Cloning and Expression of a Perilla frutescens Cytochrome P450 Enzyme Catalyzing the Hydroxylation of Phenylpropenes
Abstract
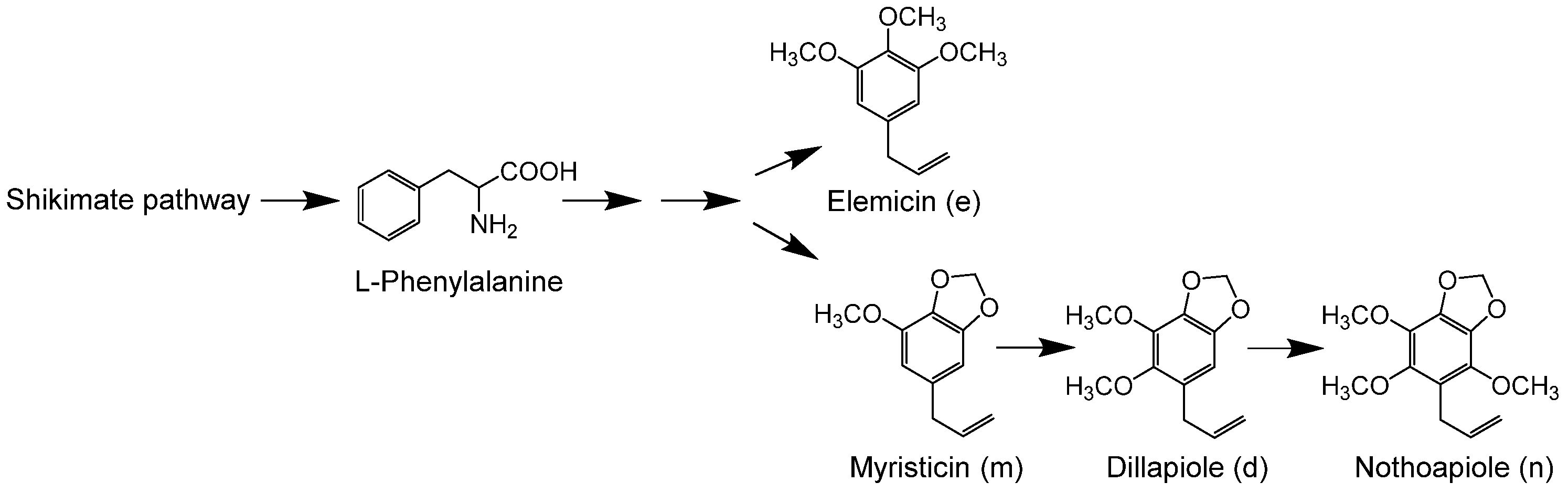
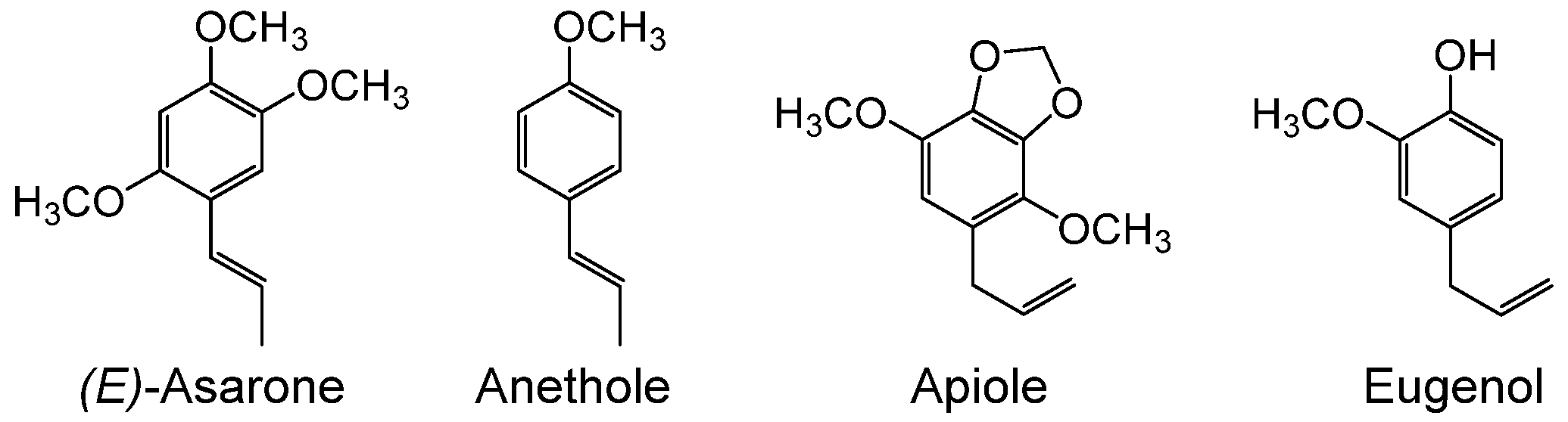
1. Introduction
2. Results
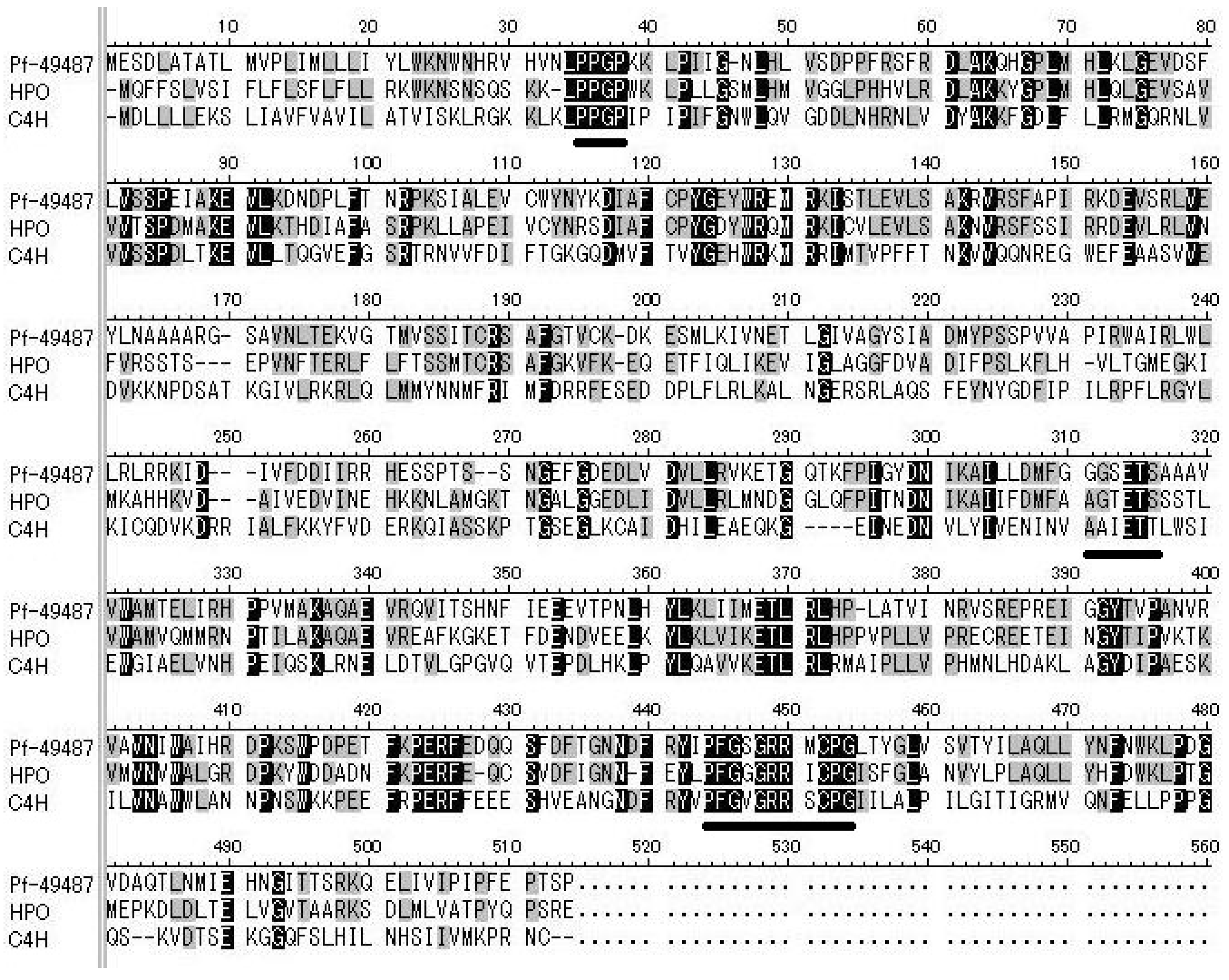
2.1. Isolation of a P450 Sequence from Perilla
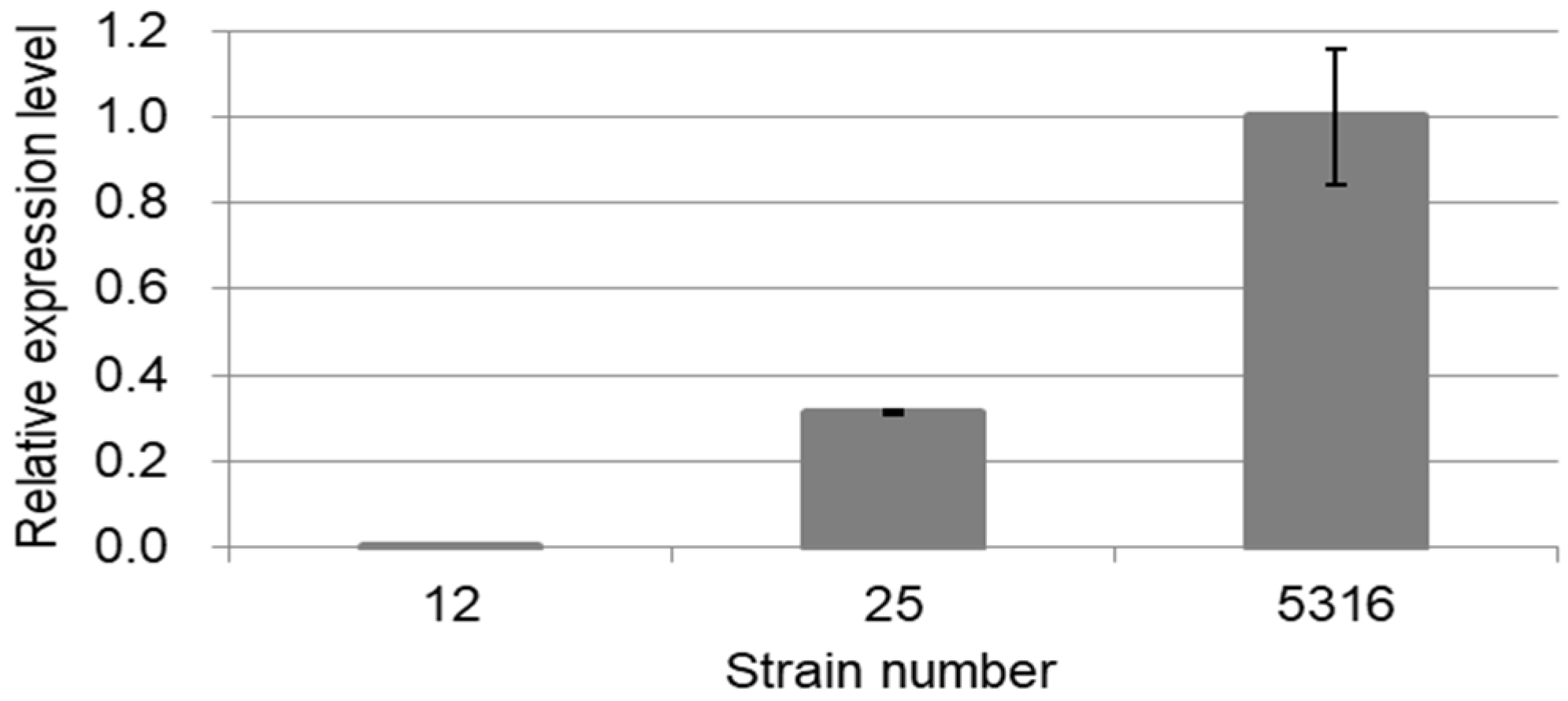
2.2. Comparison of Pf-49487 Expression among Strains of Phenylpropene (PP)-Type Perilla
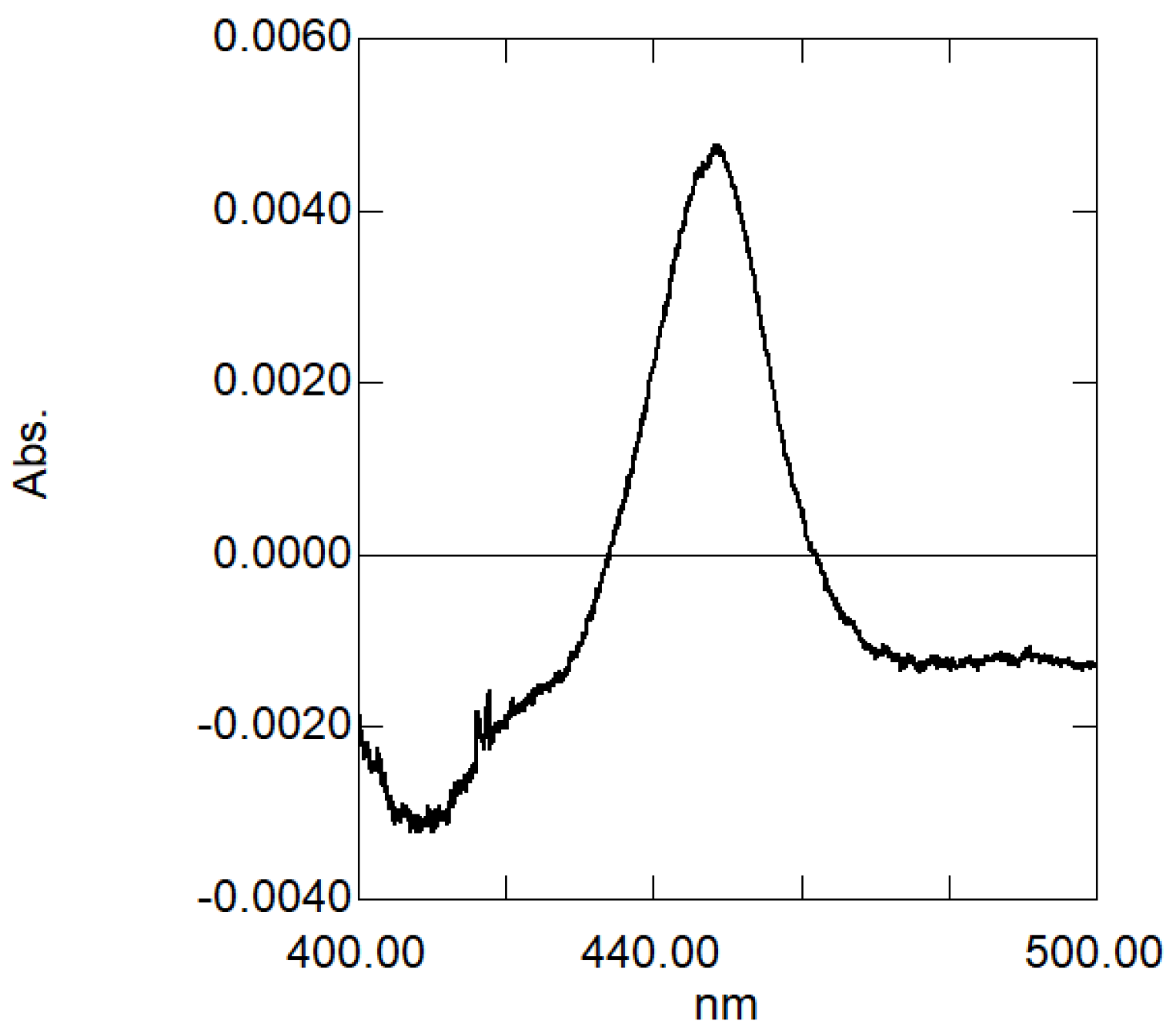
2.3. Heterologous Expression and Functional Analysis of Pf-49487
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Construction of the Expressed Sequence Tag (EST) Library and Cloning of Pf-49487
4.3. Heterologous Expression of Pf-49487 in Saccharomyces cerevisiae
4.4. Enzymatic Assays and Gas Chromatography-Mass Spectrometry (GC-MS) Analyses
4.5. Quantitative RT-PCR
4.6. GC-MS Analysis of Perilla Oil
4.7. Chemicals
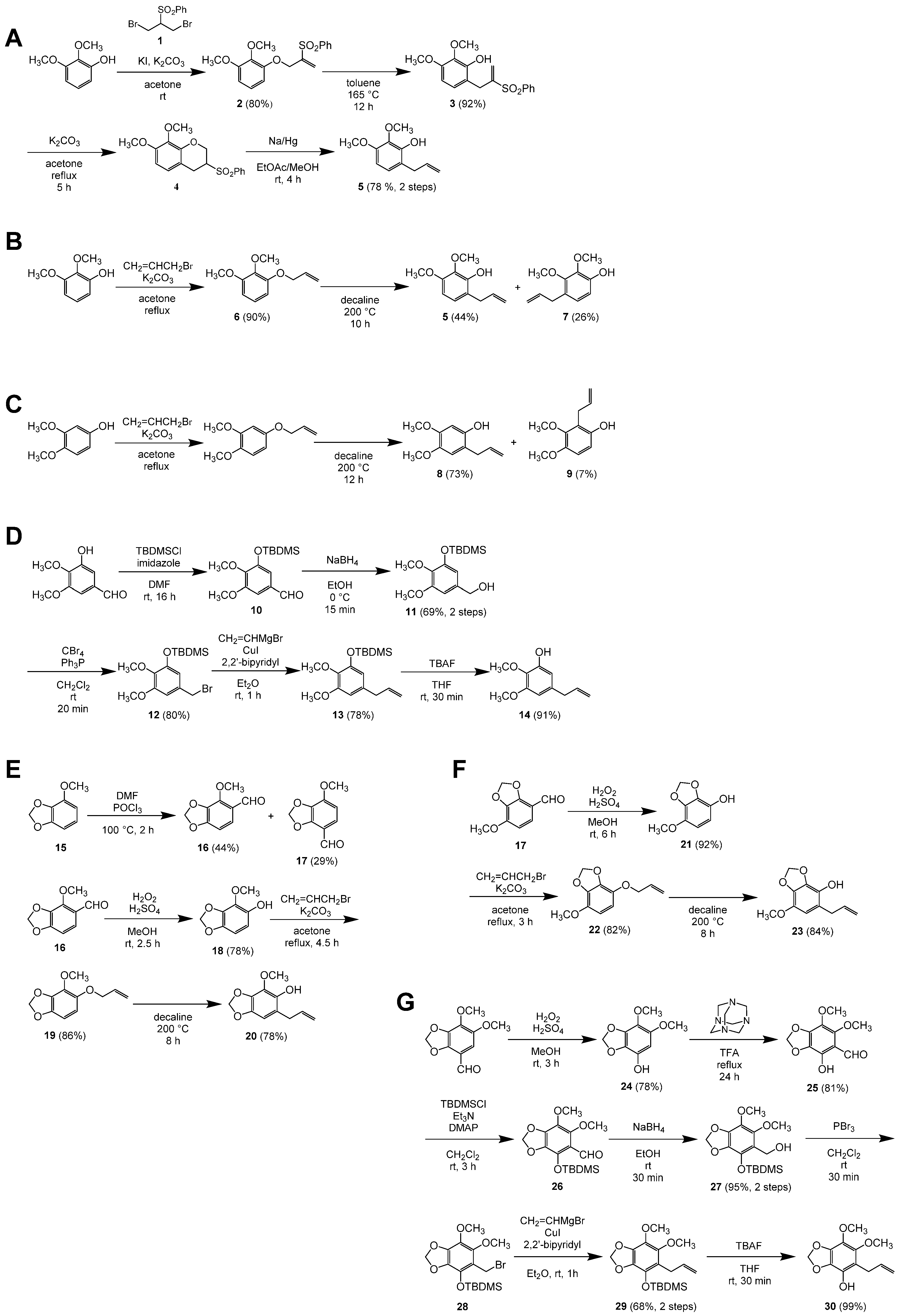
4.7.1. Synthesis of 6-Allyl-2,3-dimethoxyphenol (5)
- 1-(2-Benzenesulfonylallyloxy)-2,3-dimethoxybenzene (2): 2,3-Dimethoxyphenol (262 mg, 1.70 mmol) was dissolved in dry acetone (17 mL), and to the solution was added K2CO3 (705 mg, 5.10 mmol). The resulting suspension was stirred at room temperature for 10 min, and 2-benzenesulfonyl-1,3-dibromopropane (1) (640 mg, 1.87 mmol) and KI (7 mg, 0.04 mmol) were added to the suspension. After 3 h, the mixture was filtered through Celite, and the residue was successively washed with EtOAc. The combined filtrate and washings were concentrated in vacuo and purified by column chromatography (hexane/EtOAc 4:1) to give 2,3-dimethoxyphenol (45 mg, 17%) and the title compound (453 mg, 80%) as pale-yellow oils.
- 6-(2-Benzenesulfonylallyl)-2,3-dimethoxyphenol (3): 1-(2-Benzenesulfonylallyloxy)-2,3-dimethoxybenzene (2) (436 mg, 1.30 mmol) was dissolved in toluene (1 mL) and heated at 165 °C for 12 h under microwave irradiation. The mixture was concentrated in vacuo and purified by column chromatography (hexane/EtOAc 7:3) to give the title compound (398 mg, 92%) as a pale-yellow solid: mp 108–109 °C (EtOAc/hexane).
- 3-Benzenesulfonyl-7,8-dimethoxychromane (4): To a solution of 6-(2-benzenesulfonylallyl)-2,3-dimethoxyphenol (3) (144 mg, 0.430 mmol) in dry acetone (4 mL), was added K2CO3 (89 mg, 0.64 mmol), and the mixture was heated under reflux for 5 h. The mixture was diluted with EtOAc and washed with H2O. The aqueous layer was extracted with EtOAc, and the combined organic layers were washed with brine, dried over Na2SO4, and concentrated in vacuo to give the title compound as a white solid (140 mg). The crude product was used in the next step without further purification. The title compound was characterized after recrystallization from EtOAc/hexane, which gave colorless needles of mp 132–134 °C.
- 6-Allyl-2,3-dimethoxyphenol [450357-58-1] (5): To a solution of the crude 3-benzenesulfonyl-7,8-dimethoxychromane (4) (140 mg) in EtOAc/MeOH (2:1, 4.5 mL), was added 5% sodium amalgam (0.93 g, 2.0 mmol) at room temperature, and the mixture was stirred for 4 h. The reaction was quenched by the addition of solid citric acid (0.39 g), and the mixture was diluted with EtOAc, washed with saturated aqueous NaHCO3, dried over Na2SO4, and concentrated in vacuo. The residue was purified by column chromatography (hexane/EtOAc 9:1) to give the title compound (65 mg, 78% over 2 steps) as a pale-yellow oil.
4.7.2. Alternative Synthesis of 6-Allyl-2,3-dimethoxyphenol (5)
- 1-(Allyloxy)-2,3-dimethoxybenzene [380621-78-3] (6): The title compound was prepared from 2,3-dimethoxyphenol (982 mg, 6.37 mmol) according to the reported procedure [28]. After purification by column chromatography (hexane/EtOAc 9:1), the title compound (1.11 g, 90%) was obtained as a colorless oil.
- 6-Allyl-2,3-dimethoxyphenol [450357-58-1] (5) and 4-Allyl-2,3-dimethoxyphenol [29445-64-5] (7): A solution of 1-allyloxy-2,3-dimethoxtbenzene (6) (528 mg, 2.72 mmol) in decaline (0.5 mL) was heated at 200 °C under microwave irradiation for 10 h. After cooled to ambient temperature, the solution was directly purified by column chromatography (hexane/EtOAc 9:1) to give 6-allyl-2,3-dimethoxyphenol (103 mg, 20%) as a pale-yellow oil and a 63:37 mixture of 6-allyl-(5) and 4-allyl-2,3-dimethoxyphenol (7) (367 mg, 44% and 26%, respectively) as a pale-yellow oil. 4-Allyl-2,3-dimethoxyphenol (7) was characterized as a mixture with the other isomer.
4.7.3. Synthesis of 2-Allyl-4,5-dimethoxyphenol (8)
4.7.4. Synthesis of 5-Allyl-2,3-dimethoxyphenol (14)
- 3-tert-Butyldimethylsiloxy-4,5-dimethoxybenzaldehyde [122271-47-0] (10): To a solution of 3-hydroxy-4,5-dimethoxybenzaldehyde (500 mg, 2.74 mmol) in dry N,N-dimethylformamide (DMF) (3 mL), were added tert-butylchlorodimethylsilane (496 mg, 3.29 mmol) and imidazole (466 mg, 6.84 mmol). The mixture was stirred at room temperature for 16 h, diluted with EtOAc, washed with saturated aqueous NaHCO3 and brine, dried over Na2SO4, and concentrated in vacuo. The resulting pale-yellow oil, containing mainly the title compound, was used for the next step without further purification. The 1H NMR was consistent with that reported [31].
- 3-tert-Butyldimethylsiloxy-4,5-dimethoxybenzenemethanol [111394-55-9] (11): To a solution of the crude 3-tert-butyldimethylsiloxy-4,5-dimethoxybenzaldehyde (10) in EtOH (24 mL) cooled in an ice–water bath, was portion-wise added NaBH4 (104 mg, 2.75 mmol). After 15 min, water was added to the mixture, and most of EtOH was removed from the mixture by evaporation. The mixture was extracted three times with EtOAc, and the combined organic layers were washed with brine, dried over Na2SO4, and concentrated in vacuo. The resulting residue was purified by column chromatography (hexane/EtOAc 3:1) to give the title compound (563 mg, 69% over 2 steps) as a white solid. Recrystallization from hexane gave colorless needles of mp 57–58 °C. The 13C NMR was identical to that reported, while all the 1H NMR chemical shifts differed by 0.18 ppm from the reported values [32].
- 3-tert-Butyldimethylsiloxy-4,5-dimethoxybenzyl Bromide [111394-56-0] (12): To a solution of 3-tert-butyldimethylsiloxy-4,5-dimethoxybenzenemethanol (11) (315 mg, 1.06 mmol) in dry CH2Cl2 (10 mL) cooled in an ice–water bath, were added CBr4 (420 mg, 1.27 mmol) and Ph3P (333 mg, 1.27 mmol). The cooling bath was removed, and the mixture was stirred at room temperature for 20 min. Volatile materials were removed from the mixture by evaporation, and the residue was purified by column chromatography (hexane to hexane/EtOAc 97:3) to give the title compound (305 mg, 80%) as a pale-yellow oil.
- 5-Allyl-1-tert-butyldimethylsiloxy-2,3-dimethoxybenzene (13): To a mixture of 3-tert-butyldimethylsiloxy-4,5-dimethoxybenzyl bromide (12) (396 mg, 1.10 mmol), CuI (21 mg, 0.11 mmol), and 2,2’-bipyridine (17 mg, 0.11 mmol) in Et2O (1 mL) cooled in an ice–water bath, was dropwise added a 1.0 M tetrahydrofuran (THF) solution of vinylmagnesium bromide (1.6 mL, 1.6 mmol). The cooling bath was removed, and the mixture was stirred at room temperature for 1 h. The reaction was quenched by the addition of saturated aqueous NH4Cl and then 28% aqueous NH3. After stirred for 30 min, the whole was extracted three times with Et2O. The combined organic layers were washed with brine, dried over Na2SO4, and concentrated in vacuo. The residue was purified by column chromatography (hexane/EtOAc 49:1) to give the title compound (266 mg, 78%) as a pale-yellow oil.
- 5-Allyl-2,3-dimethoxyphenol [76773-99-4] (14): To a stirred solution of 5-allyl-1-tert-butyldimethylsiloxy-2,3-dimethoxybenzene (13) (74 mg, 0.24 mmol) in THF (1 mL), was added a 1.0 M THF solution of tetrabutylammonium fluoride (TBAF) (0.24 mL, 0.24 mmol). After 30 min, 0.5 M aqueous citric acid (1 mL) was added to the mixture, and the whole was extracted three times with EtOAc. The combined organic layers were washed with saturated aqueous NaHCO3 and brine, dried over Na2SO4, and concentrated in vacuo. The residue was purified by column chromatography (hexane/EtOAc 9:1) to give the title compound (43 mg, 91%) as a pale-yellow solid of mp 38–40 °C.
4.7.5. Synthesis of 6-Allyl-4-methoxy-1,3-benzodioxol-5-ol (20)
- 4-Methoxy-1,3-benzodioxole [1817-95-4] (15): The title compound was prepared from 3-methoxycatechol according to the reported procedure [33] in 85% yield as colorless blocks of mp 41–42 °C.
- 4-Methoxy-1,3-benzodioxole-5-carbaldehyde [5779-99-7] (16) and 7-Methoxy-1,3-benzodioxole-4-carbaldehyde [23731-55-7] (17): Dry DMF (0.64 mL, 8.3 mmol) and freshly distilled POCl3 (0.77 mL, 8.3 mmol) were mixed at 100 °C for 2 h. To the mixture, 4-methoxy-1,3-benzodioxole (15) (500 mg, 3.29 mmol) was added, and the mixture was heated at 100 °C. After 2 h, the reaction was quenched by the addition of ice, and the whole was extracted three times with Et2O. The combined organic layers were washed with water, saturated aqueous NaHCO3, and brine, dried over Na2SO4, and concentrated in vacuo to give a crude mixture of the title compounds (3:2) as a pale brown solid. The two isomers were separated by column chromatography (hexane/Et2O 4:1, Rf = 0.24 and 0.12, respectively). 4-Methoxy-1,3-benzodioxole-5-carbaldehyde (16): 44% yield. Colorless needles of mp 107–108 °C (EtOH). 7-Methoxy-1,3-benzodioxole-4-carbaldehyde (17): 29% yield. Colorless needles of mp 85–86 °C (EtOH).
- 4-Methoxy-1,3-benzodioxol-5-ol [23504-78-1] (18): To a solution of 4-methoxy-1,3-benzodioxole-5-carbaldehyde (16) (100 mg, 0.555 mmol) in MeOH (1 mL), were added conc. H2SO4 (0.01 mL, 0.2 mmol) and 30% H2O2 (0.085 mL, 0.83 mmol). The mixture was stirred at room temperature for 2.5 h, and saturated aqueous NaHCO3 was added to the mixture. The whole was extracted three times with EtOAc, and the combined organic layers were washed with brine, dried over Na2SO4, and concentrated in vacuo. The residual solid was purified by column chromatography (hexane/EtOAc 9:1) to give the title compound (72 mg, 78%) as a white solid: Rf = 0.29 (hexane/EtOAc 4:1). Colorless plates of mp 58–59 °C (Et2O/hexane).
- 5-Allyloxy-4-methoxy-1,3-benzodioxole [23731-59-1] (19): A mixture of 4-methoxy-1,3-benzodioxol-5-ol (18) (67 mg, 0.40 mmol), K2CO3 (111 mg, 0.800 mmol), and allyl bromide (0.05 mL, 0.6 mmol) in dry acetone (4 mL) was heated under reflux for 4.5 h, and water was added to the mixture. The whole was extracted three times with EtOAc, and the combined organic layers were washed with 15% aqueous NaOH twice and brine, dried over Na2SO4, and concentrated in vacuo. The residue was purified by column chromatography (hexane/EtOAc 95:5) to give the title compound (71.6 mg, 86%) as a pale-yellow oil: Rf = 0.25 (hexane/EtOAc 19:1).
- 6-Allyl-4-methoxy-1,3-benzodioxol-5-ol [23731-60-4] (20): A solution of 5-allyloxy-4-methoxy-1,3-benzodioxole (19) (58 mg, 0.28 mmol) in a 1:2 mixture of N-methyl-2-pyrrolidone (NMP) and decaline (1.5 mL) was heated at 200 °C under microwave irradiation for 8 h. After cooled to ambient temperature, the mixture was diluted with EtOAc, washed three times with water and with brine, dried over Na2SO4, and concentrated in vacuo. The residue was purified by column chromatography (hexane/EtOAc 9:1) to give the title compound (46 mg, 78%) as a pale-yellow oil: Rf = 0.44 (hexane/EtOAc 4:1).
4.7.6. Synthesis of 5-Allyl-7-methoxy-1,3-benzodioxol-4-ol (23)
- 7-Methoxy-1,3-benzodioxol-4-ol [23812-54-6] (21): To a solution of 7-methoxy-1,3-benzodioxole-4-carbaldehyde (17) (100 mg, 0.555 mmol) in MeOH (1 mL), conc. H2SO4 (0.01 mL, 0.2 mmol) and 30% H2O2 (0.085 mL, 0.83 mmol) were added. The mixture was stirred at room temperature for 6 h, and saturated aqueous NaHCO3 was added to the mixture. The whole was extracted three times with EtOAc, and the combined organic layers were washed with brine, dried over Na2SO4, and concentrated in vacuo. The residual solid was purified by column chromatography (hexane/EtOAc 3:1) to give the title compound (85 mg, 92%) as a white solid: Rf = 0.13 (hexane/EtOAc 4:1). Colorless needles of mp 104–105 °C (CHCl3/hexane).
- 4-Allyloxy-7-methoxy-1,3-benzodioxole [23731-70-6] (22): A mixture of 7-methoxy-1,3-benzodioxol-4-ol (21) (82 mg, 0.49 mmol), K2CO3 (135 mg, 0.977 mmol), and allyl bromide (63 μL, 0.73 mmol) in dry acetone (2.5 mL) was heated under reflux for 3 h, and water was added to the mixture. The whole was extracted three times with EtOAc, and the combined organic layers were washed with 15% aqueous NaOH twice and brine, dried over Na2SO4, and concentrated in vacuo. The residue was purified by column chromatography (hexane/EtOAc 95:5) to give the title compound (83.7 mg, 82%) as a pale-yellow oil: Rf = 0.20 (hexane/EtOAc 19:1).
- 5-Allyl-7-methoxy-1,3-benzodioxol-4-ol [76773-99-4] (23): A solution of 4-allyloxy-7-methoxy-1,3-benzodioxole (22) (82 mg, 0.39 mmol) in a 1:4 mixture of NMP and decaline (1 mL) was heated at 200 °C under microwave irradiation for 8 h. After cooled to ambient temperature, the mixture was diluted with EtOAc, washed three times with water and with brine, dried over Na2SO4, and concentrated in vacuo. The residue was purified by column chromatography (hexane/EtOAc 4:1) to give the title compound (68 mg, 84%) as a pale-yellow solid: Rf = 0.24 (hexane/EtOAc 4:1). Colorless needles of mp 84–85 °C (EtOAc/hexane).
4.7.7. Synthesis of 5-Allyl-6,7-dimethoxy-1,3-benzodioxol-4-ol (30)
- 6,7-Dimethoxy-1,3-benzodioxol-4-ol [22934-71-0] (24): A mixture of 6,7-dimethoxy-1,3-benzodioxole-4-carbaldehyde (368 mg, 1.75 mmol), 30% H2O2 (0.27 mL, 2.6 mmol), and conc. H2SO4 (0.12 mL, 2.3 mmol) in MeOH (3.5 mL) was stirred at room temperature for 3 h. After addition of saturated aqueous NaHCO3, the whole was extracted three times with EtOAc, and the combined organic layers were washed with brine, dried over Na2SO4, and concentrated in vacuo. The residual pale-yellow solid (1.38 g, 78%) was used as a crude title compound without further purification to the next step.: Rf = 0.22 (hexane/EtOAc 2:1). Colorless needles of mp 111–112 °C (EtOAc/hexane).
- 4-Hydroxy-6,7-dimethoxy-1,3-benzodioxol-5-carbadehyde (25): A mixture of 6,7-dimethoxy-1,3-benzodioxol-4-ol (24) (589 mg, 2.97 mmol) and hexamethylenetetramine (834 mg, 5.94 mmol) in refluxing trifluoroacetic acid (TFA) (3 mL) was stirred for 24 h [34]. H2O was added, and the solution was stirred for further 30 min at 60 °C. After being cooled to room temperature, the whole was extracted three times with EtOAc, and the combined organic layers were washed with brine, dried over Na2SO4, and concentrated in vacuo. The residual solids were purified by column chromatography (hexane/EtOAc 7:1) to give the title compound (546 mg, 81%) as a pale-yellow solid: Rf = 0.25 (hexane/EtOAc 7:1). Colorless powder of mp 85–87 °C (EtOAc/hexane).
- 4-tert-Butyldimethylsilyloxy-6,7-dimethoxy-1,3-benzodioxole-5-carbadehyde (26): To a mixture of 4-hydroxy-6,7-dimethoxy-1,3-benzodioxole-5-carbaldehyde (25) (1.20 g, 5.31 mmol), 4-dimethylaminopyridine (DMAP) (130 mg, 1.06 mmol), and Et3N (1.5 mL, 11 mmol) in CH2Cl2 (27 mL), was added tert-butylchlorodimethylsilane (1.20 g, 7.96 mmol). The mixture was stirred at room temperature for 3 h. After addition of saturated aqueous NaHCO3, the whole was extracted three times with EtOAc, and the combined organic layers were washed with brine, dried over Na2SO4, and concentrated in vacuo. The residual pale-yellow oil (2.14 g) was used as a crude title compound without further purification to the next step.
- 4-tert-Butyldimethylsilyloxy-6,7-dimethoxy-1,3-benzodioxole-5-methanol (27): To a solution of the crude 4-tert-butyldimethylsiloxy-6,7-dimethoxy-1,3-benzodioxole-5-carbaldehyde (26) (2.14 g) in EtOH (30 mL) was added NaBH4 (714 mg, 18.8 mmol). The mixture was stirred at room temperature for 30 min. After addition of water, the whole was extracted three times with EtOAc, and the combined organic layers were washed with brine, dried over Na2SO4, and concentrated in vacuo. The residual solids were purified by column chromatography (hexane/EtOAc 9:1 to 5:1) to give the title compound (1.73 g, 95% in two steps) as a colorless oil: Rf = 0.23 (hexane/EtOAc 7:1).
- 5-Bromomethyl-4-tert-butyldimethylsilyloxy-6,7-dimethoxy-1,3-benzodioxole (28): To a mixture of 4-tert-butyldimethylsiloxy-6,7-dimethoxy-1,3-benzodioxole-5-methanol (27) (450 mg, 1.31 mmol) in CH2Cl2 (13 mL) was added PBr3 (0.14 mL, 1.4 mmol). The mixture was stirred at room temperature for 30 min. After addition of water, the whole was extracted three times with CHCl3, and the combined organic layers were washed with brine, dried over Na2SO4, and concentrated in vacuo. The residual pale-yellow oil (552 mg) was used as a crude title compound without further purification to the next step.
- 5-Allyl-4-tert-butyldimethylsilyloxy-6,7-dimethoxy-1,3-benzodioxole (29): To a mixture of the crude 5-bromomethyl-4-tert-butyldimethylsiloxy-6,7-dimethoxy-1,3-benzodioxole (28) (552 mg, 1.56 mmol), CuI (50 mg, 0.26 mmol), and 2,2’-bipyridine (41 mg, 0.26 mmol) in Et2O (4 mL) was added a 1.0 M THF solution of vinylmagnesium bromide (2.0 mL, 2.0 mmol). The mixture was stirred at room temperature for 30 min. After addition of water, the whole was extracted three times with EtOAc, and the combined organic layers were washed with brine, dried over Na2SO4, and concentrated in vacuo. The residual solids were purified by column chromatography (hexane/EtOAc 50:1) to give the title compound (313 mg, 68% over 2 steps) as a pale-yellow oil: Rf = 0.52 (hexane/EtOAc 7:1).
- 5-Allyl-6,7-dimethoxy-1,3-benzodioxol-4-ol (30): To a solution of 5-allyl-4-tert-butyldimethylsiloxy-6,7-dimethoxy-1,3-benzodioxole (29) (690 mg, 1.96 mmol) in THF (20 mL) was added a 1.0 M THF solution of TBAF (1.96 mL, 2.0 mmol). The mixture was stirred at room temperature for 30 min. After addition of water, the whole was extracted three times with EtOAc, and the combined organic layers were washed with brine, dried over Na2SO4, and concentrated in vacuo. The residual solids were purified by column chromatography (hexane/EtOAc 5:1 to 4:1) to give the title compound (462 mg, 99%) as a pale-yellow solid: Rf = 0.28 (hexane/EtOAc 4:1). Colorless needles of mp 65–67 °C (EtOAc/hexane).
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ito, M. Studies on Perilla Relating to Its Essential Oil and Taxonomy; Phytochemistry Research Progress; Nova Science Publishers, Inc.: New York, NY, USA, 2008; pp. 13–30. [Google Scholar]
- Koezuka, Y.; Honda, G.; Tabata, M. Genetic control of the chemical composition of volatile oils in Perilla frutescens. Phytochemistry 1986, 25, 859–863. [Google Scholar] [CrossRef]
- Ito, M.; Honda, G. A taxonomic study of Japanese wild Perilla (Labiatae). J. Phytogeogr. Taxon. 1996, 44, 43–52. [Google Scholar]
- Ito, M.; Toyoda, M.; Honda, G. Chemical composition of the essential oil of Perilla frutescens. Nat. Med. 1999, 53, 32–36. [Google Scholar]
- Yuba, A.; Yazaki, K.; Honda, G.; Croteau, R. cDNA cloning, characterization, and functional expression of 4S-(−)-limonene synthase from Perilla frutescens. Arch. Biochem. Biophys. 1996, 332, 280–287. [Google Scholar] [CrossRef]
- Masumoto, N.; Korin, M.; Ito, M. Gereniol and linalool synthases from wild species of perilla. Phytochemistry 2010, 71, 1068–1075. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Ito, M. Molecular cloning and characterization of a Perilla frutescens cytochrome P450 enzyme that catalyzes the later steps of perillaldehyde biosynthesis. Phytochemistry 2017, 134, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.; Rueff, J.; Rodrigues, A.S. Genotoxic alkenylbenzene flavourings, a contribution to risk assessment. Food Chem. Toxicol. 2018, 118, 861–879. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.H. Cytotoxic effects of dillapiole on embryonic development of mouse blastocysts in vitro and in vivo. Int. J. Mol. Sci. 2014, 15, 10751–10765. [Google Scholar] [CrossRef] [PubMed]
- Koeduka, T.; Baiga, T.J.; Noel, J.P.; Pichersky, E. Biosynthesis of t-anethole in anise: Characterization of t-anol/isoeugenol synthase and an o-methyltransferase specific for a C7-C8 propenyl side chain. Plant Physiol. 2009, 149, 384–394. [Google Scholar] [CrossRef]
- Pharmaceutical and Medical Device Regulatory Science Society of Japan (PMRJ). The Japanese Pharmacopoeia Technical Information; Jiho, Inc.: Tokyo, Japan, 2016; pp. 1241–1242. [Google Scholar]
- Wei, C.L.; Guo, B.L. Advances in research of volatile oil and its different chemotypes in leaves of Perilla frutescens. Zhongguo Zhong Yao Za Zhi 2015, 40, 2937–2944. [Google Scholar]
- Hwang, S.H.; Kwon, S.H.; Kang, Y.H.; Lee, J.Y.; Lim, S.S. Rapid high performance liquid chromatography determination and optimization of extraction parameters of the α-asarone isolated from Perilla frutescens L. Molecules 2017, 22, 270. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Kim, S.I.; Ahn, Y.J. Insecticidal activity of asarones identified in Acrous gramineus rhizome against three coleopteran stored-product insects. J. Stored Prod. Res. 2003, 39, 333–342. [Google Scholar] [CrossRef]
- Haupenthal, S.; Berg, K.; Gründken, M.; Vallicotti, S.; Hemgesberg, M.; Sak, K.; Schrenk, D.; Esselen, M. In vitro genotoxicity of carcinogenic asarone isomers. Food Funct. 2017, 8, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Toyoda, M.; Yuba, A.; Honda, G. Genetic analysis of nothoapiol formation in Perilla frutescens. Biol. Pharm. Bull. 1999, 22, 598–601. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ikezawa, N.; Iwasa, K.; Sato, F. Molecular cloning and characterization of methylenedioxy bridge-forming enzymes involved in stylopine biosynthesis in Eschscholzia californica. FEBS J. 2007, 274, 1019–1035. [Google Scholar] [CrossRef]
- Ito, M.; Honda, G. Geraniol synthases from perilla and their taxonomical significance. Phytochemistry 2007, 68, 446–453. [Google Scholar] [CrossRef]
- Kitada, C.; Gong, Z.; Tanaka, Y.; Yamazaki, M.; Saito, K. Differential expression of two cytochrome P450s involved in the biosynthesis of flavones and anthocyanins in chemo-varietal forms of Perilla frutescens. Plant Cell Physiol. 2001, 42, 1338–1344. [Google Scholar] [CrossRef]
- Takahashi, S.; Yeo, Y.S.; Zhao, Y.; O’Maille, P.E.; Greenhagen, B.T.; Noel, J.P.; Coates, R.M.; Chappell, J. Functional characterization of premnaspirodiene oxygenase, a cytochrome P450 catalyzing regio-and stereo-specific hydroxylations of diverse sesquiterpene substrates. J. Biol. Chem. 2007, 282, 31744–31754. [Google Scholar] [CrossRef]
- Wüst, M.; Little, D.B.; Schalk, M.; Croteau, R. Hydroxylation of limonene enantiomers and analogs by recombinant (2)-limonene 3-and 6-Hydroxylases from mint (Mentha) species: Evidence for catalysis within sterically constrained active sites. Arch. Biochem. Biophys. 2001, 387, 125–136. [Google Scholar] [CrossRef]
- Gang, D.R.; Lavid, N.; Zubieta, C.; Chen, F.; Beuerle, T.; Lewinsohn, E.; Noel, J.P.; Pichersky, E. Characterization of phenylpropene O-methyltransferases from sweet basil: Facile change of substrate specificity and convergent evolution within a plant O-methyltransferase family. Plant Cell 2002, 14, 505–519. [Google Scholar] [CrossRef]
- Ikezawa, N.; Tanaka, M.; Nagayoshi, M.; Shinkyo, R.; Sakaki, T.; Inouye, K.; Sato, F. Molecular cloning and characterization of CYP719, a methylenedioxy bridge-forming enzyme that belongs to a novel P450 family, from cultured Coptis japonica cells. J. Biol. Chem. 2003, 278, 38557–38565. [Google Scholar] [CrossRef] [PubMed]
- Omura, T.; Sato, R. The carbon monoxide-binding pigment of liver microsomes. I. Evidence for its hemoprotein nature. J. Biol. Chem. 1964, 239, 2370–2378. [Google Scholar] [PubMed]
- Bochicchio, A.; Cefola, R.; Choppin, S.; Colobert, F.; Di Noia, M.A.; Funicello, M.; Hanquet, G.; Pisano, I.; Todisco, S. Selective Claisen rearrangement and iodination for the synthesis of polyoxygenated allyl phenol derivatives. Tetrahedron Lett. 2016, 37, 4053–4055. [Google Scholar] [CrossRef]
- Auvray, P.; Knochel, P.; Normant, J.F. 3-Bromo-2-t-butysulfonyl-1-propene: A versatile multi-coupling reagent part I. Tetrahedron 1998, 44, 4495–4508. [Google Scholar] [CrossRef]
- Pettus, T.R.R.; Inoue, M.; Chen, X.T.; Danishefsky, S.J. A fully synthetic route to the neurotrophic illicinones: Syntheses of tricycloillicinone and bicycloillicinone aldehyde. J. Am. Chem. Soc. 2000, 122, 6160–6168. [Google Scholar] [CrossRef]
- Allen, C.F.H.; Gates, J.W., Jr. o-Eugenol. Org. Synth. 1945, 25, 49–50. [Google Scholar]
- Argüelles, N.; Sánchez-Sandoval, E.; Mendieta, A.; Villa-Tanaca, L.; Garduño-Siciliano, L.; Jiménez, F.; del Carmen Cruz, M.; Medina-Franco, J.L.; Chamorro-Cevallos, G.; Tamariz, J. Design, synthesis, and docking of highly hypolipidemic agents: Schizosaccharomyces pombe as a new model for evaluating α-asarone-based HMG-CoA reductase inhibitors. Bioorg. Med. Chem. 2010, 18, 4238–4248. [Google Scholar] [CrossRef]
- Büchi, G.; Chu, P.S. The synthesis of megaphone. J. Am. Chem. Soc. 1981, 103, 2718–2721. [Google Scholar] [CrossRef]
- Singh, S.B.; Pettit, G.R. Isolation, structure, and synthesis of combretastatin C-1. J. Org. Chem. 1989, 54, 4105–4114. [Google Scholar] [CrossRef]
- Pettit, G.R.; Thornhill, A.; Melody, N.; Knight, J.C. Antineoplastic agents. 578. Synthesis of stilstatins 1 and 2 and their water-soluble prodrugs. J. Nat. Prod. 2009, 72, 380–388. [Google Scholar] [CrossRef]
- Loriot, M.; Robin, J.P.; Brown, E. Syntheses totales et etudes de lignanes biologiquement actifs—6: Syntheses totales de la (±)-iso-β-peltatine et de ses analogues. Tetrahedron 1984, 40, 2529–2535. [Google Scholar] [CrossRef]
- Olia, M.B.A.; Zavras, A.; Schiesser, C.H.; Alexander, S.A. Blue ‘turn-on’ fluorescent probes for the direct detection of free radicals and nitric oxide in Pseudomonas aeruginosa biofilms. Org. Biomol. Chem. 2016, 14, 2272–2281. [Google Scholar] [CrossRef] [PubMed]


| Strain (Oil Type) | ||||||
|---|---|---|---|---|---|---|
| Contig | 10 (PP-em) | 12 (PP-m) | 16 (PP-md) | 25 (PP-emd) | 5316 (PP-mdn) | 5717 (C) |
| 49487 | 0.0824 | 0.0968 | 68.4 | 23.7 | 56.7 | 0.325 |
| 77404 | 0.260 | 0.459 | 1.10 | 1.40 | 1.70 | 0.446 |
| Formula | Name (Compound Number) | Mass Spectra | Retention Time (min) |
|---|---|---|---|
 | 6-Allyl-2,3-dimethoxyphenol (5) |  | 26 |
 | 2-Allyl-4,5-dimethoxyphenol (8) | 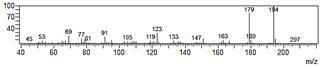 | 50 |
 | 5-Allyl-2,3-dimethoxyphenol (14) |  | 29 |
 | 6-Allyl-4-methoxy-1,3-benzodioxol-5-ol (20) | 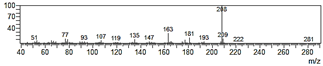 | 37 |
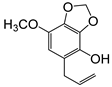 | 5-allyl-7-methoxy-1,3-benzodioxol-4-ol (23) | 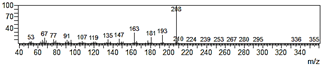 | 66 |
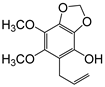 | 5-Allyl-6,7-dimethoxy-1,3-benzodioxol-4-ol (30) |  | 77 |
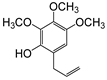 | 6-Allyl-2,3,4-trimethoxyphenol (31) | 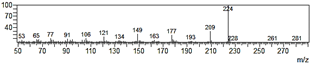 | 31 |
| Formula | Substrate | Activity (%) |
|---|---|---|
 | Myristicin | 100 |
 | Methyl eugenol | 91 |
 | Elemicin | 22 |
 | Dillapiole | 0 |
 | Eugenol | 0 |
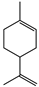 | Limonene | 0 |
 | Geraniol | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baba, M.; Yamada, K.-i.; Ito, M. Cloning and Expression of a Perilla frutescens Cytochrome P450 Enzyme Catalyzing the Hydroxylation of Phenylpropenes. Plants 2020, 9, 577. https://doi.org/10.3390/plants9050577
Baba M, Yamada K-i, Ito M. Cloning and Expression of a Perilla frutescens Cytochrome P450 Enzyme Catalyzing the Hydroxylation of Phenylpropenes. Plants. 2020; 9(5):577. https://doi.org/10.3390/plants9050577
Chicago/Turabian StyleBaba, Mariko, Ken-ichi Yamada, and Michiho Ito. 2020. "Cloning and Expression of a Perilla frutescens Cytochrome P450 Enzyme Catalyzing the Hydroxylation of Phenylpropenes" Plants 9, no. 5: 577. https://doi.org/10.3390/plants9050577
APA StyleBaba, M., Yamada, K.-i., & Ito, M. (2020). Cloning and Expression of a Perilla frutescens Cytochrome P450 Enzyme Catalyzing the Hydroxylation of Phenylpropenes. Plants, 9(5), 577. https://doi.org/10.3390/plants9050577






