Effect of Gibberellic Acid on Production of Biomass, Polyphenolics and Steviol Glycosides in Adventitious Root Cultures of Stevia rebaudiana (Bert.)
Abstract
1. Introduction
2. Results
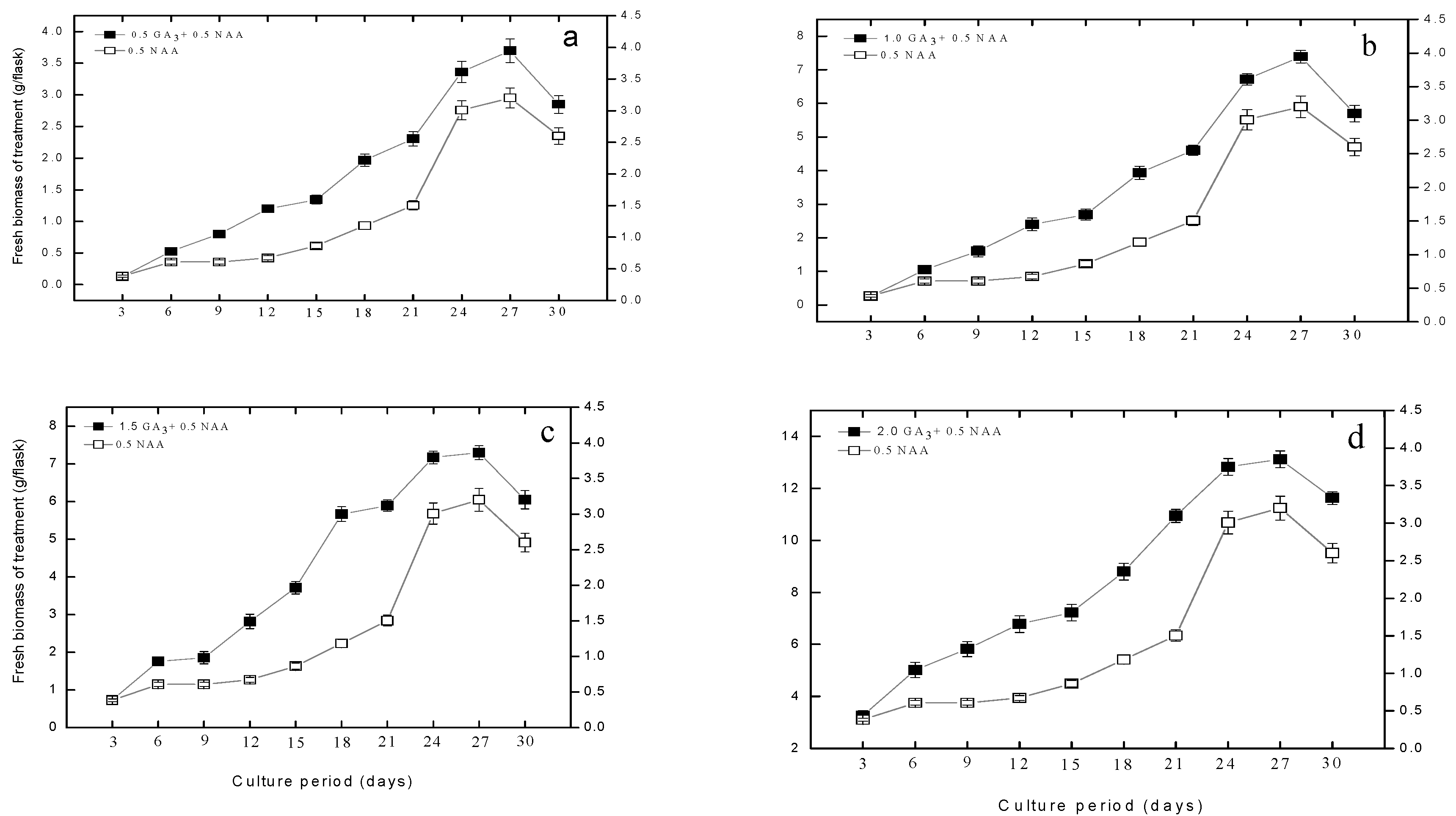
2.1. Effect of Gibberellic Acid on Adventitious Roots Biomass and Growth Kinetics
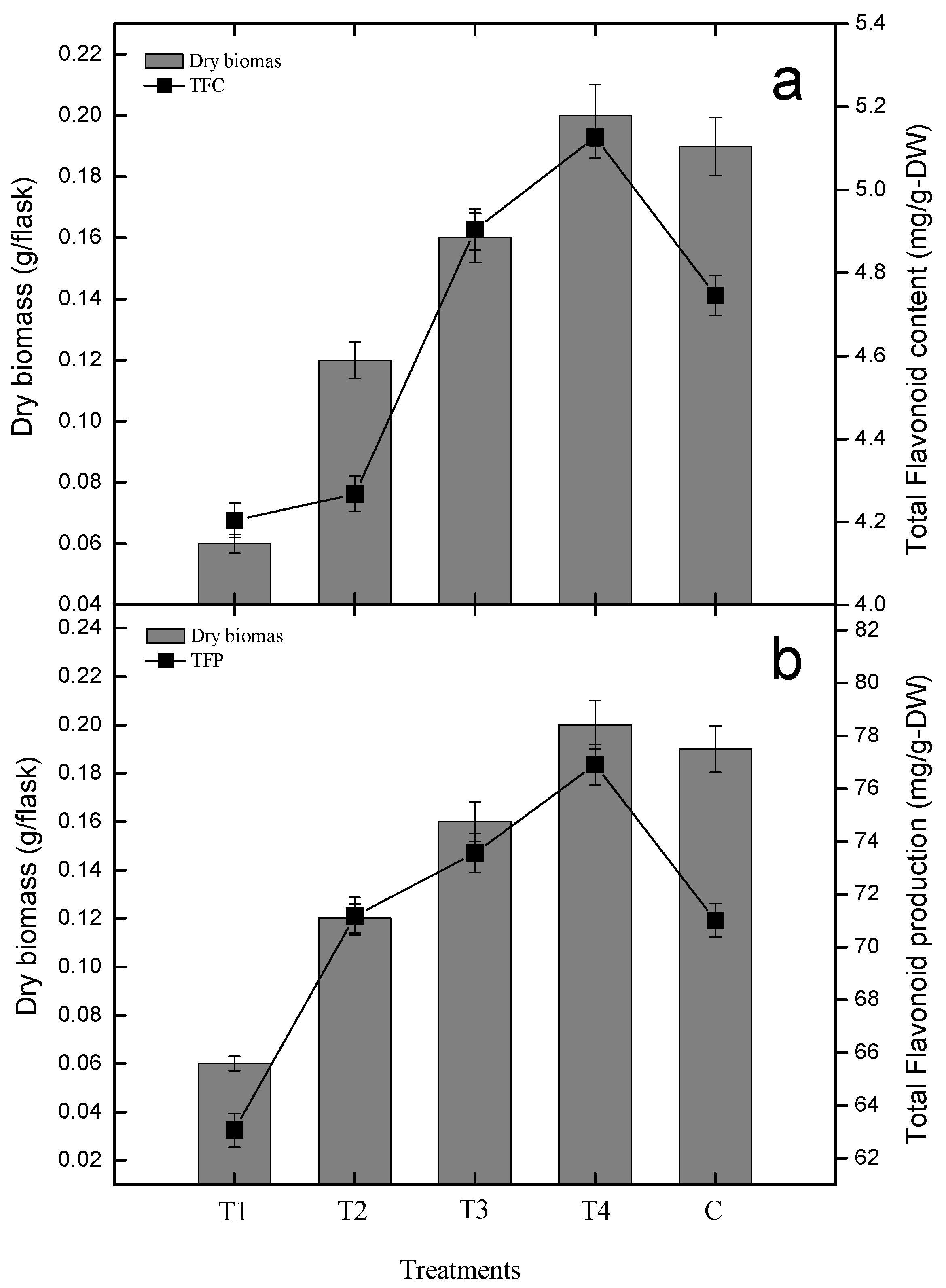
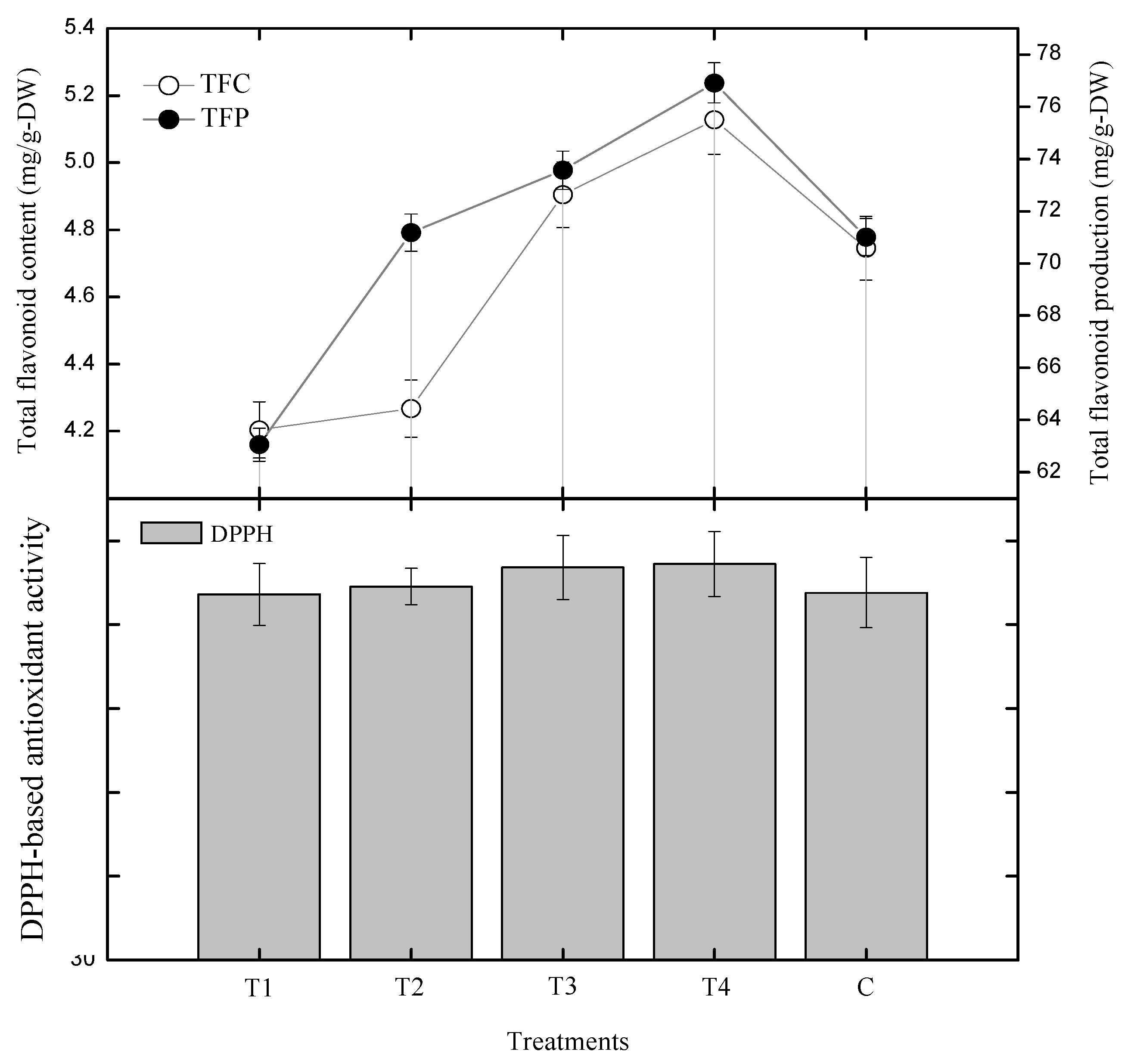
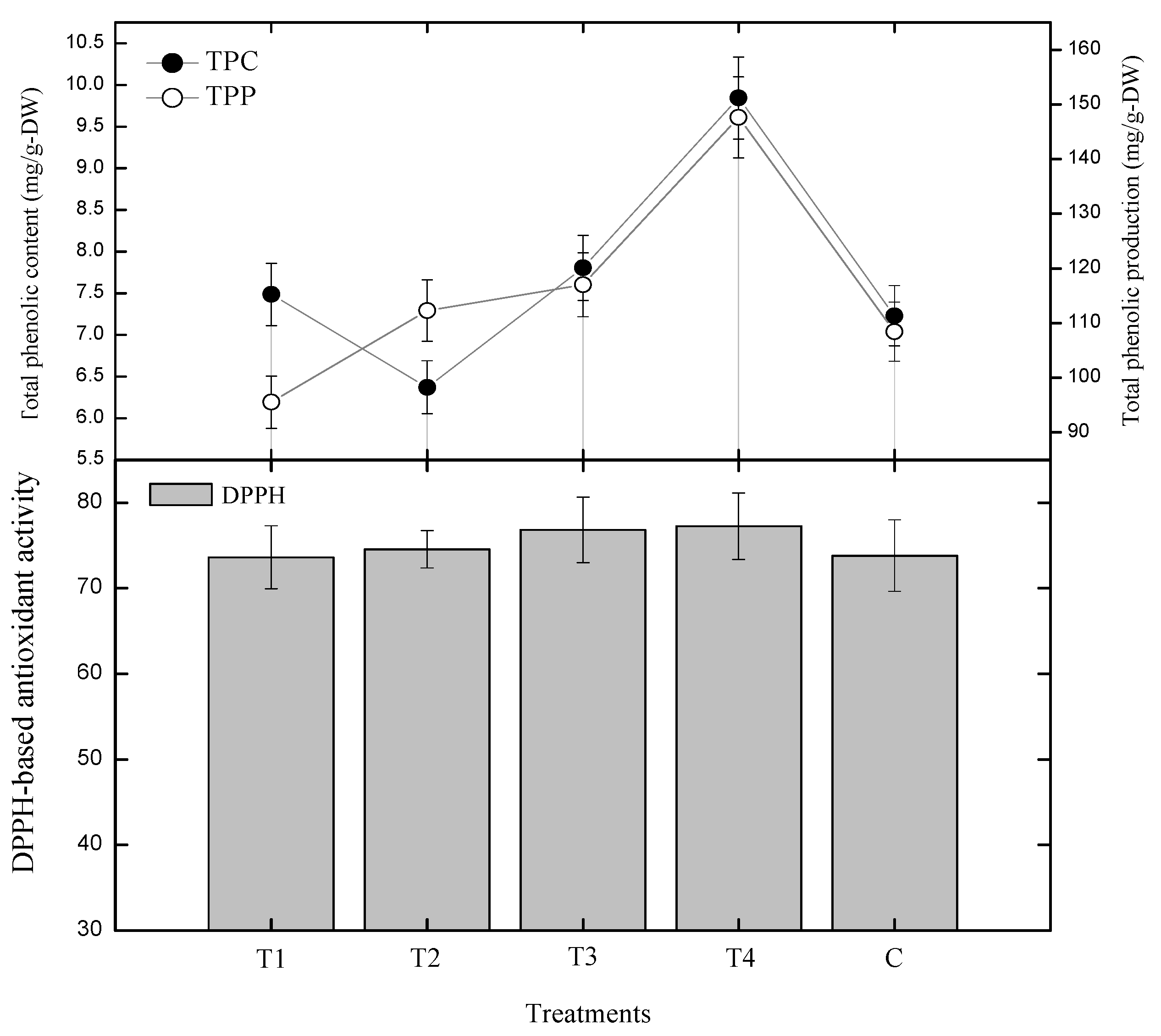
2.2. Effect of Gibberellic Acid on Polyphenolics and Antioxidant Activity
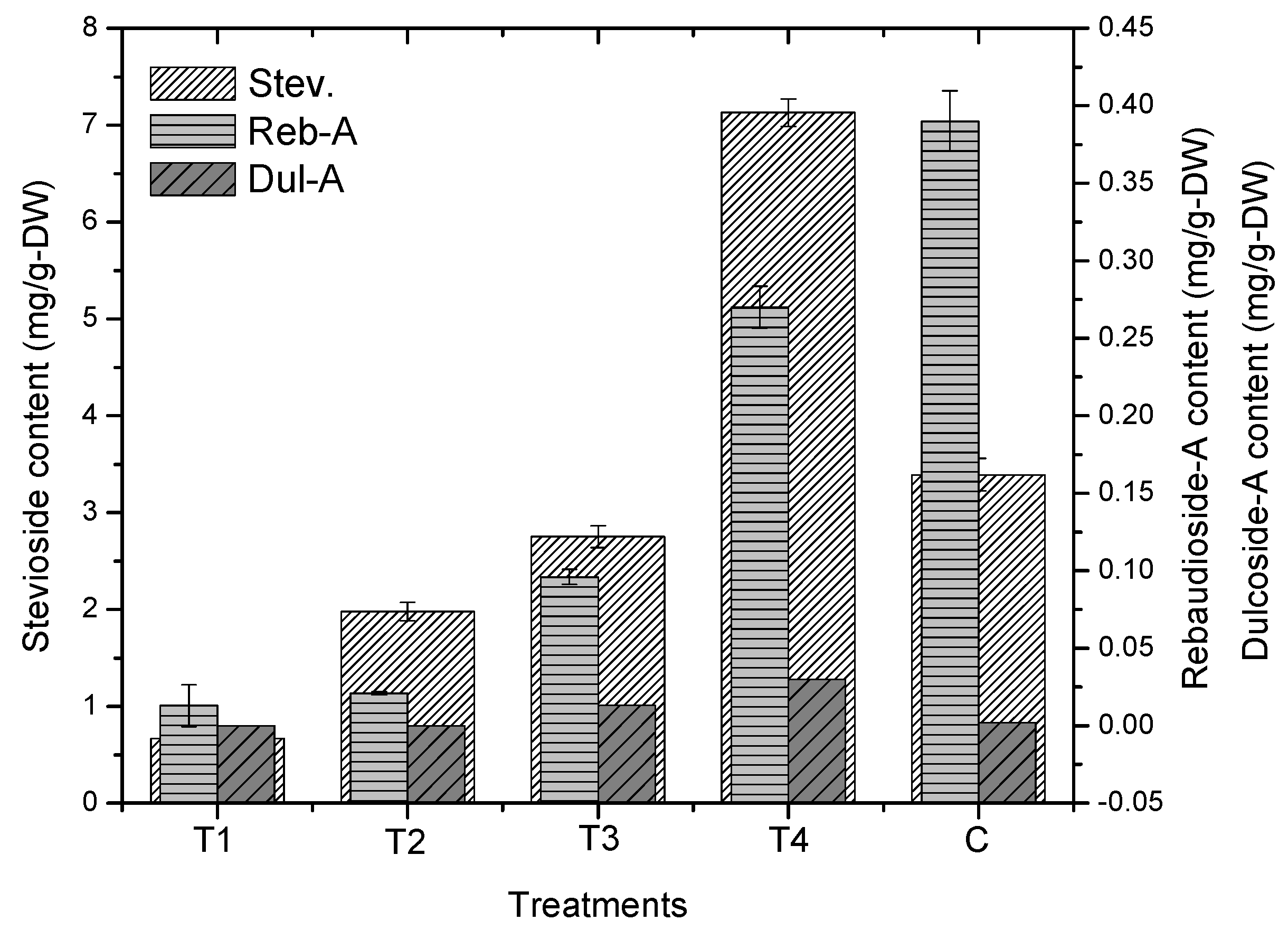
2.3. Effect of Gibberellic Acid on Steviol Glycosides
3. Discussion
4. Materials and Methods
4.1. Seed Collection and Germination
4.2. Development of Adventitious Roots Cultures
4.3. Investigation of Biomass of Stevia Adventitious Root Cultures
4.4. Biochemical Analysis
4.4.1. Extract Preparation
4.4.2. Determination of Total Phenolic and Total Flavonoid Contents
4.4.3. DPPH-Based Antioxidant Activity
4.4.4. Quantification of Steviol Glycosides in Adventitious Root Cultures
4.5. Formatting of Mathematical Components
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rashid, Z.; Rashid, M.; Inamullah, S.; Rasool, S.; Bahar, F.A. Effect of different levels of farmyard manure and nitrogen on the yield and nitrogen uptake by stevia (Stevia rebaudiana Bertoni). AFR 2013, 8, 3941–3945. [Google Scholar]
- Katayama, O.; Sumida, T.; Hayashi, H.; Mitsuhashi, H. The Practical Application of Stevia and Research and Development Data; ISU Company: Tokyo, Japan, 1976. [Google Scholar]
- Madan, S.; Ahmad, S.; Singh, G.; Kohli, K.; Kumar, Y.; Singh, R.; Garg, M. Stevia rebaudiana (Bert.) Bertoni-a review. Indian J. Nat. Prod. Resour. 2010, 1, 267–286. [Google Scholar]
- Carakostas, M.; Curry, L.; Boileau, A.; Brusick, D. Overview: The history, technical function and safety of rebaudioside A, a naturally occurring steviol glycoside, for use in food and beverages. Food Chem Toxicol. 2008, 46, S1–S10. [Google Scholar] [CrossRef]
- Mathur, S.; Shekhawat, G.S. Establishment and characterization of Stevia rebaudiana (Bertoni) cell suspension culture: An in vitro approach for production of stevioside. Acta Physiol. Plant. 2013, 35, 931–939. [Google Scholar] [CrossRef]
- Reis, R.V.; Borges, A.P.P.L.; Chierrito, T.P.C.; de Souto, E.R.; de Souza, L.M.; Iacomini, M.; de Oliveira, A.J.B.; Gonçalves, R.A.C. Establishment of adventitious root culture of Stevia rebaudiana Bertoni in a roller bottle system. Plant. Cell Tiss. Org. Cult. 2011, 106, 329–335. [Google Scholar] [CrossRef]
- Ahmad, N.; Fazal, H.; Zamir, R.; Khalil, S.A.; Abbasi, B.H. Callogenesis and shoot organogenesis from flowers of Stevia rebaudiana (Bert.). Sugar Tech. 2011, 13, 174–177. [Google Scholar] [CrossRef]
- Chan, P.; Tomlinson, B.; Chen, Y.J.; Liu, J.C.; Hsieh, M.H.; Cheng, J.T. A double-blind placebo-controlled study of the effectiveness and tolerability of oral stevioside in human hypertension. Br. J. Clin. 2000, 50, 215–220. [Google Scholar] [CrossRef]
- Chatsudthipong, V.; Muanprasat, C. Stevioside and related compounds: Therapeutic benefits beyond sweetness. Pharmacol. Ther. 2009, 121, 41–54. [Google Scholar] [CrossRef]
- Ramesh, K.; Singh, V.; Megeji, N.W. Cultivation of stevia [Stevia rebaudiana (Bert.) Bertoni]: A comprehensive review. Adv. Agron. 2006, 89, 137–177. [Google Scholar]
- Brandle, J.; Starratt, A.; Gijzen, M. Stevia rebaudiana: Its agricultural, biological, and chemical properties. Can. J. Plant. Sci. 1998, 78, 527–536. [Google Scholar] [CrossRef]
- Ferrazzano, G.F.; Cantile, T.; Alcidi, B.; Coda, M.; Ingenito, A.; Zarrelli, A.; Pollio, A. Is Stevia rebaudiana Bertoni a non cariogenic sweetener? A review. Molecules 2016, 21, 38. [Google Scholar] [CrossRef]
- Curi, R.; Alvarez, M.; Bazotte, R.; Botion, L.; Godoy, J.; Bracht, A. Effect of Stev/A Reba Ud/ANA on glucose tolerance in normal adult humans. Braz. J. Med. Biol. Res. 1986, 19, 771–774. [Google Scholar]
- Lu, C.-Y. The use of thidiazuron in tissue culture. Vitro Cell. Dev. 1993, 29, 92–96. [Google Scholar] [CrossRef]
- Tomita, T.; Sato, N.; Arai, T.; Shiraishi, H.; Sato, M.; Takeuchi, M.; Kamio, Y. Bactericidal activity of a fermented hot-water extract from Stevia rebaudiana Bertoni towards enterohemorrhagic Escherichia coli O157: H7 and other food-borne pathogenic bacteria. Microbiol. Immunol. 1997, 41, 1005–1009. [Google Scholar] [CrossRef]
- Debnath, M.; Malik, C.; Bisen, P.S. Micropropagation: A tool for the production of high quality plant-based medicines. Curr. Pharm. Biotechno 2006, 7, 33–49. [Google Scholar] [CrossRef]
- Khalil, S.A.; Kamal, N.; Sajid, M.; Ahmad, N.; Zamir, R.; Ahmad, N.; Ali, S. Synergism of polyamines and plant growth regulators enhanced morphogenesis, stevioside content, and production of commercially important natural antioxidants in Stevia rebaudiana Bert. Curr. Pharm. Biotechnol. 2016, 52, 174–184. [Google Scholar] [CrossRef]
- Fazal, H.; Abbasi, B.H.; Ahmad, N. Optimization of adventitious root culture for production of biomass and secondary metabolites in Prunella vulgaris L. Appl. Biochem. Biotechnol. 2014, 174, 2086–2095. [Google Scholar] [CrossRef]
- Verpoorte, R.; Van Der Heijden, R.; Ten Hoopen, H.; Memelink, J. Metabolic engineering of plant secondary metabolite pathways for the production of fine chemicals. Biotechnol. Lett. 1999, 21, 467–479. [Google Scholar] [CrossRef]
- Wu, C.-H.; Tewari, R.K.; Hahn, E.-J.; Paek, K.-Y. Nitric oxide elicitation induces the accumulation of secondary metabolites and antioxidant defense in adventitious roots of Echinacea purpurea. J. Plant. Biol. 2007, 50, 636–643. [Google Scholar] [CrossRef]
- Bourgaud, F.; Gravot, A.; Milesi, S.; Gontier, E. Production of plant secondary metabolites: A historical perspective. Plant. Sci. 2001, 161, 839–851. [Google Scholar] [CrossRef]
- Baque, M.A.; Elgirban, A.; Lee, E.-J.; Paek, K.-Y. Sucrose regulated enhanced induction of anthraquinone, phenolics, flavonoids biosynthesis and activities of antioxidant enzymes in adventitious root suspension cultures of Morinda citrifolia (L.). Acta Physiol. Plant. 2012, 34, 405–415. [Google Scholar] [CrossRef]
- Rao, S.R.; Ravishankar, G. Plant cell cultures: Chemical factories of secondary metabolites. Biotechnol. Adv. 2002, 20, 101–153. [Google Scholar] [PubMed]
- Ahmad, N.; Abbasi, B.H.; Ur Rahman, I.; Fazal, H. Piper nigrum: Micropropagation, antioxidative enzyme activities, and chromatographic fingerprint analysis for quality control. Appl. Biochem. Biotechnol. 2013, 169, 2004–2015. [Google Scholar] [CrossRef]
- Joo, S.-S.; Kim, Y.-B.; Lee, D.-I. Antimicrobial and antioxidant properties of secondary metabolites from white rose flower. Plant. Pathol. J. 2010, 26, 57–62. [Google Scholar] [CrossRef]
- Ali, M.; Abbasi, B.H. Thidiazuron-induced changes in biomass parameters, total phenolic content, and antioxidant activity in callus cultures of Artemisia absinthium L. Appl. Biochem. Biotechnol. 2014, 172, 2363–2376. [Google Scholar] [CrossRef]
- Roberts, S.C.; Shuler, M.L. Large-scale plant cell culture. Curr. Opin Biotechnol. 1997, 8, 154–159. [Google Scholar] [CrossRef]
- Dörnenburg, H.; Knorr, D. Strategies for the improvement of secondary metabolite production in plant cell cultures. Enzyme Microb. Tech. 1995, 17, 674–684. [Google Scholar] [CrossRef]
- Gomi, K.; Matsuoka, M. Gibberellin signalling pathway. Curr. Opin. Plant. Biol. 2003, 6, 489–493. [Google Scholar] [CrossRef]
- Bertea, C.; Freije, J.; Van der Woude, H.; Verstappen, F.; Perk, L.; Marquez, V.; De Kraker, J.-W.; Posthumus, M.; Jansen, B.; De Groot, A. Identification of intermediates and enzymes involved in the early steps of artemisinin biosynthesis in Artemisia annua. Planta. Medica. 2005, 71, 40–47. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; YE, H.C.; LI, G.F. Advances in the plant isoprenoid biosynthesis pathway and its metabolic engineering. J. Integr. Plant. Biol 2005, 47, 769–782. [Google Scholar] [CrossRef]
- Hedden, P.; Phillips, A.L. Gibberellin metabolism: New insights revealed by the genes. Trends Plant. Sci 2000, 5, 523–530. [Google Scholar] [CrossRef]
- Abbasi, B.H.; Stiles, A.R.; Saxena, P.K.; Liu, C.-Z. Gibberellic acid increases secondary metabolite production in Echinacea purpurea hairy roots. Appl. Biochem. Biotechnol. 2012, 168, 2057–2066. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Huang, L.-q.; Cui, G.-h.; Mao, Y.; He, X.-r. Effect of gibberellins and its synthetic inhibitor on metabolism of tanshinones. Chinese J. Exp. Trad. Med. Formulae 2008, 6, 2. [Google Scholar]
- Smith, T.C.; Weathers, P.J.; Cheetham, R.D. Effects of gibberellic acid on hairy root cultures of Artemisia annua: Growth and artemisinin production. Vitro Cell. Dev. 1997, 33, 75–79. [Google Scholar] [CrossRef]
- Banyai, W.; Mii, M.; Supaibulwatana, K. Enhancement of artemisinin content and biomass in Artemisia annua by exogenous GA 3 treatment. Plant. Growth Regul. 2011, 63, 45–54. [Google Scholar] [CrossRef]
- Ali, M.; Abbasi, B.H.; Ali, G.S. Elicitation of antioxidant secondary metabolites with jasmonates and gibberellic acid in cell suspension cultures of Artemisia absinthium L. Plant. Cell Tiss Org. 2015, 120, 1099–1106. [Google Scholar] [CrossRef]
- Ohkawa, H.; Kamada, H.; Sudo, H.; Harada, H. Effects of gibberellic acid on hairy root growth in Datura innoxia. J. Plant. Physiol. 1989, 134, 633–636. [Google Scholar] [CrossRef]
- Subroto, M.A.; Doran, P.M. Production of steroidal alkaloids by hairy roots of Solanum aviculare and the effect of gibberellic acid. Plant. Cell Tiss Org. 1994, 38, 93–102. [Google Scholar] [CrossRef]
- Baluška, F.; Parker, J.; Barlow, P. A role for gibberellic acid in orienting microtubules and regulating cell growth polarity in the maize root cortex. Planta 1993, 191, 149–157. [Google Scholar] [CrossRef]
- Teszlák, P.; Gaál, K.; Nikfardjam, M.S.P. Influence of grapevine flower treatment with gibberellic acid (GA3) on polyphenol content of Vitis vinifera L. wine. Anal. Chim. 2005, 543, 275–281. [Google Scholar] [CrossRef]
- Alonso-Ramírez, A.; Rodríguez, D.; Reyes, D.; Jiménez, J.A.; Nicolás, G.; López-Climent, M.; Gómez-Cadenas, A.; Nicolás, C. Evidence for a role of gibberellins in salicylic acid-modulated early plant responses to abiotic stress in Arabidopsis seeds. J. Plant. Physiol. 2009, 150, 1335–1344. [Google Scholar] [CrossRef] [PubMed]
- Jaleel, C.A.; Gopi, R.; Manivannan, P.; Sankar, B.; Kishorekumar, A.; Panneerselvam, R. Antioxidant potentials and ajmalicine accumulation in Catharanthus roseus after treatment with giberellic acid. Colloids Surf. B Biointerfaces 2007, 60, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Aman, N.; Hadi, F.; Khalil, S.A.; Zamir, R.; Ahmad, N. Efficient regeneration for enhanced steviol glycosides production in Stevia rebaudiana (Bertoni). C R Biol. 2013, 336, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Kundu, S.; Bandyopadhyay, A.; Bhattacharjee, A. Efficient micropropagation and chlorocholine chloride induced stevioside production of Stevia rebaudiana Bertoni. C R Biol. 2013, 336, 17–28. [Google Scholar] [CrossRef]
- Ladygin, V.; Bondarev, N.; Semenova, G.; Smolov, A.; Reshetnyak, O.; Nosov, A. Chloroplast ultrastructure, photosynthetic apparatus activities and production of steviol glycosides in Stevia rebaudiana in vivo and in vitro. Biol. Plant. 2008, 52, 9–16. [Google Scholar] [CrossRef]
- Bondarev, N.; Reshetnyak, O.; Nosov, A. Effects of nutrient medium composition on development of Stevia rebaudiana shoots cultivated in the roller bioreactor and their production of steviol glycosides. Plant. Sci. 2003, 165, 845–850. [Google Scholar] [CrossRef]
- Zamir, R.; Khalil, S.A.; Ahmad, N.; Rab, A.; Shah, S.T.; Jabeen, N.; Ali, S.; Ali, M. The synergistic effects of sucrose and plant growth regulators on morphogenesis and evaluation of antioxidant activities in regenerated tissues of Caralluma tuberculata. Acta. Physiol. Plant. 2016, 38, 200. [Google Scholar] [CrossRef]

| Different Treatments Used in the Present Study | ||
|---|---|---|
| Treatments | NAA Concentration | GA3 Concentration |
| T1 | 0.5 mg/L | 0.5 mg/L |
| T2 | 0.5 mg/L | 1.0 mg/L |
| T3 | 0.5 mg/L | 1.5 mg/L |
| T4 | 0.5 mg/L | 2.0 mg/L |
| Control | 0.5 mg/L | 0.0 mg/L |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, A.; Ali, H.; Khan, H.; Begam, A.; Khan, S.; Ali, S.S.; Ahmad, N.; Fazal, H.; Ali, M.; Hano, C.; et al. Effect of Gibberellic Acid on Production of Biomass, Polyphenolics and Steviol Glycosides in Adventitious Root Cultures of Stevia rebaudiana (Bert.). Plants 2020, 9, 420. https://doi.org/10.3390/plants9040420
Ahmad A, Ali H, Khan H, Begam A, Khan S, Ali SS, Ahmad N, Fazal H, Ali M, Hano C, et al. Effect of Gibberellic Acid on Production of Biomass, Polyphenolics and Steviol Glycosides in Adventitious Root Cultures of Stevia rebaudiana (Bert.). Plants. 2020; 9(4):420. https://doi.org/10.3390/plants9040420
Chicago/Turabian StyleAhmad, Ashfaq, Haider Ali, Habiba Khan, Almas Begam, Sheraz Khan, Syed Shujait Ali, Naveed Ahmad, Hina Fazal, Mohammad Ali, Christophe Hano, and et al. 2020. "Effect of Gibberellic Acid on Production of Biomass, Polyphenolics and Steviol Glycosides in Adventitious Root Cultures of Stevia rebaudiana (Bert.)" Plants 9, no. 4: 420. https://doi.org/10.3390/plants9040420
APA StyleAhmad, A., Ali, H., Khan, H., Begam, A., Khan, S., Ali, S. S., Ahmad, N., Fazal, H., Ali, M., Hano, C., Ahmad, N., & Abbasi, B. H. (2020). Effect of Gibberellic Acid on Production of Biomass, Polyphenolics and Steviol Glycosides in Adventitious Root Cultures of Stevia rebaudiana (Bert.). Plants, 9(4), 420. https://doi.org/10.3390/plants9040420







