Mechanical Signaling in the Sensitive Plant Mimosa pudica L.
Abstract
1. Introduction
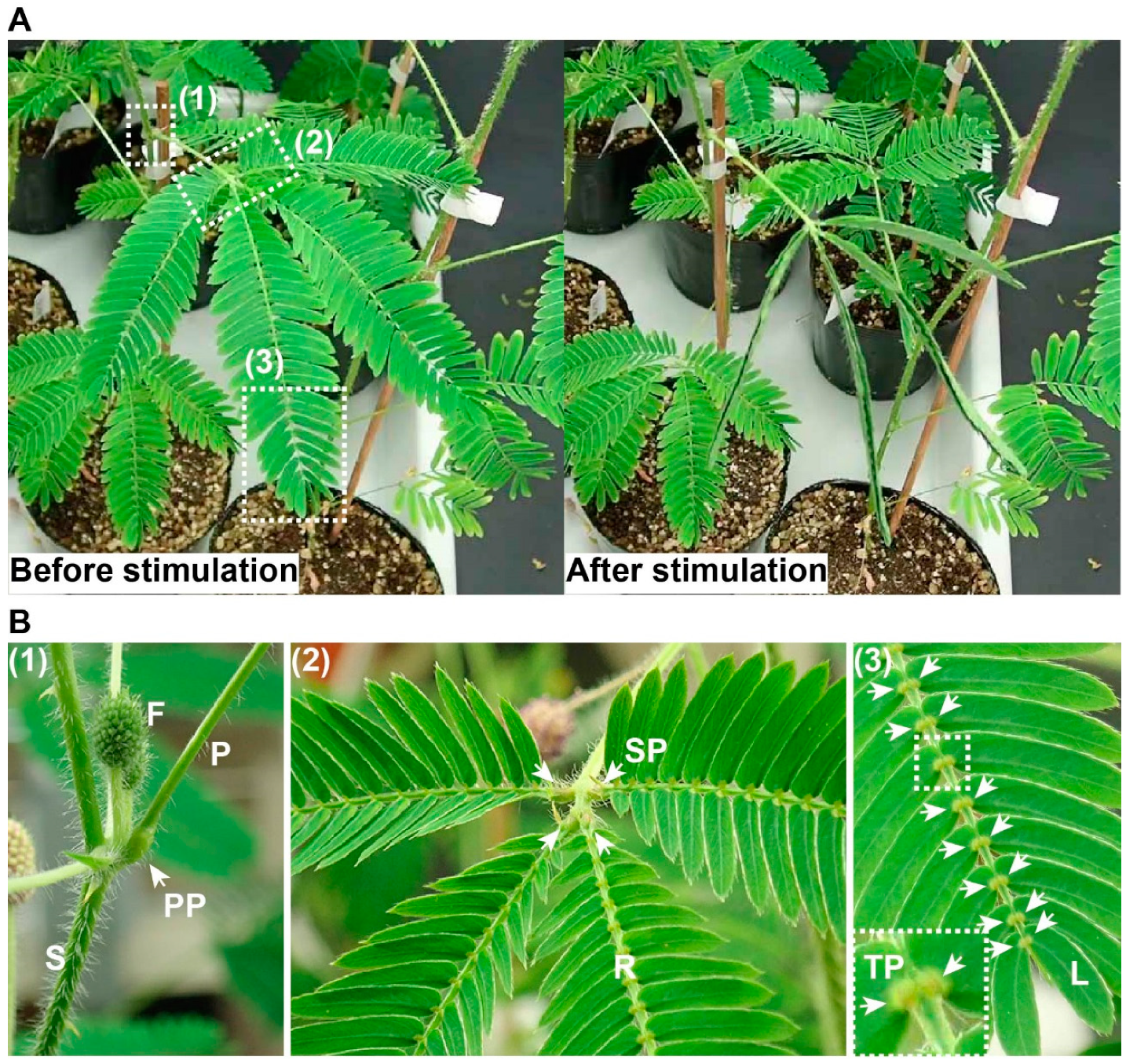
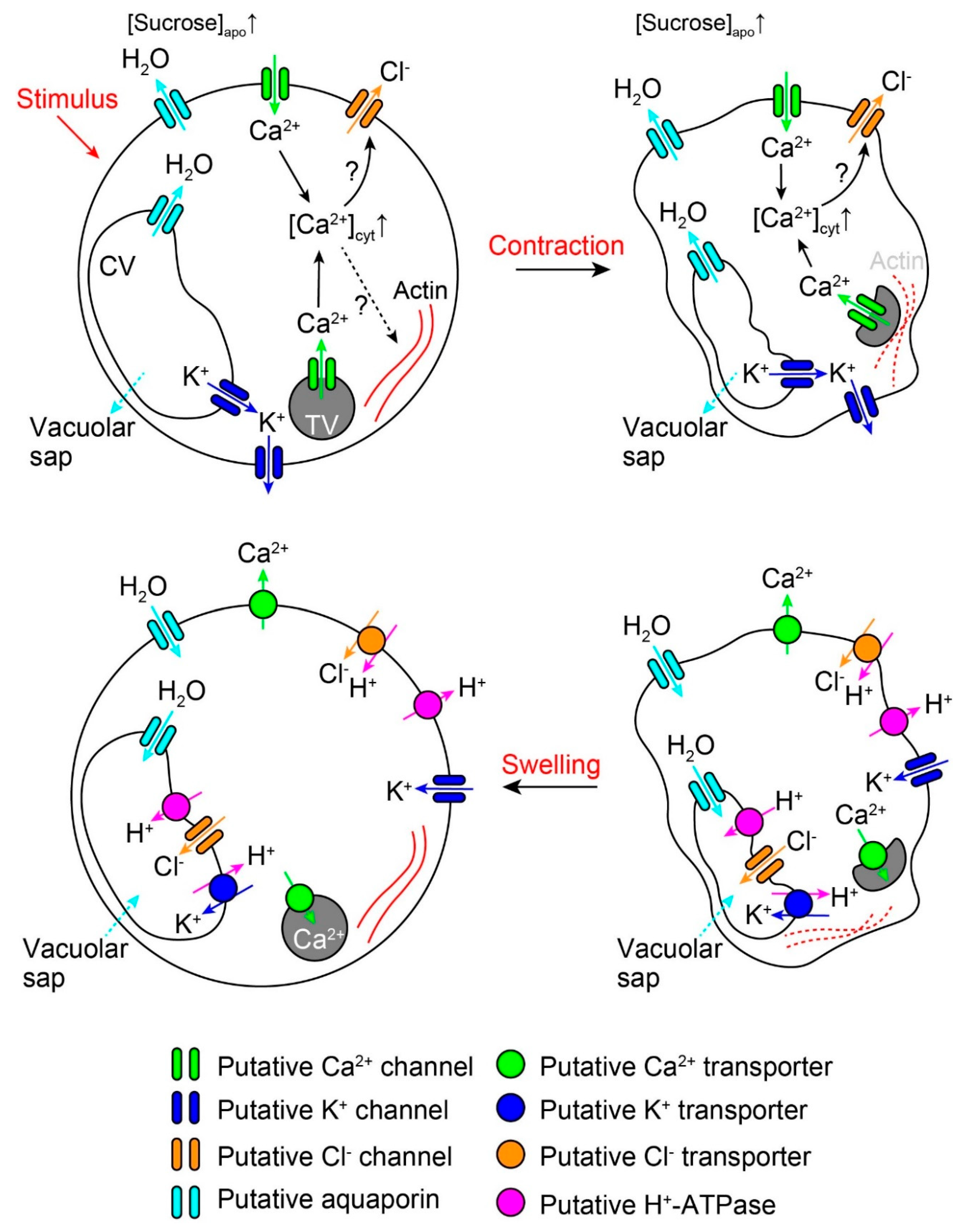
2. Rapid Movements
3. Long-Distance Signaling
4. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Christie, J.M.; Murphy, A.S. Shoot phototropism in higher plants: New light through old concepts. Am. J. Bot. 2013, 100, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Minorsky, P.V. The functions of foliar nyctinasty: A review and hypothesis. Biol. Rev. 2019, 94, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Darwin, C.; Darwin, F. The Power of Movement in Plants; John Murray: London, UK, 1880; p. 592. [Google Scholar]
- Snow, R. Conduction of excitation in stem and leaf of Mimosa pudica. Proc. R. Soc. B 1924, 96, 349–374. [Google Scholar]
- Ball, N.G. Rapid conduction of stimuli in Mimosa pudica. New Phytol. 1927, 26, 148–170. [Google Scholar] [CrossRef]
- Houwink, A.L. The conduction of excitation in Mimosa pudica. Recl. Trav. Bot. Neerl. 1935, 32, 51–91. [Google Scholar]
- Sibaoka, T. Action potentials in plant organs. Symp. Soc. Exp. Biol. 1966, 20, 49–73. [Google Scholar] [PubMed]
- Malone, M. Wound-induced hydraulic signals and stimulus transmission in Mimosa pudica L. New Phytol. 1994, 128, 49–56. [Google Scholar] [CrossRef]
- Asprey, G.F.; Palmer, J.H. A new interpretation of the mechanics of pulvinar movement. Nature 1955, 175, 1122. [Google Scholar] [CrossRef]
- Tamiya, T.; Miyazaki, T.; Ishikawa, H.; Iriguchi, N.; Maki, T.; Matsumoto, J.J.; Tsuchiya, T. Movement of water in conjunction with plant movement visualized by NMR imaging. J. Biochem. 1988, 104, 5–8. [Google Scholar] [CrossRef]
- Cote, G.G. Signal transduction in leaf movement. Plant Physiol. 1995, 109, 729–734. [Google Scholar] [CrossRef]
- Toriyama, H. Observational and experimental studies of sensitive plants VI. The migration of potassium in the primary pulvinus. Cytologia 1955, 20, 367–377. [Google Scholar] [CrossRef][Green Version]
- Allen, R.D. Mechanism of the seismonastic reaction in Mimosa pudica. Plant Physiol. 1969, 44, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Samejima, M.; Sibaoka, T. Changes in the extracellular ion concentration in the main pulvinus of Mimosa pudica during rapid movement and recovery. Plant Cell Physiol. 1980, 21, 467–479. [Google Scholar]
- Roblin, G.; Fleurat-Lessard, P. Redistribution of potassium, chloride and calcium during the gravitropically induced movement of Mimosa pudica pulvinus. Planta 1987, 170, 242–248. [Google Scholar] [CrossRef]
- Stoeckel, H.; Takeda, K. Plasmalemmal, voltage-dependent ionic currents from excitable pulvinar motor cells of Mimosa pudica. J. Membr. Biol. 1993, 131, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Fromm, J.; Eschrich, W. Transport processes in stimulated and non-stimulated leaves of Mimosa pudica I. The movement of 14C-labelled photoassimilates. Trees 1988, 2, 7–17. [Google Scholar] [CrossRef]
- Uehlein, N.; Kaldenhoff, R. Aquaporins and plant leaf movements. Ann. Bot. 2008, 101, 1–4. [Google Scholar] [CrossRef]
- Fleurat-Lessard, P.; Frangne, N.; Maeshima, M.; Ratajczak, R.; Bonnemain, J.L.; Martinoia, E. Increased expression of vacuolar aquaporin and H+-ATPase related to motor cell function in Mimosa pudica L. Plant Physiol. 1997, 114, 827–834. [Google Scholar] [CrossRef][Green Version]
- Temmei, Y.; Uchida, S.; Hoshino, D.; Kanzawa, N.; Kuwahara, M.; Sasaki, S.; Tsuchiya, T. Water channel activities of Mimosa pudica plasma membrane intrinsic proteins are regulated by direct interaction and phosphorylation. FEBS Lett. 2005, 579, 4417–4422. [Google Scholar] [CrossRef]
- Toriyama, H. Observational and Experimental Studies of Sensitive Plants VIII. The migration of colloidal substance in the primary pulvinus. Cytologia 1957, 22, 184–192. [Google Scholar] [CrossRef][Green Version]
- Bose, J. Comparative Electro-Physiology; Longmans, Green and Co.: London, UK, 1907. [Google Scholar]
- Abe, T.; Oda, K. Resting and action potentials of excitable cells in the main pulvinus of Mimosa pudica. Plant Cell Physiol. 1976, 17, 1343–1346. [Google Scholar]
- Samejima, M.; Sibaoka, T. Membrane potentials and resistances of excitable cells in the petiole and main pulvinus of Mimosa pudica. Plant Cell Physiol. 1982, 23, 459–465. [Google Scholar] [CrossRef]
- Oda, K.; Abe, T. Action potential and rapid movement in the main pulvinus of Mimosa pudica. Bot. Mag. Tokyo 1972, 85, 135–145. [Google Scholar] [CrossRef]
- Visnovitz, T.; Vilagi, I.; Varro, P.; Kristof, Z. Mechanoreceptor cells on the tertiary pulvini of Mimosa pudica L. Plant Signal. Behav. 2007, 2, 462–466. [Google Scholar] [CrossRef]
- Toyota, M.; Gilroy, S. Gravitropism and mechanical signaling in plants. Am. J. Bot. 2013, 100, 111–125. [Google Scholar] [CrossRef]
- Choi, H.W.; Klessig, D.F. DAMPs, MAMPs, and NAMPs in plant innate immunity. BMC Plant Biol. 2016, 16, 232. [Google Scholar] [CrossRef]
- Volkov, A.G.; Foster, J.C.; Baker, K.D.; Markin, V.S. Mechanical and electrical anisotropy in Mimosa pudica pulvini. Plant Signal. Behav. 2010, 5, 1211–1221. [Google Scholar] [CrossRef]
- Toriyama, H.; Jaffe, M.J. Migration of calcium and its role in the regulation of seismonasty in the motor cell of Mimosa pudica L. Plant Physiol. 1972, 49, 72–81. [Google Scholar] [CrossRef][Green Version]
- Campbell, N.A.; Thomson, W.W. Effects of lanthanum and ethylenediaminetetraacetate on leaf movements of Mimosa. Plant Physiol. 1977, 60, 635–639. [Google Scholar] [CrossRef]
- Turnquist, H.M.; Allen, N.S.; Jaffe, M.J. A pharmacological study of calcium flux mechanisms in the tannin vacuoles of Mimosa pudica L. motor cells. Protoplasma 1993, 176, 91–99. [Google Scholar] [CrossRef]
- Yao, H.; Xu, Q.; Yuan, M. Actin dynamics mediates the changes of calcium level during the pulvinus movement of Mimosa pudica. Plant Signal. Behav. 2008, 3, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Moysset, L.; Llambrich, E.; Simon, E. Calcium changes in Robinia pseudoacacia pulvinar motor cells during nyctinastic closure mediated by phytochromes. Protoplasma 2019, 256, 615–629. [Google Scholar] [CrossRef] [PubMed]
- Kanzawa, N.; Hoshino, Y.; Chiba, M.; Hoshino, D.; Kobayashi, H.; Kamasawa, N.; Kishi, Y.; Osumi, M.; Sameshima, M.; Tsuchiya, T. Change in the actin cytoskeleton during seismonastic movement of Mimosa pudica. Plant Cell Physiol. 2006, 47, 531–539. [Google Scholar] [CrossRef]
- Yamashiro, S.; Kameyama, K.; Kanzawa, N.; Tamiya, T.; Mabuchi, I.; Tsuchiya, T. The gelsolin/fragmin family protein identified in the higher plant Mimosa pudica. J. Biochem. 2001, 130, 243–249. [Google Scholar] [CrossRef]
- Kameyama, K.; Kishi, Y.; Yoshimura, M.; Kanzawa, N.; Sameshima, M.; Tsuchiya, T. Tyrosine phosphorylation in plant bending. Nature 2000, 407, 37. [Google Scholar] [CrossRef]
- Ueda, M.; Nakamura, Y. Chemical basis of plant leaf movement. Plant Cell Physiol. 2007, 48, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Fleurat-Lessard, P.; Bouche-Pillon, S.; Leloup, C.; Bonnemain, J.L. Distribution and activity of the plasma membrane H+-ATPase in Mimosa pudica L. in relation to ionic fluxes and leaf movements. Plant Physiol. 1997, 113, 747–754. [Google Scholar] [CrossRef]
- Kim, H.Y.; Cote, G.G.; Crain, R.C. Effects of light on the membrane potential of protoplasts from Samanea saman pulvini : Involvement of K+ channels and the H+-ATPase. Plant Physiol. 1992, 99, 1532–1539. [Google Scholar] [CrossRef]
- Eisenach, C.; De Angeli, A. Ion transport at the vacuole during stomatal movements. Plant Physiol. 2017, 174, 520–530. [Google Scholar] [CrossRef]
- Sibaoka, T. Excitable cells in Mimosa. Science 1962, 137, 226. [Google Scholar] [CrossRef]
- Samejima, M.; Sibaoka, T. Identification of the excitable cells in the petiole of Mimosa pudica by intracellular injection of procion yellow. Plant Cell Physiol. 1983, 24, 33–39. [Google Scholar] [CrossRef]
- Fromm, J.; Eschrich, W. Transport processes in stimulated and non-stimulated leaves of Mimosa pudica II. Energesis and transmission of seismic stimulations. Trees 1988, 2, 18–24. [Google Scholar] [CrossRef]
- Somers, D.A.; Samac, D.A.; Olhoft, P.M. Recent advances in legume transformation. Plant Physiol. 2003, 131, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Mano, H.; Fujii, T.; Sumikawa, N.; Hiwatashi, Y.; Hasebe, M. Development of an Agrobacterium-mediated stable transformation method for the sensitive plant Mimosa pudica. PLoS ONE 2014, 9, e88611. [Google Scholar] [CrossRef]
- DeFalco, T.A.; Toyota, M.; Phan, V.; Karia, P.; Moeder, W.; Gilroy, S.; Yoshioka, K. Using GCaMP3 to study Ca2+ signaling in Nicotiana Species. Plant Cell Physiol. 2017, 58, 1173–1184. [Google Scholar] [CrossRef]
- Vincent, T.R.; Avramova, M.; Canham, J.; Higgins, P.; Bilkey, N.; Mugford, S.T.; Pitino, M.; Toyota, M.; Gilroy, S.; Miller, A.J.; et al. Interplay of plasma membrane and vacuolar ion channels, together with BAK1, elicits rapid cytosolic calcium elevations in Arabidopsis during aphid feeding. Plant Cell 2017, 29, 1460–1479. [Google Scholar] [CrossRef]
- Toyota, M.; Spencer, D.; Sawai-Toyota, S.; Jiaqi, W.; Zhang, T.; Koo, A.J.; Howe, G.A.; Gilroy, S. Glutamate triggers long-distance, calcium-based plant defense signaling. Science 2018, 361, 1112–1115. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hagihara, T.; Toyota, M. Mechanical Signaling in the Sensitive Plant Mimosa pudica L. Plants 2020, 9, 587. https://doi.org/10.3390/plants9050587
Hagihara T, Toyota M. Mechanical Signaling in the Sensitive Plant Mimosa pudica L. Plants. 2020; 9(5):587. https://doi.org/10.3390/plants9050587
Chicago/Turabian StyleHagihara, Takuma, and Masatsugu Toyota. 2020. "Mechanical Signaling in the Sensitive Plant Mimosa pudica L." Plants 9, no. 5: 587. https://doi.org/10.3390/plants9050587
APA StyleHagihara, T., & Toyota, M. (2020). Mechanical Signaling in the Sensitive Plant Mimosa pudica L. Plants, 9(5), 587. https://doi.org/10.3390/plants9050587




