In Vitro Propagation of Gastrochilus matsuran (Makino) Schltr., an Endangered Epiphytic Orchid
Abstract
1. Introduction
2. Results
2.1. Impact of Culture Media on Seed Germination
2.2. Impact of CW and PGRs on Seed Germination
2.3. Impact of Thidiazuron (TDZ) on Secondary Protocorm Induction
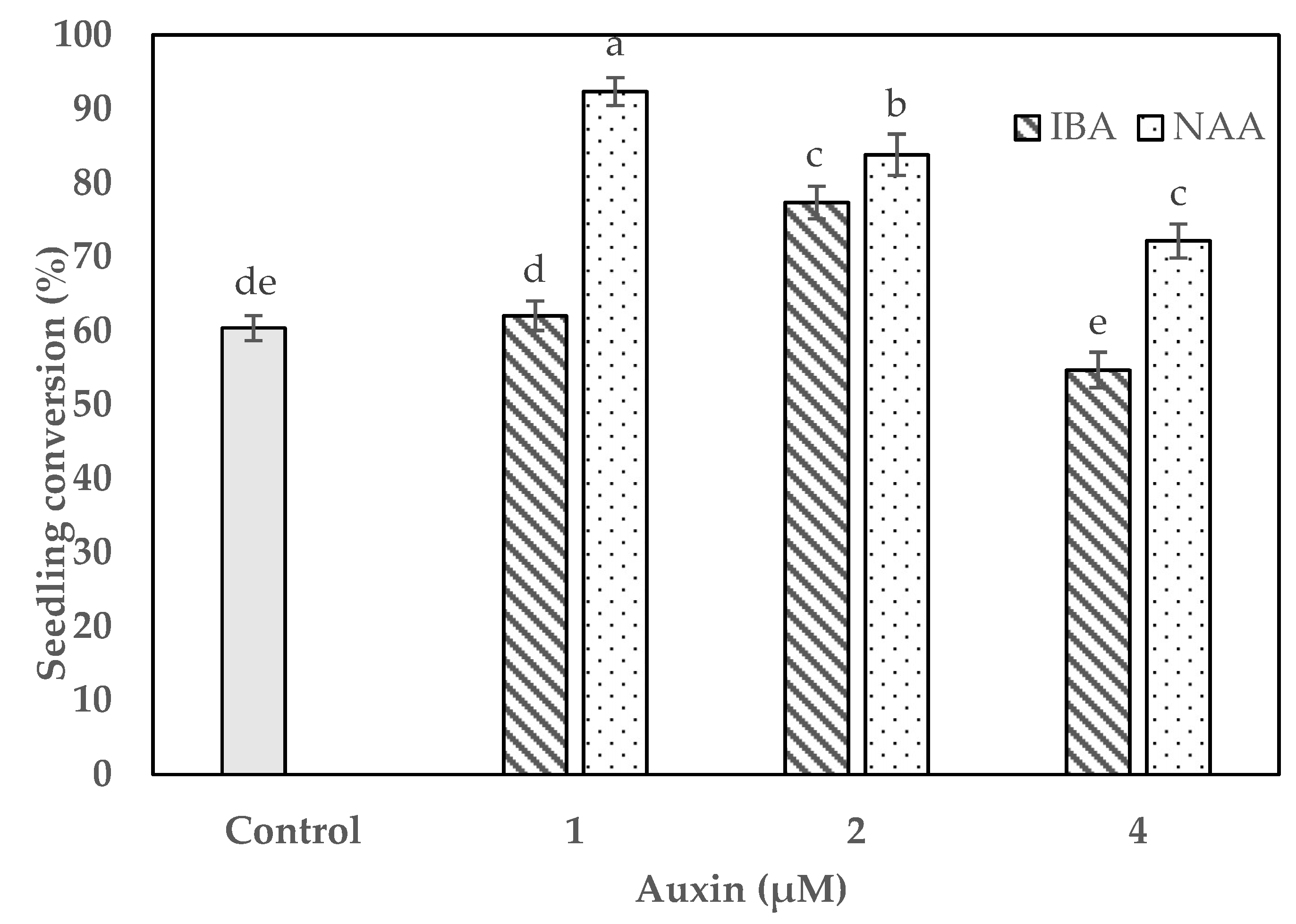
2.4. Impact of Auxins on Protocorm Conversion and Seedling Development
2.5. Acclimatization and Reintroduction
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Surface Disinfection
4.2. Impact of Culture Media on Seed Germination
4.3. Impact of CW and PGRs on Seed Germination
4.4. Impact of TDZ on Secondary Protocorm Induction
4.5. Impact of IBA and NAA on Protocorm Conversion and Seedling Development
4.6. Acclimatization and Reintroduction
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kim, J.Y.; Do, Y.; Im, R.-Y.; Kim, G.-Y.; Joo, G.-J. Use of large web-based data to identify public interest and trends related to endangered species. Biodivers. Conserv. 2014, 23, 2961–2984. [Google Scholar] [CrossRef]
- Kauth, P.J.; Dutra, D.; Johnson, T.R.; Stewart, S.L.; Kane, M.E.; Vendrame, V. Techniques and applications of in vitro orchid seed germination. In Floriculture, Ornamental and Plant Biotechnology: Advances and Topics Issues; da Silva, J.A.T., Ed.; Global Science Books: Isleworth, UK, 2008; pp. 375–391. [Google Scholar]
- Park, H.Y.; Kang, K.W.; Kim, D.H.; Sivanesan, I. In vitro propagation of Cymbidium goeringii Reichenbach fil. through direct adventitious shoot regeneration. Physiol. Mol. Biol. Plants 2018, 24, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S. In vitro regeneration of shoots from nodal explants of Dendrobium chrysotoxum Lindl. J. Hortic. Res. 2017, 25, 27–34. [Google Scholar] [CrossRef][Green Version]
- Shen, H.J.; Chen, J.T.; Chung, H.H.; Chang, W.C. Plant regeneration via direct somatic embryogenesis from leaf explants of Tolumnia Louise Elmore ‘Elsa’. Bot. Stud. 2018, 59, 4. [Google Scholar] [CrossRef] [PubMed]
- Bustam, S.; Sinniah, U.R.; Swamy, M.K. Simple and efficient in vitro method of storing Dendrobium sw Shavin White protocorm like bodies (PLBs). Bangladesh J. Bot. 2017, 46, 439–449. [Google Scholar]
- Chookoh, N.; Chiu, Y.; Chang, C.; Hu, W.; Dai, T. Micropropagation of Tolumnia orchids through induction of protocorm-like bodies from leaf segments. Hortscience 2019, 54, 1230–1236. [Google Scholar] [CrossRef]
- Mehraj, H.; Alam, M.M.; Habiba, S.U.; Mehbub, H. LEDs combined with CHO sources and CCC priming PLB regeneration of Phalaenopsis. Horticulture 2019, 5, 34. [Google Scholar] [CrossRef]
- Cardoso, J.C.; Zanello, C.A.; Chen, J.-T. An overview of orchid protocorm-like bodies: Mass propagation, biotechnology, molecular aspects, and breeding. Int. J. Mol. Sci. 2020, 21, 985. [Google Scholar] [CrossRef]
- Jiang, H.; Chen, M.-C.; Lee, Y.-I. In vitro germination and low-temperature seed storage of Cypripedium lentiginosum P.J. Cribb & S.C. Chen, a rare and endangered lady’s slipper orchid. Sci. Hortic. 2017, 225, 471–479. [Google Scholar]
- Pathak, P.; Piri, H.; Vij, S.P.; Mahant, K.C.; Chauhan, S. In vitro propagation and mass scale multiplication of critically endangered epiphytic orchid, Gastrochilus calceolaris (Buch.-ham ex. J.Sm.) D. Don using immature seeds. Indian J. Exp. Biol. 2011, 49, 711–716. [Google Scholar]
- Kim, D.H.; Kang, K.W.; Sivanesan, I. In vitro germination and seedling development of Gastrochilus japonicus (Makino) Schltr. Propag. Ornam. Plants 2019, 19, 61–65. [Google Scholar]
- Kim, D.H.; Kang, K.W.; Enkhtaivan, G.; Jan, U.; Sivanesan, I. Impact of activated charcoal, culture medium strength and thidiazuron on non-symbiotic in vitro seed germination of Pecteilis radiata (Thunb.) Raf. S. Afr. J. Bot. 2019, 124, 144–150. [Google Scholar] [CrossRef]
- Seon, K.M.; Kim, D.H.; Kang, K.W.; Sivanesan, I. Highly competent in vitro propagation of Thrixspermum japonicum (Miq.) Rchb.f., a rare epiphytic orchid. In Vitro Cell. Dev. Biol. Plant 2018, 54, 302–308. [Google Scholar] [CrossRef]
- Knudson, L. A new nutrient solution for germination of orchid seed. Am. Orchid. Soc. Bull. 1946, 15, 214–217. [Google Scholar]
- Abraham, S.; Augustine, J.; Thomas, T.D. Asymbiotic seed germination and in vitro conservation of Coelogyne nervosa A. Rich. an endemic orchid to western Ghats. Physiol. Mol. Biol. Plants 2012, 18, 245–251. [Google Scholar] [CrossRef][Green Version]
- Kim, D.H.; Kang, K.W.; Sivanesan, I. In vitro propagation of Cymbidium hybrid. Propag. Ornam. Plants 2017, 17, 48–54. [Google Scholar]
- Huh, Y.S.; Lee, J.K.; Nam, S.Y.; Paek, K.Y.; Suh, G.U. Improvement of asymbiotic seed germination and seedling development of Cypripedium macranthos Sw. with organic additives. J. Plant Biotechnol. 2016, 43, 138–145. [Google Scholar] [CrossRef]
- Zhou, X.; Gao, J.Y. Highly compatible Epa-01 strain promotes seed germination and protocorm development of Papilionanthe teres (Orchidaceae). Plant Cell Tissue Organ Cult. 2016, 125, 479–493. [Google Scholar] [CrossRef]
- Bembemcha, P.; Kishor, R.; Bai, N. In vitro immature embryo germination and propagation of Vanda stangeana Rchb. f., an orchid endemic to India. Hortic. Environ. Biotechnol. 2016, 57, 615–624. [Google Scholar]
- Molnár, Z.; Virág, E.; Ördög, V. Natural substances in tissue culture media of higher plants. Acta Biol. Szeged. 2011, 55, 123–127. [Google Scholar]
- Miyoshi, K.; Mii, M. Phytohormone pretreatment for the enhancement of seed germination and protocorm formation by the terrestrial orchid, Calanthe discolor (Orchidaceae), in asymbiotic culture. Sci. Hortic. 1995, 63, 263–267. [Google Scholar] [CrossRef]
- Manrique, J.P.; Fernandex-Lizarazo, C.; Suarez-Silva, A. Evaluation of the effect of three growth regulators in the germination of Comparetia falcate seeds under in vitro conditions. In Vitro Cell. Dev. Biol. Plant 2005, 41, 838–843. [Google Scholar] [CrossRef]
- Heirati, H.K.; Onsinejad, R.; Yari, F. The effect of pollination time and gibberellic acid (GA3) on the production and seed germination of Phalaenopsis orchids. J. Ornam. Plants 2015, 5, 83–89. [Google Scholar]
- Bhattacharyya, P.; Kumar, V.; Grúz, J.; Doležal, K.; Van Staden, J. Deciphering the phenolic acid reserves and antioxidant activity within the protocorm like bodies of Ansellia africana: A vulnerable medicinal orchid. Ind. Crops Prod. 2019, 135, 21–29. [Google Scholar] [CrossRef]
- Vogel, I.N.; Macedo, A.F. Influence of IAA, TDZ, and light quality on asymbiotic germination, protocorm formation, and plantlet development of Cyrtopodium glutiniferum Raddi, a medicinal orchid. Plant Cell Tissue Organ Cult. 2011, 104, 147–155. [Google Scholar] [CrossRef]
- Parthibhan, S.; Rao, M.V.; Kumar, T.S. In vitro regeneration from protocorms in Dendrobium aqueum Lindley—An imperiled orchid. J. Genet. Eng. Biotechnol. 2015, 13, 227–233. [Google Scholar] [CrossRef]
- Korah, P.L.; Shylaraj, K.S. In vitro proliferation competence of protocorm-like-bodies for direct induction of shoot buds for multiplication of Vanda ‘Robert’s Delight. Propag. Ornam. Plants 2018, 18, 115–123. [Google Scholar]
- Novak, S.D.; Luna, L.J.; Gamage, R.N. Role of auxin in orchid development. Plant Signal. Behav. 2014, 9, e972277. [Google Scholar] [CrossRef]
- Zeng, S.J.; Chen, Z.L.; Wu, K.L.; Zhang, J.X.; Bai, C.K.; Teixeira da Silva, J.A.; Duan, J. Asymbiotic seed germination, induction of calli and protocorm-like bodies, and in vitro seedling development of the rare and endangered Nothodoritis zhejiangensis Chinese orchid. HortScience 2011, 46, 460–465. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Vacin, E.; Went, F. Some pH changes in nutrient solution. Bot. Gazette 1949, 110, 605–613. [Google Scholar] [CrossRef]



| CW (%) | IAA (µM) | NAA (µM) | GA3 (µM) | Seed Germination (%) |
|---|---|---|---|---|
| 0 | - | - | - | 43.4 ± 1.6 i |
| 2.5 | - | - | - | 53.9 ± 2.3 gh |
| 5.0 | - | - | - | 62.0 ± 3.0 ef |
| 7.5 | - | - | - | 57.2 ± 2.2 fg |
| 10.0 | - | - | - | 47.8 ± 2.0 hi |
| 5.0 | 0.5 | - | - | 58.3 ± 3.1 fg |
| 5.0 | 1.0 | - | - | 66.7 ± 2.4 de |
| 5.0 | 2.0 | - | - | 72.8 ± 2.9 cd |
| 5.0 | 4.0 | - | - | 51.4 ± 2.4 gh |
| 5.0 | - | 0.5 | - | 67.9 ± 1.6 cde |
| 5.0 | - | 1.0 | - | 80.1 ± 2.3 b |
| 5.0 | - | 2.0 | - | 73.1 ± 2.4 bcd |
| 5.0 | - | 4.0 | - | 49.2 ± 2.5 hi |
| 5.0 | - | 1.0 | 1.0 | 87.8 ± 2.6 a |
| 5.0 | - | 1.0 | 1.5 | 93.3 ± 1.6 a |
| 5.0 | - | 1.0 | 2.0 | 75.2 ± 2.5 bc |
| 5.0 | - | 1.0 | 3.0 | 69.9 ± 2.5 cd |
| TDZ(µM) | Secondary Protocorm Induction (%) | Number of Secondary Protocorms |
|---|---|---|
| 0 | 35.3 ± 1.2 d | 2.8 ± 0.4 c |
| 1.0 | 59.3 ± 2.1 b | 4.6 ± 0.6 b |
| 2.0 | 86.7 ± 2.4 a | 8.3 ± 0.6 a |
| 4.0 | 64.9 ± 1.7 b | 5.1 ± 0.7 b |
| 8.0 | 43.3 ± 2.2 c | 3.7 ± 0.7 bc |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, H.; Kang, K.W.; Kim, D.H.; Sivanesan, I. In Vitro Propagation of Gastrochilus matsuran (Makino) Schltr., an Endangered Epiphytic Orchid. Plants 2020, 9, 524. https://doi.org/10.3390/plants9040524
Kang H, Kang KW, Kim DH, Sivanesan I. In Vitro Propagation of Gastrochilus matsuran (Makino) Schltr., an Endangered Epiphytic Orchid. Plants. 2020; 9(4):524. https://doi.org/10.3390/plants9040524
Chicago/Turabian StyleKang, Hyeonjeong, Kyung Won Kang, Doo Hwan Kim, and Iyyakkannu Sivanesan. 2020. "In Vitro Propagation of Gastrochilus matsuran (Makino) Schltr., an Endangered Epiphytic Orchid" Plants 9, no. 4: 524. https://doi.org/10.3390/plants9040524
APA StyleKang, H., Kang, K. W., Kim, D. H., & Sivanesan, I. (2020). In Vitro Propagation of Gastrochilus matsuran (Makino) Schltr., an Endangered Epiphytic Orchid. Plants, 9(4), 524. https://doi.org/10.3390/plants9040524






