Abstract
Cracking of sweet cherry (Prunus avium L.) fruits is caused by rain events close to harvest. This problem has occurred in most cherry growing regions with significant economic losses. Several orchard management practices have been applied to reduce the severity of this disorder, like the foliar application of minerals or growth regulators. In the present study, we hypothesized that preharvest spray treatments improve the physiological performance of sweet cherry trees and could also mitigate environmental stressful conditions. Effects of repeated foliar spraying of calcium (Ca), gibberellic acid (GA3), abscisic acid (ABA), salicylic acid (SA), glycine betaine (GB), and the biostimulant Ascophyllum nodosum (AN) on the physiological and biochemical performance of ‘Skeena’ sweet cherry trees during two consecutive years (without Ca in 2015 and in 2016 with addition of Ca) were studied. Results showed that in general spray treatments improved the physiological performance and water status of the trees. AN and ABA sprays were demonstrated to be the best compounds for increasing yield and reducing cherry cracking as well as improving photosynthetic performance and leaf metabolites content. In conclusion, AN and ABA might be promising tools in the fruit production system.
1. Introduction
The most recent climate projections [1] point to a decrease in water availability, an increase in air temperature, and the occurrence of extreme phenomena, such as excessive rainfall near the harvest period, which may increase the incidence of fruit cracking in sweet cherry (Prunus avium L.). Consequently, significant economic losses occur, due to a strong reduction of the commercial value of the cherries. Under the current climate changing scenario [1] and also due to the increase of global trade in fruit to meet consumer demand for regular supply of high quality fruit, it is important to understand the relationship between preharvest treatments with calcium (Ca) and growth regulators and the physiological parameters of sweet cherry trees. This information can provide new insights into the putative potential measures to mitigate environmental stressful conditions.
Ca is an important macronutrient, which is involved in the regulation of the main physiological processes in plants, contributing to the strength of the cell walls and membranes and reducing cherry cracking [2,3,4,5,6]. Under drought conditions, growth and physiological performance are improved by Ca sprays of Zoysia japonica and Zea mays plants [7,8]. Gibberellic acid (GA3) has been used as a compound to promote growth, which regulates plant growth processes, like seed germination, flower, and fruit development [9,10,11]. However, the impact of GA3 spray treatment on fruit cracking incidence is sometimes contradictory [12,13,14,15,16]. Other plant hormones, like salicylic acid (SA) and abscisic acid (ABA), are signaling phytohormones with different regulatory roles in plant metabolism and adaptation to abiotic stresses [17,18]. The yield increase in olive [19], peach [20], and strawberry [21] is associated with SA application, as well as the quality improvement of cherry fruits [22,23]. ABA stimulates stomatal closure and minimizes water loss by transpiration [24]. Additonally, Balbontín et al. [25] mentioned that ABA foliar sprays reduced cracking in ‘Bing’ cherries. Therefore, exogenous ABA application can have a great interest in water conservation in agricultural settings.
Although no consistent literature is available about the effect of preharvest substances, such as glycine betaine (GB) and Ascophyllum nodosum (AN), on the physiological performance of sweet cherry trees, these compounds might be a new and innovative solution to increase the crop ability to tolerate stressful environments. The accumulation of osmolytes such as GB (quaternary ammonium compound) in cells can stabilize the structures by maintaining the integrity of membranes against the damaging effects of abiotic stresses via osmoregulation or osmoprotection [26]. Seaweed based biostimulants, like AN, are composed of several components, such as plant hormones, proteins, sugars, vitamins, humic substances, and phenolic compounds [27,28]. Several published reports suggest that biostimulants improve plant productivity by increasing the minerals assimilation and the photosynthetic rate, reducing the transpiration and the fruit cracking incidence [27,29,30,31]. Despite these well-documented effects, no consistent results are yet available, at least to our knowledge, about the influence of plant growth regulators, with the addition or no addition of Ca, on the performance of sweet cherry trees.
Therefore, the objective of this study was to assess the effect of plant growth regulators, with the addition or no addition of Ca, on the plant physiological and biochemical responses, namely plant water status, photosynthetic performance, and leaf metabolites, as well as on the yield of sweet cherry trees and cherry cracking incidence.
2. Material and Methods
2.1. Experimental Site and Plant Material
Experiments were conducted in Carrazedo de Montenegro, Portugal (latitude 41°33′ N, longitude 7°17′ W, altitude 682 m), in 2015 and 2016, on a six-year-old late-maturing ‘Skeena’ sweet cherry orchard grafted on ‘Gisela 6’ rootstock. The soil characteristics were: 13 g kg−1 of organic matter content, high K2O (125 mg kg−1) and medium P2O5 (75 mg kg−1) contents, medium texture, and pH 5.5. Trees were trained under a vertical axis system with a spacing of 4.5 m between rows and 2.0 m in the row (about 864 trees ha−1) [32]. Between May and September, trees were daily drip-irrigated for 4 h per day (drippers 1 m apart in line with a 4 L h−1 flow rate) and summer pruned. According to recommendations provided by a certified soil analysis laboratory of University of Trás-os-Montes e Alto Douro (UTAD), trees were also periodically fertilized.
Meteorological data [air temperature (°C), rainfall (mm), and solar radiation (W m−2)] for both years (Figure 1) were recorded by a standard weather station (IMT280, iMETOS, Weiz, Austria) located near the experimental site. In 2015, the mean air temperature between March and June was, on average, about 2.6 °C higher than 2016. Additionally, the mean solar radiation until June was also higher in 2015 than 2016. Annual rainfall in 2015 was 470 mm against the long-term rainfall (30 years) of 923 mm. The year 2016 experienced higher annual rainfall (1140 mm), mainly in spring (Figure 1), corresponding to the final phase of the flower development and the fruit development of cherry trees.

Figure 1.
Mean air temperature (°C), rainfall (mm), and mean solar radiation (W m−2) in 2015 and 2016 measured at Carrazedo de Montenegro.
2.2. Experimental Design and Treatments
Six trees from each treatment were selected, a total of 42 trees. In 2015, the experiment included the following treatments: 0.5 mL L−1 biostimulant Ascophyllum nodosum (AN), 10 mg L−1 gibberellic acid (GA3), 10 µM abscisic acid (ABA), 1 mM salicylic acid (SA), 1 mL L−1 glycine betaine (GB), 5 g kg−1 calcium (CaCl2), and control (distilled water). In 2016, the same seven treatments were applied to the same trees selected in the previous year, but now including 5 g kg−1 CaCl2, except for the control treatment. All cherry trees were sprayed with 2.5 L of spraying solution per tree. Wetting agent (1 mL L−1) was mixed in control and treatment solutions. Foliar treatments, except CaCl2, were applied 30, 49, and 56 days after full bloom (DAFB), corresponding to the shuck split (beginning of fruit development), the transition from green to yellow color, and from yellow to orange color. CaCl2 was added at 56, 62, and 69 DAFB, corresponding to the first application at the transition from yellow to orange color and the other applications were applied one week later.
The gas exchange and relative water content (RWC) determinations were performed at midday on 08 July 2015 and 15 July 2016 (corresponding to the harvest date of the cherries) in healthy, fully expanded mature leaves that were well exposed to the sun. For each treatment, fifty fruits were harvested per tree to determine cracking index, and yield was determined for each tree. Furthermore, leaves were collected for biochemical analyses (photosynthetic pigments, total soluble sugars, starch, and soluble proteins) and immediately frozen in liquid nitrogen and then stored at −80 °C until analysis.
2.3. Leaf Gas Exchange
Leaf gas exchange measurements were performed using a portable LCpro+ Infrared Gas Analyzer System (IRGA) (ADC Bioscientific Ltd, Hoddesdon, England), with a 2.5 cm2 leaf chamber (ADC-PLC), operating in the open mode, at midday (13:00–14:30 h) in both years. Incident photosynthetic photon flux density (PPFD) on the leaves was always greater than 1500 μmol m−2 s−1. Net CO2 assimilation rate (A, μmol m−2 s−1), transpiration rate (E, mmol m−2 s−1), and stomatal conductance (gs, mmol m−2 s−1) were calculated using the equations developed by von Caemmerer and Farquhar [33]. Intrinsic water-use efficiency was calculated as the ratio of A to gs (A/gs, µmol mol−1), according to Düring [34]. All results are expressed as the average of six replicates with standard error (SE) shown.
2.4. Leaf Water Status
After the midday gas exchange measurements, sweet cherry leaves were detached and immediately placed into air-tight tubes, and the following parameters were studied: fresh weight (FW in g), weight at full turgor (TW in g, measured after immersing the leaf petioles in deionized water for 24 h at 4 °C in the dark), and dry weight (DW in g, measured after drying at 70 °C to a constant weight). The relative water content (RWC in %) was calculated as follows: RWC = (FW−DW)/(TW−DW) × 100. Results are expressed as the average of six replicates with SE shown.
2.5. Metabolite Composition Determination
2.5.1. Photosynthetic Pigments
For chlorophyll (Chl) and carotenoid (Carot) determination, leaf discs (0.8 cm diameter) were ground with mortar and pestle using acetone/distilled-water (80/20, v/v) as extraction solvent. Analyses were performed under the dim light to avoid chlorophyll degradation. Determination of total chlorophyll (Chltotal) and total carotenoids (Carottotal) were performed according to Šesták et al. [35] and Lichtenthaler [36], respectively. The results were expressed as mg g−1 DW as the mean ± SE of six replicates.
2.5.2. Total Soluble Sugars and Starch
Total soluble sugars (SS) quantification was performed using the methodology of Irigoyen et al. [37], by heating foliar discs in ethanol/distilled-water (80/20, v/v) for 1 h, at 80 °C. After the reaction of the alcoholic extract with fresh anthrone in a boiling water bath for 10 min, SS were quantified by recorded absorbance values at 625 nm. Afterwards, starch (St) was extracted from the same solid fraction by heating leaf discs in 30% perchloric acid at 60 °C for 1 h, using the methodology of Osaki et al. [38]. The St concentration was determined by the anthrone method described above. Glucose was used as a standard for both SS and St quantification. The results were expressed as mg g−1 DW as the mean ± SE of six replicates.
2.5.3. Soluble Proteins
Total soluble proteins (SP) determination was performed using phosphate buffer (pH 7.5), 0.1 mM ethylenediaminetetraacetic acid (EDTA), 100 mM phenyl-methylsulfonyl fluoride (PMSF), and 20 g L−1 polyvinylpyrolli-done (PVP). The absorbance values were recorded at 595 nm, using bovine serum albumin (BSA) as standard [39]. The results were expressed as mg g−1 DW as the mean ± SE of six replicates.
2.6. Yield Determination
Sweet cherries were harvested at optimum maturity stage and yield per tree was recorded in kilograms as the mean ± SE of six replicates.
2.7. Fruit Cracking Index Determination
The cracking index (CI in %) was determined according to Christensen [40]. Fifty fruits without defects were selected and immersed in 2 L plastic containers filled with distilled water (20 ± 1 °C) for 6 h. Cracked fruits were removed, counted, and fruits without cracks were reincubated. After 2, 4, and 6 h, the fruits were observed for macroscopic cracks, with the CI calculated according to the following formula: CI = [(5a + 3b + c) × 100]/250, where a, b, and c represent the number of cracked fruits after 2, 4, and 6 h, respectively. The measurements are presented as average values (n = 3) with SE.
2.8. Statistical Analysis
The statistical analysis was carried out using the statistical software program SPSS V.25 (SPSS-IBM, Orchard Road-Armonk, New York, NY, USA). Statistical differences were evaluated by one-way analysis of variance (ANOVA) followed by the post hoc Duncan’s multiple range test (P < 0.05), establishing treatment effect. The ANOVA requirements, namely the normal distribution of the residuals and the homogeneity of variance, were evaluated by means of the Shapiro–Wilk’s test and Bartlett’s tests, respectively. Dependent variables were analyzed using ANOVA with or without Welch correction, depending if homogeneity of variances was observed or not. For the relationship between parameters, Pearson’s correlation was performed.
3. Results
3.1. Leaf Gas Exchange Parameters
The results show that for both years, most of the gas exchange parameters were significantly affected (P < 0.05) by the spray treatments (Figure 2), except the transpiration rate (E) (data not shown).
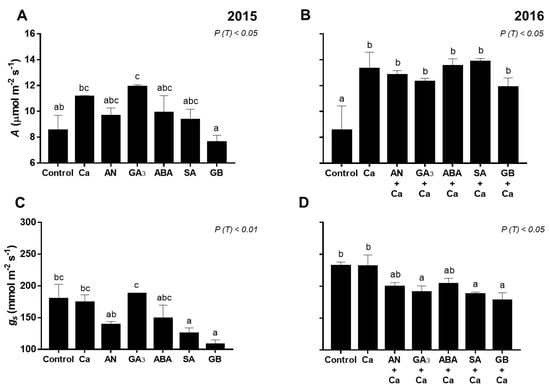

Figure 2.
Net CO2 assimilation (A) (A,B), stomatal conductance (gs) (C,D), and intrinsic water-use efficiency (A/gs) (E,F) of fully exposed leaves of ‘Skeena’ cherry treated after spray treatments application in 2015–2016. Each column is expressed as mean ± SE (n = 6). Different letters indicate significant differences (P < 0.05) among treatments by Duncan’s test.
In 2015, GA3 sprays increased (P < 0.05) photosynthetic rate (A) compared to the control (H2O) (Figure 2a). In turn, SA and GB spray treatments decreased (P < 0.01) gs (Figure 2c). All spray treatments increased (P < 0.01) intrinsic water-use efficiency (A/gs) compared to the control (Figure 2e).
In 2016, spray treatments increased (P < 0.05) A, with the highest value for SA+Ca-treated cherry trees (13.85 µmol m−2 s−1), whereas control trees recorded the minimum rate (Figure 2b). GA3+Ca-, SA+Ca-, and GB+Ca-treated cherry trees presented significantly lower (P < 0.05) gs values than control and Ca-treated cherry trees (Figure 2d). AN+Ca, GA3+Ca, ABA+Ca, SA+Ca, and GB+Ca spray treatments increased (P < 0.05) A/gs compared to the control (Figure 2f).
3.2. Leaf Water Status
In both years, relative water content (RWC) was affected by the spray treatments (P < 0.05) (Figure 3). In 2015, treatments with GA3, AN, and GB increased (P < 0.05) RWC up to 3%, 3.4%, and 4.8%, respectively, compared with the control (Figure 3A). In 2016, all spray treatments presented higher (P < 0.001) RWC values in comparison with control plants. The highest value was obtained in cherry trees treated with ABA+Ca, about 4% higher than control (Figure 3B).

Figure 3.
Relative water content (RWC, %) of ‘Skeena’ leaf cherry after spray treatments application in 2015 (A) and 2016 (B). Each column is expressed as mean ± SE (n = 6). Different letters indicate significant differences (P < 0.05) among treatments by Duncan’s test.
3.3. Leaf Photosynthetic Pigments and Metabolites
The content of photosynthetic pigments Chltotal and Carottotal was affected by spray treatments (P < 0.05) in 2015, while in 2016 significant differences (P < 0.05) were only found for the content of Chltotal (Table 1). Compared with the control, the highest Chltotal content (10.56 mg g−1 DW) was observed with the ABA treatment in 2015 and both AN+Ca and GB+Ca treatments in 2016. In relation to the Carottotal content in 2015, cherry trees treated with Ca, AN, GA3, SA, and GB presented higher (P < 0.001) values than the control. Both years presented similar Chltotal contents for control and Ca-treated plants. In turn, Carottotal concentration seems to be influenced by the year, being higher (P < 0.01) in 2016.

Table 1.
Total chlorophyll content (Chltotal), total carotenoids content (Carottotal), soluble sugars content (SS), starch content (St), and soluble protein content (SP) of ‘Skeena’ leaf cherry after spray treatments application in 2015–2016.
The results also indicated that spray treatments increased leaf metabolites in cherry leaves (Table 1). Soluble sugars (SS) content was significantly affected by the spray treatments (P < 0.05) in both years. In 2015, AN and ABA sprays increased (P < 0.05) SS content in relation to the control. On the other hand, addition of Ca to GA3, ABA, and SA usually resulted in higher SS concentration in 2016. Significant differences were also observed among spray treatments for soluble proteins (SP) and starch (St) concentration in the first and second year of the experiment, respectively. In 2015, the highest SP concentration was recorded in cherry trees sprayed with AN, GA3, SA, and GB. In 2016, the combined treatments AN+Ca, ABA+Ca, SA+Ca, and GB+Ca showed a higher (P < 0.01) St content.
3.4. Yield of Sweet Cherry Trees
Significant yield differences were found among spray treatments (P < 0.05) in both years (Figure 4). In 2015, cherry trees sprayed with AN and ABA exhibited the highest (P < 0.05) yield, averaging 45% and 41%, respectively, compared to the control (Figure 4A). This behavior was also observed in 2016, although the highest production was obtained for GB+Ca-treated cherry trees, up to 40% compared to the control (Figure 4B).
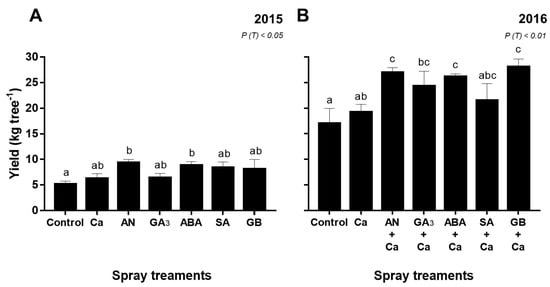
Figure 4.
Yield (kg tree−1) of ‘Skeena’ cherry trees after spray treatments application in 2015 (A) and 2016 (B). Each column is expressed as mean ± SE (n = 6). Different letters indicate significant differences (P < 0.05) among treatments by Duncan’s test.
3.5. Cracking Incidence
Spray treatments did not affect significantly (P > 0.05) the fruit cracking index (CI) in both years (Figure 5). However, cherries treated with Ca, AN, ABA, SA, and GB spray treatments showed a decreasing trend of CI in both years, compared to the control. In contrast, GA3/GA3+Ca sprays increased the CI, in both years.

Figure 5.
Cracking index (%) of ‘Skeena’ fruits after spray treatments application in 2015 (A) and 2016 (B). Each column is expressed as mean ± SE (n = 3, each with 50 fruits).
4. Discussion
4.1. Spray Treatments Modulate Leaf Gas Exchange and Water Status of Sweet Cherry Trees
Overall, spray treatments improved the physiological behavior of sweet cherry trees in both years (Figure 2). A presented a positive correlation with gs (r = 0.54, P < 0.001), as observed previously by Gonçalves et al. [41] in sweet cherry. Higher gs values were noticed in 2016 compared to 2015, which may be due to the higher solar radiation recorded in July 2016, near the harvest, and consequently, the values of A were also higher in 2016 (Figure 1 and Figure 2). Our findings are in agreement with previous studies, which demonstrated an increase of A in broad beans (Vicia faba) and grapevine (Vitis vinifera L.) treated with GA3 [42]. Grapevine treated with GA3 also resulted in favorable A/gs [43]. Other studies with Brassica juncea, corn, and soybean treated with SA also showed an improvement of A, A/gs and gs adjustment [44,45,46], which agrees with our results. The first sign of plant defense for maintaining the water status is the stomatal closure [47]. Indeed, our results indicate that GB sprays improved A and also increased stomata closure by the reduction of gs (Figure 2), which might be considered a strategy for enhancing tolerance to various abiotic stresses [48]. Moreover, GB-treated olive trees under drought showed an enhancement of A [49]. Tradescantia virginiana plants treated with ABA and grown under well-watered conditions had lower gs and an improvement in A/gs, while the A was unaffected [50]. Although, in both years of our study, the application of ABA increased A/gs, gs was not affected, and an improvement in A was observed, mainly in 2016. Additionally, A and A/gs values also increased in response to AN+Ca treatment (in 2016) (Figure 2). This finding is in agreement with previous works, which revealed an increase of A, a reduction of E, and gs parameters in plants treated with biostimulant products [29,30].
The leaf RWC was around 90% in both years, suggesting sufficient drip irrigation. Nevertheless, in treated-plants with foliar compounds, the RWC values were mostly higher in both years (Figure 3). The water balance in plants is estimated by calculation of RWC, which is positively correlated with the photosynthetic efficiency of plants [51]. This relationship is established by the correlation between RWC and A, mainly in 2016 (r = 0.54, P < 0.05). Similarly with our results, a significant improvement of RWC in different crops exposed to several biotic stresses and treated with biostimulant [29,52,53,54], GA3 [55], ABA [56], SA [57], and GB [49,58] was previously reported.
4.2. Photosynthetic Pigments’ and Metabolites’ Behavior in Response to Spray Treatments
Chltotal exhibited the same tendency as A, displaying higher contents compared to the control in both years (Table 1). ABA sprays increased Chltotal content, which is in accordance with a previous study that revealed that the exogenous application of ABA increases the synthesis of chlorophylls in ‘micro’ tomato leaf tissue [59]. Photosynthetic pigments contents, Chltotal and Carottotal, also increased in AN-, SA-, and GB-treated cherry trees (Table 1). Similarly, Kabiri et al. [60], mentioned that the SA application in Nigella sativa increased the content of chlorophylls and carotenoids. According to Hayat and Ahmad [61], this increase improved the antioxidant capacity of plants and it was related to the synthesis of protective compounds. Additionally, overaccumulation of GB due to the introduction of the betaine aldehyde dehydrogenase (BADH) gene can increase the protection of chlorophylls and carotenoids and enhance the photosynthetic rate [62]. Several biostimulants, like AN, have been stated to stimulate plant growth by increasing photosynthetic pigments [28]. These findings can be related to the preservation of carotenoids as a mechanism of photoprotection [63] and amelioration of the leaf water retention. Our study indicates that spray treatments, mainly AN, SA, and GB induced the increased chlorophylls and carotenoids levels in the leaf tissue, which can improve the antioxidant capacity of plants to abiotic stress.
The spray treatments also affected the SP content in cherry leaves, showing higher levels in AN-, GA3-, SA-, and GB-treated trees, up to 34% with SA spray treatment (Table 1). These treatments might induce the growing of antioxidant responses as described for SA [18,61]. The higher accumulation of SS in leaves observed in response to AN and ABA sprays in 2015 and to GA3+Ca, ABA+Ca, and SA+Ca sprays in 2016 (Table 1) can be a protective mechanism to preserve cell homeostasis, indicating that these spray treatments provided a better cherry tree photosynthetic performance. Interestingly, the highest SS accumulation in cherry leaves was found for ABA spray treatment for around 20% in both years (Table 1). Exogenous application of ABA is reported to increase the maturity index and anthocyanin content in cherries [64] and the soluble sugars in grapes [65]. Although our previous works reported that ABA application increased anthocyanin content in cherry, no significant effect was observed on the maturation [66]. The highest St content observed in 2016 might be related to the higher photosynthetic efficiency determined in the same year (Table 1). Overall, the present study suggests a relation between acclimation of photosynthesis (A) and St accumulation (r = 0.46, P < 0.05). Indeed, several authors have suggested that a decrease of St in leaf was correlated with the acclimation of photosynthesis [67,68]. In 2016, the higher accumulation of St in mainly AN+Ca-, ABA+Ca-, SA+Ca-, and GB+Ca-treated cherry trees may have a positive effect on tree production, since St is a crucial storage carbohydrate that is frequently mobilized in the form of SS [69]. The St accumulation in leaves of ABA+Ca-, SA+Ca-, and GB+Ca-treated cherry trees could be associated with the increase in weight observed in the fruits collected from the plants treated with these spray treatments [23]. In addition, ABA might be related to control of the enzymes responsible for St degradation, regulating the St accumulation during osmotic stress in plants [70].
4.3. Effect of Spray Treatments on Yield of Sweet Cherry Trees and Fruit Cracking Incidence
Yield and cracking incidence evaluation are important parameters in the effectiveness of spray treatments. However, fruit yield is a function of several factors, such as meteorological conditions, rootstock vigor, irrigation, and pruning [71]. Our previous work reported that on average, fruit weight was reduced in 2016 by 33% compared to 2015 [23]. On the other hand, yield was higher in 2016 compared to 2015 (Figure 4), which was due to a higher crop load rather than larger fruit size. This may also be because of a winter pruning in 2014, which will have reduced the number of flowers in the following year (2015); in turn, in 2015 no pruning was performed. Nevertheless, AN and ABA sprays were related to the increase in yield in both years, while in 2016 also GA3- and GB-treated cherry trees showed a significant increase of yield (Figure 4). Although cherry cracking incidence did not significantly decrease with spray treatments in both years, it was observed that also the AN- and ABA-treated fruits showed the least cracking index in 2015 and 2016 (Figure 5). Reduced cracking index for ABA-treated ‘Bing’ cherry was reported by Balbontín et al. [25]. An additional promising strategy to mitigate cherry cracking is AN, as had been reported by our research group for ‘Skeena’ and ‘Sweetheart’ cherry fruits [31]. On the other hand, foliar application of GA3 increased ‘Skeena’ cherry cracking, and this finding corroborates with previous studies [12]. Biostimulants, like AN, have offered a potentially novel approach in plants to stimulate growth and to increase yield, as reported by Basak [72], Colavita et al. [73], and Jannin et al. [30]. Quiroga et al. [74] reported that ABA foliar application in the field-grown grapevine could improve yield per plant. Our data suggest that the application of GA3 and GB seems to benefit with the combination of Ca in increasing of cherry yield. A positive impact of exogenous GB application on plant growth and final crop yield has been reported on sunflower (Helianthus annuus L.) and maize (Zea mays) under drought [75,76]. As observed in our study, foliar application of GA3 increased fruit yield in several crops, such as in tomato [77,78] and cucumber (Cucumis sativus L.) [79]. Our data also indicated a positive correlation between yield and A (r = 0.54, P < 0.01), as observed by Parry et al. [80] and Zhu et al. [81] in other species. Positive correlations were also observed between yield and SS (r = 0.50, P < 0.001), St (r = 0.83, P < 0.001), SP (r = 0.46, P < 0.01), and RWC (r = 0.66, P < 0.001). Indeed, other studies have also reported that yield is correlated positively with SS and RWC in Sorghum bicolor L. [82] and in several cultivars of banana fruits [83].
5. Conclusions
Foliar spraying of growth regulators and calcium was associated with an enhancement of the physiological performance and yield of the ‘Skeena’ sweet cherry trees. Among the treatments evaluated, foliar application of AN and ABA were more effective in increasing yield and in reducing the incidence of cherry cracking. Therefore, these two foliar sprays are attractive compounds to improve physiological performance and yield of sweet cherry trees and might be a promising cherry-cracking mitigation approach. Although AN and ABA foliar application might be a cultural practice in the near future, further studies on the influence of environmental conditions, concentrations, and time of foliar compounds application in order to develop new cultivar-specific farming strategies will be required.
Author Contributions
All the authors contributed in a significant way to the activities of the paper. S.C., F.Q., A.P.S. and B.G. conceived and designed the experiment. S.C., F.Q., H.F., M.C.M. and S.A. performed foliar treatments, field work, and the experiments. S.C. analyzed results and wrote the manuscript. All authors reviewed and approved the final manuscript.
Funding
This work was funded by the R&D Project CHERRYCRACKLESS—Cherry cracking & mitigation strategies: towards their understanding using a functional metabolic approach, with reference POCI-01-145-FEDER-016805 and PTDC/AGR-PRO/7028/2014, financed by the European Regional Development Fund (ERDF) through COMPETE 2020 - Operational Program for Competitiveness and Internationalization (POCI) and by the Foundation for Science and Technology (FCT).
Acknowledgments
The author Sofia Correia acknowledges the financial support provided by the Portuguese Foundation for Science and Technology (FCT) (SFRH/BD/52541/2014), under the Doctoral Programme ‘Agricultural Production Chains—from fork to farm’ (PD/00122/2012). The authors also acknowledge the support of National Funds by FCT, under the project UIDB/04033/2020. The authors are grateful to the late Manuel Aires from the Frumont company for providing access to his orchard; João Santos for providing the climate data; Ana Monteiro, Cristiana Teixeira, Ivo Oliveira, Linton Dinis, Sara Bernardo and Silvina Morais for their support in collecting cherry samples and gas exchange measurements in the orchard and help on the laboratory work.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- IPCC. Global Warming of 1.5 °C. An. IPCC Special Report on the Impacts of Global Warming of 1.5 °C Above Pre-Industrial Levels and Related Global Greenhouse Gas. Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; Masson-Delmotte, V., Zhai, P., Pörtner, H.O., Roberts, D., Skea, J., Shukla, P.R., Pirani, A., Moufouma-Okia, W., Péan, C., Pidcock, R., et al., Eds.; IPCC: Geneva, Switzerland, 2018. [Google Scholar]
- Hanson, J.B. The function of calcium in plant nutrition. In Advances in Plant Nutrition; Tinker, P.B., Lauchli, A., Eds.; Praeger: New York, NY, USA, 1984; pp. 149–208. [Google Scholar]
- Demirsoy, L.; Bilgener, S. The effects of preharvest calcium hydroxide applications on cracking in ‘0900 Ziraat’, ‘Lambert’ and ‘Van’ sweet cherries. Acta Hortic. 1998, 468, 657–662. [Google Scholar] [CrossRef]
- Vangdal, E.; Hovland, K.L.; Børve, J.; Sekse, L.; Slimestad, R. Foliar application of calcium reduces postharvest decay in sweet cherry by various mechanisms. Acta Hortic. 2008, 768, 143–148. [Google Scholar] [CrossRef]
- Wójcik, P.; Akgül, H.; Demirtaş, I.; Sarısu, C.; Aksu, M.; Gubbuk, H. Effect of preharvest sprays of calcium chloride and sucrose on cracking and quality of Burlat sweet cherry fruit. J. Plant. Nutr. 2013, 36, 1453–1465. [Google Scholar] [CrossRef]
- Erogul, D. Effect of preharvest calcium treatments on sweet cherry fruit quality. Not. Bot. Hort. Agrobot. 2014, 42, 150–153. [Google Scholar] [CrossRef]
- Xu, C.; Li, X.; Zhang, L. The effect of calcium chloride on growth, photosynthesis, and antioxidant responses of Zoysia japonica under drought conditions. PLoS ONE 2013, 8, e68214. [Google Scholar] [CrossRef]
- Naeem, M.; Naeem, M.S.; Ahmad, R.; Ihsan, M.Z.; Ashraf, M.Y.; Hussain, Y.; Fahad, S. Foliar calcium spray confers drought stress tolerance in maize via modulation of plant growth, water relations, proline content and hydrogen peroxide activity. Arch. Agron. Soil Sci. 2017, 64, 116–131. [Google Scholar] [CrossRef]
- Razem, F.A.; Baron, K.; Hill, R.D. Turning on gibberellin and abscisic acid signaling. Curr. Opin. Plant. Biol. 2006, 9, 454–459. [Google Scholar] [CrossRef]
- Erogul, D.; Sen, F. Effects of gibberellic acid treatments on fruit thinning and fruit quality in Japanese plum (Prunus salicina Lindl.). Sci. Hortic. 2015, 186, 137–142. [Google Scholar] [CrossRef]
- Ozkan, Y.; Ucar, M.; Yildiz, K.; Ozturkc, B. Pre-harvest gibberellic acid (GA3) treatments play an important role on bioactive compounds and fruit quality of sweet cherry cultivars. Sci. Hortic. 2016, 211, 358–362. [Google Scholar] [CrossRef]
- Cline, J.A.; Trought, M. Effect of gibberellic acid on fruit cracking and quality of ‘Bing’ and ‘Sam’ sweet cherries. Can. J. Plant. Sci. 2007, 87, 545–550. [Google Scholar] [CrossRef]
- Horvitz, S.; López Camelo, A.F.; Yommi, A.; Godoy, C. Application of gibberellic acid to ‘sweetheart’ sweet cherries: Effects on fruit quality at harvest and during cold storage. Acta Hortic. 2003, 628, 311–316. [Google Scholar] [CrossRef]
- Hoppe, F.; Huyskens-Keil, S.; Ulrichs, C.; Hanrahan, I. Assessment of susceptibility and prevention of cracking of ‘Skeena’ sweet cherry. In Proceedings of the II International Symposium on Horticulture in Europe 1099, Angers, France, 1–5 July 2012; pp. 819–826. [Google Scholar]
- Usenik, V.; Kastelec, D.; Stampar, F. Physicochemical changes of sweet cherry fruits related to application of gibberellic acid. Food Chem. 2005, 90, 663–671. [Google Scholar] [CrossRef]
- Suran, P.; Vavra, R.; Zeleny, L. Effectiveness of potential products to reduce rain cracking of cherry fruit. Acta Hortic. 2016, 1137, 183–186. [Google Scholar] [CrossRef]
- Leung, J.; Giraudat, J. Abcisic acid signal transduction. Annu. Rev. Plant. Physiol. Plant. Mol. Biol. 1998, 49, 199–222. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant. Sci. 2015, 6, 462. [Google Scholar] [CrossRef]
- Brito, C.; Dinis, L.T.; Silva, E.; Gonçalves, A.; Matos, C.; Rodrigues, M.A.; Moutinho-Pereira, J.; Barros, A.; Correia, C. Kaolin and salicylic acid foliar application modulate yield, quality and phytochemical composition of olive pulp and oil from rainfed trees. Sci. Hortic. 2018, 237, 176–183. [Google Scholar] [CrossRef]
- El-Shazly, S.M.; Eisa, A.M.; Moảtamed, A.M.H.; Kotb, H.R.M. Effect of some agrochemicals preharvest foliar application on yield and fruit quality of ‘Swelling’ peach trees. Alex. J. Agric. Res. 2013, 58, 219–229. [Google Scholar]
- Mohamed, R.A.; Abdelbaset, A.K.; Abd-Elkader, D.Y. Salicylic acid effects on growth, yield, and fruit quality of strawberry cultivars. J. Med. Act. Plants 2017, 6, 1–11. [Google Scholar]
- Giménez, M.J.; Valverde, J.M.; Valero, D.; Guillén, F.; Martínez-Romero, D.; Serrano, M.; Castillo, S. Quality and antioxidant properties on sweet cherries as affected by preharvest salicylic and acetylsalicylic acids treatments. Food Chem. 2014, 160, 226–232. [Google Scholar] [CrossRef]
- Correia, S.; Queirós, F.; Ribeiro, C.; Vilela, A.; Aires, A.; Barros, A.I.; Schouten, R.; Silva, A.P.; Gonçalves, B. Effects of calcium and growth regulators on sweet cherry (Prunus avium L.) quality and sensory attributes at harvest. Sci. Hortic. 2019, 248, 231–240. [Google Scholar] [CrossRef]
- Jiang, F.; Hartung, W. Long-distance signalling of abscisic acid (ABA): The factors regulating the intensity of the ABA signal. J. Exp. Bot. 2008, 59, 37–43. [Google Scholar] [CrossRef]
- Balbontín, C.; Gutiérrez, C.; Wolff, M.; Figueroa, C.R. Effect of abscisic acid and methyl jasmonate preharvest applications on fruit quality and cracking tolerance of sweet cherry. Chil. J. Agric. Res. 2018, 78, 438–446. [Google Scholar] [CrossRef]
- Ma, X.L.; Wang, Y.J.; Xie, S.L.; Wang, C.; Wang, W. Glycine betaine application ameliorates negative effects of drought stress in tobacco. Russ. J. Plant. Physiol. 2007, 54, 472–479. [Google Scholar] [CrossRef]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front. Plant. Sci. 2017, 7, 2049. [Google Scholar] [CrossRef]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed extracts as biostimulants of plant growth and development. J. Plant. Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Jannin, L.; Arkoun, M.; Etienne, P.; Laîné, P.; Goux, D.; Garnica, M.; Fuentes, M.; Francisco, S.S.; Baigorri, R.; Cruz, F. Brassica napus growth is promoted by Ascophyllum nodosum (L.) seaweed extract: Microarray analysis and physiological characterization of N, C, and S metabolisms. J. Plant. Growth Regul. 2013, 32, 31–52. [Google Scholar] [CrossRef]
- Correia, S.; Oliveira, I.; Queirós, F.; Ribeiro, C.; Ferreira, L.; Luzio, A.; Silva, A.P.; Gonçalbes, B. Preharvest application of seaweed based biostimulant reduced cherry (Prunus avium L.) cracking. In Proceedings of the Environmental Sciences-Agriculture and Climate Change—Adapting Crops to Increased Uncertainty (AGRI 2015), Amsterdam, The Netherlands, 15–17 February 2015; Volume 29, pp. 251–252. [Google Scholar]
- Ayala, M.; Lang, G.A. Chapter 12: Morphology, cropping physiology and canopy training. In Cherries: Botany, Production and Uses; Quero-García, J., Iezzoni, A., Puławska, J., Lang, G., Eds.; CABI: Wallingford, UK, 2017; pp. 269–304. [Google Scholar]
- Von Caemmerer, S.; Farquhar, G.D. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 1981, 153, 376–387. [Google Scholar] [CrossRef]
- Düring, H. Photosynthesis of ungrafted and grafted grapevines: Effects of rootstock genotype and plant age. Am. J. Enol. Vitic. 1994, 45, 297–299. [Google Scholar]
- Šesták, Z.; Castky, J.; Jarvis., P.G. Plant. Photosynthetic Production. Manual of Methods; Dr. W. Junk Publishers: Haia, The Netherlands, 1971; p. 818. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Method Enzymol. 1987, 148, 350–382. [Google Scholar]
- Irigoyen, J.J.; Einerich, D.W.; Sánchez-Díaz, M. Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativa) plants. Physiol. Plant. 1992, 84, 55–60. [Google Scholar] [CrossRef]
- Osaki, M.; Shinano, T.; Tadano, T. Redistribution of carbon and nitrogen compounds from the shoot to the harvesting organs during maturation in field crops. Soil Sci. Plant. Nutr. 1991, 37, 117–128. [Google Scholar] [CrossRef]
- Bradford, M. A rapid and sensitive method for the quantification of microgram quantities of protein using the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Christensen, J.V. Cracking in cherries III: Determination of cracking susceptibility. Acta Agric. Scand. 1972, 22, 128–136. [Google Scholar] [CrossRef]
- Gonçalves, B.; Moutinho-Pereira, J.; Santos, A.; Silva, A.P.; Bacelar, E.; Correia, C.; Rosa, E. Scion-rootstock interaction affects the physiology and fruit quality of sweet cherry. Tree Physiol. 2005, 26, 93–104. [Google Scholar] [CrossRef]
- Yuan, L.; Xu, D.Q. Stimulation effect of gibberellic acid short-term treatment on leaf photosynthesis related to the increase in Rubisco content in broad bean and soybean. Photosyn. Res. 2001, 68, 39–47. [Google Scholar] [CrossRef]
- Teszlák, P.; Kocsis, M.; Gaal, K.; Nikfardjam, M.P. Regulatory effects of exogenous gibberellic acid (GA3) on water relations and CO2 assimilation among grapevine (Vitis vinifera L.) cultivars. Sci. Hortic. 2013, 159, 41–51. [Google Scholar] [CrossRef]
- Kumar, P.; Lakshmi, N.J.; Mani, V.P. Interactive effects of salicylic acid and phytohormones on photosynthesis and grain yield of soybean (Glycine max L. Merrill). Physiol. Mol. Biol. Plants 2000, 6, 179–186. [Google Scholar]
- Fariduddin, Q.; Hayat, S.; Ahmad, A. Salicylic acid influences net photosynthetic rate, carboxylation efficiency, nitrate reductase activity and seed yield in Brassica juncea. Photosynthetica 2003, 41, 281–284. [Google Scholar] [CrossRef]
- Khan, W.; Prithviraj, B.; Smith, D.L. Photosynthetic responses of corn and soybean to foliar application of salicylates. J. Plant. Physiol. 2003, 160, 485–492. [Google Scholar] [CrossRef]
- Suzy, Y.R.; Dennis, H.G.; Hutton, R.J.; Clarke, S.J. Transpiration efficiency of the grapevine cv. Semillon is tied to VPD in warm climates. Ann. Appl. Biol. 2011, 158, 106–114. [Google Scholar]
- Giri, J. Glycinebetaine and abiotic stress tolerance in plants. Plant. Signal. Behav. 2011, 11, 1746–1751. [Google Scholar] [CrossRef] [PubMed]
- Denaxa, N.K.; Roussos, P.A.; Damvakaris, T.; Stournaras, V. Comparative effects of exogenous glycine betaine, kaolin clay particles and ambiol on photosynthesis, leaf sclerophylly indexes and heat load of olive cv. Chondrolia Chalkidikis under drought. Sci. Hortic. 2012, 137, 87–94. [Google Scholar] [CrossRef]
- Mikiciuk, G.; Mikiciuk, M.; Ptak, P. The effects of anitranspirant di-1-p-menthene on some physiological traits of strawberry. Ecol. Eng. 2015, 16, 161–167. [Google Scholar] [CrossRef]
- Franks, P.J.; Farquhar, G.D. The effect of exogenous abscisic acid on stomatal development, stomatal mechanics, and leaf gas exchange in Tradescantia virginiana. Plant. Physiol. 2001, 125, 935–942. [Google Scholar] [CrossRef]
- Xu, C.; Leskovar, D.I. Effects of A. nodosum seaweed extracts on spinach growth, physiology and nutrition value under drought stress. Sci. Hortic. 2015, 183, 39–47. [Google Scholar] [CrossRef]
- El-Mageed, T.A.; Semida, W.M.; Radyc, M.M. Moringa leaf extract as biostimulant improves water use efficiency, physio-biochemical attributes of squash plants under deficit irrigation. Agr. Water Manag. 2017, 193, 46–54. [Google Scholar] [CrossRef]
- Cabo, S.; Morais, M.C.; Aires, A.; Carvalho, R.; Pascual-Seva, N.; Silva, A.P.; Gonçalves, B. Kaolin and seaweed-based extracts can be used as middle and long-term strategy to mitigate negative effects of climate change in physiological performance of hazelnut tree. J. Agro. Crop. Sci. 2019, 206, 28–42. [Google Scholar] [CrossRef]
- Ali, H.M.; Siddiqui, M.H.; Basalah, M.O.; Al-Whaibi, M.H.; Sakran, A.M.; Al-Amri, A. Effects of gibberellic acid on growth and photosynthetic pigments of Hibiscus sabdariffa L. under salt stress. Afr. J. Biotechnol. 2012, 11, 800–804. [Google Scholar]
- Hussain, S.; Saleem, M.F.; Ashraf, M.Y.; Cheema, M.A.; Haq, M.A. Abscisic acid, a stress hormone helps in improving water relations and yield of sunflower (Helianthus annuus L.) hybrids under drought. Pak. J. Bot. 2010, 42, 2177–2189. [Google Scholar]
- Brito, C.; Dinis, L.T.; Luzio, A.; Silva, E.; Gonçalves, A.; Meijón, M.; Escandón, M.; Arrobas, M.; Rodrigues, M.A.; Moutinho-Pereira, J.; et al. Kaolin and salicylic acid alleviate summer stress in rainfed olive orchards by modulation of distinct physiological and biochemical responses. Sci. Hortic. 2019, 246, 201–211. [Google Scholar] [CrossRef]
- Ragab, M.E.; Helal, N.A.S.; Sawan, O.M.; Fawzy, Z.F.; El-Sawy, S.M. Foliar application of glycine betaine for alleviating water stress of tomato plants grown under sandy soil conditions. Int. J. Chem. Tech. Res. 2015, 8, 52–67. [Google Scholar]
- Barickman, T.C.; Kopsell, D.A.; Sams, C.E. Abscisic acid increases carotenoid and chlorophyll concentrations in leaves and fruit of two tomato genotypes. J. Amer. Soc. Hort. Sci. 2014, 139, 261–266. [Google Scholar] [CrossRef]
- Kabiri, R.; Naisibi, F.; Farahbakhsh, H. Effect of exogenous salicylic acid on some physiological parameters and alleviation of drought stress in Nigella sativa plant under hydroponic culture. Plant. Protect. Sci. 2014, 50, 43–51. [Google Scholar] [CrossRef]
- Hayat, S.; Ahmad, A. Salicylic Acid a Plant. Hormone; Springer Publishers: Dordrecht, The Netherlands, 2007. [Google Scholar]
- Tian, F.; Wang, W.; Liang, C.; Wang, X.; Wang, G.; Wang, W. Overaccumulation of glycine betaine makes the function of the thylakoid membrane better in wheat under salt stress. Crop. J. 2017, 5, 73–82. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Gitelson, A.A. Why and what for the leaves are yellow inautumm? On the interpretation of optical spectra of senescing leaves (Acerplatanoides L.). J. Plant. Physiol. 1995, 145, 315–320. [Google Scholar] [CrossRef]
- Luo, H.; Dai, S.; Ren, J.; Zhang, C.; Ding, Y.; Li, Z.; Sun, Y.; Ji, K.; Wang, Y.; Li, Q.; et al. The role of ABA in the maturation and postharvest life of a nonclimacteric sweet cherry fruit. J. Plant. Growth Regul. 2014, 33, 373–383. [Google Scholar] [CrossRef]
- Murcia, G.; Pontin, M.; Reinoso, H.; Baradi, R.; Bertazza, G.; Gómez-Talquenca, S.; Bottini, R.; Piccolia, P.N. ABA and GA3 increase carbon allocation in different organs of grapevine plants by inducing accumulation of non-structural carbohydrates in leaves, enhancement of phloem area and expression of sugar transporters. Physiol. Plant. 2016, 156, 323–337. [Google Scholar] [CrossRef]
- Correia, S.; Aires, A.; Queirós, F.; Carvalho, R.; Schouten, R.; Silva, A.P.; Gonçalves, B. Climate conditions and spray treatments induce shifts in health promoting compounds in cherry (Prunus avium L.) fruits. Sci. Hortic. 2020, 263, 109147. [Google Scholar] [CrossRef]
- Sasek, T.W.; De Lucía, E.H.; Strain, B.R. Reversibility of photosynthetic inhibition in cotton after long-term exposure to elevated CO2 concentrations. Plant. Physiol. 1985, 78, 612–622. [Google Scholar] [CrossRef]
- Sicher, R.C.; Bunce, J.A. Adjustments of net photosynthesis in Solanum tuberosum in response to reciprocal changes in ambient and elevated growth CO2 partial pressures. Physiol. Plantarum 2001, 112, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Rosa, M.; Prado, C.; Podazza, G.; Interdonato, R.; González, J.A.; Hilal, M.; Prado, F.E. Soluble sugars-metabolism, sensing and abiotic stress a complex network in the life of plants. Plant. Signal. Behav. 2009, 4, 388–393. [Google Scholar] [CrossRef]
- Thalmann, M.; Pazmino, D.; Seung, D.; Horrer, D.; Nigro, A.; Meier, T.; Kölling, K.; Pfeifhofer, H.W.; Zeeman, S.C.; Santelia, D. Regulation of leaf starch degradation by abscisic acid is important for osmotic stress tolerance in plants. Plant. Cell 2016, 28, 1860–1878. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, C.M.; Díaz, J.B.R. A statistical model to estimate potential yields in peach before bloom. J. Am. Soc. Hortic. Sci. 2003, 128, 297–301. [Google Scholar] [CrossRef]
- Basak, A. Effect of preharvest treatment with seaweed products, KelpakR and Goëmar BM 86R, on fruit quality in apple. Int. J. Fruit Sci. 2008, 8, 1–14. [Google Scholar] [CrossRef]
- Colavita, G.M.; Spera, N.; Blackhall, V.; Sepulveda, G.M. Effects of seaweed extract on pear fruit quality and yield. Acta Hortic. 2011, 909, 601–607. [Google Scholar] [CrossRef]
- Quiroga, A.; Berli, F.; Moreno, D.; Cavagnaro, J.; Bottini, R. Abscisic acid sprays significantly increase yield per plant in vineyard-grown wine grape (Vitis vinifera L.) cv. Cabernet Sauvignon through increased berry set with no negative effects on anthocyanin content and total polyphenol index of both juice and wine. J. Plant. Growth Regul. 2009, 28, 28–35. [Google Scholar]
- Iqbal, N.; Ashraf, M. Glycine betaine, an osmolyte of interest to improve water stress tolerance in sunflower (Helianthus annuus L.): Water relations and yield. S. Afr. J. Bot. 2008, 74, 274–281. [Google Scholar] [CrossRef]
- Anjum, S.A.; Farooq, M.; Wang, L.C.; Xue, L.L.; Wang, S.G.; Wang, L.; Zhang, S.; Chen, M. Gas exchange and chlorophyll synthesis of maize cultivars are enhanced by exogenously-applied glycine betaine under drought conditions. Plant. Soil Environ. 2011, 57, 326–331. [Google Scholar] [CrossRef]
- Khan, M.M.A.; Gautam, A.C.; Mohammad, F.; Siddiqui, M.H.; Naeem, M.; Khan, M.N. Effect of gibberellic acid spray on performance of tomato. Turk. J. Biol. 2006, 30, 11–16. [Google Scholar]
- Uddain, J.; Hossain, A.K.M.; Mostafa, M.G.; Rahman, M.J. Effect of different plant growth regulators on growth and yield of tomato. Int. J. Sustain. Agric. 2009, 1, 58–63. [Google Scholar]
- Pal, P.; Yadav, K.; Kumar, K.; Singh, N. Effect of gibberellic acid and potassium foliar sprays on productivity and physiological and biochemical parameters of parthenocarpic cucumber Cv. ‘Seven Star F1’. J. Hortic. Res. 2016, 24, 93–100. [Google Scholar] [CrossRef]
- Parry, M.A.J.; Reynolds, M.; Salvucci, M.E.; Raines, C.; Andralojc, P.J.; Zhu, X.G.; Furbank, R.T. Raising yield potential of wheat. II. Increasing photosynthetic capacity and efficiency. J. Exp. Bot. 2011, 62, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.G.; Long, S.P.; Ort, D.R. Improving photosynthetic efficiency for greater yield. Ann. Rev. Plant. Biol. 2010, 61, 235–261. [Google Scholar] [CrossRef]
- Getnet, Z.; Husen, A.; Fetene, M.; Yemata, G. Growth, water status, physiological, biochemical and yield response of stay green sorghum (Sorghum bicolor (L.) Moench) varieties—A field trial under drought-prone area in Amhara regional state Ethiopia. J. Agron. 2015, 14, 188–202. [Google Scholar]
- Surendar, K.K.; Devil, D.D.; Ravi, I.; Jeyakumar, P.; Velayudham, K. Studies on the impact of water deficit on morphological, physiological and yield of banana (Musa spp.) cultivars and hybrids. Int. J. Agr. Sci. 2013, 3, 473–482. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).