N.G. Biosynthetic Pathway to the Cancer Chemopreventive Secoisolariciresinol Diglucoside− Hydroxymethyl Glutaryl Ester-Linked Lignan Oligomers in Flax (Linum usitatissimum) Seed. J. Nat. Prod. 2001, 64, 1388–1397. [Google Scholar] [CrossRef]
- Barvkar, V.T.; Pardeshi, V.C.; Kale, S.M.; Kadoo, N.Y.; Gupta, V.S. Phylogenomic analysis of UDP glycosyltransferase 1 multigene family in Linum usitatissimum identified genes with varied expression patterns. BMC Genom. 2012, 13, 175. [Google Scholar] [CrossRef] [Green Version]
- Hano, C.; Martin, I.; Fliniaux, O.; Legrand, B.; Gutierrez, L.; Arroo, R.; Mesnard, F.; Lamblin, F.; Lainé, E. Pinoresinol–lariciresinol reductase gene expression and secoisolariciresinol diglucoside accumulation in developing flax (Linum usitatissimum) seeds. Planta 2006, 224, 1291–1301. [Google Scholar] [CrossRef]
- Ghose, K.; Selvaraj, K.; McCallum, J.; Kirby, C.W.; Sweeney-Nixon, M.; Cloutier, S.J.; Deyholos, M.; Datla, R.; Fofana, B. Identification and functional characterization of a flax UDP-glycosyltransferase glucosylating secoisolariciresinol (SECO) into secoisolariciresinol monoglucoside (SMG) and diglucoside (SDG). BMC Plant Biol. 2014, 14, 82. [Google Scholar] [CrossRef] [Green Version]
- Touré, A.; Xueming, X. Flaxseed lignans: Source, biosynthesis, metabolism, antioxidant activity, bio-active components, and health benefits. Compr. Rev. Food Sci. Food Saf. 2010, 9, 261–269. [Google Scholar] [CrossRef]
- Qu, H.; Madl, R.L.; Takemoto, D.J.; Baybutt, R.C.; Wang, W. Lignans are involved in the antitumor activity of wheat bran in colon cancer SW480 cells. J. Nutr. 2005, 135, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Rajesha, J.; Rao, A.R.; Madhusudhan, B.; Karunakumar, M. Antibacterial properties of secoisolariciresinol diglucoside isolated from Indian flaxseed cultivars. Curr. Trends Biotechnol. Pharm. 2010, 4, 551–560. [Google Scholar]
- Pilar, B.; Güllich, A.; Oliveira, P.; Ströher, D.; Piccoli, J.; Manfredini, V. Protective role of flaxseed oil and flaxseed lignan secoisolariciresinol diglucoside against oxidative stress in rats with metabolic syndrome. J. Food Sci. 2017, 82, 3029–3036. [Google Scholar] [CrossRef]
- Kezimana, P.; Dmitriev, A.A.; Kudryavtseva, A.V.; Romanova, E.V.; Melnikova, N.V. Secoisolariciresinol Diglucoside of Flaxseed and Its Metabolites: Biosynthesis and Potential for Nutraceuticals. Front. Genet. 2018, 9, 641. [Google Scholar] [CrossRef] [Green Version]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Ali, A.; Mohammad, S.; Khan, M.A.; Raja, N.I.; Arif, M.; Kamil, A.; Mashwani, Z.U.R. Silver nanoparticles elicited in vitro callus cultures for accumulation of biomass and secondary metabolites in Caralluma tuberculata. Artif. Cells Nanomed. Biotechnol. 2019, 47, 715–724. [Google Scholar] [CrossRef] [Green Version]
- Liu, N.; Lin, Z.; Guan, L.; Gaughan, G.; Lin, G. Antioxidant enzymes regulate reactive oxygen species during pod elongation in Pisum sativum and Brassica chinensis. PLoS ONE 2014, 9, e87588. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, B.H.; Khan, M.A.; Mahmood, T.; Ahmad, M.; Chaudhary, M.F.; Khan, M.A. Shoot regeneration and free-radical scavenging activity in Silybum marianum L. Plant Cell Tissue Organ Cult. 2010, 101, 371–376. [Google Scholar] [CrossRef]
- Sarajlija, H.; ČukelJ, N.; Mršić, G.N.D.; Brnčić, M.; Ćurić, D. Preparation of flaxseed for lignan determination by gas chromatography-mass spectrometry method. Czech J. Food Sci. 2012, 30, 45–52. [Google Scholar] [CrossRef] [Green Version]
| Shoot Regeneration Frequency (%) | |||||
|---|---|---|---|---|---|
| PGRs | Concentration (mg/L) | Leaf Explants | Cotyledon Explants | Hypocotyl Explants | Root Explants |
| TDZ | 0.5 | 20.54 ± 0.82 d | 15.45 ± 0.76 d | 86.54 ± 6.93 a | 12.54 ± 0.86 cd |
| TDZ | 1.0 | 44.87 ± 2.45 bc | 30.54 ± 1.17 c | 81.23 ± 6.91 ab | 19.59 ± 0.78 c |
| TDZ | 1.5 | 27.68 ± 0.93 c | 41.75 ± 2.12 bc | 56 ± 2.66 c | 28.59 ± 0.95 bc |
| Kn | 0.5 | 57.76 ± 3.74 a | 24.36 ± 0.90 cd | 64.65 ± 4.47 bc | 32.43 ± 1.33 b |
| Kn | 1.0 | 46.12 ± 2.62 b | 26.75 ± 0.92 cd | 67.23 ± 4.71 b | 28.65 ± 0.95 bc |
| Kn | 1.5 | 40.43 ± 2.05 bc | 32.80 ± 1.33 c | 57.87 ± 3.75 c | 16.86 ± 0.77 c |
| TDZ + Kn | 0.5:0.5 | 50.42 ± 3.01 ab | 42.23 ± 2.45 bc | 86.87 ± 6.93 a | 45.76 ± 2.54 a |
| TDZ + Kn | 1.0:1.0 | 59.43 ± 3.95 a | 56.54 ± 3.62 a | 82.54 ± 6.92 ab | 38.94 ± 1.84 ab |
| TDZ + Kn | 1.5:1.5 | 48.76 ± 2.85 b | 47.65 ± 2.75 b | 80.83 ± 6.01 ab | 44.70 ± 2.44 a |
| PGRs | Concentration (mg/L) | Days to Shoot Initiation | Frequency of Shoot Initiation | Avg. No of Shoots | Max. Shoot Length | Avg. Shoot Length | Avg. FW of Callus Developed at the Base (mg) | FW (Full Plant) mg | DW (Full Plant) mg |
|---|---|---|---|---|---|---|---|---|---|
| TDZ | 0.5 | 7.1 ± 1.05 d | 86.54 ± 6.93 a | 1.33 ± 0.38 cd | 2.9 ± 0.15 e | 2.76 ± 0.12 cd | 140 ± 10.32 d | 770 ± 7.34 de | 638.8 ± 5.3 de |
| TDZ | 1.0 | 5.2 ± 0.85 f | 81.23 ± 6.91 ab | 1 ± 0.11 d | 3.3 ± 0.28 de | 2.96 ± 0.14 c | 750 ± 7.63 b | 1591 ± 9.25 a | 1336 ± 2.3 a |
| TDZ | 1.5 | 7.8 ± 0.90 c | 56 ± 2.66 e | 1 ± 0.11 d | 3.5 ± 0.39 d | 3.25 ± 0.23 bc | 1033 ± 11.01 a | 1333 ± 9.13 b | 1161.7 ± 4.9 b |
| Kn | 0.5 | 10 ± 0.81 a | 64.65 ± 4.47 d | 1.66 ± 0.57 c | 4.2 ± 0.34 cd | 3.9 ± 0.26 b | 0 ± 0 | 189 ± 1.6 f | 128 ± 1.9 f |
| TDZ + Kn | 0.5:0.5 | 8.6 ± 1.21 b | 86.87 ± 6.93 a | 6.3 ± 0.36 a | 6.5 ± 0.54 a | 4.56 ± 0.25 a | 40 ± 5.77 f | 858 ± 5.7 c | 742.7 ± 2.5 c |
| TDZ + Kn | 1.0:0.5 | 6.5 ± 0.55 e | 82.54 ± 6.92 ab | 2 ± 0.25 bc | 4.6 ± 0.44 c | 4.15 ± 0.32 ab | 91.4 ± 7.63 e | 422.8 ± 0.013 e | 276 ± 6.4 e |
| TDZ + Kn | 1.5:0.5 | 7.3 ± 0.95 cd | 80.83 ± 6.01 ab | 2.5 ± 0.46 b | 5.5 ± 0.44 b | 4.25 ± 0.42 ab | 500 ± 12.58 c | 789.3 ± 4.6 d | 657.8 ± 2.4 d |
| Media | PGRs | Concentration (mg/L) | Frequency of Root Induction (%) | Days to Root Initiation | Avg. Root Length |
|---|---|---|---|---|---|
| Full strength MS media | IBA | 0.1 | 27.6 ± 0.95 cd | 10 ± 0.81 b | 3.1 ± 0.21 b |
| IBA | 0.5 | 36.9 ± 1.84 b | 10 ± 0.81 b | 3.1 ± 0.21 b | |
| IBA | 1.0 | 21.5 ± 0.82 d | 10 ± 0.81 b | 3.1 ± 0.21 b | |
| Half strength MS media | IBA | 0.1 | 45.7 ± 2.54 a | 8 ± 0.20 c | 3.5 ± 0.46 a |
| IBA | 0.5 | 42.2 ± 2.45 ab | 8 ± 0.20 c | 3.5 ± 0.46 a | |
| IBA | 1.0 | 32.43 ± 1.33 bc | 8 ± 0.20 c | 3.5 ± 0.46 a | |
| MS0 | No PGRs | 0 | 33.16 ± 1.34 c | 13 ± 0.95 a | 1.3 ± 0.16 c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, I.; Khan, M.A.; Shehzad, M.A.; Ali, A.; Mohammad, S.; Ali, H.; Alyemeni, M.N.; Ahmad, P. Micropropagation and Production of Health Promoting Lignans in Linum usitatissimum. Plants 2020, 9, 728. https://doi.org/10.3390/plants9060728
Khan I, Khan MA, Shehzad MA, Ali A, Mohammad S, Ali H, Alyemeni MN, Ahmad P. Micropropagation and Production of Health Promoting Lignans in Linum usitatissimum. Plants. 2020; 9(6):728. https://doi.org/10.3390/plants9060728
Chicago/Turabian StyleKhan, Irfan, Mubarak Ali Khan, Muhammad Amir Shehzad, Amir Ali, Sher Mohammad, Huma Ali, Mohammed Nasser Alyemeni, and Parvaiz Ahmad. 2020. "Micropropagation and Production of Health Promoting Lignans in Linum usitatissimum" Plants 9, no. 6: 728. https://doi.org/10.3390/plants9060728
APA StyleKhan, I., Khan, M. A., Shehzad, M. A., Ali, A., Mohammad, S., Ali, H., Alyemeni, M. N., & Ahmad, P. (2020). Micropropagation and Production of Health Promoting Lignans in Linum usitatissimum. Plants, 9(6), 728. https://doi.org/10.3390/plants9060728






