Nitro-Oleic Acid in Seeds and Differently Developed Seedlings of Brassica napus L.
Abstract
1. Introduction
2. Results and Discussion
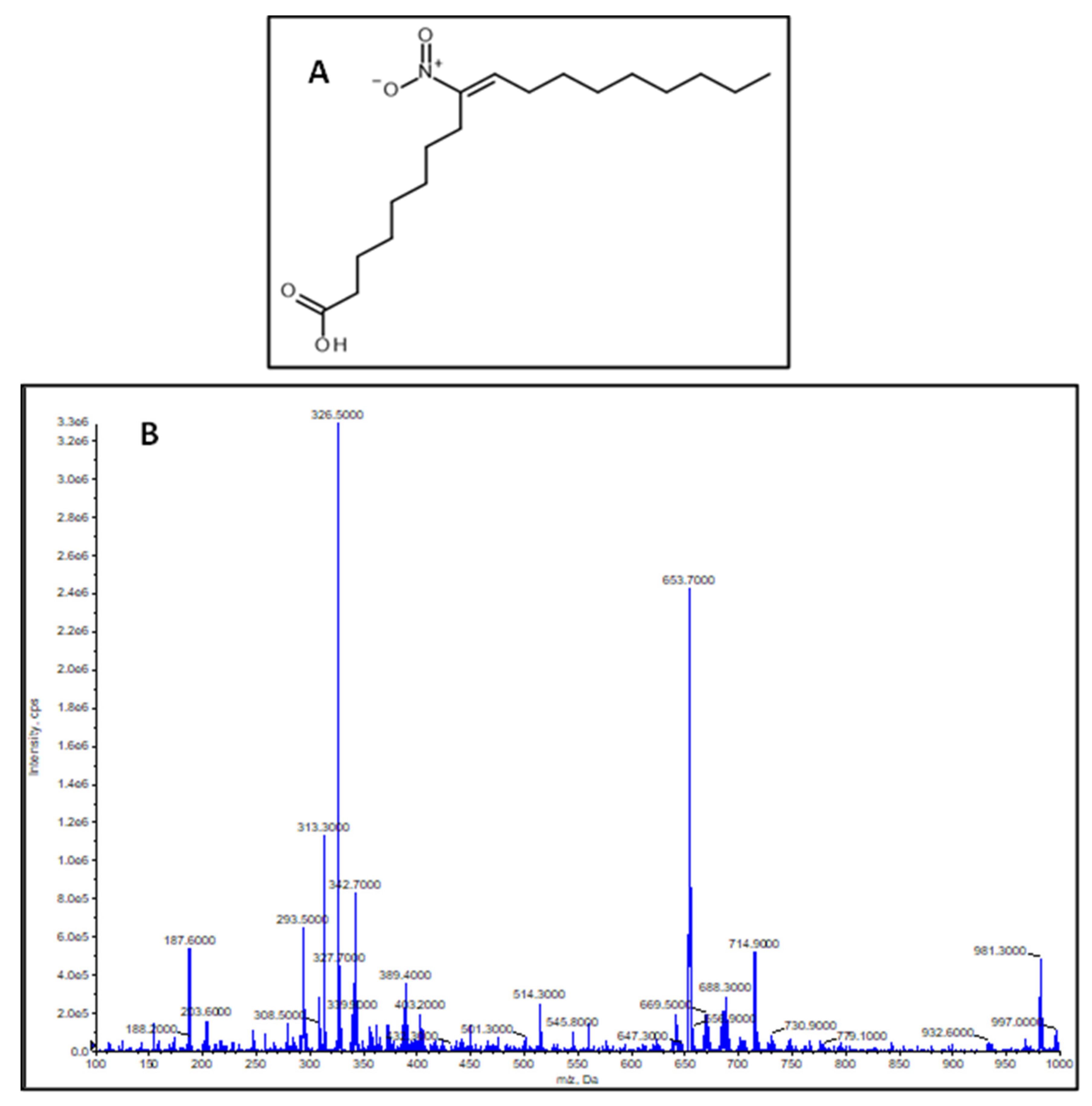
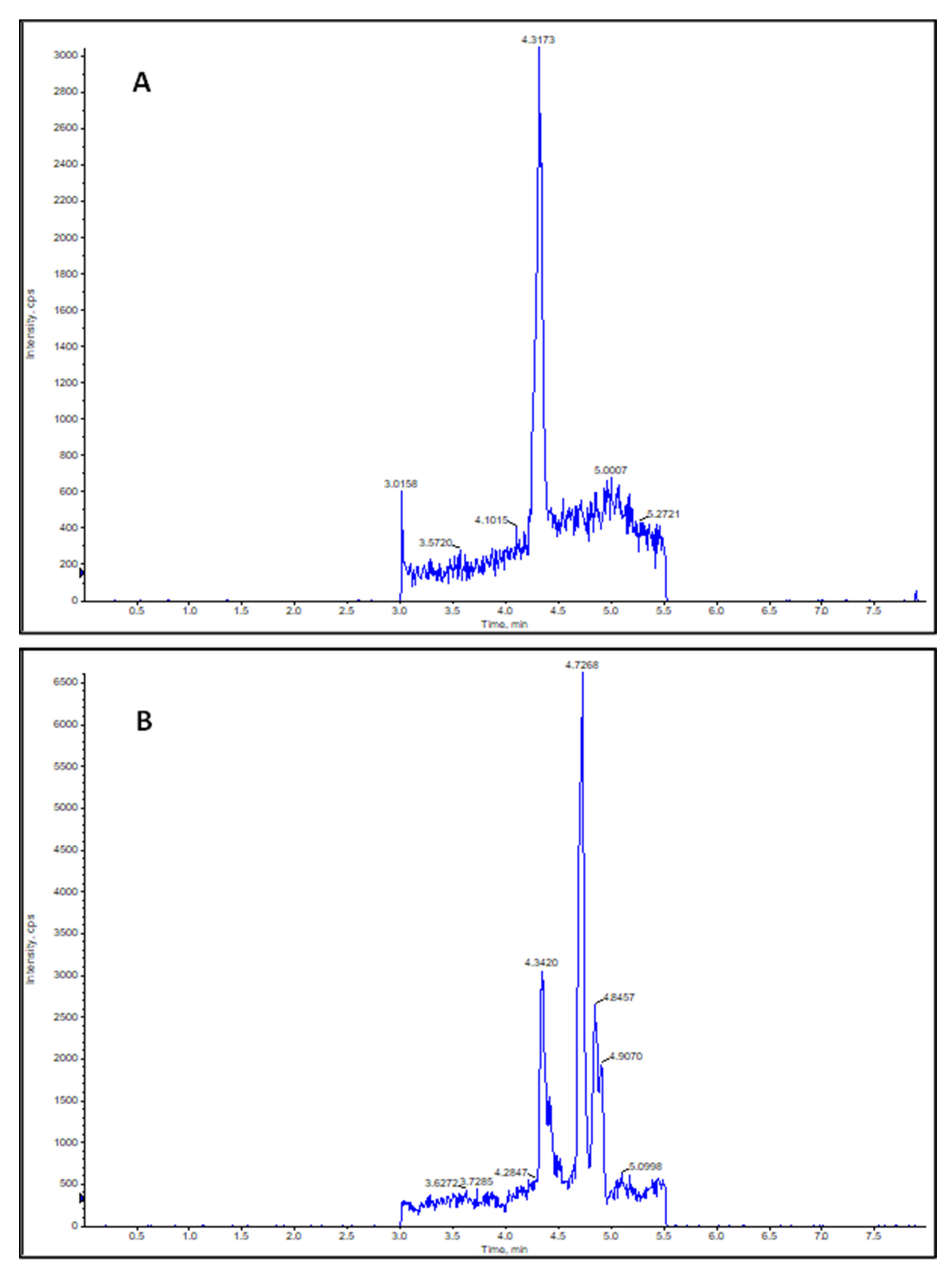
2.1. Characterization of the Synthesized NO2-OA Standard
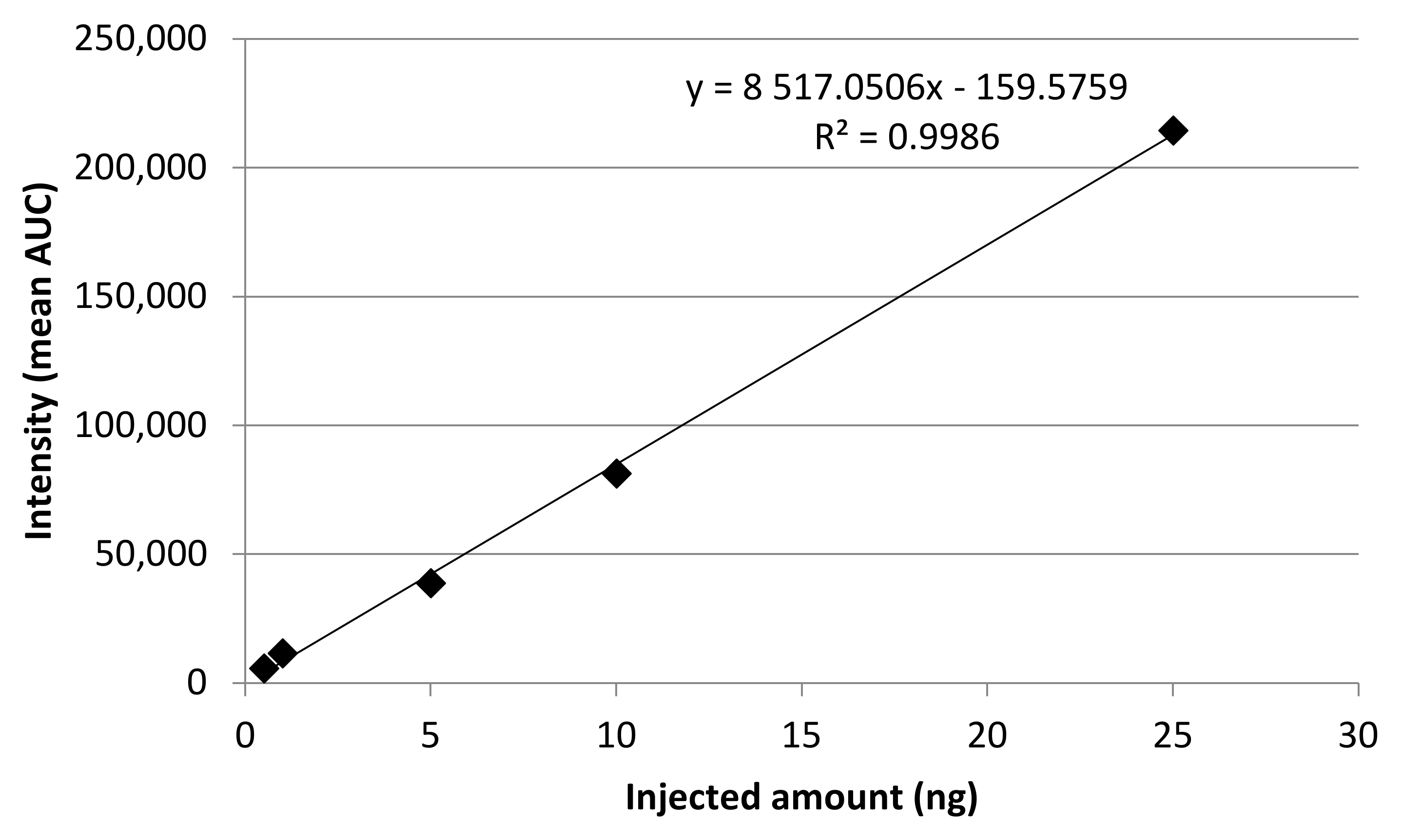
2.2. Calibration
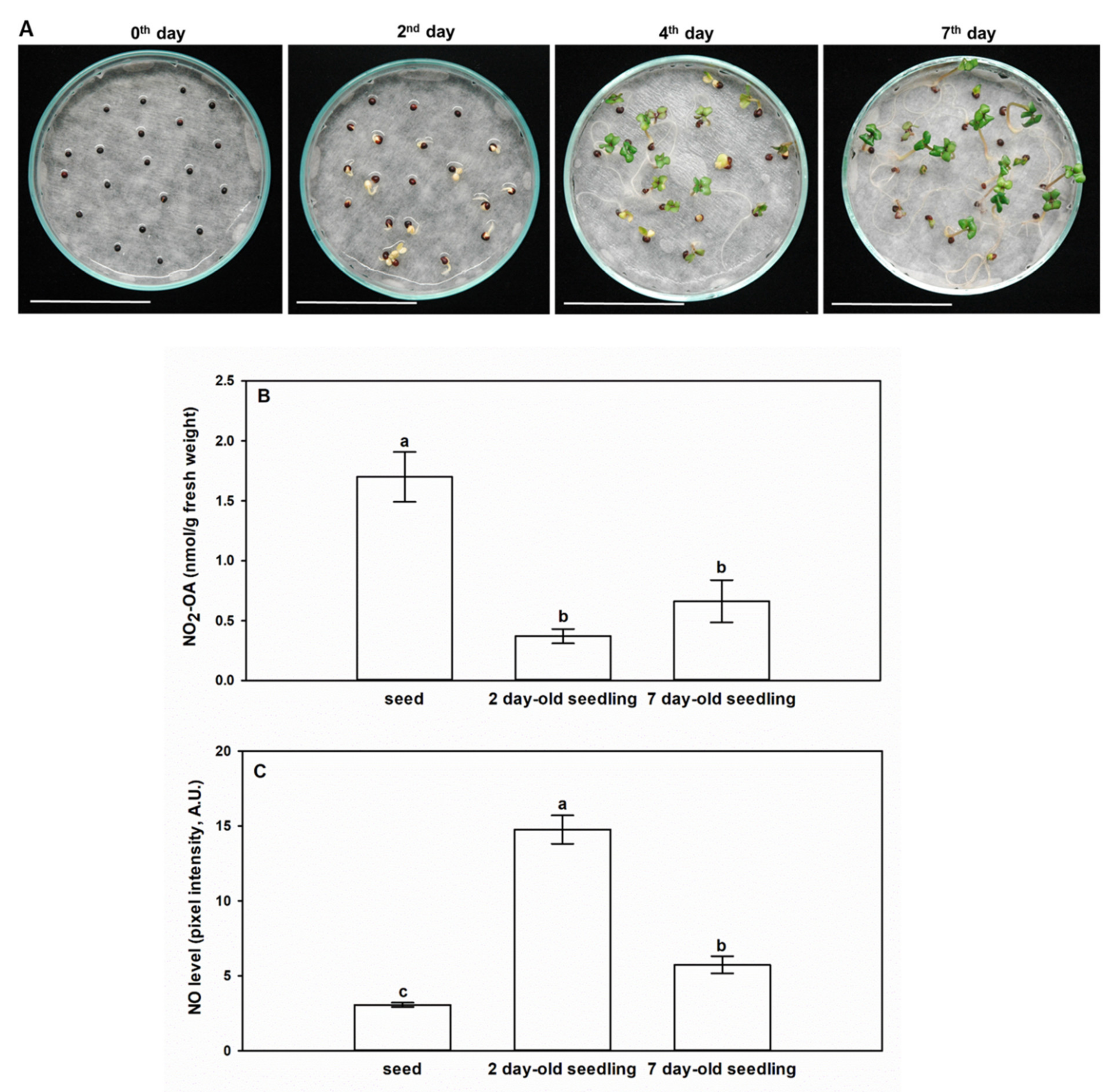
2.3. NO2-OA Content of Brassica napus at the Seed and Seedling Stages
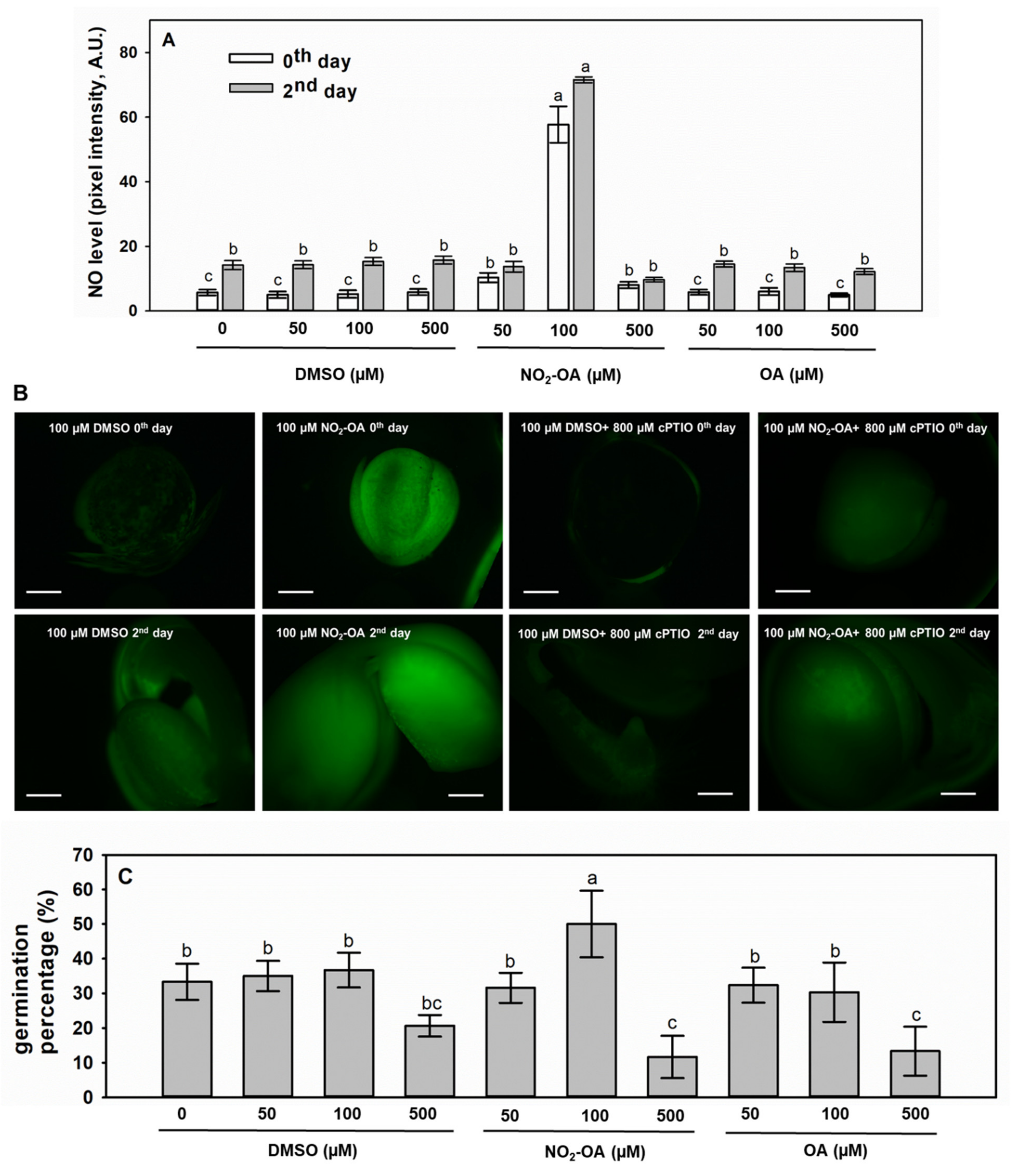
2.4. Exogenous NO2-OA Treatment of Brassica Seeds Positively Influences •NO Levels and Germination Capacity
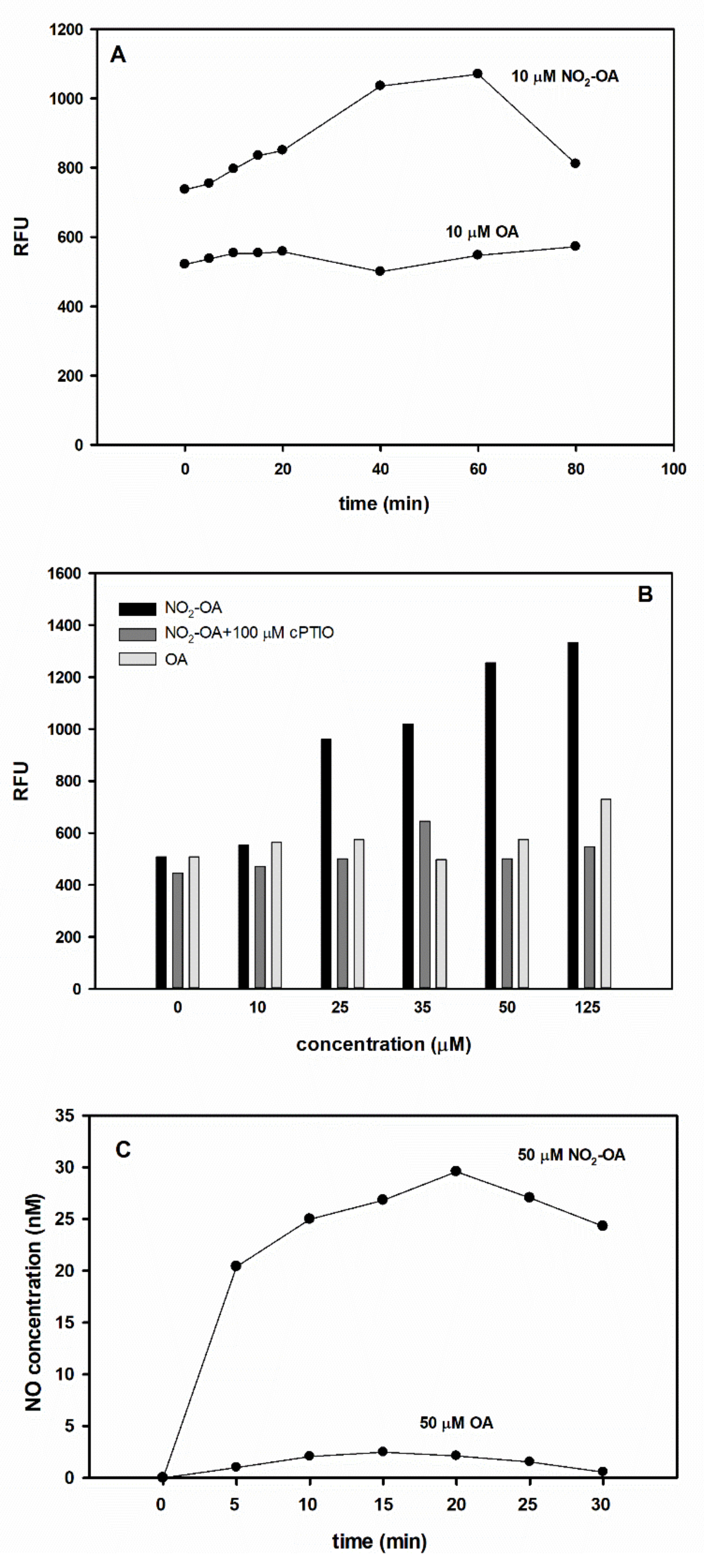
2.5. NO2-OA Releases •NO in Vitro
3. Materials and Methods
3.1. Plant Material and Growing Conditions
3.2. Synthesis and Structure Determination of 9-Nitro-Oleic Acid Standard
3.3. LC-MS Quantification of NO2-OA in Brassica Seeds and Seedlings
3.4. •NO Detection in Brassica napus Seeds and Seedlings
3.5. NO2-OA Treatment of Brassica napus Seeds
3.6. Spectrofluorometric Determination of •NO Levels
3.7. Measurement of •NO Concentration by •NO-Specific Electrode
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Melo, T.; Montero-Bullón, J.F.; Domingues, P.; Domingues, M.R. Discovery of bioactive nitrated lipids and nitro-lipid-protein adducts using mass spectrometry-based approaches. Redox Biol. 2019, 23, 101–106. [Google Scholar] [CrossRef]
- Buchan, G.J.; Bonacci, G.; Fazzari, M.; Salvatore, S.R.; Gelhaus Wendell, S. Nitro-fatty acid formation and metabolism. Nitric Oxid. 2018, 79, 38–44. [Google Scholar] [CrossRef]
- Pryor, W.A.; Lightsey, J.W.; Church, D.F. Reaction of nitrogen dioxide with alkenes and polyunsaturated fatty acids: Addition and hydrogen abstraction mechanisms. J. Am. Chem. Soc. 1982, 104, 6685–6692. [Google Scholar] [CrossRef]
- d’Ischia, M.; Napolitano, A.; Manini, P.; Panzella, L. Secondary targets of nitrite-derived reactive nitrogen species: Nitrosation/nitration pathways, antioxidant defense mechanisms and toxicological implications. Chem. Res. Toxicol. 2011, 24, 2071–2092. [Google Scholar] [CrossRef]
- Fazzari, M.; Trostchansky, A.; Schopfer, F.J.; Salvatore, S.R.; Sánchez-Calvo, B.; Vitturi, D.; Valderrama, R.; Barroso, J.B.; Radi, R.; Freeman, J.B.; et al. Olives and olive oil are sources of electrophilic fatty acid nitroalkenes. PLoS ONE 2014, 9, e84884. [Google Scholar] [CrossRef] [PubMed]
- Mata-Pérez, C.; Sánchez-Calvo, B.; Padilla, M.N.; Begara-Morales, J.C.; Luque, F.; Melguizo, M.; Jiménez-Ruiz, J.; Fierro-Risco, J.; Peñas-Sanjuán, A.; Valderrama, R.; et al. Nitro-fatty acids in plant signaling: Nitro-linolenic acid induces the molecular chaperone network in Arabidopsis. Plant Physiol. 2016, 170, 686–701. [Google Scholar] [CrossRef] [PubMed]
- Mata-Pérez, C.; Sánchez-Calvo, B.; Padilla, M.N.; Begara-Morales, J.C.; Valderrama, R.; Corpas, F.J.; Barroso, J.B. Nitro-fatty acids in plant signaling: New key mediators of nitric oxide metabolism. Redox Biol. 2017, 11, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Di Palma, A.A.; Di Fino, L.M.; Salvatore, S.R.; D’Ambrosio, J.M.; Grozeff, G.E.G.; García-Mata, C.; Schopfer, F.J.; Laxalt, A.M. Nitro-oleic acid induced reactive oxygen species formation and plant defense signaling in tomato cell suspensions. BioRxiv 2018, in press. [Google Scholar] [CrossRef]
- Di Palma, A.A.; Di Fino, L.M.; Salvatore, S.R.; D’Ambrosio, J.M.; García-Mata, C.; Schopfer, F.J.; Laxalt, A.M. Nitro-oleic acid triggers ROS production via NADPH oxidase activation in plants: A pharmacological approach. J. Plant Physiol. 2020, 246–247, 153128, in press. [Google Scholar] [CrossRef]
- Rudolph, V.; Rudolph, T.K.; Schopfer, F.J.; Bonacci, G.; Woodcock, S.R.; Cole, M.P.; Baker, P.R.S.; Ramani, R.; Freeman, B.A. Endogenous generation and protective effects of nitro-fatty acids in a murine model of focal cardiac ischaemia and reperfusion. Cardiovasc. Res. 2010, 85, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Baker, L.M.; Baker, P.R.; Golin-Bisello, F.; Schopfer, F.J.; Fink, M.; Woodcock, S.R.; Branchaud, B.P.; Radi, R.; Freeman, B.A. Nitro-fatty acid reaction with glutathione and cysteine. Kinetic analysis of thiol alkylation by a Michael addition reaction. J. Biol. Chem. 2007, 282, 31085–31093. [Google Scholar] [CrossRef] [PubMed]
- Aranda-Caño, L.; Sánchez-Calvo, B.; Begara-Morales, J.C.; Chaki, M.; Mata-Pérez, C.; Padilla, M.N.; Valderrama, R.; Barroso, J.B. Post-translational modification of proteins mediated by nitro-fatty acids in plants: Nitroalkylation. Plants 2019, 8, 82. [Google Scholar] [CrossRef]
- Rubbo, H.; Parthasarathy, S.; Barnes, S.; Kirk, M.; Kalyanaraman, B.; Freeman, B.A. Nitric oxide inhibition of lipoxygenase-dependent liposome and low-density lipoprotein oxidation: Termination of radical chain propagation reactions and formation of nitrogen-containing oxidized lipid derivatives. Arch. Biochem. Biophys. 1995, 324, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Lima, É.S.; Bonini, M.G.; Augusto, O.; Barbeiro, H.V.; Souza, H.P.; Abdalla, D.S.P. Nitrated lipids decompose to nitric oxide and lipid radicals and cause vasorelaxation. Free Radic. Biol. Med. 2005, 39, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Schopfer, F.J.; Baker, P.R.S.; Giles, G.; Chumley, P.; Batthyany, C.; Crawford, J.; Patel, R.P.; Hogg, N.; Branchaud, B.P.; Lancaster, J.R.; et al. Fatty acid transduction of nitric oxide signaling: Nitrolinoleic acid is a hydrophobically stabilized nitric oxide donor. J. Biol. Chem. 2005, 280, 19289–19297. [Google Scholar] [CrossRef]
- Mata-Pérez, C.; Sánchez-Calvo, B.; Begara-Morales, J.C.; Padilla, M.N.; Valderrama, R.; Corpas, F.J.; Barroso, J.B. Nitric oxide release from nitro-fatty acids in Arabidopsis roots. Plant Signal. Behav. 2016, 11, e1154255. [Google Scholar] [CrossRef]
- Villacorta, L.; Zhang, J.; Garcia-Barrio, M.T.; Chen, X.; Freeman, B.A.; Chen, Y.E.; Cui, T. Nitro-linoleic acid inhibits vascular smooth muscle cell proliferation via the Keap1/Nrf2 signaling pathway. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, 770–776. [Google Scholar] [CrossRef]
- Nie, H.; Xue, X.; Li, J.; Liu, X.; Lv, S.; Guan, G.; Liu, H.; Liu, G.; Liu, S.; Chen, Z. Nitro-oleic acid attenuates ogd/r-triggered apoptosis in renal tubular cells via inhibition of Bax mitochondrial translocation in a PPAR-γ-dependent manner. Cell. Physiol. Biochem. 2015, 35, 1201–1218. [Google Scholar] [CrossRef]
- Kansanen, E.; Kuosmanen, S.M.; Ruotsalainen, A.K.; Hynynen, H.; Levonen, A.L. Nitro-oleic acid regulates endothelin signaling in human endothelial cells. Mol. Pharmacol. 2017, 92, 481–490. [Google Scholar] [CrossRef]
- Yang, R.X.; Fan, J.G. Nitro-oleic acid as a new drug candidate for non-alcoholic steatohepatitis. EBioMedicine 2019, 42, 32–33. [Google Scholar] [CrossRef]
- Liu, H.; Jia, Z.; Soodvilai, S.; Guan, G.; Wang, M.H.; Dong, Z.; Symons, J.D.; Yang, T. Nitro-oleic acid protects the mouse kidney from ischemia and reperfusion injury. Am. J. Physiol. Renal Physiol. 2008, 295, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Rom, O.; Xu, G.; Guo, Y.; Zhu, Y.; Wang, H.; Zhang, J.; Fan, Y.; Liang, W.; Lu, H.; Liu, Y.; et al. Nitro-fatty acids protect against steatosis and fibrosis during development of nonalcoholic fatty liver disease in mice. EBioMedicine 2019, 41, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Márton, M.; Mándoki, Z.S.; Csapó-Kiss, Z.S.; Csapó, J. The role of sprouts in human nutrition. A review. Acta Univ. Sapientiae Aliment. 2010, 3, 81–117. [Google Scholar]
- Sharafi, Y.; Majidi, M.M.; Goli, S.A.H.; Rashidi, F. Oil content and fatty acids composition in Brassica species. Int. J. Food Proper. 2015, 18, 2145–2154. [Google Scholar] [CrossRef]
- Gorczynski, M.J.; Huang, J.; King, S.B. Regio- and stereospecific syntheses and nitric oxide donor properties of (E)-9- and (E)-10-nitrooctadec-9-enoic acids. Org. Lett. 2006, 8, 2305–2308. [Google Scholar] [CrossRef]
- Sánchez-Calvo, B.; Barroso, J.B.; Corpas, F.J. Hypothesis: Nitro-fatty acids play a role in plant metabolism. Plant Sci. 2013, 199, 1–6. [Google Scholar] [CrossRef]
- Mata-Pérez, C.; Sánchez-Calvo, B.; Begara-Morales, J.C.; Carreras, A.; Padilla, M.N.; Melguizo, M.; Valderrama, R.; Corpas, F.J.; Barroso, J.B. Nitro-linoleic acid is a nitric oxide donor. Nitric Oxid. 2016, 57, 57–63. [Google Scholar] [CrossRef]
- Signorelli, S.; Considine, M.J. Nitric oxide enables germination by a four-pronged attack on ABA-induced seed dormancy. Front. Plant Sci. 2018, 9, 296. [Google Scholar] [CrossRef]
- Woodcock, S.R.; Bonacci, G.; Gelhaus, S.L.; Schopfer, F.J. Nitrated fatty acids: Synthesis and measurement. Free Radic. Biol. Med. 2013, 59, 14–26. [Google Scholar] [CrossRef]
- Woodcock, S.R.; Marwitz, A.J.V.; Bruno, P.; Branchaud, B.P. Synthesis of nitrolipids. all four possible diastereomers of nitrooleic acids: (E)- and (Z)-, 9- and 10-nitro-octadec-9-enoic acids. Org. Lett. 2006, 8, 3931–3934. [Google Scholar] [CrossRef]
- Kolbert, Z.S.; Pető, A.; Lehotai, N.; Feigl, G.; Ördög, A.; Erdei, L. In Vivo and In Vitro studies on fluorophore-specificity. Acta Biol. Szeged. 2012, 65, 37–41. [Google Scholar]
- Zhang, X. Real time and In Vivo monitoring of nitric oxide by electrochemical sensors from dream to reality. Front. Biosci. 2004, 9, 3434–3446. [Google Scholar] [CrossRef] [PubMed]
| Sample | NO2-OA Concentration (nmol/g Fresh Weight) | Mean NO2-OA Concentration (nmol/g Fresh Weight) | RSD (%) |
|---|---|---|---|
| Seed 1 | 1.4582 | 1.6987 | 12.2753 |
| Seed 2 | 1.8293 | ||
| Seed 3 | 1.8086 | ||
| Seedling 2nd day 1 | 0.4378 | 0.3701 | 16.1281 |
| Seedling 2nd day 2 | 0.3475 | ||
| Seedling 2nd day 3 | 0.3250 | ||
| Seedling 4th day 1 | N/A | ||
| Seedling 4th day 2 | |||
| Seedling 4th day 3 | |||
| Seedling 7th day 1 | 0.5569 | 0.6622 | 26.6930 |
| Seedling 7th day 2 | 0.8663 | ||
| Seedling 7th day 3 | 0.5635 | ||
| Shoot 7th day 1 | 0.3124 | 0.3470 | 12.2250 |
| Shoot 7th day 2 | 0.3943 | ||
| Shoot 7th day 3 | 0.3343 | ||
| Root 7th day 1 | 0.4681 | 0.4260 | 9.4828 |
| Root 7th day 2 | 0.3876 | ||
| Root 7th day 3 | 0.4221 | ||
| Plant Species | Organ or Xperimental System | Type of NO2-FA Detected | Concentration of NO2-FA Detected (pmol/g Fresh Weight) | Refs. |
|---|---|---|---|---|
| Arabidopsis thaliana | seed | NO2-Ln | 11.18 | [6] |
| 14-day-old seedling | NO2-Ln | 3.84 | ||
| 30-day-old leaves | NO2-Ln | 0.36 | ||
| 45 day-old leaves | NO2-Ln | 0.54 | ||
| 9-day-old ACSC | NO2-Ln | 0.28 | ||
| Pisum sativum | root | NO2-Ln | 0.072 | [7] |
| leaf | NO2-Ln | 0.084 | ||
| mitochondria | NO2-Ln | 0.282 | ||
| peroxisomes | NO2-Ln | |||
| Oryza sativa | leaf | NO2-Ln | 0.748 | |
| Solanum lycopersicum | cell suspension treated with NO2-OA (0.5, 5, 10, 12.5, 25, 50 µM, 1 h or 6 h) | NO2-OA | ~2500 | [8] [9] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vollár, M.; Feigl, G.; Oláh, D.; Horváth, A.; Molnár, Á.; Kúsz, N.; Ördög, A.; Csupor, D.; Kolbert, Z. Nitro-Oleic Acid in Seeds and Differently Developed Seedlings of Brassica napus L. Plants 2020, 9, 406. https://doi.org/10.3390/plants9030406
Vollár M, Feigl G, Oláh D, Horváth A, Molnár Á, Kúsz N, Ördög A, Csupor D, Kolbert Z. Nitro-Oleic Acid in Seeds and Differently Developed Seedlings of Brassica napus L. Plants. 2020; 9(3):406. https://doi.org/10.3390/plants9030406
Chicago/Turabian StyleVollár, Martin, Gábor Feigl, Dóra Oláh, Attila Horváth, Árpád Molnár, Norbert Kúsz, Attila Ördög, Dezső Csupor, and Zsuzsanna Kolbert. 2020. "Nitro-Oleic Acid in Seeds and Differently Developed Seedlings of Brassica napus L." Plants 9, no. 3: 406. https://doi.org/10.3390/plants9030406
APA StyleVollár, M., Feigl, G., Oláh, D., Horváth, A., Molnár, Á., Kúsz, N., Ördög, A., Csupor, D., & Kolbert, Z. (2020). Nitro-Oleic Acid in Seeds and Differently Developed Seedlings of Brassica napus L. Plants, 9(3), 406. https://doi.org/10.3390/plants9030406








