Why Seed Physiology Is Important for Genebanking
Abstract
1. Introduction
2. Understanding Why a Seed Lot Is Showing No or Low Germination
2.1. Viability versus Germinability
2.2. Seed Dormancy and Germination Behaviour
2.3. Desiccation Tolerance
2.4. Seed Ageing
3. Understanding How Long a Seed Lot Will Continue to Show Good Germination and the Factors That Influence That Period
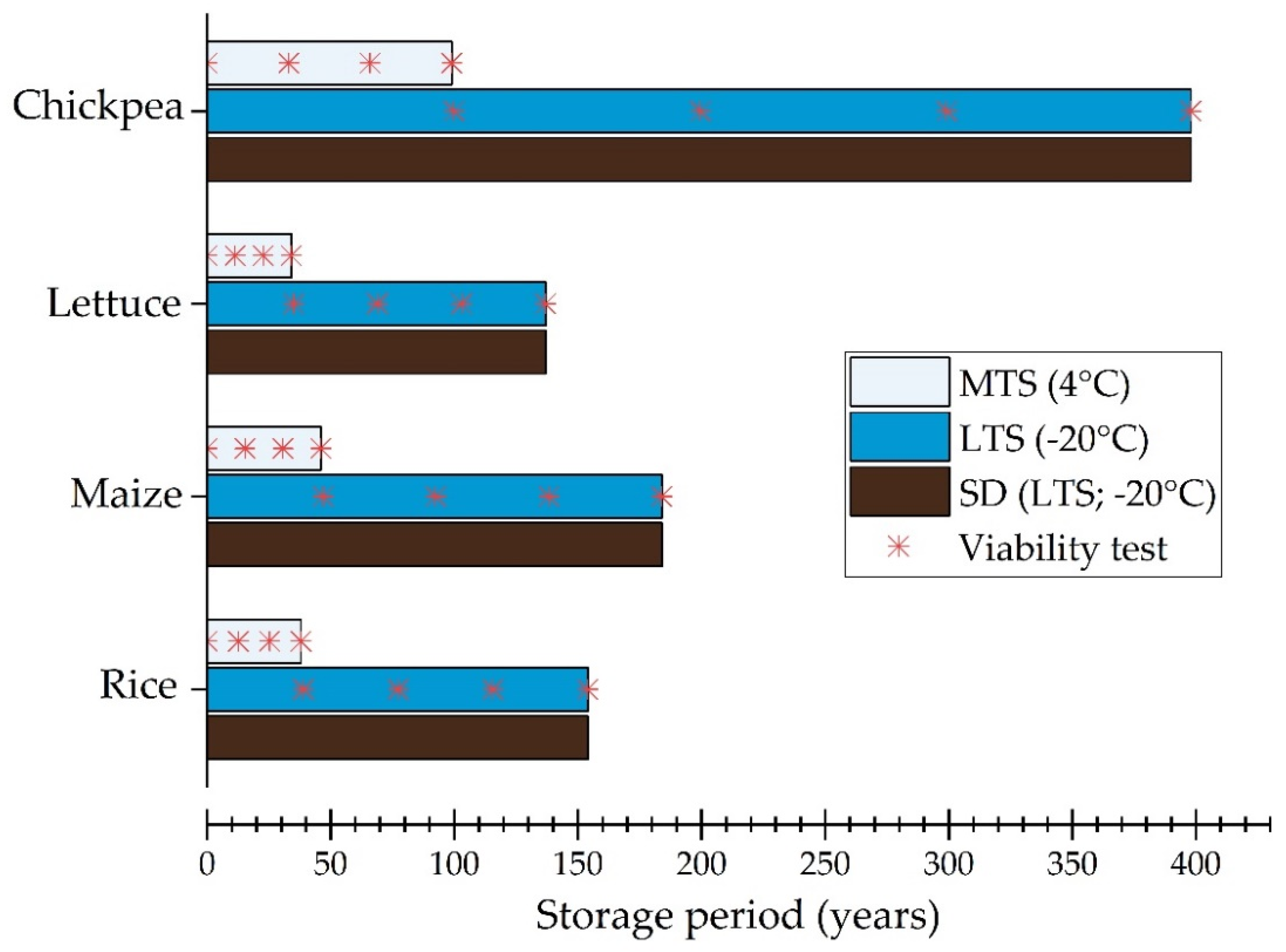
3.1. Historical Viability Monitoring Data
3.2. Seed Longevity Predictions
3.3. Comparative Seed Longevity Studies
3.4. Maturity at Harvest
3.5. Post-Harvest Handling
3.6. Length of Time Before Storage
4. Statistics of Seed Testing
4.1. Comparing Two Germination/Viability Results
4.2. Analysis of a Factorial Germination Experiment
4.3. Analysing a Series of Germination Results
5. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smith, R.D.; Dickie, J.B.; Linington, S.H.; Pritchard, H.W.; Probert, R.J. (Eds.) Seed Conservation: Turning Science into Practice; Royal Botanic Gardens Kew: Richmond, UK, 2003. [Google Scholar]
- Hay, F.R.; Probert, R.J. Advances in seed conservation of wild plant species: A review of recent research. Cons. Phys. 2013, 1, cot030. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.D.; Linington, S.; Ellis, R.H. Seed storage behaviour: A compendium. In Handbook for Genebanks No. 4; International Plant Genetic Resources: Rome, Italy, 1996. [Google Scholar]
- Sackville Hamilton, N.R.; Chorlton, K.H. Regeneration of accessions in seed collections: A decision guide. In Handbook for Genebanks No. 5; International Plant Genetic Resources Institute: Rome, Italy, 1997. [Google Scholar]
- ISTA. International Rules for Seed Testing; International Seed Testing Association: Basserdorf, Switzerland, 2020. [Google Scholar]
- Pritchard, H.W. Determination of orchid seed viability using fluorescein diacetate. Plant Cell Environ. 1985, 8, 727–730. [Google Scholar]
- Wood, C.B.; Pritchard, H.W. Determination of intra-specific variation in orchid seed viability using fluorescein diacetate. Seed Sci. Technol. 2004, 32, 629–635. [Google Scholar] [CrossRef]
- Noland, T.L.; Mohammed, G.H. Fluorescein diacetate as a viability stain for tree roots and seeds. New For. 1997, 14, 221–232. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. When breaking seed dormancy is a problem. Try a move-along experiment. NPJ 2003, 4, 17–21. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds, Ecology, Biogeography, and Evolution of Dormancy and Germination, 2nd ed.; Academic Press: San Diego, CA, USA; London, UK; Waltham, MA, USA, 2014. [Google Scholar]
- Ellis, R.H.; Hong, T.D.; Roberts, E.H. Handbook of Seed Technology for Genebanks. Volume I. Principles and Methodology; International Board for Plant Genetic Resources: Rome, Italy, 1985. [Google Scholar]
- Ellis, R.H.; Hong, T.D.; Roberts, E.H. Handbook of Seed Technology for Genebanks. Volume II. Compendium of Specific Germination Information and Test Recommendations; International Board for Plant Genetic Resources: Rome, Italy, 1985. [Google Scholar]
- Royal Botanic Gardens Kew: Seed Information Database (SID). Version 7.1. Available online: http://data.kew.org/sid/ (accessed on 10 March 2020).
- Crawford, A.D.; Steadman, K.J.; Plummer, J.A.; Cochrane, A.; Probert, R.J. Analysis of seed-bank data confirms suitability of international seed-storage standards for the Australian flora. Aus. J. Bot. 2007, 55, 18–29. [Google Scholar] [CrossRef]
- Pérez-García, F.; Gómez-Campo, C.; Ellis, R.H. Successful long-term ultra-dry storage of seed of 15 species of Brassicaceae in a genebank: Variation in ability to germinate over 40 years and dormancy. Seed Sci. Technol. 2009, 37, 640–649. [Google Scholar] [CrossRef]
- González-Benito, M.E.; Pérez-García, F.; Tejeda, G.; Gómez-Campo, C. Effect of gaseous environment and water content on seed viability of four Brassicaceae species after 36 years. Seed Sci. Technol. 2011, 39, 443–451. [Google Scholar] [CrossRef]
- Wyse, S.V.; Dickie, J.B. Predicting the global incidence of seed desiccation sensitivity. J. Ecol. 2017, 105, 1082–1093. [Google Scholar] [CrossRef]
- Ellis, R.H. Temporal patterns of seed quality development, decline, and timing of maximum quality during seed development and maturation. Seed Sci. Res. 2019, 29, 135–142. [Google Scholar] [CrossRef]
- Hay, F.R.; Probert, R.J. Seed maturity and the effects of different drying conditions on desiccation tolerance and seed longevity in foxglove (Digitalis purpurea L.). Ann. Bot. 1995, 76, 639–647. [Google Scholar] [CrossRef]
- Bewley, J.D.; Bradford, J.K.; Hilhorst, H.W.M.; Nonogaki, H. Seeds. Physiology of development, Germination and Dormancy, 3rd ed.; Springer: New York, NY, USA, 2013. [Google Scholar]
- Leprince, O.; Deltour, R.; Thorpe, P.C.; Atherton, N.M.; Hendry, G.A.F. The role of free radicals and radical processing systems in loss of desiccation tolerance in germinating maize (Zea mays L.). New Phytol. 1990, 116, 573–580. [Google Scholar] [CrossRef]
- Buitink, J.; Leprince, O.; Hoekstra, F.A. Dehydration-induced redistribution of amphiphilic molecules between cytoplasm and lipids is associated with desiccation tolerance in seeds. Plant Physiol. 2000, 124, 1413–1425. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, F.A.; Golovina, E.A.; Buitink, J. Mechanisms of plant desiccation tolerance. Trends Plant Sci. 2001, 6, 431–438. [Google Scholar] [CrossRef]
- Vertucci, C.W.; Leopold, A.C. Bound water in soybean seed and its relation to respiration and imbibitional damage. Plant Physiol. 1984, 75, 114–117. [Google Scholar] [CrossRef]
- Vertucci, C.W.; Leopold, A.C. Physiological activities associated with hydration level in seeds. In Membranes, Metabolism and Dry Organisms; Leopold, A.C., Ed.; Cornell University: Ithaca, NY, USA, 1986; pp. 35–49. [Google Scholar]
- Vertucci, C.W. The effects of low water contents on physiological activities of seeds. Physiol. Plant. 1989, 77, 172–179. [Google Scholar] [CrossRef]
- Roberts, E.H.; Ellis, R.H. Water and seed survival. Ann. Bot. 1989, 63, 39–52. [Google Scholar] [CrossRef]
- Ellis, R.H.; Hong, T.D. Seed longevity—Moisture content relationships in hermetic and open storage. Seed Sci. Technol. 2007, 35, 423–431. [Google Scholar] [CrossRef]
- Zinsmeister, J.; Leprince, O.; Buitink, J. Molecular and environmental factors regulating seed longevity. Biochem. J. 2020, 477, 305–323. [Google Scholar] [CrossRef]
- Corbineau, F. Markers of seed quality: From present to future. Seed Sci. Res. 2012, 22, 61–68. [Google Scholar] [CrossRef]
- Waterworth, W.M.; Bray, C.M.; West, C.E. The importance of safeguarding genome integrity in germination and seed longevity. J. Exp. Bot. 2015, 66, 3549–3558. [Google Scholar] [CrossRef] [PubMed]
- Waterworth, W.; Masnavi, G.; Bhardwaj, R.M.; Jiang, Q.; Bray, C.M.; West, C.E. A plant DNA ligase is an important determinant of seed longevity. Plant J. 2010, 63, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Dona, M.; Balestrazzi, A.; Mondoni, A.; Rossi, G.; Ventura, L.; Buttafava, A.; Macavei, A.; Sabatini, M.E.; Valassi, A.; Cabonera, D. DNA profiling, telomere analysis and antioxidant properties as tools for monitoring ex situ seed longevity. Ann. Bot. 2013, 111, 987–998. [Google Scholar] [CrossRef] [PubMed]
- FAO. Genebank Standards for Plant Genetic Resources for Food and Agriculture, Rev Ed.; FAO: Rome, Italy, 2014. [Google Scholar]
- Royal Botanic Gardens Kew: Seed Conservation Standards for ‘MSB Partnership Collections’. Final Version February 2015. Updated November 2019. Available online: http://brahmsonline.kew.org/Content/Projects/msbp/resources/Training/MSBP-Seed-Conservation-Standards.pdf (accessed on 10 March 2020).
- Walters, C.; Wheeler, L.M.; Grotenhuis, J.M. Longevity of seeds stored in a Genebank: Species characteristics. Seed Sci. Res. 2005, 15, 1–20. [Google Scholar] [CrossRef]
- Lee, H.S.; Jeon, Y.A.; Lee, Y.Y.; Lee, S.Y.; Kim, Y.G. Comparison of seed viability among 42 species stored in a genebank. Korean J. Crop. Sci. 2013, 58, 432–438. [Google Scholar] [CrossRef]
- Van Treuren, R.; de Groot, E.C.; van Hintum, T.J.L. Preservation of seed viability during 25 years of storage under standard genebank conditions. Genet. Resour. Crop Evol. 2013, 60, 1407–1421. [Google Scholar] [CrossRef]
- Van Treuren, R.; Bas, N.; Kodde, J.; Groot, S.P.C.; Kik, C. Rapid loss of seed viability in ex situ conserved wheat and barley at 4 °C as compared to −20 °C storage. Conserv. Physiol. 2018, 6, coy033. [Google Scholar] [CrossRef]
- Hay, F.R.; de Guzman, F.; Sackville Hamilton, N.R. Viability monitoring intervals for genebank samples of Oryza sativa. Seed Sci. Technol. 2015, 43, 218–237. [Google Scholar] [CrossRef]
- Ellis, R.H.; Nasehzadeh, M.; Hanson, J.; Woldemariam, Y. Medium-term seed storage of 50 genera of forage legumes and evidence-based genebank monitoring intervals. Genet. Resour. Crop Evol. 2018, 65, 207–623. [Google Scholar] [CrossRef]
- Ellis, R.H.; Nasehzadeh, M.; Hanson, J.; Ndiwa, N.; Woldemariam, Y. Medium-term seed storage of diverse genera of forage grasses, evidence-based genebank monitoring intervals, and regeneration standards. Genet. Resour. Crop. Evol. 2019, 66, 723–734. [Google Scholar] [CrossRef]
- Brüning, R.S.; Oppermann, M.; Willner, E.; Börner, A.; Nagel, M. Application of probit analysis and neural networks to predict seed longevity. In Proceedings of the ISTA Seed Symposium of the 32nd ISTA Congress, Hyderabad, India, 26–28 June 2019; pp. 2–16. [Google Scholar]
- Roberts, E.H. Predicting the storage life of seeds. Seed Sci. Technol. 1973, 1, 499–514. [Google Scholar]
- Ellis, R.H.; Roberts, E.H. Improved equations for the prediction of seed longevity. Ann. Bot. 1980, 45, 13–30. [Google Scholar] [CrossRef]
- Dickie, J.B.; Ellis, R.H.; Kraak, H.L.; Ryder, K.; Tompsett, P.B. Temperature and seed storage longevity. Ann. Bot. 1990, 65, 197–204. [Google Scholar] [CrossRef]
- Hay, F.R.; Mead, A.; Manger, K.; Wilson, F.J. One-step analysis of seed storage data and the longevity of Arabidopsis thaliana seeds. J. Exp. Bot. 2003, 54, 737–746. [Google Scholar] [CrossRef]
- Hay, F.R.; Whitehouse, K.J. Rethinking the approach to viability monitoring in seed genebanks. Conserv. Physiol. 2017, 5. [Google Scholar] [CrossRef]
- Lee, J.S.; Velasco-Punzalan, M.; Pacleb, M.; Valdez, R.; Kretzschmar, T.; McNally, K.L.; Ismail, A.M.; Sta Cruz, P.C.; Sackville Hamilton, N.R.; Hay, F.R. Variation in seed longevity among diverse Indica rice varieties. Ann. Bot. 2019, 124, 447–460. [Google Scholar] [CrossRef]
- Whitehouse, K.J.; Hay, F.R.; Ellis, R.H. Improvement in rice seed storage longevity from high-temperature drying is a consistent positive function of harvest moisture content above a critical value. Seed Sci. Res. 2018, 28, 332–339. [Google Scholar] [CrossRef]
- Butler, L.H.; Hay, F.R.; Ellis, R.H.; Smith, R.D.; Murray, T.B. Priming and re-drying improve the survival of mature seeds of Digitalis purpurea during storage. Ann. Bot. 2009, 103, 1261–1270. [Google Scholar] [CrossRef]
- Whitehouse, K.J.; Hay, F.R.; Ellis, R.H. Increases in desiccation-phase developing rice seeds: Response to high-temperature drying depends on harvest moisture content. Ann. Bot. 2015, 116, 247–259. [Google Scholar] [CrossRef]
- Ellis, R.H.; Hong, T.D. Temperature sensitivity of the low-moisture-content limit to negative seed longevity–moisture content relationships in hermetic storage. Ann. Bot. 2006, 97, 785–791. [Google Scholar] [CrossRef]
- Kameswara Rao, N.; Dulloo, M.E.; Engels, J.M.M. A review of factors that influence the production of quality seed for long-term conservation in genebanks. Genet. Resour. Crop. Evol. 2017, 64, 1061–1074. [Google Scholar] [CrossRef]
- Ellis, R.H.; Hong, T.D.; Roberts, E.H.; Tao, K.-L. Low moisture content limits to relations between seed longevity and moisture. Ann. Bot. 1990, 65, 493–504. [Google Scholar] [CrossRef]
- FAO/IPGRI. Genebank Standards; Food and Agriculture Organization of the United Nations, Rome, International Plant Genetic Resources Institute: Rome, Italy, 1994. [Google Scholar]
- Gold, K.; Manger, K. Measuring Seed Moisture Status Using a Hygrometer. Millennium Seed Bank Partnership Technical Information Sheet 5. Royal Botanic Gardens Kew, UK. 2014. Available online: http://brahmsonline.kew.org/Content/Projects/msbp/resources/Training/05-eRH-moisture-measurement.pdf (accessed on 15 April 2020).
- Hung, L.Q.; Hong, T.D.; Ellis, R.H. Constant, fluctuating and effective temperature and seed longevity: A tomato (Lycopersicon esculentum Mill.) exemplar. Ann. Bot. 2001, 88, 465–470. [Google Scholar] [CrossRef][Green Version]
- Newton, R.; Hay, F.R.; Probert, R. A Protocol for Comparative Seed Longevity Testing. Millennium Seed Bank Partnership Technical Information Sheet 1. Royal Botanic Gardens Kew, UK. 2014. Available online: http://brahmsonline.kew.org/Content/Projects/msbp/resources/Training/01-Comparative-longevity.pdf (accessed on 15 April 2020).
- Hay, F.R.; Valdes, R.; Lee, J.S.; Sta Cruz, P.C. Seed longevity phenotyping: Recommendations on research methodology. J. Exp. Bot. 2019, 70, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Probert, R.J.; Adams, J.; Coneybeer, J.; Crawford, A.; Hay, F. Seed quality for conservation is critically affected by pre-storage factors. Aus. J. Bot. 2007, 55, 326–335. [Google Scholar] [CrossRef]
- Merritt, D.J.; Martyn, A.J.; Ainsley, P.; Young, R.E.; Seed, L.U.; Thorpe, M.; Hay, F.R.; Commander, L.E.; Shackelford, N.; Offord, C.A.; et al. A continental-scale study of seed lifespan in storage reveals seed, plant, and environmental traits associated with longevity. Bio. Conserv. 2014, 23, 1081–1104. [Google Scholar] [CrossRef]
- Mondoni, A.; Probert, R.J.; Rossi, G.; Vegini, E.; Hay, F.R. Seeds of alpine plants are short lived: Implications for long-term conservation. Ann. Bot. 2011, 107, 171–179. [Google Scholar] [CrossRef]
- Hay, F.R.; Smith, R.D.; Ellis, R.H.; Butler, L.H. Developmental changes in the germinability, desiccation tolerance, hardseededness, and longevity of individual seeds of Trifolium ambiguum. Ann. Bot. 2010, 105, 1035–1052. [Google Scholar] [CrossRef]
- Leprince, O.; Pellizzaro, A.; Berriri, S.; Buitink, J. Late seed maturation: Drying without drying. J. Exp. Bot. 2017, 68, 827–841. [Google Scholar]
- Pereira Lima, J.J.; Buitink, J.; Lalanne, D.; Rossi, R.F.; Pelletier, S.; da Silva, E.A.A.; Leprince, O. Molecular characterisation of the acquisition of longevity during seed maturation in soybean. PLoS ONE 2017, 12. [Google Scholar] [CrossRef]
- Basso, D.P.; Hoshino-Bezerra, A.A.; Sartori, M.M.P.; Buitink, J.; Leprince, O.; de Silva, E.E.A. Late seed maturation improves the preservation of seedling emergence during storage in soybean. J. Seed Sci. 2014, 40, 185–192. [Google Scholar] [CrossRef]
- Ellis, R.H.; Yadav, G. Effect of simulated rainfall during wheat seed development and maturation on subsequent seed longevity is reversible. Seed Sci. Res. 2016, 26, 67–76. [Google Scholar] [CrossRef]
- Ellis, R.H.; Pieta Filho, C. The development of seed quality in spring and winter cultivars of barley and wheat. Seed Sci. Res. 1992, 2, 9–15. [Google Scholar] [CrossRef]
- Hong, T.D.; Gedebo, A.; Ellis, R.H. Accumulation of sugars during the onset and development of desiccation tolerance in immature seeds of Norway maple (Acer platanoides L.) stored moist. Seed Sci. Res. 2000, 10, 147–152. [Google Scholar] [CrossRef]
- Butler, L.H.; Hay, F.R.; Ellis, R.H.; Smith, R.D. Post-abscission pre-dispersal seeds of Digitalis purpurea L. remain in a developmental state that is not terminated by desiccation ex planta. Ann. Bot. 2009, 103, 785–794. [Google Scholar] [CrossRef]
- Hay, F.R.; Smith, R.D. Seed maturity: When to collect seeds from wild plants. In Seed Conservation. Turning Science into Practice; Smith, R.D., Dickie, J.B., Linington, S.H., Pritchard, H.W., Probert, R.J., Eds.; The Royal Botanic Gardens Kew: Richmond, UK, 2003; pp. 97–133. [Google Scholar]
- Hay, F.R.; Timple, S.; van Duijn, B. Can chlorophyll fluorescence be used to determine the optimal time to harvest rice seeds for long-term genebank storage? Seed Sci. Res. 2015, 25, 321–334. [Google Scholar] [CrossRef]
- Hay, F.R.; Klin, J.; Probert, R.J. Can a post-harvest ripening treatment extend the longevity of Rhododendron L. seeds? Sci. Hortic. 2006, 111, 80–83. [Google Scholar] [CrossRef]
- Hay, F.R.; Probert, R.J. Collecting and handling seeds in the field. In Collecting Plant Genetic Diversity: Technical Guidelines–2011 Update; Guarino, L., Goldberg, E., Eds.; Bioversity International: Rome, Italy, 2011. [Google Scholar]
- Whitehouse, K.J.; Hay, F.R.; Ellis, R.H. High-temperature stress during drying improves subsequent rice (Oryza sativa L.) seed longevity. Seed Sci. Res. 2017, 27, 281–291. [Google Scholar] [CrossRef]
- Whitehouse, K.J.; Owoborode, O.F.; Adebayo, O.O.; Oyatomi, O.A.; Olaniyan, A.B.; Abberton, M.T.; Hay, F.R. Further evidence that the genebank standards for drying orthodox seeds may not be optimal for subsequent seed longevity. Biopreserv. Biobank. 2018, 16, 327–336. [Google Scholar] [CrossRef]
- Radawan, A.; Hara, M.; Kleinwächter Selmar, D. Dehydrin expression in seeds and maturation drying: A paradigm change. Plant Biol. 2014, 16, 853–855. [Google Scholar] [CrossRef]
- Nellist, M.E. Safe drying temperatures for seed grain. In Seed Production; Hebblewaite, P.D., Ed.; Butterworth: London, UK, 1980; pp. 371–388. [Google Scholar]
- Cromarty, A.S.; Ellis, R.H.; Roberts, E.H. The Design of Seed Storage Facilities for Genetic Conservation; International Board for Plant Genetic Resources: Rome, Italy, 1982. [Google Scholar]
- McDonald, M.B.; Copeland, L. Seed Production: Principles and Practices; Chapman & Hall: New York, NY, USA, 1997. [Google Scholar]
- Sileshi, G.W. A critique of current trends in the statistical analysis of seed germination and viability data. Seed Sci. Res. 2012, 22, 145–159. [Google Scholar] [CrossRef]
- Hay, F.R.; Mead, A.; Bloomberg, M. Modelling seed germination in response to continuous variables: Use and limitations of probit analysis and alternative approaches. Seed Sci. Res. 2014, 24, 165–186. [Google Scholar] [CrossRef]
- Newton, R.J.; Hay, F.R.; Ellis, R.H. Temporal patterns of seed germination in early spring-flowering temperate woodland geophytes are modified by warming. Ann. Bot. 2020. [Google Scholar] [CrossRef] [PubMed]
- Gianinetti, A. Basic features of the analysis of germination data with generalized linear mixed models. Data 2020, 5, 6. [Google Scholar] [CrossRef]
- Gianinetti, A.; Cohn, M.A. Seed dormancy in red rice. XII: Population-based analysis of dry- afterripening with a hydrotime model. Seed Sci. Res. 2007, 17, 253–271. [Google Scholar] [CrossRef]
- Bradford, K.J.; Benech-Arnold, R.L.; Côme, D.; Corbineau, F. Quantifying the sensitivity of barley seed germination to oxygen, abscisic acid, and gibberellin using a population-based threshold model. J. Exp. Bot. 2008, 59, 335–347. [Google Scholar] [CrossRef]
- Joosen, R.V.L.; Kodde, J.; Willems, L.A.J.; Ligerink, W.; van der Plas, L.H.W.; Hilhorst, H.W.M. GERMINATOR: A software package for high-throughput scoring and curve fitting of Arabidopsis seed germination. Plant J. 2010, 62, 148–159. [Google Scholar] [CrossRef]
- Genebank Reviews. Available online: https://www.genebanks.org/resources/genebank-reviews/ (accessed on 15 April 2020).
| Optimum Strategy from a Genebank Management Perspective | More Scientifically Sound or of Interest from a Scientific Perspective |
|---|---|
| Only one sample of each accession in the active and base collections. | To compare the physiological response of seed lots produced in different crop seasons or environments; to have seeds of different ages to test at the same time (e.g., to understand seed longevity). |
| Once-over harvesting strategy. | Harvest seeds as they reach maturity. |
| Stop monitoring seeds once the seed lot that represents an accession has been replaced. | Continue monitoring viability to collect more data to inform seed longevity. This is better, not just because there are more data, but also because more of the data will cover the range where viability is expected to decline faster, enabling more robust model fitting. |
| Only consider one or a few different dormancy-breaking treatments at a time. | Factorial dormancy breaking/germination experiment, with different treatments and/or germination temperatures and treatment combinations. This should be a priority for ‘new’ species where there is little information on dormancy and germination requirements. |
| Initial viability test to confirm initial seed quality is sufficient. | Initial seed storage experiment to estimate initial seed storage potential, for setting seed lot-based monitoring intervals, and/or for confirming that the ranking of seed lots for longevity based on experimental storage corresponds with the ranking in genebank storage. |
| Minimal viability monitoring tests, e.g., only test a subset from each harvest season. | Monitor the viability of all samples at frequent intervals to get more data on relative seed longevity of different samples and of the same samples in different storage environments (e.g., medium- vs. long-term storage). |
| Only score for germination once or twice during a viability monitoring test. | Regular scoring of germination during a germination viability monitoring test to get information on speed of germination (vigour measures) and how vigour declines as seeds age. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Whitehouse, K.J.; Hay, F.R.; Lusty, C. Why Seed Physiology Is Important for Genebanking. Plants 2020, 9, 584. https://doi.org/10.3390/plants9050584
Whitehouse KJ, Hay FR, Lusty C. Why Seed Physiology Is Important for Genebanking. Plants. 2020; 9(5):584. https://doi.org/10.3390/plants9050584
Chicago/Turabian StyleWhitehouse, Katherine J., Fiona R. Hay, and Charlotte Lusty. 2020. "Why Seed Physiology Is Important for Genebanking" Plants 9, no. 5: 584. https://doi.org/10.3390/plants9050584
APA StyleWhitehouse, K. J., Hay, F. R., & Lusty, C. (2020). Why Seed Physiology Is Important for Genebanking. Plants, 9(5), 584. https://doi.org/10.3390/plants9050584





