Bioactive Compounds and Aroma Profile of Some Lamiaceae Edible Flowers
Abstract
1. Introduction
2. Results
2.1. Bioactive Compounds
2.2. Phytochemical Analyses
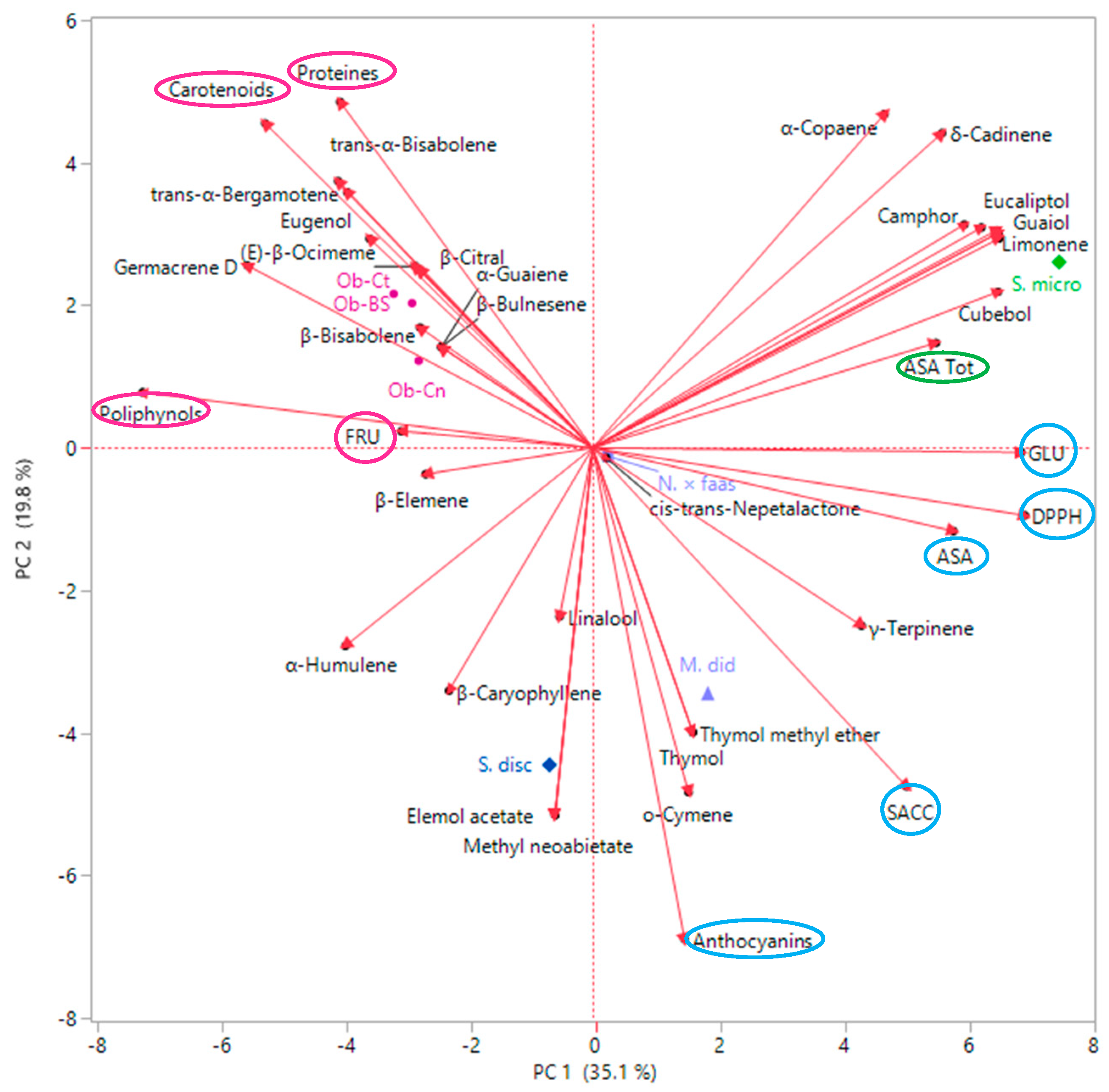
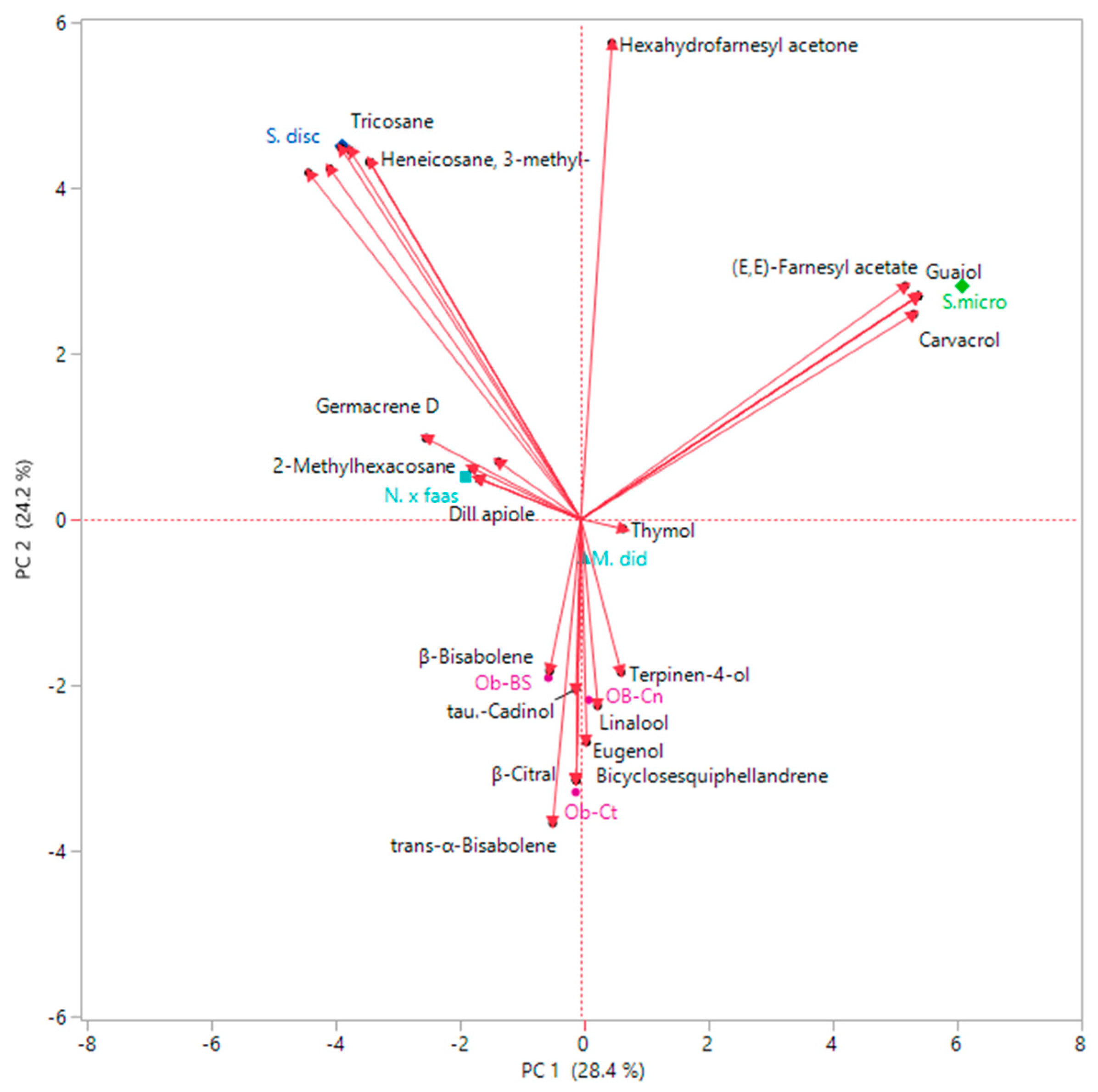
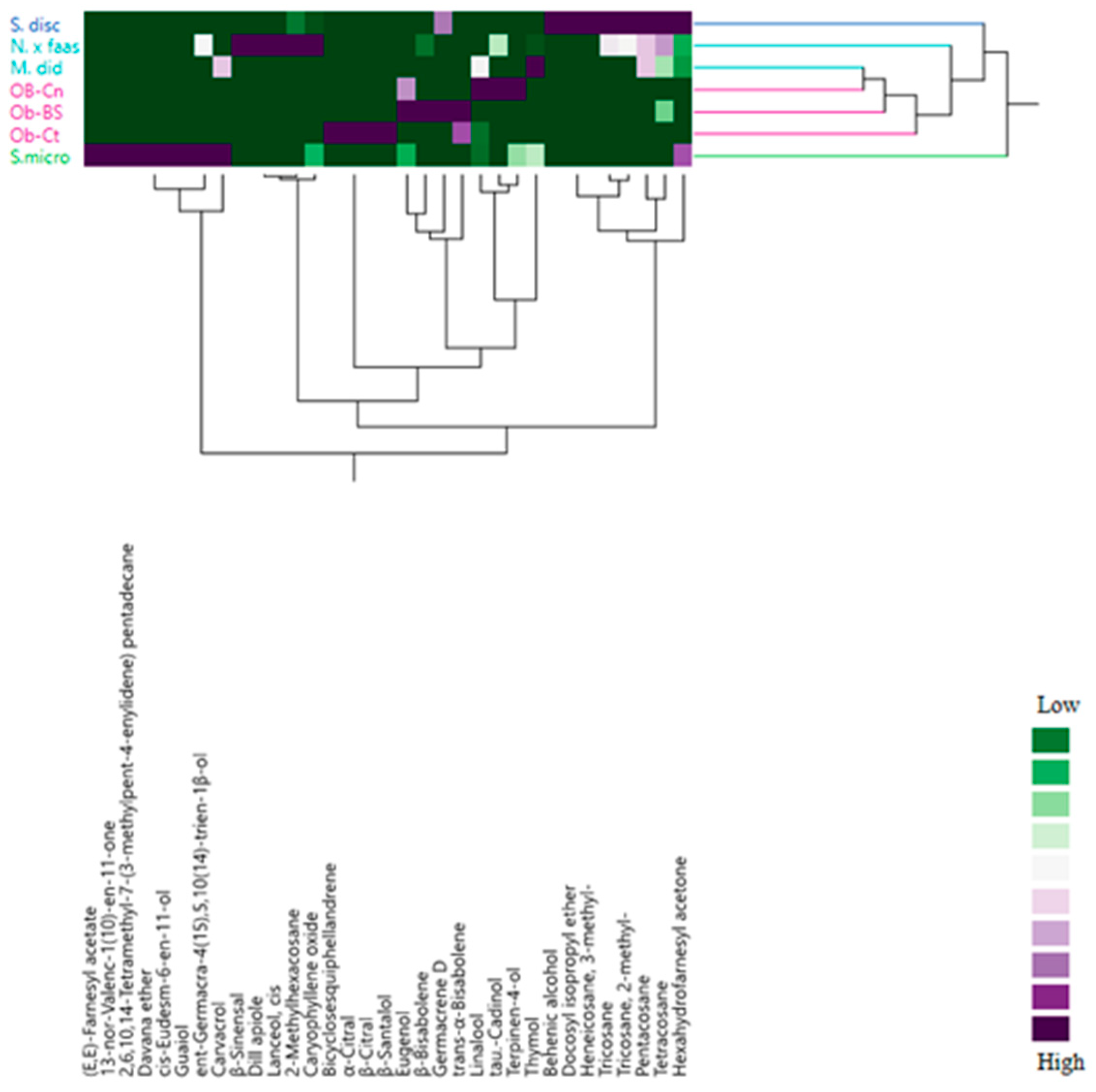
2.3. Multivariate Explorer Analyses
2.4. Essential Oil (EO) Analysis
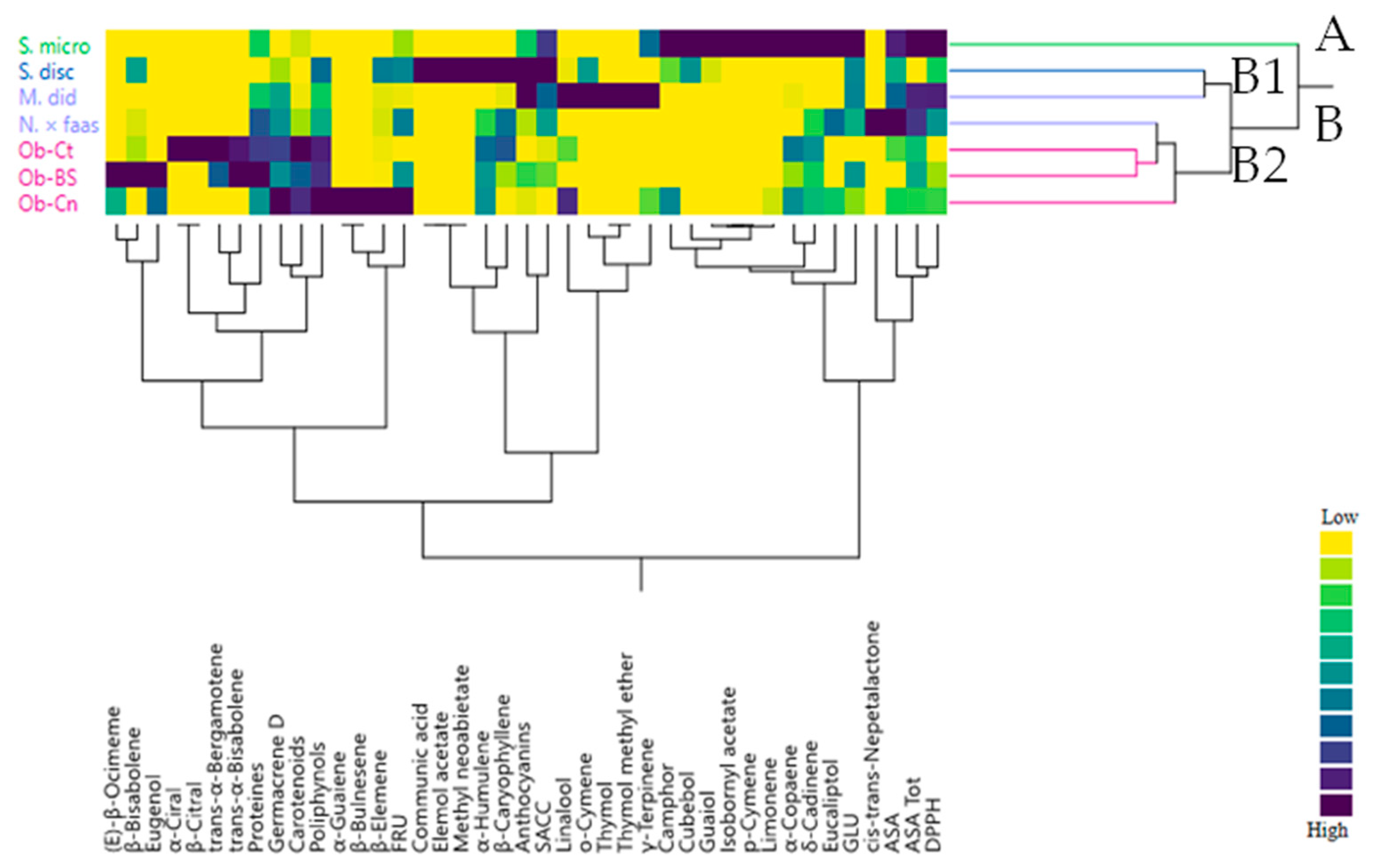
2.5. Multivariate Explorer Analyses
3. Discussion
3.1. Bioactive Compounds
3.2. Spountaneous Emissions
3.3. Essential Oils
4. Materials and Methods
4.1. Plant Material and Cultivation
4.2. Biochemical Analyses
4.3. Phytochemical Analysis
4.4. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Azzazy, M.F. Systematic Importance of Pollen Morphology of Some Plants of (Lamiaceae). Curr. Bot. 2016, 7, 5. [Google Scholar] [CrossRef]
- Karpiński, T.M. Essential Oils of Lamiaceae Family Plants as Antifungals. Biomolecules 2020, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- Gul, S.; Ahmad, M.; Zafar, M.; Bahadur, S.; Sultana, S.; Begum, N.; Shah, S.N.; Zaman, W.; Ullah, F.; Ayaz, A.; et al. Taxonomic study of subfamily Nepetoideae (Lamiaceae) by polynomorphological approach. Microsc. Res. Tech. 2019, 82, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Echeverría, J.; Niemeyer, H.M. Essential oil of Kurzamra pulchella (Clos) Kuntze (Lamiaceae, Nepetoideae, Mentheae, Menthinae): Relationship with chemotype groups in the subtribe Menthinae. Nat. Prod. Res. 2017, 31, 108–112. [Google Scholar] [CrossRef]
- Wojdylo, A.; Oszmianski, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Bonesi, M.; Loizzo, M.R.; Acquaviva, R.; Malfa, G.A.; Aiello, F.; Tundis, R. Anti-inflammatory and antioxidant agents from Salvia genus (Lamiaceae): An assessment of the current state of knowledge. Antiinflamm. Antiallergy Agents Med. Chem. 2017, 16, 70–86. [Google Scholar] [CrossRef]
- Süntar, I.; Nabavi, S.M.; Barreca, D.; Fischer, N.; Efferth, T. Pharmacological and chemical features of Nepeta L. genus: Its importance as a therapeutic agent. Phyther. Res. 2018, 32, 185–198. [Google Scholar] [CrossRef]
- Elansary, H.O.; Szopa, A.; Kubica, P.; Ekiert, H.; El-Ansary, D.O.; Al-Mana, F.A.; Mahmoud, E.A. Saudi Rosmarinus officinalis and Ocimum basilicum L. Polyphenols and Biological Activities. Processes 2020, 8, 446. [Google Scholar] [CrossRef]
- Mamadalieva, N.Z.; Akramov, D.K.; Böhmdorfer, S.; Azimova, S.S.; Rosenau, T. Extractives and biological activities of Lamiaceae species growing in Uzbekistan. Holzforschung 2020, 74, 96–115. [Google Scholar] [CrossRef]
- Caser, M.; D’Angiolillo, F.; Chitarra, W.; Lovisolo, C.; Ruffoni, B.; Pistelli, L.; Pistelli, L.; Scariot, V. Ecophysiological and phytochemical responses of Salvia sinaloensis Fern. to drought stress. Plant Growth Regul. 2018, 84, 383–394. [Google Scholar] [CrossRef]
- Trivellini, A.; Lucchesini, M.; Maggini, R.; Mosadegh, H.; Villamarin, T.S.S.; Vernieri, P.; Mensuali-Sodi, A.; Pardossi, A. Lamiaceae phenols as multifaceted compounds: Bioactivity, industrial prospects and role of “positive-stress”. Ind. Crop. Prod. 2016, 83, 241–254. [Google Scholar] [CrossRef]
- Mishra, L.K.; Sarkar, D.; Shetty, K. Human health-relevant bioactives and associated functionalities of herbs in the Lamiaceae family. In Functional Foods and Biotechnology: Sources of Functional Foods and Ingredients; CRC Press, Taylor & Francis Group, LLC: Boca Raton, FL, USA, 2019; pp. 115–131. [Google Scholar] [CrossRef]
- Fernandes, L.; Casal, S.; Pereira, J.A.; Saraiva, J.A.; Ramalhosa, E. Edible flowers: A review of the nutritional, antioxidant, antimicrobial properties and effects on human health. J. Food Compos. Anal. 2017, 60, 38–50. [Google Scholar] [CrossRef]
- Mlcek, J.; Rop, O. Fresh edible flowers of ornamental plants—A new source of nutraceutical foods. Trends Food Sci. Technol. 2011, 22, 561–569. [Google Scholar] [CrossRef]
- Husti, A.; Cantor, M.; Buta, E.; Horţ, D. Current trends of using ornamental plants in culinary arts. ProEnvironment 2013, 6, 52–58. [Google Scholar]
- Lu, B.; Li, M.; Yin, R. Phytochemical Content, Health Benefits, and Toxicology of Common Edible Flowers: A Review (2000–2015). Crit. Rev. Food Sci. Nutr. 2016, 56, S130–S148. [Google Scholar] [CrossRef]
- Najar, B.; Marchioni, I.; Ruffoni, B.; Copetta, A.; Pistelli, L.; Pistelli, L. Volatilomic Analysis of Four Edible Flowers from Agastache Genus. Molecules 2019, 24, 4480. [Google Scholar] [CrossRef]
- Pires, T.C.S.P.; Barros, L.; Santos-Buelga, C.; Ferreira, I.C.F.R. Edible flowers: Emerging components in the diet. Trends Food Sci. Technol. 2019, 93, 244–258. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Pugliese, A.; Bonesi, M.; Tenuta, M.C.; Menichini, F.; Xiao, J.; Tundis, R. Edible flowers: A rich source of phytochemicals with antioxidant and hypoglycemic properties. J. Agric. Food Chem. 2016, 64, 2467–2474. [Google Scholar] [CrossRef]
- Nowicka, P.; Wojdyło, A. Anti-hyperglycemic and anticholinergic effects of natural antioxidant contents in edible flowers. Antioxidants 2019, 8, 308. [Google Scholar] [CrossRef]
- Fernandes, L.; Ramalhosa, E.; Pereira, J.A.; Saraiva, J.A.; Casal, S. The unexplored potential of edible flowers lipids. Agriculture 2018, 8, 146. [Google Scholar] [CrossRef]
- Oladeji, O.; Amusan, T. Proximate, vitamin and mineral assays of an underutilised indigenous vegetable in West Africa Salvia elegans Vahl (Lamiales: Lamiaceae) in enhancing diet diversification. Rev. Bras. Gestão Ambient. E Sustentabilidade 2016, 3, 327–336. [Google Scholar] [CrossRef][Green Version]
- Rop, O.; Mlcek, J.; Jurikova, T.; Neugebauerova, J.; Vabkova, J. Edible Flowers—A New Promising Source of Mineral Elements in Human Nutrition. Molecules 2012, 17, 6672–6683. [Google Scholar] [CrossRef] [PubMed]
- Grzeszczuk, M.; Stefaniak, A.; Meller, E.; Wysocka, G. Mineral composition of some edible flowers. J. Elem. 2018, 23, 151–162. [Google Scholar] [CrossRef]
- Fernandes, L.; Casal, S.; Pereira, J.A.; Malheiro, R.; Rodrigues, N.; Saraiva, J.A.; Ramalhosa, E. Borage, calendula, cosmos, Johnny Jump up, and pansy flowers: Volatiles, bioactive compounds, and sensory perception. Eur. Food Res. Technol. 2019, 245, 593–606. [Google Scholar] [CrossRef]
- Chen, N.-H.; Wei, S. Factors influencing consumers’ attitudes towards the consumption of edible flowers. Food Qual Prefer. 2017, 56, 93–100. [Google Scholar] [CrossRef]
- Makri, O.; Kintzios, S. Ocimum sp.(basil): Botany, cultivation, pharmaceutical properties, and biotechnology. J. Herbs Spices Med. Plants 2008, 13, 123–150. [Google Scholar] [CrossRef]
- Poonkodi, K. Chemical composition of essential oil of Ocimum basilicum L. (Basil) and its biological activities-an overview. J. Crit. Rev. 2016, 3, 56–62. [Google Scholar]
- Beatovic, D.; Krstic-Milosevic, D.; Trifunovic, S.; Siljegovic, J.; Glamoclija, J.; Ristic, M.; Jelacic, S. Chemical composition, antioxidant and antimicrobial activities of the essential oils of twelve Ocimum basilicum L. cultivars grown in Serbia. Rec. Nat. Prod 2015, 9, 62–75. [Google Scholar]
- Copetta, A.; Lingua, G.; Berta, G. Effects of three AM fungi on growth, distribution of glandular hairs, and essential oil production in Ocimum basilicum L. var. Genovese. Mycorrhiza 2006, 16, 485–494. [Google Scholar] [CrossRef]
- Lim Ah Tock, M.J.; Kamatou, G.P.P.; Combrinck, S.; Sandasi, M.; Viljoen, A.M. A chemometric assessment of essential oil variation of three Salvia species indigenous to South Africa. Phytochemistry 2020, 172, 112249. [Google Scholar] [CrossRef]
- Talebi, S.M.; Behzadpour, S.; Matsyura, A. Morphological and essential oil variations among Iranian populations of Salvia chloroleuca (Lamiaceae). Biosyst. Divers. 2019, 27, 233–237. [Google Scholar] [CrossRef]
- Giffen, J.E.; Lesiak, A.D.; Dane, A.J.; Cody, R.B.; Musah, R.A. Rapid species-level identification of salvias by chemometric processing of ambient ionisation mass spectrometry-derived chemical profiles. Phytochem. Anal. 2017, 28, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Landi, M.; Ruffoni, B.; Combournac, L.; Guidi, L. Nutraceutical value of edible flowers upon cold storage. Ital. J. Food Sci. 2017, 30, 1–18. [Google Scholar] [CrossRef]
- Jenks, A.A.; Kim, S.C. Medicinal plant complexes of Salvia subgenus Calosphace: An ethnobotanical study of new world sages. J. Ethnopharmacol. 2013, 146, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Sharopov, F.; Valiev, A.; Sobeh, M.; Arnold, E.; Winka, M. Bioactivity of three Salvia species in relation to their total phenolic and flavonoid contents. Pharm. Chem. J. 2018, 52, 596–600. [Google Scholar] [CrossRef]
- Asadollahi, M.; Firuzi, O.; Heidary Jamebozorgi, F.; Alizadeh, M.; Jassbi, A.R. Ethnopharmacological studies, chemical composition, antibacterial and cytotoxic activities of essential oils of eleven Salvia in Iran. J. Herb. Med. 2019, 17–18, 100250. [Google Scholar] [CrossRef]
- Karafakıoglu, Y.; Aksoy, L. Evaluation of Minerals, Phenolics, Radical Scavenging Activity, Total Oxidant Status and Total Antioxidant Status of Nepeta Viscida Boiss. Int. J. Agric. Life Sci. 2019, 3, 98–105. [Google Scholar]
- Anishchenko, I.E.; Zhigunov, O.Y.U. On biology of some reprentatives of the genus Nepeta L. under cultivationconditions in the bashkir cis-Urals. B. Acad. Sci. 2016, 1, 32–37. [Google Scholar]
- Salehi, B.; Valussi, M.; Jugran, A.K.; Martorell, M.; Ramírez-Alarcón, K.; Stojanović-Radić, Z.Z.; Antolak, H.; Kręgiel, D.; Mileski, K.S.; Sharifi-Rad, M.; et al. Nepeta species: From farm to food applications and phytotherapy. Trends Food Sci. Technol. 2018, 80, 104–122. [Google Scholar] [CrossRef]
- Radulović, N.; Blagojević, P.D.; Rabbitt, K.; de Sousa Menezes, F. Essential oil of Nepeta x faassenii Bergmans ex Stearn (N. mussinii Spreng. x N. nepetella L.): A comparison study. Nat. Prod. Commun. 2011, 6, 1015–1022. [Google Scholar] [CrossRef]
- Laquale, S.; Avato, P.; Argentieri, M.P.; Bellardi, M.G.; D’Addabbo, T. Nematotoxic activity of essential oils from Monarda species. J. Pest Sci. 2018, 91, 1115–1125. [Google Scholar] [CrossRef]
- Mattarelli, P.; Epifano, F.; Minardi, P.; Di Vito, M.; Modesto, M.; Barbanti, L.; Bellardi, M.G. Chemical composition and antimicrobial activity of essential oils from aerial parts of Monarda didyma and Monarda fistulosa cultivated in Italy. J. Essent. Oil-Bearing Plants. 2017, 20, 76–86. [Google Scholar] [CrossRef]
- Trettel, J.R.; Gazim, Z.C.; Gonçalves, J.E.; Stracieri, J.; Magalhães, H.M. Volatile essential oil chemical composition of basil (Ocimum basilicum L. ‘Green’) cultivated in a greenhouse and micropropagated on a culture medium containing copper sulfate. In Vitro Cell. Dev. Biol. Plant. 2017, 53, 631–640. [Google Scholar] [CrossRef]
- Guimarães, R.; Barros, L.; Carvalho, A.M.; Ferreira, I.C. Studies on chemical constituents and bioactivity of Rosa micrantha: An alternative antioxidants source for food, pharmaceutical, or cosmetic applications. J. Agric. Food Chem. 2010, 58, 6277–6284. [Google Scholar] [CrossRef] [PubMed]
- Petanidou, T. Sugars in Mediterranean floral nectars: An ecological and evolutionary approach. J. Chem. Ecol. 2005, 31, 1065–1088. [Google Scholar] [CrossRef]
- Cresswell, J.E. How and why do nectar-foraging bumblebees initiate movements between inflorescences of wild bergamot Monarda fistulosa (Lamiaceae)? Oecologia 1990, 82, 450–460. [Google Scholar]
- Mačukanović-Jocić, M.; Stevanović, Z.D.; Mladenović, M.; Jocić, G. Flower morphophysiology of selected Lamiaceae species in relation to pollinator attraction. J. Apic. Res. 2011, 50, 89–101. [Google Scholar] [CrossRef]
- Stefaniak, A.; Grezeszczuk, M.E. Nutritional and biological value of five edible flower species. Not. Bot. Horti Agrobo. 2019, 47, 128–134. [Google Scholar] [CrossRef]
- Shanaida, M.; Kernychna, I.; Shanaida, Y. Chromatographic analysis of organic acids, amino acids, and sugars in Ocimum americanum L. Acta Pol. Pharm. Drug Res. 2017, 74, 729–732. [Google Scholar]
- Grzeszczuk, M.; Wesolowska, A.; Jadczak, D.; Jakubowska, B. Nutritional value of chive edible flowers. Acta Sci. Pol. Hortoru. 2011, 10, 85–94. [Google Scholar]
- Sotelo, A.; López-García, S.; Basurto-Peña, F. Content of nutrient and antinutrient in edible flowers of wild plants in Mexico. Plant Foods Hum. Nutr. 2007, 62, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Bourgaud, F.; Gravot, A.; Milesi, S.; Gontier, E. Production of plant secondary metabolites: A historical perspective. Plant Sci. 2001, 161, 839–851. [Google Scholar] [CrossRef]
- Daayf, F.; Lattanzio, V. Recent Advances in Polyphenol Research; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Theis, N.; Lerdau, M. The evolution of function in plant secondary metabolites. Int. J. Plant Sci. 2003, 164, S93–S102. [Google Scholar] [CrossRef]
- Mol, J.; Grotewold, E.; Koes, R. How genes paint flowers and seeds. Trends Plant Sci. 1998, 3, 212–217. [Google Scholar]
- Zhu, C.; Bai, C.; Sanahuja, G.; Yuan, D.; Farré, G.; Naqvi, S.; Shi, L.; Capell, T.; Christou, P. The regulation of carotenoid pigmentation in flowers. Arch. Biochem. Biophys. 2010, 504, 132–141. [Google Scholar] [CrossRef]
- Gandía-Herrero, F.; García-Carmona, F. Biosynthesis of betalains: Yellow and violet plant pigments. Trends Plant Sci. 2013, 18, 334–343. [Google Scholar] [CrossRef]
- Chanwitheesuk, A.; Teerawutgulrag, A.; Rakariyatham, N. Screening of antioxidant activity and antioxidant compounds of some edible plants of Thailand. Food Chem. 2005, 92, 491–497. [Google Scholar] [CrossRef]
- Grzeszczuk, M.; Stefaniak, A.; Pachlowska, A. Biological value of various edible flower species. Acta Sci. Pol-Hortoru. 2016, 15, 109–119. [Google Scholar] [CrossRef]
- Benvenuti, S.; Bortolotti, E.; Maggini, R. Antioxidant power, anthocyanin content and organoleptic performance of edible flowers. Sci. Hortic. 2016, 199, 170–177. [Google Scholar] [CrossRef]
- Srivastava, S.; Adholeya, A.; Conlan, X.A.; Cahill, D.M. Acidic potassium permanganate chemiluminescence for the determination of antioxidant potential in three cultivars of Ocimum basilicum. Plant Foods Hum. Nutr. 2016, 71, 72–80. [Google Scholar] [CrossRef]
- Simon, J.E. Phytochemical Analysis and Anti-Inflammatory Activity of Nepeta cataria Accessions. J. Med. Active Plants 2018, 7, 19–27. [Google Scholar]
- Seladji, M.; Bekhechi, C.; Beddou, F.; Hanane, D.I.B.; Bendimerad, N. Antioxidant activity and phytochemical screening of Nepeta nepetella aqueous and methanolic extracts from Algeria. J. Appl. Pharm. Sci. 2014, 4, 12. [Google Scholar] [CrossRef]
- Iqbal, K.; Khan, A.; Khattak, M.M.A.K. Biological significance of ascorbic acid (vitamin C) in human health—A review. Pak. J. Nutr. 2004, 3, 5–13. [Google Scholar]
- Nishikimi, M.; Fukuyama, R.; Minoshima, S.; Shimizu, N.; Yagi, K. Cloning and chromosomal mapping of the Human nonfunctional gene for L-gulono-gamma-lactone oxidase, the enzyme for L-ascorbic acid biosynthesis missing in man. J. Biol. Chem. 1994, 269, 13685–13688. [Google Scholar] [PubMed]
- European Parliament. Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011, Daily Reference Intakes for vitamins and minerals (adults), Annex XII; European Parliament: Strasbourg, France, 2011. [Google Scholar]
- Carović-Stanko, K.; PeteK, M.; Martina, G.; Pintar, J.; Bedeković, D.; Ćustić, M.H.; Šatović, Z. Medicinal Plants of the Family Lamiaceaeas Functional Foods-a Review. Czech J. Food Sci. 2016, 34, 377. [Google Scholar] [CrossRef]
- Cavaiuolo, M.; Cocetta, G.; Ferrante, A. The antioxidants changes in ornamental flowers during development and senescence. Antioxidants 2013, 2, 132–155. [Google Scholar] [CrossRef]
- Houta, O.; Akrout, A.; Neffati, M.; Amri, H. Phenolic contents, antioxidant and antimicrobial potentials of Crithmum maritimum cultivated in Tunisia arid zones. J. Biol. Active Prod. Nat. 2011, 1, 138–143. [Google Scholar] [CrossRef]
- Anvari, D.; Jamei, R. A comparative study between the leaf and flowers of some Asteraceae plants with respect to their antioxidant activity compounds. Curr. Nutr. Food Sci. 2016, 12, 296–303. [Google Scholar] [CrossRef]
- Siatka, T.; Kašparová, M. Seasonal variation in total phenolic and flavonoid contents and DPPH scavenging activity of Bellis perennis L. flowers. Molecules 2010, 15, 9450–9461. [Google Scholar] [CrossRef]
- Butnariu, M.; Coradini, C.Z. Evaluation of biologically active compounds from Calendula officinalis flowers using spectrophotometry. Chem. Cent. J. 2012, 6, 35. [Google Scholar] [CrossRef]
- Zeng, Y.; Deng, M.; Lv, Z.; Peng, Y. Evaluation of antioxidant activities of extracts from 19 Chinese edible flowers. SpringerPlus 2014, 3, 315. [Google Scholar] [CrossRef]
- Tarchoune, I.; Baâtour, O.; Harrathi, J.; Cioni, P.L.; Lachaâl, M.; Flamini, G.; Ouerghi, Z. Essential oil and volatile emissions of basil (Ocimum basilicum) leaves exposed to NaCl or Na2SO4 salinity. J. Plant Nutr. Soil Sci. 2013, 176, 748–755. [Google Scholar] [CrossRef]
- Ronga, D.; Pellati, F.; Brighenti, V.; Laudicella, K.; Laviano, L.; Fedailaine, M.; Benvenuti, S.; Pecchioni, N.; Francia, E. Testing the influence of digestate from biogas on growth and volatile compounds of basil (Ocimum basilicum L.) and peppermint (Mentha x piperita L.) in hydroponics. J. Appl. Res. Med. Aromat. Plants. 2018, 11, 18–26. [Google Scholar] [CrossRef]
- Matłok, N.; Gorzelany, J.; Stępień, A.E.; Figiel, A.; Balawejder, M. Effect of fertilization in selected phytometric features and contents of bioactive compounds in dry matter of two varieties of basil (Ocimum basilicum L.). Sustainability 2019, 11, 6590. [Google Scholar] [CrossRef]
- Khairun Fadila, S.; Chun Hui, A.; Sook Mei, K.; Cheng Hock, C. Chemical constituents and antioxidant capacity of Ocimum basilicum and Ocimum sanctum. Iran. J. Chem. Chem. Eng. 2019, 38, 139–152. [Google Scholar]
- Açıkgöz, M. Establishment of cell suspension cultures of Ocimum basilicum L. and enhanced production of pharmaceutical active ingredients. Ind. Crop. Prod. 2020, 148, 112278. [Google Scholar] [CrossRef]
- Klimánková, E.; Holadová, K.; Hajšlová, J.; Čajka, T.; Poustka, J.; Koudela, M. Aroma profiles of five basil (Ocimum basilicum L.) cultivars grown under conventional and organic conditions. Food Chem. 2008, 107, 464–472. [Google Scholar] [CrossRef]
- Nestorović, J.; Mišić, D.; Šiler, B.; Soković, M.; Glamočlija, J.; Ćirić, A.; Maksimović, V.; Grubišić, D. Nepetalactone content in shoot cultures of three endemic Nepeta species and the evaluation of their antimicrobial activity. Fitoterapia 2010, 81, 621–626. [Google Scholar] [CrossRef]
- Yayli, B.; Tosun, G.; Karaköse, M.; Renda, G.; Yayli, N. SPME/GC-MS analysis of volatile organic compounds from three lamiaceae species (Nepeta conferta Hedge & Lamond, Origanum onites L. and Satureja cuneifolia Ten.) growing in Turkey. Asian J. Chem. 2014, 26, 2541–2544. [Google Scholar] [CrossRef]
- Barhoumi, L. Volatile organic compounds and essential oil composition of selected organs of nepeta curviflora collected from two regions in Jordan. Jordan J. Chem. 2017, 12, 101–112. [Google Scholar]
- Lušić, D.; Koprivnjak, O.; Ćurić, D.; Sabatini, A.G.S.; Conte, L.S. Volatile profile of croatian lime tree (Tilia sp.), fir honeydew (Abies alba) and sage (Salvia officinalis) honey. Food Technol. Biotechnol. 2007, 45, 156–165. [Google Scholar]
- Znini, M.; Majidi, L.; Desjobert, J.M.; Paolini, J.; Costa, J. GC-MS analysis and comparison of volatile compounds of Salvia aucheri Boiss. var. mesatlantica Maire. obtained by hydrodistillation and headspace solid phase microextraction (HS-SPME). Acta Chromatogr. 2014, 26, 495–505. [Google Scholar] [CrossRef]
- D’Auria, M.; Racioppi, R. The Effect of Drying of the Composition of Volatile Organic Compounds in Rosmarinus officinalis, Laurus nobilis, Salvia officinalis and Thymus serpyllum. A HS-SPME-GC-MS Study. J. Essent. Oil-Bearing Plants. 2015, 18, 1209–1223. [Google Scholar] [CrossRef]
- Mohammadhosseini, M. Chemical composition of the volatile fractions from flowers, leaves and stems of Salvia mirzayanii by HS-SPME-GC-MS. J. Essent. Oil Bear. Plants 2015, 18, 464–476. [Google Scholar] [CrossRef]
- Mohammadhosseini, M. Chemical composition of the essential oils and volatile fractions from flowers, stems and roots of Salvia multicaulis Vahl. by Using MAHD, SFME and HS-SPME Methods. J. Essent. Oil Bear. Plants. 2015, 18, 1360–1371. [Google Scholar] [CrossRef]
- Al Jaber, H. Salvia ceratophylla from Jordan: Volatile Organic Compounds, Essential oil composition and antioxidant activity. Jordan J. Chem. 2016, 11, 108–119. [Google Scholar]
- Cozzolino, R.; Ramezani, S.; Martignetti, A.; Mari, A.; Piacente, S.; De Giulio, B. Determination of volatile organic compounds in the dried leaves of Salvia species by solid-phase microextraction coupled to gas chromatography mass spectrometry. Nat. Prod. Res. 2016, 30, 841–848. [Google Scholar] [CrossRef]
- Hatipoglu, S.D.; Zorlu, N.; Dirmenci, T.; Goren, A.C.; Ozturk, T.; Topcu, G. Determination of volatile organic compounds in fourty five Salvia species by thermal desorption-GC-MS technique. Rec. Nat. Prod. 2016, 10, 659–700. [Google Scholar]
- Koutsaviti, A.; Tzini, D.I.; Tzakou, O. Greek Salvia sclarea L. essential oils: Effect of hydrodistillation time, comparison of the aroma chemicals using hydrodistillation and HS-SPME techniques. Rec. Nat. Prod. 2016, 10, 800–805. [Google Scholar]
- Ascrizzi, R.; Cioni, P.L.; Amadei, L.; Maccioni, S.; Flamini, G. Geographical patterns of in vivo spontaneously emitted volatile organic compounds in Salvia species. Microchem. J. 2017, 133, 13–21. [Google Scholar] [CrossRef]
- Nekoei, M.; Mohammadhosseini, M. Chemical Composition of the Essential Oils and Volatiles of Salvia leriifolia by Three Different Extraction Methods Prior to Gas Chromatographic-Mass Spectrometric Determination: Comparison of HD with SFME and HS-SPME. J. Essent. Oil Bear. Plants. 2017, 20, 410–425. [Google Scholar] [CrossRef]
- Padalia, R.C.; Verma, R.S.; Chauhan, A.; Chanotiya, C.S. Changes in aroma profiles of 11 Indian Ocimum taxa during plant ontogeny. Acta Physiol. Plant. 2013, 35, 2567–2587. [Google Scholar] [CrossRef]
- Opalchenova, G.; Obreshkova, D. Comparative studies on the activity of basil - An essential oil from Ocimum basilicum L. - Against multidrug resistant clinical isolates of the genera Staphylococcus, Enterococcus and Pseudomonas by using different test methods. J. Microbiol. Methods. 2003, 54, 105–110. [Google Scholar] [CrossRef]
- Hussain, A.I.; Anwar, F.; Hussain Sherazi, S.T.; Przybylski, R. Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Food Chem. 2008, 108, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Pozzatti, P.; Scheid, L.A.; Spader, T.B.; Atayde, M.L.; Santurio, J.M.; Alves, S.H. in vitro activity of essential oils extracted from plants used as spices against fluconazole-resistant and fluconazole-susceptible Candida spp. Can. J. Microbiol. 2008, 54, 950–956. [Google Scholar] [CrossRef]
- Zhang, J.-W.; Li, S.-K.; Wu, W.-J. The main chemical composition and in vitro antifungal activity of the essential oils of Ocimum basilicum Linn. var. pilosum (Willd.) Benth. Molecules. 2009, 14, 273–278. [Google Scholar] [CrossRef]
- Soković, M.; Glamočlija, J.; Marin, P.D.; Brkić, D.; Griensven, L.J.L.D. antibacterial effects of the essential oils of commonly consumed medicinal herbs using an in vitro model. Molecules 2010, 15, 7532–7546. [Google Scholar] [CrossRef]
- Orhan, I.E.; Ozcelik, B.; Kan, Y.; Kartal, M. Inhibitory effects of various essential oils and individual components against extended-spectrum Beta-Lactamase (ESBL) produced by Klebsiella pneumoniae and their chemical compositions. J. Food Sci. 2011, 76. [Google Scholar] [CrossRef]
- Rajeswara Rao, B.R.; Kothari, S.K.; Rajput, D.K.; Patel, R.P.; Darokar, M.P. Chemical and biological diversity in fourteen selections of four Ocimum species. Nat. Prod. Commun. 2011, 6, 1705–1710. [Google Scholar] [CrossRef]
- Govindarajan, M.; Sivakumar, R.; Rajeswary, M.; Yogalakshmi, K. Chemical composition and larvicidal activity of essential oil from Ocimum basilicum (L.) against Culex tritaeniorhynchus, Aedes albopictus and Anopheles subpictus (Diptera: Culicidae). Exp. Parasitol. 2013, 134, 7–11. [Google Scholar] [CrossRef]
- Nardoni, S.; Giovanelli, S.; Pistelli, L.; Mugnaini, L.; Profili, G.; Pisseri, F.; Mancianti, F. in vitro activity of twenty commercially available, plant-derived essential oils against selected dermatophyte species. Nat. Prod. Commun. 2015, 10, 1473–1478. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, N.N.R.; Alviano, C.S.; Blank, A.F.; Romanos, M.T.V.; Fonseca, B.B.; Rozental, S.; Rodrigues, I.A.; Alviano, D.S. Synergism Effect of the Essential Oil from Ocimum basilicum var. Maria Bonita and its major components with fluconazole and its influence on ergosterol biosynthesis. evidence-based complement. Altern. Med. 2016, 1–12. [Google Scholar] [CrossRef]
- Snoussi, M.; Dehmani, A.; Noumi, E.; Flamini, G.; Papetti, A. Chemical composition and antibiofilm activity of Petroselinum crispum and Ocimum basilicum essential oils against Vibrio spp. strains. Microb. Pathog. 2016, 90, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Sajjadi, S.E. Analysis of the essential oils of two cultivated basil (Ocimum basilicum L.) from Iran. Daru J. 2006, 3, 128–130. [Google Scholar]
- Ahmed, A.; Hussein, K.; Alsyari, A. Chemotaxonomy and Spectral Analysis (GC/MS and FT-IR) of Essential Oil Composition of Two Ocimum basilicum L. Varietiesv and their Morphological Characterization. Jordan J. Chem. 2017, 12, 147–160. [Google Scholar]
- Tsasi, G.; Mailis, T.; Daskalaki, A.; Sakadani, E.; Razis, P.; Samaras, Y.; Skaltsa, H. The effect of harvesting on the composition of essential oils from five varieties of Ocimum basilicum L. cultivated in the Island of Kefalonia, Greece. Plants 2017, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Telci, I.; Bayram, E.; Yilmaz, G.; Avci, B. Variability in essential oil composition of Turkish basils (Ocimum basilicum L.). Biochem. Syst. Ecol. 2006, 34, 489–497. [Google Scholar] [CrossRef]
- Tansi, S.; Nacar, S. First cultivation trials of lemon basil (Ocimum basilicum var. citriodorum) in Turkey. Pak. J. Biol. Sci. 2000, 3, 395–397. [Google Scholar] [CrossRef]
- Avetisyan, A.; Markosian, A.; Petrosyan, M.; Sahakyan, N.; Babayan, A.; Aloyan, S.; Trchounian, A. Chemical composition and some biological activities of the essential oils from basil Ocimum different cultivars. BMC Complement. Altern. Med. 2017, 17. [Google Scholar] [CrossRef]
- Raina, A.P.; Gupta, V. Chemotypic characterization of diversity in essential oil composition of Ocimum species and varieties from India. J. Essent. Oil Res. 2018, 30, 444–456. [Google Scholar] [CrossRef]
- Fraternale, D.; Giamperi, L.; Bucchini, A.; Ricci, D.; Epifano, F.; Burini, G.; Curini, M. Chemical composition, antifungal and in vitro antioxidant properties of Monarda didyma L. Essent. Oil. J. Essent. Oil Res. 2006, 18, 581–585. [Google Scholar] [CrossRef]
- Adebayo, O.; Bélanger, A.; Khanizadeh, S. Variable inhibitory activities of essential oils of three Monarda species on the growth of Botrytis cinerea. Can. J. Plant Sci. 2013, 93, 987–995. [Google Scholar] [CrossRef]
- Ricci, D.; Epifano, F.; Fraternale, D. The essential oil of Monarda didyma L. (Lamiaceae) exerts phytotoxic activity in vitro against various weed seeds. Molecules 2017, 22, 222. [Google Scholar] [CrossRef]
- Regnier, F.E.; Eeisenbraun, E.J.; Waller, G.R. Nepetalactone and epinepetalactone from Nepeta cataria L. Phytochemistry 1967, 6, 1271–1280. [Google Scholar] [CrossRef]
- Saharkhiz, M.J.; Zadnour, P.; Kakouei, F. Essential oil analysis and phytotoxic activity of catnip (Nepeta cataria L.). Am. J. Essent. Oils Nat. Prod. 2016, 4, 40–45. [Google Scholar]
- Dmitrović, S.; Perišić, M.; Stojić, A.; Živković, S.; Boljević, J.; Nestorović Živković, J.; Aničić, N.; Ristić, M.; Mišić, D. Essential oils of two Nepeta species inhibit growth and induce oxidative stress in ragweed (Ambrosia artemisiifolia L.) shoots in vitro. Acta Physiol. Plant. 2015, 37, 1–15. [Google Scholar] [CrossRef]
- Baranauskienė, R.; Bendžiuvienė, V.; Ragažinskienė, O.; Venskutonis, P.R. Essential oil composition of five Nepeta species cultivated in Lithuania and evaluation of their bioactivities, toxicity and antioxidant potential of hydrodistillation residues. Food Chem. Toxicol. 2019, 129, 269–280. [Google Scholar] [CrossRef]
- Formisano, C.; Rigano, D.; Arnold, N.A.; Piozzi, F.; Senatore, F. GC and GC-MS analysis of the essential oil of Nepeta cilicica Boiss. ex Benth. From Lebanon. Nat. Prod. Res. 2013, 27, 1975–1981. [Google Scholar] [CrossRef]
- Musso, L.; Scaglia, B.; Al Haj, G.; Arnold, N.A.; Adani, F.; Scarì, G.; Dallavalle, S.; Iriti, M. Chemical characterization and nematicidal activity of the essential oil of Nepeta nuda L. ssp. pubescens and Nepeta curviflora boiss. From Lebanon. J. Essent. Oil-Bear. Plants 2017, 20, 1424–1433. [Google Scholar] [CrossRef]
- Ali, A.; Tabanca, N.; Demirci, B.; Blythe, E.K.; Baser, K.H.C.; Khan, I.A. Chemical composition and biological activity of essential oils from four Nepeta species and hybrids against Aedes aegypti (L.) (Diptera: Culicidae). Rec. Nat. Prod. 2015, 10, 137–147. [Google Scholar]
- Chialva, F.; Monguzzi, F.; Manitto, P. Composition of the Essential Oils of Five Salvia Species. J. Ess. Oil Res. 1992, 4, 447–455. [Google Scholar] [CrossRef]
- Aydoǧmuş, Z.; Yeşilyurt, V.; Topcu, G. Constituents of Salvia microphylla. Nat. Prod. Res. 2006, 20, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Lima, R.K.; Cardoso, M.D.G.; Andrade, M.A.; Guimarães, P.L.; Batista, L.R.; Nelson, D.L. Bactericidal and Antioxidant Activity of Essential Oils from Myristica fragrans Houtt and Salvia microphylla H.B.K. J. Am. Oil Chem. Soc. 2012, 89, 523–528. [Google Scholar] [CrossRef]
- Koutsaviti, A.; Antonopoulou, V.; Vlassi, A.; Antonatos, S.; Michaelakis, A.; Papachristos, D.P.; Tzakou, O. Chemical composition and fumigant activity of essential oils from six plant families against Sitophilus oryzae (Col: Curculionidae). J. Pest Sci. 2018, 91, 873–886. [Google Scholar] [CrossRef]
- Wróblewska, K.; Szumny, A.; Żarowska, B.; Kromer, K.; Dębicz, R.; Fabian, S. Impact of mulching on growth essential oil composition and its biological activity in Monarda didyma L. Ind. Crop. Prod. 2019, 129, 299–308. [Google Scholar] [CrossRef]
- Sharopov, F.S.; Satyal, P.; Setzer, W.N.; Wink, M. Chemical compositions of the essential oils of three Salvia species cultivated in Germany. Am. J. Essent. Oils Nat. Prod. 2015, 3, 26–29. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Method. Enzym. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Lebensm. Wiss. Technol. 1995, 28, 25–30. [Google Scholar]
- Jones, J.B., Jr.; Wolf, B.; Mills, H.A. Plant Analysis Handbook: A Practical SAMPLING, preparation, Analysis, and Interpretation Guide; Micro-Macro Publishing, Inc.: Athens, Greece, 1991. [Google Scholar]
- European Pharmacopoeia. European Pharmacopoeia, 9th ed.; EDQM, Council of Europe: Strasbourg, France, 2017. [Google Scholar]
- NIST 14/EPA/NIH. Mass Spectra Library. I; Willy and Sons, Inc.: Hoboken, NJ, USA, 2014. [Google Scholar]
- Adams, R.P. Identification of essential oil components by gas chromatography/mass spectrometry. Biochem. Syst. Ecol. 1996, 24, 594. [Google Scholar]
- Davies, N.W. Gas chromatographic retention indices of monoterpenes and sesquiterpenes on methyl silicon and Carbowax 20M phases. J. Chromatogr. 1990, A 503, 1–24. [Google Scholar] [CrossRef]
- Jennings, W.; Shibamoto, T. Qualitative Analysis of Flavor and Fragrance Volatiles by Glass Capillary Gas Chromatography, Food/Nahrung; Academic Press: Cambridge, MA, USA, 1982. [Google Scholar]
- Masada, Y. Analysis of Essential Oils by Gas Chromatography and Mass Spectrometry; John Wiley & Sons, Inc.: New York, NY, USA, 1976. [Google Scholar]
- Stenhagen, E.; Abrahamsson, S.; McLafferty, F.W. Registry of Mass Spectral Data; Wiley & Sons: New York, NY, USA, 1974. [Google Scholar]
- Swigar, A.A.; Silverstein, R.M. Monoterpenes; Aldrich Chemical Company: Milwaukee, WI, USA, 1981. [Google Scholar]
- Lee, S.; Lee, D.K. What is the proper way to apply the multiple comparison test? Korean J. Anesthesiol. 2018, 71, 353. [Google Scholar] [CrossRef] [PubMed]
- Moita Neto, J.M.; Moita, G.C. An introduction analysis exploratory multivariate date. Quimica Nova 1998, 21, 467–469. [Google Scholar] [CrossRef]

| Acronyms | Species/Hybrid | Variety/Genotype | English Name | Flowering Period | Taste * |
|---|---|---|---|---|---|
| M. did | Monarda didyma L. | Fireball | Bee balm | Jun-Aug | Sweet oregano |
| N. × faas | Nepeta × faassenii Bergmans ex Stearn | Six Hills Giant | Catmint | Mar-Nov | Strong aromatic |
| Ob-BS | Ocimum basilicum L. | Blue Spice | - | Apr-Nov | Spice |
| Ob-Cn | Ocimum basilicum L. | Cinnamon | Cinnamon basil | Apr-Nov | Cinnamon |
| Ob-Ct | Ocimum × citriodorum Vis | - | Thai lemon basil | Apr-Nov | Lemon peel |
| S. disc | Salvia discolor Kunth | - | Andean sage | Jan-Nov | Black currant and pine nut |
| S. micro | Salvia microphylla Kunth | Hot Lips | - | Feb-Oct | Floral and fruity |
| Parameters | Monarda didyma ‘Fireball’ (1) | Nepeta × faassenii ‘Six Hills Giant (2) | Ocimum basilicum ‘Blue Spice (3) | Ocimum basilicum ‘Cinnamon’ (4) | Ocimum × citriodorum (5) | Salvia discolor (6) | Salvia microphylla ‘Hot Lips’ (7) | Sig. |
|---|---|---|---|---|---|---|---|---|
| Primary metabolites | ||||||||
| D-Glucose (GLU) mg/g FW | 5.07 ± 0.16 | 4.36 ± 0.40 | 4.70 ± 0.35 | 3.49 ± 0.12 | 3.03 ± 0.11 | 5.02 ± 0.19 | 7.60 ± 0.50 | 1vs4,5,7//2vs7//3vs5,7// 4vs1,6,7//5vs1,3,6,7 6vs4,5,7//7vs1,2,3,4,5,6 |
| D-Fructose (FRU) mg/g FW | 2.19 ± 0.22 | 4.11 ± 0.46 | 3.58 ± 0.15 | 6.85 ± 0.64 | 2.10 ± 0.08 | 3.96 ± 0.21 | 2.46 ± 0.27 | 1vs2,4,6//2vs1,4,5//3vs4// 4vs1,2,3,5,6,7//5vs2,4,6// 6vs1,4,5//7vs4 |
| Sucrose (SUC) mg/g FW | 6.66 ± 0.56 | 4.46 ± 0.02 | 2.44 ± 0.29 | 1.27 ± 0.11 | 1.60 ± 0.05 | 9.60 ± 0.84 | 7.91 ± 0.43 | 1vs2,3,4,5,6//2vs1,4,5,6,7// 3vs1,6,7//4vs1,2,6,7//5vs1,2,6,7// 6vs1,2,3,4,5//7vs2,3,4,5 |
| Crude protein (% DW) | 6.79 ± 0.16 | 12.69 ± 0.25 | 16.16 ± 0.16 | 9.62 ± 0.12 | 13.81 ± 0.00 | 3.19 ± 0.31 | 6.29 ± 0.16 | 1vs2,3,4,5,6//2vs1,3,4,5,6,7// 3vs1,2,4,5,6,7//4vs1,2,3,5,6,7// 5vs1,2,3,4,6,7//6vs1,2,3,4,5,7// 7vs2,3,4,5,6 |
| Secondary metabolites | ||||||||
| Total carotenoids (TCar) μg/ g FW | 1.91 ± 0.02 | 6.92 ± 0.98 | 51.59 ± 6.48 | 68.33 ± 3.10 | 81.86 ± 1.48 | 61.34 ± 0.09 | 4.25 ± 0.53 | 1vs3,4,5,6//2vs3,4,5,6// 3vs1,2,4,5,7 4vs1,2,3,7//5vs1,2,3,6,7 6vs1,2,5,7//7vs3,4,5,6 |
| Total anthocyanins (TAnth) mg ME/g FW | 0.98 ± 0.04 | 0.09 ± 0.00 | 0.16 ± 0.00 | 0.06 ± 0.00 | 0.03 ± 0.00 | 0.98 ± 0.08 | 0.20 ± 0.02 | 1vs2,3,4,5,7//2vs1,3,4,5,6,7 3vs1,2,4,5,6//4vs1,2,3,6,7 5vs1,2,3,6,7//6vs2,3,4,5,7 7vs1,2,4,5,6 |
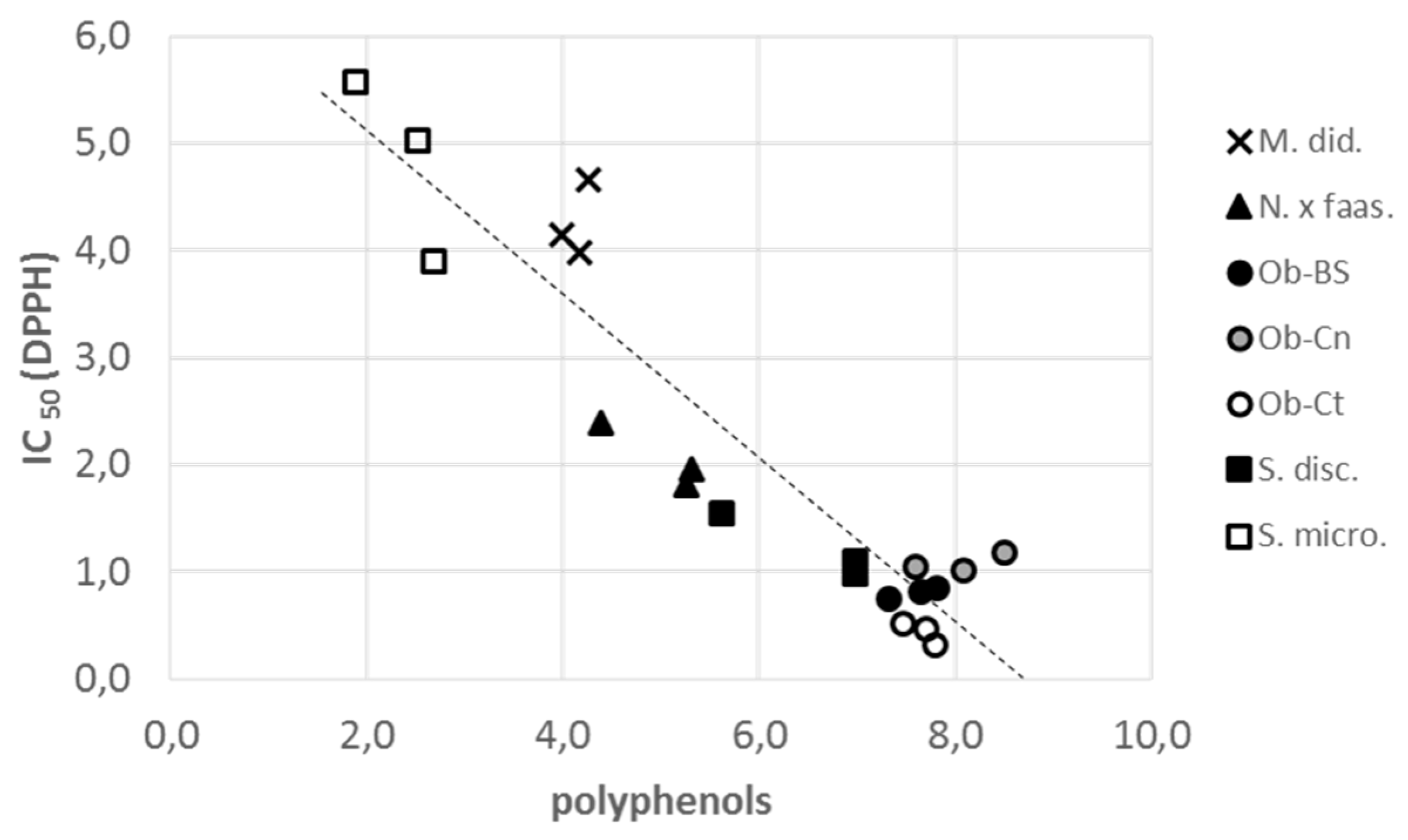
| Total polyphenols (TPC) mg GAE/g FW | 4.14 ± 0.08 | 5.11 ± 0.21 | 7.42 ± 0.13 | 8.06 ± 0.18 | 7.63 ± 0.14 | 6.53 ± 0.29 | 2.41 ± 0.18 | 1vs3,4,5,6,7//2vs3,4,5,6,7 3vs1,2,7//4vs1,2,6,7//5vs1,2,7 6vs1,2,4,7//7vs1,2,3,4,5,6 |
| Ascorbic acid reduced form (ASA) mg AsA/100 g FW | 1.36 ± 0.07 | 1.77 ± 0.05 | 0.56 ± 0.03 | 0.81 ± 0.05 | 0.77 ± 0.10 | 0.99 ± 0.05 | 1.64 ± 0.05 | 1vs2,3,4,5,6//2vs1,3,4,5,6 3vs1,2,6,7//4vs1,2,7//5vs1,2,7 6vs1,2,3,7//7vs3,4,5,6 |
| Total ascorbic acid (AsATOT) mg AsATOT /100 g FW | 2.42 ± 0.03 | 2.34 ± 0.44 | 1.76 ± 0.07 | 1.45 ± 0.21 | 1.61 ± 0.05 | 1.14 ± 0.07 | 2.57 ± 0.31 | 1vs4,5,6//2vs4,6//3vs7 4vs1,2,7//5vs1,7 6vs1,2,7//7vs3,4,5,6 |
| Radical scavenging assay (IC50 DPPH-mg/mL) | 4.26 ± 0.20 | 2.05 ± 0.17 | 0.81 ± 0.03 | 1.08 ± 0.05 | 0.43 ± 0.05 | 1.20 ± 0.17 | 4.83 ± 0.49 | 1vs2,3,4,5,6//2vs1,3,4,5,7 3vs1,2,7//4vs1,2,7//5vs1,2,7 6vs1,7//7vs2,3,4,5,6 |
| Compounds | Class | RI (esp) | RI (lit) | M. did. | N. × faas | Ob-BS | Ob-Cn | Ob-Ct | S. disco | S. micro | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Relative Abundance % | |||||||||||
| 1 | ethyl isovalerate | NT | 854 | 856 $ | - | - | 0.5 ± 0.35 * | - | - | - | - |
| 2 | β-myrcene | MH | 991 | 988 | 0.8 ± 0.23 | - | - | 0.1 ± 0.07 | - | - | - |
| 3 | oxime, methoxy phenyl | NT-N | 926 | - | - | - | - | - | - | 1.1 ± 0.55 | - |
| 4 | α-thujene | MH | 929 | 924 | - | - | - | 0.1 ± 0.10 | - | - | - |
| 5 | α-pinene | MH | 937 | 932 | - | - | - | 0.1 ± 0.10 | - | - | 0.4 ± 0.40 |
| 6 | camphene | MH | 952 | 946 | - | - | - | 0.1 ± 0.08 | - | - | - |
| 7 | β-thujene | MH | 966 | 971 $ | - | - | - | - | - | 0.4 ± 0.37 | - |
| 8 | β-myrcene | MH | 991 | 988 | - | - | - | 0.1 ± 0.10 | - | - | - |
| 9 | α-phellandrene | MH | 1005 | 1002 | - | - | - | 0.1 ± 0.06 | - | - | - |
| 10 | (+)-4-carene | MH | 1009 | 1004 $ | 1.6 ± 0.19 | - | - | - | - | - | - |
| 11 | (E,E)-2,4-nonadiene | NT | 1014 | 1014 $ | - | - | - | 0.1 ± 0.08 | - | - | - |
| 12 | α-terpinene | MH | 1017 | 1014 | - | - | - | 0.1 ± 0.10 | - | - | - |
| 13 | o-cymene | MH | 1022 | 1022 | 13.3 ± 3.98 | - | - | - | - | 2.0 ± 0.96 | - |
| 14 | p-cymene | MH | 1025 | 1020 | - | - | - | - | - | - | 4.0 ± 0.23 |
| 15 | limonene | MH | 1030 | 1224 | - | - | - | 0.5 ± 0.09 | - | - | 25.8 ± 2.11 |
| 16 | eucaliptol | OM | 1032 | 1026 | - | 2.3 ± 0.17 | - | 0.6 ± 0.06 | - | - | 4.8 ± 0.10 |
| 17 | (Z)-β-ocimeme | MH | 1038 | 1032 | - | - | 0.2 ± 0.02 | 0.2 ± 0.05 | - | - | - |
| 18 | (E)-β-ocimeme | MH | 1049 | 1044 | - | - | 19.8 ± 0.25 | 2.4 ± 0.78 | 0.3 ± 0.28 | - | - |
| 19 | γ-terpinene | MH | 1060 | 1054 | 13.3 ± 3.08 | - | - | 1.0 ± 0.48 | - | - | 6.0 ± 0.34 |
| 20 | cis-sabinene hydrate | OM | 1070 | 1065 | 0.3 ± 0.27 | - | - | 0.2 ± 0.20 | - | - | - |
| 21 | 1-octanol | NT | 1071 | 1063 | - | - | - | - | 0.1 ± 0.08 | - | - |
| 22 | terpinolene | MH | 1088 | 1086 | - | - | - | 0.6 ± 0.06 | - | - | - |
| 23 | benzoic acid, methyl ester | NT | 1094 | 1091 $ | 0.2 ± 0.17 | - | - | - | - | - | - |
| 24 | linalool | OM | 1099 | 1095 | 17.1 ± 0.92 | - | - | 13.7 ± 0.75 | 1.6 ± 0.10 | 0.3 ± 0.07 | - |
| 25 | n-nonanal | NT | 1100 | 1100 | 0.2 ± 0.09 | - | - | - | - | - | 0.6 ± 0.10 |
| 26 | (E)-myroxide | OM | 1141 | 1140 | - | - | - | 0.7 ± 0.27 | 0.4 ± 0.05 | - | - |
| 27 | camphor | OM | 1145 | 1141 | - | - | - | 1.7 ± 0.68 | - | 0.4 ± 0.08 | 6.5 ± 0.28 |
| 28 | borneol | OM | 1167 | 1165 | - | - | - | 0.3 ± 0.14 | - | - | - |
| 29 | isoneral | OM | 1170 | 1175 $ | - | - | - | - | 0.1 ± 0.02 | - | - |
| 30 | terpinen-4-ol | OM | 1177 | 1174 | - | - | - | 2.5 ± 0.20 | - | - | - |
| 31 | isogeranial | OM | 1185 | 1184 $ | - | - | - | - | 0.3 ± 0.05 | - | - |
| 32 | α-terpineol | OM | 1189 | 1186 | - | - | - | 0.1 ± 0.06 | - | - | - |
| 33 | 3,7-octadiene-2,6-diol,2,6-dimethyl- | OM | 1190 | 1189 $ | - | - | - | 0.1 ± 0.06 | - | - | - |
| 34 | methyl salicylate | NT | 1192 | 1190 | 0.4 ± 0.03 | - | - | - | - | - | - |
| 35 | n-decanal | NT | 1206 | 1201 | 1.9 ± 0.35 | - | - | - | - | 0.5 ± 0.04 | 0.7 ± 0.11 |
| 36 | ethanol, 2-phenoxy- | NT | 1226 | 1221 $ | 0.1 ± 0.10 | - | - | - | - | - | - |
| 37 | nerol | OM | 1228 | 1227 | - | - | - | - | 1.8 ± 0.44 | - | - |
| 38 | 6-octenol, 7-methyl-3-methylene- | NT | 1229 | 1221 | - | - | - | - | 0.1 ± 0.09 | - | - |
| 39 | thymol methyl ether | OM | 1235 | 1232 | 19.9 ± 1.45 | - | - | - | - | - | - |
| 40 | β-citral | OM | 1240 | 1235 | - | - | - | - | 5.5 ± 0.53 | - | - |
| 41 | geraniol | OM | 1255 | 1249 | - | - | - | - | 1.4 ± 0.32 | - | - |
| 42 | chavicol | PP | 1256 | 1247 | - | - | 0.2 ± 0.07 | - | - | - | - |
| 43 | α-citral | OM | 1270 | 1264 | - | - | - | - | 9.2 ± 0.34 | - | - |
| 44 | bornyl acetate | OM | 1285 | 1284 | - | - | - | 0.9 ± 0.03 | - | - | - |
| 45 | isobornyl acetate | OM | 1286 | 1283 | - | - | - | - | - | - | 14.3 ± 1.66 |
| 46 | thymol | OM | 1292 | 1289 | 19.4 ± 1.59 | - | - | - | - | - | - |
| 47 | carvacrol | OM | 1299 | 1298 | 0.6 ± 0.10 | - | - | - | - | - | - |
| 48 | tridecane | NT | 1300 | 1300 | - | - | - | - | 0.1 ± 0.10 | - | - |
| 49 | elemene isomer | SH | 1344 | 1343 $ | - | - | - | 0.1 ± 0.07 | - | - | - |
| 50 | α-cubebene | SH | 1351 | 1345 | - | - | - | 0.4 ± 0.01 | 0.2 ± 0.01 | - | - |
| 51 | eugenol | PP | 1357 | 1356 | - | - | 6.9 ± 1.80 | 3.4 ± 1.14 | - | - | - |
| 52 | neryl acetate | OM | 1364 | 1359 | - | - | - | - | 0.1 ± 0.08 | - | - |
| 53 | α-copaene | SH | 1376 | 1374 | 0.1 ± 0.05 | - | 0.4 ± 0.01 | 1.8 ± 0.10 | 2.6 ± 0.13 | - | 6.3 ± 1.02 |
| 54 | cis-trans-nepetalactone | OM | 1377 | 1386 | - | 64.2 ± 0.47 | - | - | - | - | - |
| 55 | β-bourbonene | SH | 1384 | 1387 | - | - | - | - | 0.1 ± 0.06 | - | - |
| 56 | β-cubebene | SH | 1385 | 1387 | - | - | - | 0.1 ± 0.02 | 0.1 ± 0.03 | - | - |
| 57 | β-cubebene | SH | 1389 | 1387 | - | - | 0.6 ± 0.16 | 0.4 ± 0.09 | 1.2 ± 0.23 | - | - |
| 58 | β-elemene | SH | 1391 | 1389 | 0.3 ± 0.08 | 0.4 ± 0.15 | 0.1 ± 0.04 | 16.8 ± 1.69 | 0.2 ± 0.02 | 5.7 ± 0.43 | - |
| 59 | sesquithujene | SH | 1402 | 1405 | - | - | 0.2 ± 0.03 | - | 0.1 ± 0.03 | - | - |
| 60 | α-gurjunene | SH | 1409 | 1409 | - | - | - | 0.1 ± 0.06 | - | - | - |
| 61 | isodihydronepetalactone | OM | 1413 | 1414 § | - | 0.3 ± 0.13 | - | - | - | - | - |
| 62 | β-caryophyllene | SH | 1419 | 1417 | 3.1 ± 1.15 | 19.0 ± 1.17 | 4.6 ± 0.19 | 2.5 ± 1.03 | 23.7 ± 2.00 | 36.2 ± 7.93 | 2.2 ± 0.27 |
| 63 | β-copaene | SH | 1432 | 1430 | - | 0.2 ± 0.03 | 0.4 ± 0.25 | 1.1 ± 0.84 | 0.7 ± 0.41 | - | - |
| 64 | β-gurjunene | SH | 1434 | 1431 | - | - | - | 0.1 ± 0.08 | - | - | - |
| 65 | cis-geranylacetone | AC | 1435 | 1445 $ | - | - | - | - | - | - | 0.6 ± 0.08 |
| 66 | trans-α-bergamotene | SH | 1435 | 1432 | - | - | 6.4 ± 0.03 | - | 11.6 ± 0.57 | - | - |
| 67 | α-guaiene | SH | 1439 | 1437 | - | - | - | 9.0 ± 0.03 | - | - | - |
| 68 | (Z)-β-farnesene | SH | 1444 | 1440 | - | - | 1.4 ± 0.10 | - | - | - | - |
| 69 | isogermacrene D | SH | 1448 | 1446 § | - | - | - | - | 0.8 ± 0.11 | - | - |
| 70 | trans-geranylacetone | AC | 1453 | 1452 $ | 0.2 ± 0.19 | - | - | - | - | - | - |
| 71 | cis-muurola-3,5-diene | SH | 1454 | 1448 | - | - | - | 1.0 ± 0.40 | - | - | - |
| 72 | α-humulene | SH | 1455 | 1452 | - | 0.8 ± 0.11 | 1.9 ± 0.05 | 2.1 ± 0.31 | 3.2 ± 0.36 | 6.0 ± 0.93 | - |
| 73 | (E)-β-famesene | SH | 1457 | 1454 | - | 0.5 ± 0.23 | 2.5 ± 0.11 | - | 0.3 ± 0.02 | 0.7 ± 0.21 | - |
| 74 | cis-muurola-4(14),5-diene | SH | 1463 | 1465 | - | - | 0.3 ± 0.11 | 1.4 ± 0.18 | 0.5 ± 0.04 | - | - |
| 75 | γ-muurolene | SH | 1477 | 1478 | - | - | 0.1 ± 0.07 | 0.3 ± 0.05 | 0.3 ± 0.04 | - | 0.9 ± 0.39 |
| 76 | germacrene D | SH | 1481 | 1484 | 6.7 ± 0.73 | 8.0 ± 2.13 | 8.4 ± 1.25 | 17.3 ± 1.07 | 13.4 ± 1.35 | 1.7 ± 0.70 | - |
| 77 | 2-isopropenyl-4a,8-dimethyl-1,2,3,4,4a,5,6,7-octahydronaphtalene | SH | 1485 | 1485 $ | - | - | - | 0.4 ± 0.09 | - | - | - |
| 78 | β-selinene | SH | 1486 | 1489 | - | - | - | 0.3 ± 0.03 | - | 0.7 ± 0.09 | - |
| 79 | bicyclosesquiphellandrene | SH | 1489 | 1488 $ | - | - | - | 0.1 ± 0.04 | 0.2 ± 0.03 | - | - |
| 80 | bicyclo[7.2.0undec-4-ene,4,11,11-trimethyl-8-methylene- | NT | 1490 | 1504 $ | - | - | 0.8 ± 0.06 | - | 1.1 ± 0.06 | - | - |
| 81 | (Z,E)-α-farnesene | SH | 1491 | 1498 $ | - | - | - | - | - | 0.8 ± 0.07 | - |
| 82 | cis-muurola-4(14),5-diene | SH | 1492 | 1491 § | - | - | - | - | 0.2 ± 0.04 | - | - |
| 83 | valencene | SH | 1493 | 1496 | - | - | - | - | - | - | - |
| 84 | epi-cubebol | OS | 1493 | 1493 | - | - | - | - | - | - | 1.5 ± 0.19 |
| 85 | α-zingiberene | SH | 1495 | 1493 | - | 1.0 ± 0.25 | - | - | - | 0.9 ± 0.09 | - |
| 86 | γ-amorphene | SH | 1496 | 1495 | - | - | - | - | 0.1 ± 0.10 | - | - |
| 87 | aciphyllene | SH | 1499 | 1501 | - | - | - | 1.1 ± 0.19 | - | - | - |
| 88 | β-bulnesene | SH | 1505 | 1508 $ | - | - | - | 9.5 ± 0.68 | - | - | - |
| 89 | cis-α-bisabolene | SH | 1507 | 1506 | - | - | 0.1 ± 0.00 | - | - | - | - |
| 90 | β-bisabolene | SH | 1509 | 1505 | - | 0.5 ± 0.16 | 26.2 ± 1.56 | - | 0.9 ± 0.06 | 4.0 ± 1.60 | - |
| 91 | γ-cadinene | SH | 1513 | 1513 | - | 0.2 ± 0.01 | - | 2.9 ± 0.33 | 0.6 ± 0.09 | - | - |
| 92 | cubebol | OS | 1515 | 1514 | - | - | - | - | - | 0.5 ± 0.20 | 3.0 ± 0.50 |
| 93 | β-sesquiphellandrene | SH | 1524 | 1521 | - | - | - | - | - | 1.3 ± 0.02 | - |
| 94 | δ-cadinene | SH | 1525 | 1522 | - | 0.5 ± 0.05 | 0.6 ± 0.10 | 0.8 ± 0.01 | 1.3 ± 0.10 | - | 5.3 ± 0.55 |
| 95 | trans-γ-bisabolene | SH | 1533 | 1531 $ | 0.2 ± 0.08 | - | - | - | - | - | |
| 96 | α-cadinene | SH | 1538 | 1537 | - | - | 0.1 ± 0.06 | 0.2 ± 0.03 | 0.2 ± 0.04 | - | - |
| 97 | trans-α-bisabolene | SH | 1545 | 1545 $ | - | - | 17.3 ± 2.00 | - | 15.4 ± 0.47 | - | - |
| 98 | elemol | OS | 1549 | 1548 | - | - | - | - | - | 1.1 ± 0.28 | - |
| 99 | guaiol | OS | 1596 | 1600 | - | - | - | - | - | 0.2 ± 0.04 | 11.5 ± 0.41 |
| 100 | 10-epi-γ-eudesmol | OS | 1619 | 1622 | - | - | - | - | - | - | 0.4 ± 0.04 |
| 101 | τ-cadinol | OS | 1640 | 1638 | - | - | - | 0.2 ± 0.01 | - | - | - |
| 102 | β-eudesmol | OS | 1649 | 1649 | - | - | - | - | - | - | 1.1 ± 0.17 |
| 103 | Methyl dihydrojasmonate | NT | 1650 | 1648 § | - | - | - | - | - | 0.2 ± 0.20 | - |
| 104 | α-eudesmol | OS | 1653 | 1652 | - | - | - | - | - | - | 2.7 ± 0.27 |
| 105 | (+)-valeranone | OS | 1677 | 1674 | - | - | - | - | - | - | 1.0 ± 0.13 |
| 106 | elemol acetate | OS | 1679 | 1680 | - | - | - | - | - | 9.0 ± 1.87 | - |
| 107 | (E)-α-santalol | OS | 1680 | 1687 $ | - | - | - | 0.1 ± 0.10 | - | - | - |
| 108 | β-bisabolol | OS | 1684 | 1674 | - | - | - | - | - | 1.0 ± 0.17 | - |
| 109 | 2,2,6-trimethyl-1-(3-methylbuta-1,3-dienyl)-7-oxabicyclo[4.1.0] heptan-3-ol | NT | 1692 | 1692 $ | - | - | - | 0.2 ± 0.18 | - | - | - |
| 110 | β-sinensal | NT | 1695 | 1700 | - | 0.1 ± 0.10 | - | - | - | - | - |
| 111 | benzyl benzoate | NT | 1762 | 1759 | 0.2 ± 0.20 | - | - | - | - | - | - |
| 112 | α-sinensal | OS | 1752 | 1755 | - | - | - | - | - | 0.7 ± 0.25 | - |
| 113 | hexahydrofarnesyl acetone | AC | 1844 | 1845 $ | 0.1 ± 0.10 | - | - | - | - | - | - |
| 114 | pentylcurcumene | NT | 1950 | 1951 $ | - | 0.1 ± 0.10 | - | - | - | - | - |
| 115 | 3,7,11,15-tetramethyl-2-hexadecen-1-ol | NT | 2116 | 2116 & | - | - | - | - | - | 2.5 ± 0.12 | - |
| 116 | sandaracopimarinol | OD | 2279 | 2269 | - | - | - | - | - | 2.2 ± 0.44 | - |
| 117 | communic acid | NT | 2405 | 2365 | - | - | - | - | - | 3.6 ± 0.65 | - |
| 118 | methyl neoabietate | OD | 2435 | 2443 | - | - | - | - | - | 6.3 ± 0.89 | - |
| Number of identified peaks | 21 | 16 | 24 | 51 | 38 | 27 | 21 | ||||
| Class of Compounds | M. did. | N × faas | Ob-BS | Ob-Cn | Ob-Ct | S. disc | S. micro | ||||
| Monoterpene hydrocarbons (MH) | 29.0 ± 4.71 | 2.3 ± 0.28 | 20.0 ± 0.50 | 5.3 ± 0.44 | - | 2.4 ± 0.34 | 36.8 ± 5.91 | ||||
| Oxygenated monoterpenes (OM) | 57.3 ± 4.32 | 66.8 ± 1.35 | - | 20.8 ± 0.21 | 20.4 ± 1.52 | 0.7 ± 0.13 | 25.6 ± 3.83 | ||||
| Sesquiterpene hydrocarbons (SH) | 10.2 ± 1.55 | 31.3 ± 2.03 | 71.6 ± 0.08 | 69.8 ± 4.06 | 77.9 ± 2.21 | 58.0 ± 8.11 | 14.7 ± 0.97 | ||||
| Oxygenated sesquiterpenes (OS) | - | - | - | 0.3 ± 0.16 | - | 12.5 ± 2.61 | 21.2 ± 0.89 | ||||
| Oxygenated diterpenes (OD) | - | - | - | - | - | 8.5 ± 0.45 | - | ||||
| Phenylpropanoids (PP) | - | - | 7.1 ± 1.87 | 3.4 ± 1.14 | - | - | - | ||||
| Apocarotenoids (AC) | 0.3 ± 0.05 | - | - | - | - | - | - | ||||
| Non-terpene derivatives (NT) | 3.0 ± 0.32 | 0.2 ± 0.03 | 1.3±0.59 | 0.3 ± 0.06 | 1.4 ± 0.21 | 7.9 ± 1.27 | 1.3 ± 0.38 | ||||
| Total Identified (%) | 99.8 ± 0.20 | 98.5 ± 0.50 | 100 ± 0.00 | 99.9 ± 0.01 | 100 ± 0.00 | 90.0 ± 4.41 | 99.6 ± 0.53 | ||||
| Compounds | Class | RI (exp) | RI (lit) | M. did | N. × faas | Ob-BS | Ob-Cn | Ob-Ct | S. disco | S. micro | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Relative abudance (%) | |||||||||||
| 1 | 5,5-dimethyl-2(5H)-furanone | nt | 952 | 952 | - | - | - | - | - | - | 2.3 ± 0.38 |
| 2 | eucalyptol | om | 1032 | 1026 | - | 0.3 ± 0.09 * | - | - | - | - | - |
| 3 | 3,5-octadien-2-ol | nt | 1038 | 1037 | - | - | - | - | - | - | 1.2 ± 0.61 |
| 4 | cis-sabinene hydrate | om | 1070 | 1068 | 0.6 ± 0.06 | - | - | - | - | - | - |
| 5 | linalool | om | 1099 | 1095 | 10.2 ± 1.12 | - | - | 48.6 ± 1.64 | 1.4 ± 0.06 | - | 1.3 ± 0.90 |
| 6 | terpinen-4-ol | om | 1177 | 1074 | - | - | - | 23.7 ± 1.90 | - | - | 2.3 ± 0.12 |
| 7 | isocreosol | pp | 1201 | 1202 | - | 0.9 ± 0.32 | - | - | - | - | - |
| 8 | nordavanone | om | 1230 | 1234 | - | - | - | - | - | - | 2.4 ± 0.76 |
| 9 | pulegone | om | 1237 | 1237 | - | 0.1 ± 0.08 | - | - | - | - | - |
| 10 | β-citral | om | 1240 | 1245 | - | - | - | - | 18.8 ± 0.90 | - | - |
| 11 | camphor | om | 1245 | 1143 | - | - | - | - | - | - | 1.4 ± 0.41 |
| 12 | α-citral | om | 1270 | 1271 | - | - | - | - | 32.2 ± 1.68 | - | - |
| 13 | benzenepropanoic acid, methyl ester | nt | 1279 | 1280 | - | 0.3 ± 0.09 | - | - | - | - | - |
| 14 | isobornyl acetate | om | 1286 | 1290 | - | - | - | - | - | - | 0.6 ± 0.14 |
| 15 | thymol | om | 1291 | 1289 | 68.6 ± 3.43 | 0.4 ± 1.15 | - | - | - | - | 8.1 ± 0.95 |
| 16 | carvacrol | om | 1299 | 1298 | 4.5 ± 0.99 | - | - | - | - | - | 10.9 ± 1.95 |
| 17 | eugenol | pp | 1357 | 1356 | - | - | 17.6 ± 0.25 | 10.7 ± 0.43 | - | - | 1.6 ± 0.76 |
| 18 | cis-trans-nepetalactone | om | 1377 | 1393 | - | 0.8 ± 0.22 | - | - | - | - | - |
| 19 | β-bourbonene | sh | 1384 | 1385 | - | 0.3 ± 0.04 | - | - | - | - | - |
| 20 | β-caryophyllene | sh | 1419 | 1417 | - | 2.4 ± 0.26 | - | - | - | 1.2 ± 0.61 | - |
| 21 | α-bergamotene | sh | 1435 | 1438 | - | - | 0.5 ± 0.01 | - | - | - | - |
| 22 | 2,6,10-trimethyltridecane | nt | 1449 | 1461 | - | - | - | - | - | 0.2 ± 0.11 | - |
| 23 | α-humulene | sh | 1454 | 1452 | - | 0.4 ± 0.04 | - | - | - | 0.3 ± 0.16 | - |
| 24 | (E)-β-famesene | sh | 1457 | 1454 | - | 0.4 ± 0.05 | 0.4 ± 0.02 | - | - | - | - |
| 25 | germacrene D | sh | 1481 | 1484 | - | - | 3.4 ± 0.04 | - | - | 2.3 ± 0.51 | - |
| 26 | α-curcumene | sh | 1483 | 1486 | - | 0.8 ± 0.04 | - | - | - | - | - |
| 27 | 1-(3,6,6-trimethyl-1,6,7,7a-tetrahydrocyclopenta[c]pyran-1-yl) ethanone | nt | 1484 | - | - | 0.3 ± 0.07 | - | - | - | - | - |
| 28 | bicyclosesquiphellandrene | sh | 1489 | 1488 | - | - | - | - | 3.3 ± 0.21 | - | - |
| 29 | davana ether | os | 1490 | 1491 | - | - | - | - | - | - | 16.3 ± 1.51 |
| 30 | α-farnesene | sh | 1508 | 1509 | - | - | - | - | - | 0.2 ± 0.06 | - |
| 31 | β-bisabolene | sh | 1509 | 1505 | - | 0.8 ± 0.02 | 34.4 ± 2.02 | - | - | - | - |
| 32 | trans-α-bisabolene | sh | 1512 | 1545 $ | - | - | 38.7 ± 2.94 | - | 29.3 ± 0.80 | - | - |
| 33 | γ-cadinene | sh | 1513 | 1511 | - | 0.3 ± 0.00 | - | - | - | - | - |
| 34 | β-sesquiphellandrene | sh | 1524 | 1521 | - | 0.4 ± 0.01 | - | - | - | - | - |
| 35 | cyclohexanemethanol, 4-ethenyl-α,α,4-trimethyl-3-(1-methylethenyl)-, acetate, [1R-(1α,3α,4β)]- | nt | 1569 | 1562 | - | - | - | - | - | 1.5 ± 0.03 | - |
| 36 | cis-eudesm-6-en-11-ol | os | 1571 | 1575 | - | - | - | - | - | - | 4.1 ± 0.15 |
| 37 | caryophyllene oxide | os | 1581 | 1583 | - | 17.2 ± 1.19 | - | - | - | 0.1 ± 0.03 | 1.1 ± 0.51 |
| 38 | davanone | os | 1588 | 1586 | - | - | - | - | - | - | 2.8 ± 0.19 |
| 39 | guaiol | os | 1596 | 1597 | - | - | - | - | - | - | 4.0 ± 0.65 |
| 40 | humulene epoxide II | os | 1606 | 1608 | - | 1.1 ± 0.03 | - | - | - | - | - |
| 41 | zingiberenol | os | 1616 | 1620 | - | 1.1 ± 0.19 | - | - | - | - | - |
| 42 | dill apiole | os | 1622 | 1625 | - | 3.0 ± 0.31 | - | - | - | - | - |
| 43 | 13-nor-valenc-1(10)-en-11-one | os | 1629 | 1628 | - | - | - | - | - | 3.1 ± 0.75 | |
| 44 | selin-6-en-4α-ol | os | 1636 | 1636 | - | 0.2 ± 0.04 | - | - | - | - | - |
| 45 | tau.-cadinol | os | 1640 | 1640 | - | 1.6 ± 0.19 | - | 13.8 ± 2.94 | - | - | - |
| 46 | cubenol | os | 1642 | 1643 | - | 0.4 ± 0.06 | - | - | - | - | - |
| 47 | 10,10-dimethyl-2,6-dimethylenebicyclo[7.2.0] undecan-5β-ol | os | 1644 | 1644 | - | 1.2 ± 0.11 | - | - | - | - | - |
| 48 | β-eudesmol | os | 1649 | 1651 | - | - | - | - | - | - | 2.0 ± 0.13 |
| 49 | α-eudesmol | os | 1653 | 1652 | - | - | - | - | - | - | 2.8 ± 0.41 |
| 50 | precocene II | pp | 1658 | 1659 | - | 2.3 ± 0.30 | - | - | - | - | - |
| 51 | aromadendrene oxide-(2) | os | 1678 | 1678 | - | 2.0 ± 0.08 | - | - | - | - | - |
| 52 | α-bisabolol | os | 1684 | 1683 | - | - | 1.3 ± 0.07 | - | - | - | |
| 53 | β-sinensal | os | 1695 | 1704 | - | 4.1 ± 0.91 | - | - | - | - | - |
| 54 | germacra-4(15),5,10(14)-trien-1β-ol | os | 1695 | 1686 $ | - | 0.6 ± 0.13 | - | - | - | - | 3.5 ± 0.36 |
| 55 | heptadecane | nt | 1700 | 1700 | - | 0.6 ± 0.25 | - | - | - | 0.2 ± 0.04 | - |
| 56 | Z-α-trans-bergamotol | os | 1701 | 1708 | - | 0.1 ± 0.00 | - | - | - | - | - |
| 57 | longifolenaldehyde | os | 1707 | 1708 | - | 0.5 ± 0.07 | - | - | - | - | - |
| 58 | cuprenenol | os | 1709 | 1702 | - | - | - | - | - | - | 2.0 ± 0.06 |
| 59 | β-santalol | os | 1715 | 1720 | - | - | 12.0 ± 1.29 | - | - | ||
| 60 | cis-nuciferol | pp | 1735 | 1730 | - | 2.2 ± 0.55 | - | - | - | - | - |
| 61 | (6R,7R)-bisabolone | os | 1747 | 1737 | - | 1.2 ± 0.39 | - | - | - | - | - |
| 62 | cis-lanceol | os | 1763 | 1761 | - | 4.6 ± 1.57 | - | - | - | - | - |
| 63 | costol | os | 1778 | 1774 | - | 0.4 ± 0.15 | - | - | - | - | - |
| 64 | hexadecanal | nt | 1817 | 1818 | - | - | - | - | - | 0.1 ± 0.08 | - |
| 65 | (E,E)-farnesyl acetate | os | 1843 | 1843 | - | - | - | - | - | - | 4.4 ± 1.15 |
| 66 | hexahydrofarnesyl acetone | ac | 1844 | 1845 | 1.3 ± 0.77 | 1.6 ± 0.69 | - | - | - | 15.7 ± 2.04 | 11.9 ± 1.12 |
| 67 | 2,6,10,15-tetramethyl-benzoic acid, 2-phenylethyl ester | nt | 1856 | 1860 | - | 0.1 ± 0.08 | - | - | - | - | - |
| 68 | 3-methyl-nonadecane | nt | 1970 | 1972 | - | - | - | - | - | 0.2 ± 0.04 | - |
| 69 | octadecanal | nt | 2021 | 2021 | - | - | - | - | - | 0.2 ± 0.03 | - |
| 70 | 2,6,10,14-tetramethyl-7-(3-methylpent-4-enylidene) pentadecane | nt | 2071 | 2068 | - | - | - | - | - | - | 5.3 ± 0.88 |
| 71 | heneicosane | nt | 2100 | 2100 | - | 0.5 ± 0.35 | - | - | - | 1.5 ± 0.26 | - |
| 72 | phytol | od | 2114 | 2122 | - | 0.3 ± 0.04 | - | - | - | 0.3 ± 0.06 | 2.5 ± 0.65 |
| 73 | 1 N-phenyl-naphthalenamine | nt-N | 2135 | 2135 | - | 1.2 ± 0.80 | - | - | - | - | - |
| 74 | 3-methyl-heneicosane | nt | 2171 | 2172 | - | - | - | - | - | 9.9 ± 1.75 | - |
| 75 | docosane | nt | 2200 | 2200 | - | - | - | - | - | 0.4 ± 0.11 | - |
| 76 | eicosanal | nt | 2224 | 2224 | - | - | - | - | - | 0.4 ± 0.14 | - |
| 77 | sclareol | od | 2227 | 2225 | - | 0.3 ± 0.14 | - | - | - | - | - |
| 78 | 4-methyldocosane | nt | 2257 | 2258 | - | 0.9 ± 0.07 | - | - | - | 0.6 ± 0.21 | - |
| 79 | larixol | od | 2264 | 2265 | - | 0.1 ± 0.03 | - | - | - | - | - |
| 80 | kolavenol | od | 2297 | 2297 | - | - | - | - | - | 0.3 ± 0.12 | - |
| 81 | carbonic acid, octadecyl vinyl ester | nt | 2299 | 2299 $ | - | 1.4 ± 0.47 | - | - | - | - | - |
| 82 | tricosane | nt | 2300 | 2300 | - | 1.3 ± 0.90 | - | - | - | 5.0 ± 1.24 | - |
| 93 | 2-methyl-tricosane | nt | 2363 | 2365 | - | 0.7 ± 0.48 | - | - | - | 4.3 ± 1.52 | - |
| 84 | 1-heneicosanol | nt | 2380 | 2365 | 0.5 ± 0.07 | - | - | - | - | - | - |
| 85 | tetracosane | nt | 2400 | 2400 | 4.5 ± 0.83 | 14.7 ± 3.63 | 3.7 ± 0.53 | - | - | 24.3 ± 1.87 | - |
| 86 | undec-10-ynoic acid, dodecyl ester | nt | 2409 | 2409 $ | - | 0.3 ± 0.04 | - | - | - | - | - |
| 87 | docosanal | nt | 2430 | 2430 | - | - | - | - | - | 0.4 ± 0.05 | - |
| 88 | 2-methyltetracosane | nt | 2462 | 2456 | - | 0.2 ± 0.09 | - | - | - | - | - |
| 89 | (Z)-13-docosen-1-ol | nt | 2467 | 2466 | - | - | - | - | - | 0.2 ± 0.06 | - |
| 90 | retinol | od | 2473 | 2473 $ | - | 1.9 ± 0.45 | - | - | - | - | - |
| 91 | retinal | od | 2486 | 2486 $ | - | 0.2 ± 0.03 | - | - | - | - | - |
| 92 | behenic alcohol | nt | 2493 | 2501 | - | - | - | - | - | 3.1 ± 0.95 | - |
| 93 | pentacosane | nt | 2500 | 2500 | 6.8 ± 1.42 | 6.8 ± 1.46 | - | - | - | 14.6 ± 2.76 | - |
| 94 | docosyl isopropyl ether | nt | 2524 | - | - | - | - | - | - | 10.2 ± 0.71 | - |
| 95 | 2-methylhexacosane | nt | 2661 | 2663 | - | 6.9 ± 0.87 | - | - | - | 0.2 ± 0.03 | - |
| Number of identified peaks | 8 | 52 | 8 | 4 | 6 | 28 | 24 | ||||
| Class of compounds | M. did. | N × faas | Ob-BS | Ob-Cn | Ob-Ct | S. disco | S. micro | ||||
| Oxygenated monoterpenes (om) | 83.9 ± 2.49 | 1.6 ± 0.42 | - | 72.3 ± 3.11 | 52.4 ± 2.51 | - | 27.0 ± 1.84 | ||||
| Sesquiterpene hydrocarbons (sh) | - | 5.8 ± 0.43 | 77.4 ± 2.96 | - | 32.6 ± 1.00 | 4.0 ± 1.87 | - | ||||
| Oxygenated sesquiterpenes (os) | - | 35.2 ± 2.29 | 1.3 ± 0.07 | 13.8 ± 1.94 | 12.0 ± 1.29 | 0.1 ± 0.03 | 46.1 ± 3.98 | ||||
| Oxygenated diterpenes (od) | - | 2.8 ± 0.84 | - | - | - | 0.6 ± 0.18 | 2.5 ± 0.56 | ||||
| Apocarotenoides (ac) | 1.3 ± 0.77 | 1.6 ± 0.69 | - | - | - | 15.7 ± 2.04 | 11.9 ± 1.12 | ||||
| Non-terpenes derivatives (nt) | 11.8 ± 1.02 | 41.2 ± 4.11 | 3.7 ± 0.53 | - | - | 77.5 ± 3.87 | 8.8 ± 1.87 | ||||
| Phenylpropanoids (pp) | - | 4.5 ± 0.85 | 17.6 ± 0.25 | 10.7 ± 0.43 | - | - | 1.6 ± 0.76 | ||||
| Total Identified (%) | 97.0 ± 2.31 | 92.7 ± 3.3 | 100 ± 0.00 | 96.8 ± 0.22 | 97.0 ± 0.17 | 97.9 ± 0.01 | 97.9 ± 0.63 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchioni, I.; Najar, B.; Ruffoni, B.; Copetta, A.; Pistelli, L.; Pistelli, L. Bioactive Compounds and Aroma Profile of Some Lamiaceae Edible Flowers. Plants 2020, 9, 691. https://doi.org/10.3390/plants9060691
Marchioni I, Najar B, Ruffoni B, Copetta A, Pistelli L, Pistelli L. Bioactive Compounds and Aroma Profile of Some Lamiaceae Edible Flowers. Plants. 2020; 9(6):691. https://doi.org/10.3390/plants9060691
Chicago/Turabian StyleMarchioni, Ilaria, Basma Najar, Barbara Ruffoni, Andrea Copetta, Luisa Pistelli, and Laura Pistelli. 2020. "Bioactive Compounds and Aroma Profile of Some Lamiaceae Edible Flowers" Plants 9, no. 6: 691. https://doi.org/10.3390/plants9060691
APA StyleMarchioni, I., Najar, B., Ruffoni, B., Copetta, A., Pistelli, L., & Pistelli, L. (2020). Bioactive Compounds and Aroma Profile of Some Lamiaceae Edible Flowers. Plants, 9(6), 691. https://doi.org/10.3390/plants9060691