MCA1 and MCA2 Are Involved in the Response to Hypergravity in Arabidopsis Hypocotyls
Abstract
1. Introduction
2. Results
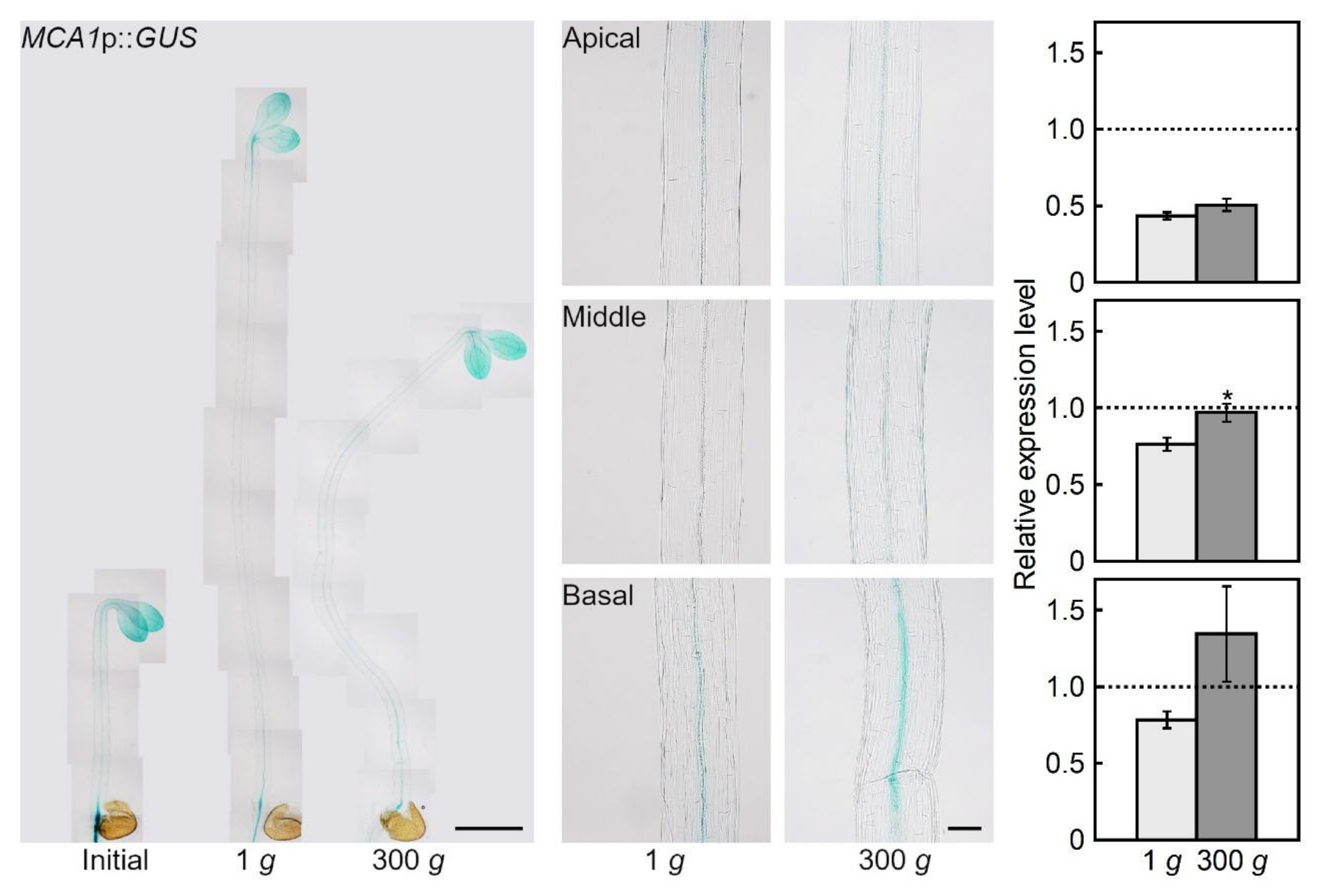
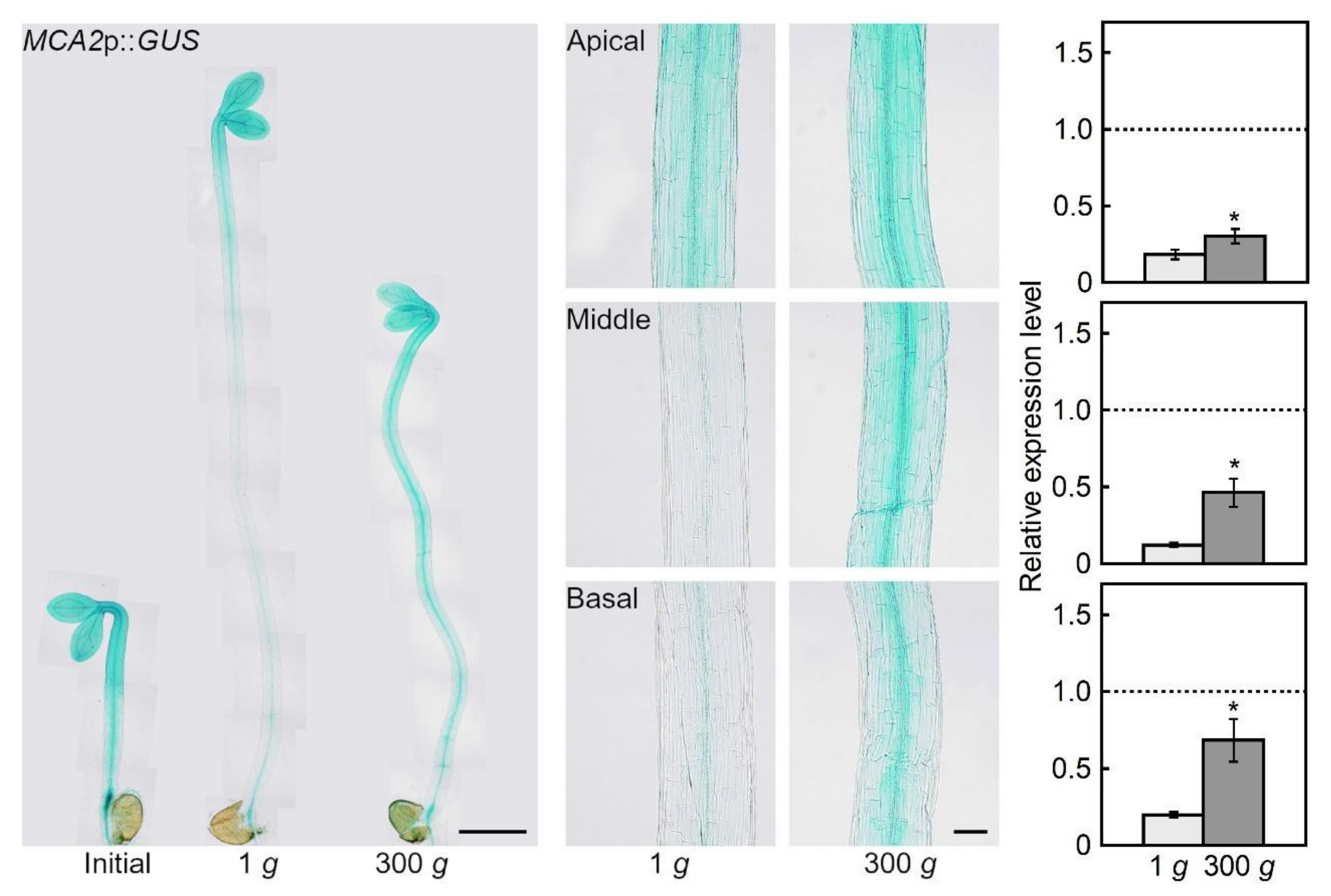
2.1. Hypergravity-induced Expression of MCA Genes
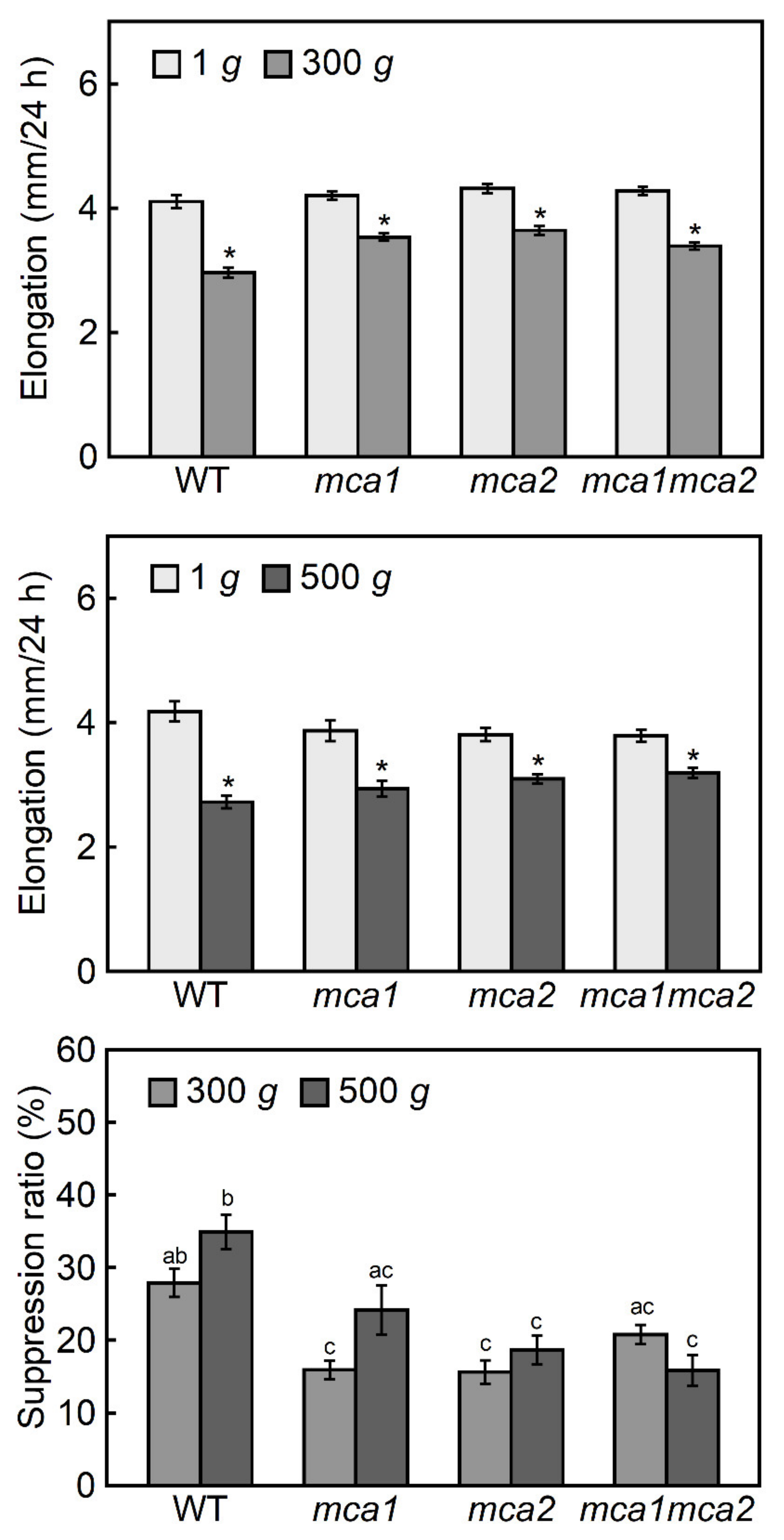
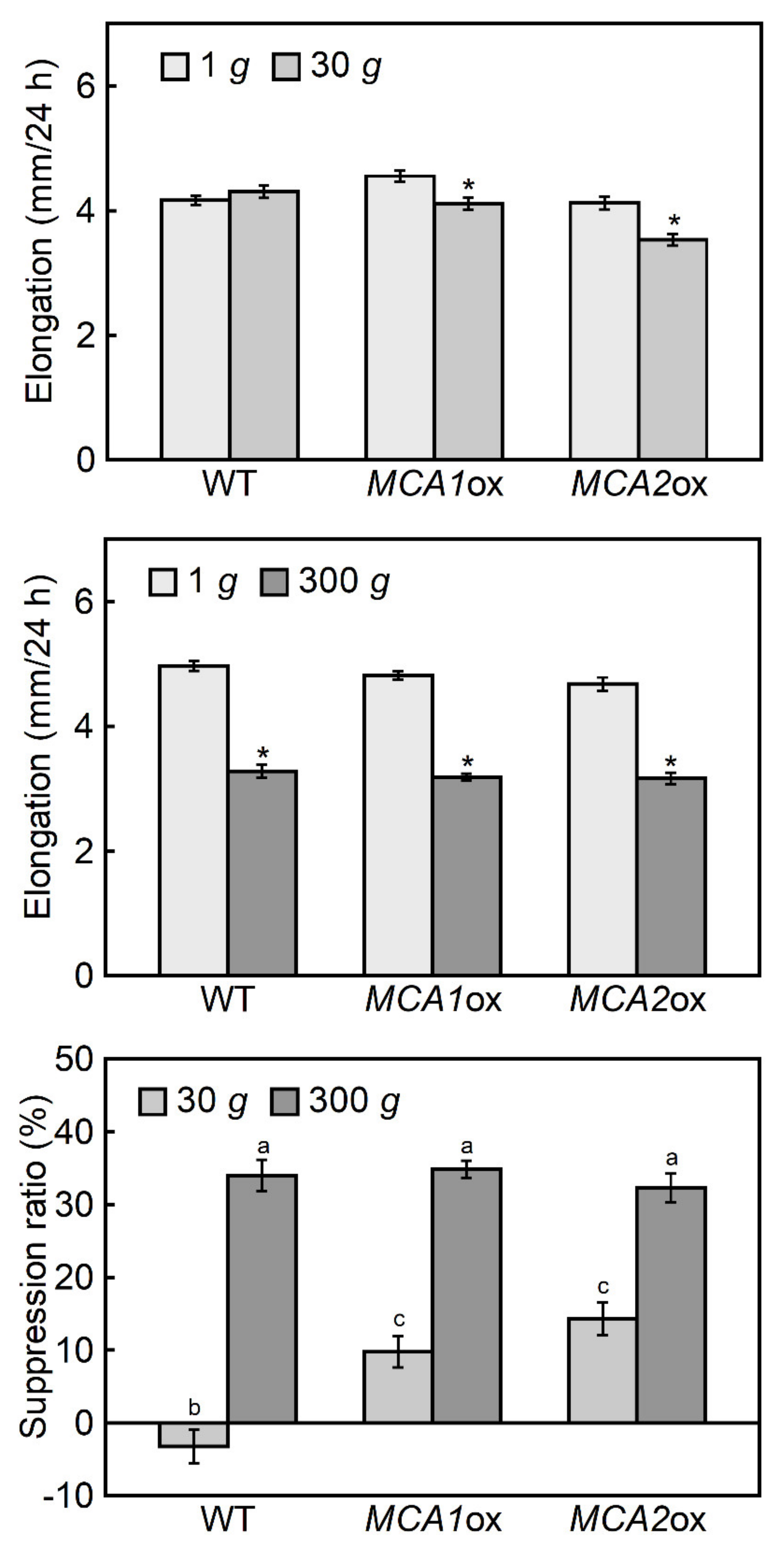
2.2. Hypergravity-Induced Growth of Mca-Null and MCA-Overexpression Mutants
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Analysis
4.2. Analysis of MCA Gene Expression
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hoson, T.; Soga, K. New aspects of gravity responses in plant cells. Int. Rev. Cytol. 2003, 229, 209–244. [Google Scholar] [PubMed]
- Hoson, T.; Saito, Y.; Soga, K.; Wakabayashi, K. Signal perception, transduction, and response in gravity resistance. Another graviresponse in plants. Adv. Space Res. 2005, 36, 1196–1202. [Google Scholar] [CrossRef]
- Hoson, T.; Wakabayashi, K. Role of the plant cell wall in gravity resistance. Phytochemistry 2015, 112, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Soga, K.; Wakabayashi, K.; Kamisaka, S.; Hoson, T. Hypergravity induces reorientation of cortical microtubules and modifies growth anisotropy in azuki bean epicotyls. Planta 2006, 224, 1485–1494. [Google Scholar] [CrossRef]
- Wasteneys, G.O.; Galway, M.E. Remodeling the cytoskeleton for growth and form: An overview with some new views. Annu. Rev. Plant Biol. 2003, 54, 691–722. [Google Scholar] [CrossRef]
- Baskin, T.I. Anisotropic expansion of the plant cell wall. Annu. Rev. Cell Dev. Biol. 2005, 21, 203–222. [Google Scholar] [CrossRef]
- Matsumoto, S.; Saito, Y.; Kumasaki, S.; Soga, K.; Wakabayashi, K.; Hoson, T. Up-regulation of tubulin genes and roles of microtubules in hypergravity-induced growth modifications in Arabidopsis hypocotyls. Adv. Space Res. 2007, 39, 1176–1181. [Google Scholar] [CrossRef]
- Soga, K.; Kotake, T.; Wakabayashi, K.; Kamisaka, S.; Hoson, T. Transient increase in the transcript levels of γ-tubulin complex genes during reorientation of cortical microtubules by gravity in azuki bean (Vigna angularis) epicotyls. J. Plant Res. 2008, 121, 493–498. [Google Scholar] [CrossRef]
- Soga, K.; Kotake, T.; Wakabayashi, K.; Kamisaka, S.; Hoson, T. The transcript level of katanin gene is increased transiently in response to changes in gravitational conditions in azuki bean epicotyls. Biol. Sci. Space 2009, 23, 23–28. [Google Scholar] [CrossRef][Green Version]
- Ding, J.P.; Pickard, B.G. Mechanosensory calcium-selective cation channels in epidermal cells. Plant J. 1993, 3, 83–110. [Google Scholar] [CrossRef]
- Fasano, J.M.; Massa, G.D.; Gilroy, S. Ionic signaling in plant responses to gravity and touch. J. Plant Growth Regul. 2002, 21, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Soga, K.; Wakabayashi, K.; Kamisaka, S.; Hoson, T. Graviperception ingrowth inhibition of plant shoots under hypergravity conditions producedby centrifugation is independent of that in gravitropism and may involve mechanoreceptors. Planta 2004, 218, 1054–1061. [Google Scholar] [PubMed]
- Soga, K.; Wakabayashi, K.; Kamisaka, S.; Hoson, T. Hypergravity inhibits elongation growth of azuki bean epicotyls independently of the direction of stimuli. Adv. Space Res. 2005, 36, 1269–1276. [Google Scholar] [CrossRef]
- Matsumoto, S.; Kumasaki, S.; Soga, K.; Wakabayashi, K.; Hashimoto, T.; Hoson, T. Gravity-induced modifications to development in hypocotyls of Arabidopsis tubulin mutants. Plant Physiol. 2010, 152, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Katagiri, T.; Shinozaki, K.; Qi, Z.; Tatsumi, H.; Furuichi, T.; Kishigami, A.; Sokabe, M.; Kojima, I.; Sato, S.; et al. Arabidopsis plasma membrane protein crucial for Ca2+ influx and touch sensing in roots. Proc. Natl. Acad. Sci. USA 2007, 104, 3639–3644. [Google Scholar] [CrossRef]
- Yamanaka, T.; Nakagawa, Y.; Mori, K.; Nakano, M.; Imamura, T.; Kataoka, H.; Terashima, A.; Iida, K.; Kojima, I.; Katagiri, T.; et al. MCA1 and MCA2 that mediate Ca2+ uptake have distinct and overlapping roles in Arabidopsis. Plant Physiol. 2010, 152, 1284–1296. [Google Scholar] [CrossRef]
- Kamano, S.; Kume, S.; Iida, K.; Lei, K.J.; Nakano, M.; Nakayama, Y.; Iida, H. Transmembrane Topologies of Ca2+-permeable Mechanosensitive Channels MCA1 and MCA2 in Arabidopsis thaliana. J. Biol. Chem. 2015, 290, 30901–30909. [Google Scholar] [CrossRef]
- Kurusu, T.; Yamanaka, T.; Nakano, M.; Takiguchi, A.; Ogasawara, Y.; Hayashi, T.; Iida, K.; Hanamata, S.; Shinozaki, K.; Iida, H.; et al. Involvement of the putative Ca²⁺-permeable mechanosensitive channels, NtMCA1 and NtMCA2, in Ca²⁺ uptake, Ca²⁺-dependent cell proliferation and mechanical stress-induced gene expression in tobacco (Nicotiana tabacum) BY-2 cells. J. Plant Res. 2012, 125, 555–568. [Google Scholar] [CrossRef]
- Furuichi, T.; Iida, H.; Sokabe, M.; Tatsumi, H. Expression of Arabidopsis MCA1 enhanced mechanosensitive channel activity in the Xenopus laevis oocyte plasma membrane. Plant Signal. Behav. 2012, 7, 1022–1026. [Google Scholar] [CrossRef]
- Mori, K.; Renhu, N.; Naito, M.; Nakamura, A.; Shiba, H.; Yamamoto, T.; Suzaki, T.; Iida, H.; Miura, K. Ca2+-permeable mechanosensitive channels MCA1 and MCA2 mediate cold-induced cytosolic Ca2+ increase and cold tolerance in Arabidopsis. Sci. Rep. 2018, 8, 550. [Google Scholar] [CrossRef]
- Hoson, T. Interaction of gravity with other environmental factors in growth and development: An introduction. Adv. Space Res. 1999, 23, 1971–1974. [Google Scholar] [CrossRef]
- Haswell, E.S.; Peyronnet, R.; Barbier-Brygoo, H.; Meyerowitz, E.M.; Frachisse, J.M. Two MscS homologs provide mechanosensitive channel activities in the Arabidopsis root. Curr. Biol. 2008, 18, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Soga, K.; Yano, S.; Matsumoto, S.; Hoson, T. Plant Gravitropism. Methods in Molecular Biology; Blancaflor, E., Ed.; Humana Press: New York, NY, USA, 2015; Volume 1309, pp. 307–319. [Google Scholar]
- Béziat, C.; Kleine-Vehn, J.; Feraru, E. Histochemical staining of β-glucuronidase and its spatial quantification. In Plant Hormones. Methods in Molecular Biology; Kleine-Vehn, J., Sauer, M., Eds.; Humana Press: New York, NY, USA, 2017; Volume 1497, pp. 73–80. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hattori, T.; Otomi, Y.; Nakajima, Y.; Soga, K.; Wakabayashi, K.; Iida, H.; Hoson, T. MCA1 and MCA2 Are Involved in the Response to Hypergravity in Arabidopsis Hypocotyls. Plants 2020, 9, 590. https://doi.org/10.3390/plants9050590
Hattori T, Otomi Y, Nakajima Y, Soga K, Wakabayashi K, Iida H, Hoson T. MCA1 and MCA2 Are Involved in the Response to Hypergravity in Arabidopsis Hypocotyls. Plants. 2020; 9(5):590. https://doi.org/10.3390/plants9050590
Chicago/Turabian StyleHattori, Takayuki, Yasuhiro Otomi, Yohei Nakajima, Kouichi Soga, Kazuyuki Wakabayashi, Hidetoshi Iida, and Takayuki Hoson. 2020. "MCA1 and MCA2 Are Involved in the Response to Hypergravity in Arabidopsis Hypocotyls" Plants 9, no. 5: 590. https://doi.org/10.3390/plants9050590
APA StyleHattori, T., Otomi, Y., Nakajima, Y., Soga, K., Wakabayashi, K., Iida, H., & Hoson, T. (2020). MCA1 and MCA2 Are Involved in the Response to Hypergravity in Arabidopsis Hypocotyls. Plants, 9(5), 590. https://doi.org/10.3390/plants9050590





