Abstract
Trachyspermum ammi (Apiaceae) plants have several medicinal and condimentary applications and are considered an aphrodisiac agent in Iranian Traditional Medicine. Thus, the present study aims to evaluate the effects of oil from Iranian T. ammi plants on the viability of spermatogonial stem cells in vitro. The essential oil of T. ammi fruits was extracted by hydrodistillation, and the amount of thymol was calculated by a gas-chromatography method. Spermatogonial stem cells were isolated from the testes of mice using enzyme digestion. Real-time polymerase chain reaction (RT-PCR) was applied to assess the gene expressions of promyelocytic leukemia zinc finger protein (Plzf), DNA-binding protein inhibitor (ID-4), tyrosine-protein kinase (c-Kit), B-cell lymphoma 2 (Bcl2) and Bcl2-associated X protein (BAX). The number and diameter of colonies were also measured in the treated cells. The amount of thymol in the oil was 130.7 ± 7.6 µg/mL. Flow cytometry analysis showed that 92.8% of all cells expressed stimulated by retinoic acid 8 (Stra8), a spermatogonial stem cell marker. Expression of Plzf and ID-4 genes significantly increased in the treatment groups, while c-Kit and BAX decreased, and Bcl2 increased in the presence of essential oil. The numbers and diameters of cells were also improved by the application of the plant oil. These data indicated that monoterpenes from the oil of T. ammi improved the quality and viability of spermatogonia cells in the cell culture.
1. Introduction
Trachyspermum ammi (L.) Sprague ex Turrill (Apiaceae) is an annual plant distributed in Iran, Afghanistan, Pakistan, India and northern Africa [1]. The plant has a striated stem with a white inflorescence compound called umbel, and is widely grown in arid and semi-arid areas [2].
As a powerful toolbox, nutraceuticals can be used in the prevention and treatment of diseases beyond diet and before drugs, especially in subjects who may not be eligible for treatment with conventional medications [3]. For several nutraceuticals in the plant kingdom, it is recognized that understanding their safety and mechanism of action will provide new vistas to new therapeutic agents [3,4]. The brown, seed-like fruits of T. ammi with their bitter and pungent taste are used as nutraceuticals for medicinal and condimentary purposes [5].
The composition of the essential oil of T. ammi fruit, a concentrated hydrophobic liquid containing volatile chemical compounds, was analyzed several times in previous studies using gas-chromatography (GC) or gas-chromatography-mass spectrometry (GC-MS) methods. The monoterpenes thymol, carvacrol, γ-terpinene, cymene, and limonene were identified as the main compounds of the oil of the fruit [6,7,8,9,10]. The content and composition of T. ammi essential oil varied in previous studies. For instance, the amount of thymol in the oil obtained from fruit ranged from 17% to 71%, indicating the effect of genotype or environmental conditions [11,12]. Salt stress caused significant reductions in the weights of the plant’s parts and its fruits, whereas the concentration of fruit oil was not affected by salt stress [12]. Aside from volatile compounds, other active primary and secondary metabolites such as carbohydrates, proteins, vitamins, minerals, tannins, carotenoids, alkaloids, steroids, saponins, and flavonoids were identified in the whole plant [13]. Water soluble constituents of the fruit have also been elucidated, including one monoterpenoid, five new monoterpenoid glucosides, two aromatic compound glucosides, and two glucides [14].
The essential oil of T. ammi fruit suppresses the growth of Gram-negative bacteria, Gram-positive bacteria, and fungi in vitro [6]. The fruit is applied traditionally for stomach disorders such as flatulence, indigestion, colic and diarrhea. Other pharmacological and biological activities, including antioxidation, antivirus, antifungal, nematicidal, anti-inflammatory, analgesic, hepatoprotective, antiepileptic, antifever, and wound healing effects, were attributed to the plant in recent investigations [7,8,13,15,16,17,18]. Powder from the fruit and the plant’s aqueous extract showed an antihelminthic effect in a dose-dependent manner, and the extract was rich in thymol [19]. Fruit extract showed antispasmodic and antihypertensive activity by the inhibition of K+-induced contraction. Additionally, the extract exhibited protective activity against both paracetamol- and CCl4-induced toxicity in mice [20].
Genetic information is transmitted to the next generation by spermatogonial stem cells, which are extensively rare, composing about 0.03% of germ cells [21]. Fertility in cancer patients may be threatened due to gonadotoxic therapies, including chemotherapy and irradiation, that can affect the spermatogenesis process.Therefore, protection of germ cells can be considered an alternative strategy to conserve fertility in these patients [22]. Previous studies revealed protective effects of extracts and natural compounds on spermatogonial cells. For instance, quercetin protected spermatogonial cells in the presence of oxidant agents [23]. Additionally, the ethanolic extract of Moringa oleifera reduced cyclophosphamide-induced damage on spermatogonial cells [24]. Results of previous studies also revealed that the essential oil of T. ammi caused morphological deformities of the sperm plasma membrane and detachment of head-to-tail coiling. This suggested that the oil is suitable to be applied as a spermicidal agent in vaginal contraceptives [25,26]. However, in Iranian Traditional Medicine the plant has a reputation of having an aphrodisiac effect, with galactagogue and diuretic properties [27]. There is no research regarding the effect of the plant on spermatogonial cells; therefore, the present study aims to evaluate the effect of the plant’s oil on the viability of spermatogonial stem cells in vitro.
2. Materials and Methods
2.1. Plant Materials
T. ammi fruit was purchased from local medicinal plant shops in Tehran Province, Iran (2014) and identified at the herbarium of the Faculty of Pharmacy, Tehran University of Medical Sciences. In order to obtain essential oil, the fruit (100 g) was subjected to the hydrodistillation method, using a Clevenger-type apparatus. The plant material was poured into a glass balloon with sufficient distilled water (about 500 mL) and heated until boiling for 4 h. The obtained oil was dried over anhydrous sodium sulfate (Merck, Darmstadt, Germany) and stored in a sealed, dark glass vial in a refrigerator (4 ºC) until further investigation.
2.2. Thymol Quantification Using GC
According to the composition previously reported in our study, thymol is the major component of the plant’s oil [11]. Therefore, the amount of thymol was analyzed quantitatively using a Dani Master GC (Dani, Italy) with an OV1 column (SE54CB, 25 m × 0.25 mm internal diameter 0.25 µm film thickness), nitrogen as a carrier gas, with a split ratio of 1:30, and a flame ionization detector (FID). Temperature programming was performed from 75 °C (42 min) to 250 °C (14 min) at 15 °C/min. Injector and detector temperatures were 250 and 260 °C, respectively. Thymol with a concentration of 12.5 to 100 µL/mL was prepared to obtain the calibration curve. The oil of the fruit was injected into the column under the same conditions, and the amount of thymol was calculated using the following equations (Equations (1) and (2)):
Y = 0.0341X−0.378
R2 = 0.98
2.3. Isolation of Spermatogonial Cells
Male laboratory mice (3-6 days old) were purchased from the National Medical Research Institute (NMRI) and maintained in accordance with the guidelines issued by Tehran University of Medical Sciences. Spermatogonial stem cells were isolated in two steps of enzymatic digestion from testis tissue. Phosphate buffered saline (PBS; Sigma, Munich, Germany) was applied to wash the collected testes of mice. Testes were minced into small pieces after removing the tunica albuginea and then digested in a medium containing collagenase type IV (1 mg/mL; Sigma), hyaluronidase (0.5 mg/mL; Sigma), and DNase (10 µg/mL; Sigma) in minimum essential medium alpha (MEMα; Sigma) for 20 min. The tubules were separated with shaking at 37 °C in 5% CO2. The cells were centrifuged at 1500× g for 5 min and then washed twice with PBS [28].
The project was approved by an ethical committee (Approval ID: IR.TUMS.NIHR.REC.1398.005) that certified the project was in accordance with ethical principles and standards for conducting research.
2.4. Cell Enrichment Percentage
Flow cytometry was performed to assess Stra8-positive cells that had been isolated from testes. A total of 106 cells were suspended in 100 µL of PBS/fetal bovine serum (FBS), and 10 µL of a primary antibody (anti-Stra8 antibody, cat. no. ab49602, Abcam, Cambridge, UK) was added to the cell suspension (20 min, 4 °C). Cells were then washed with 1 mL of PBS/FBS. Subsequently, 10 µL of a secondary antibody (donkey antirabbit, cat. no. ab6798, Abcam) conjugated with fluorescein isothiocyanate (FITC) was added (20 min, 4 °C). Cells used as controls were not treated with antibodies. Cells were kept in the dark on ice until analysis by flow cytometry [29].
2.5. Spermatogonial Cell Culture
The cells, divided into 4 groups (3 treatment groups and 1 control group), were cultured for 2 weeks. Basic medium cultures contained MEMα, nonessential amino acids (Invitrogen, Carlsbad, CA, USA), 10% FBS, 0.1 mM 2-mercaptoethanol (Sigma), 103 U/mL human recombinant leukemia inhibitory factor (LIF; B&D, Franklin Lakes, NJ, USA), 100 U/mL penicillin, and 100 µg/mL streptomycin (both from Sigma). The essential oil of T. ammi was added to the cell cultures in amounts of 10, 20, and 30 µL. The culture media were changed every other day and stored at 35 °C in an atmosphere humidified with 5% CO2. The diameter and number of colonies were evaluated using an inverted microscope (Ziess, Jena, Germany), and captured images were processed by imageJ software [29].
2.6. Real-Time Polymerase Chain Reaction (Real-Time PCR)
The pluripotency of the spermatogonial cells was examined by evaluating the expression levels of ID-4 and Plzf alongside the apoptosis regulators BAX, Bcl2, and tyrosine-protein kinase (c-Kit) using real-time PCR. The sequence of primers is indicated in Table 1. Total RNA was extracted by an RNase kit (Accuzole Total RNA Extraction Kit, K-3090, Kentucky, USA) according to the manufacturer’s instructions. One microgram of total RNA was used in the synthesis of cDNA using reverse a transcription kit (Thermo Scientific, K1621, Kentucky, USA). Real-time PCR was performed with 40 reaction amplifications using 7500 fast real-time PCR and the detection of SYBR Green (Applied Bioscience, Carlsbad, CA, USA). All samples were normalized against glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (internal control) using the comparative CT method (ΔΔCT) [28].

Table 1.
Primers used in analyses of the effects of T. ammi oil on spermatogonial cells.
2.7. Viability of Spermatogonial Cells
The cell count was performed using a hemocytometer. A cell suspension (20 µL) with phosphate buffer solution (80 µL) was colored with 100 µL of trypan blue 0.4% for 5 min. Viable and dead cells (blue colored cells) of the mixture were counted in 10 µL.
2.8. Statistical Analysis
Data are presented as mean ± standard deviation (SD) of three independent experiments. The statistical significance between the mean values was determined by one-way analysis of variance (ANOVA) followed by a Tukey post hoc test with p < 0.05 as the statistically significant criterion.
3. Results
3.1. Essential Oil
Fruits of T. ammi (100 g) yielded 3.5 mL of yellow colored essential oil with a gravity of 0.97 mg/mL. As it was indicated in our previous study, 13 monotrepenes composed 99.1% of the oil, as identified using the gas-chromatography-mass spectrometry (GC-MS) method. Thymol (74.2%), p-cymene (16%), and γ-terpinene (7.1%) were the main constituents of the oil [11]. Therefore, the amount of thymol as a major component was assayed in the oil using the GC method. According to the thymol calibration curve, the amount of thymol was 130.7 ± 7.6 µg/mL in the essential oil of T. ammi fruit.
3.2. Spermatogonial Cell Culture
Flow cytometry analysis showed that 92.8% of the isolated cells from mice testes expressed Stra8, a spermatogonial stem cell marker. The diameters of all the colonies treated with T. ammi oil were significantly higher than those of the control group (Table 2), while there was no significant difference observed between the treatment groups in the increase of colonies’ diameters. The respective diameters of the control group were 48.6 ± 0.64 and 195.57 ± 1.36 mm in the first and second weeks. In the first week, colony diameters in the treatments of 10, 20, and 30 µL of the oil were 78.42 ± 2.31, 89.26 ± 0.48, and 79.71 ± 0.68 mm, respectively. The diameters of the colonies increased similarly, and 20 µL of the oil induced colony diameters (283.53 ± 8.05 mm) that were higher than the other treatments in the second week of the study. Colony diameters in the presence of the oil (10 and 30 µL) were increased to 272.26 ± 11.23 and 276.35 ± 0.48 mm, respectively. The number of colonies was significantly increased in the presence of all concentrations of the plant oil in both weeks of observation compared with control group (p < 0.05). Similar to the diameters of the colonies, the number of colonies increased in the presence of 20 µL of oil, from 17 ± 1.1 in the first week to 28 ± 1.2 in the second week, compared to the other groups. In the other treatment groups, i.e., 10 and 30 µL of oil, the colony numbers enhanced after two weeks to 25 ± 1.7 and 26 ± 1.1, respectively.

Table 2.
Diameters and numbers of colonies of spermatogonial cells treated with three different concentrations of T. ammi oil after 2 weeks. Results are presented as mean ± SD.
3.3. Gene Expression
A significant increase in the expression of genes related to the undifferentiated state, ID-4 and Plzf, in spermatogonial cells in the treatment groups of 10, 20, and 30 µL of oil was observed, which was more than that in control group (p < 0.05). The enhancement of gene expression was higher in the treatment with 20 µL of oil in comparison to the others (Figure 1). Meanwhile, the expression of the c-Kit gene (differentiated gene) in all the treatment groups was significantly decreased compared to the control (p < 0.05), and greater amount of decrease observed in the presence of 20 µL of the oil than in the two other treatment groups. There was a significant decrease in the expression level of the BAX gene (pro-apoptotic) in the presence of T. ammi oil in comparison with the control group (p < 0.05). The highest decrease in the level of the BAX gene was obtained with 20 µL of the fruit oil, but treatments with 10 and 30 µL of the oil also considerably lowered the expression of the BAX gene. After the oil treatment, expression of Bcl2 increased significantly in the presence of the oil compared with the control group (p < 0.05). All the oil amounts raised the expression of Bcl2, and the oil at an amount of 30 µL affected the expression of Bcl2 (antiapoptotic) more than the other treatments.
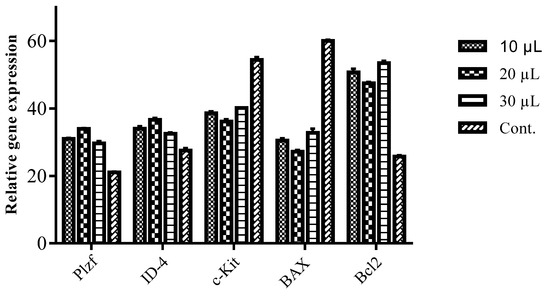
Figure 1.
Expression patterns of Plzf, ID-4, c-Kit, BAX, and Bcl2 genes after treatment with different amounts of T. ammi fruit oil (10, 20, and 30 µL). Expressions of Plzf and ID-4 were increased after treatment with oil, while the expression of c-Kit was decreased. Expression levels of BAX and Bcl2 were decreased and increased after treatment, respectively. Stars show significant differences between treatment groups and the control group (p < 0.05).
4. Discussion
Several beneficial effects have been attributed to T. ammi, such as aphrodisiac properties, which led us to evaluate the effect of T. ammi oil on the viability of spermatogonial cells in vitro. The results of the present study revealed that like previous reports, thymol composed the main part of the fruit oil of T. ammi. Different amounts of thymol were presented (17% to 71%) in previous analyses [11], while the oil tested on spermatogonial cells in the present study contained 74.2% thymol. Thus, thymol as a major component was assayed in the oil using the GC method according to the thymol calibration curve.
Stra8 was expressed in 92.8% of the cells isolated from the testes of mice, which is acceptable according to a previous study [29]. All the oil treatments (most of all, 20 µL of the oil) increased the diameter and number of colonies significantly more than the control group. Antioxidants can suppress the deteriorative effects of reactive oxygen species (ROS) and oxidative stress that can affect cell membranes, structural proteins, enzymes, and nucleic acids [30]. As it was indicated, thymol composed the main part of the tested oil, which displayed favorable effects on spermatogonial cells in the medium. Thymol exhibits antioxidation activity by inhibiting free radicals, chelating metal ions, and enhancing endogenous antioxidants, dependent or independent of enzymes [31]. In addition, γ-terpinene, which is a non-phenolic monoterpene, has antioxidant properties [32]. The results of a previous study indicated that cryopreservation and antioxidants such as α-tocopherol (belonging to the vitamin E family) and catalase (an enzyme in peroxisomes that converts H2O2 to H2O and O) increased the number and diameter of colonies. The obtained results are attributed to the presence of the mentioned antioxidants in the cell culture [22].
Gene expressions were analyzed after 2 weeks of the treatment with T. ammi oil, indicating that the oil influenced gene expressions in spermatogonial cells. The expression of BAX and Bcl2 genes was changed in favor of reduction of apoptosis in the cells. Markers of spermatogonial cells, ID-4 and Plzf, were overexpressed in the presence of the oil, whereas expression of the c-Kit gene was reduced. According to the data of another study, treatment of Chang liver cells with thymol suppressed apoptosis induced by oxidative stress, through the reduction of expression of the BAX gene and induction of the Bcl2 gene. It was concluded that thymol protected cells via the inhibition of ROS, increases in glutathione, and decreases in malonyl aldehyde [33]. Thymol’s antioxidation and free radical-scavenging abilities were associated with the protective capacity of the compound against damage induced by UV radiation on human keratinocyte cell lines (HaCaT) [34]. The compound at quantities up to 250 µM did not show mutagenic or genotoxic effects using Ames and Comet assays [35]. However, treatment of glioblastoma cells with thymol caused apoptosis via the release of Ca2+ from the endoplasmic reticulum [36]. Results of another study described that the concentration of thymol and carvacrol (isomer of thymol) is crucial in their activity as pro-oxidants or antioxidants in Caco-2 cells [37]. Both thymol and carvacrol at lower concentrations showed antioxidant activity. It is common for phenolic compounds to show antioxidant and pro-oxidant properties depending on their concentrations [37]. In our study, treatment of spermatogonial cells with 20 µL of the oil provided better results, which implies the importance of concentration in the preservation of spermatogonial cells using T. ammi oil.
5. Conclusions
The antioxidant abilities of monoterpenes such as thymol and γ-terpinene, along with other minor components in essential oil of the fruit of T. ammi, may be responsible for preserving and improving the viability of spermatogonial cells of mice through induction of Bcl2 and inhibition of BAX. Therefore, T. ammi oil can be a promising agent in the conservation of fertility in patients who suffer from malignancies and/or the fruits can be used as an appropriate nutraceutical formulation for the aforementioned purpose. Our results merely support the beneficial effect of T. ammi oil in cell culture. Further in vivo and trial studies are recommended concerning the protective properties of the plant.
Author Contributions
The authors contributed in this reaserch as followings “conceptualization, A.M. and F.A.; methodology, S.O.; software, M.V.; formal analysis, M.K.-m.; investigation, S.M.N.; resources, A.H.; writing—original draft preparation, A.M.; project administration, A.M.”. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Tehran University of Medical Sciences grant number 98-01-56-41734.
Acknowledgments
This study was supported by Tehran University of Medical Sciences (Grant number: 98-01-56-41734).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rechinger, K.H. Flora Iranica: Verlagsanstalt; Akademische Druck-u: Graz, Austria, 1982. [Google Scholar]
- Asif, H.M.; Sultana, S.; Akhtar, N. A Panoramic View on Phytochemical, Nutritional, Ethanobotanical Uses and Pharmacological Values of Trachyspermum Ammi Linn. Asian Pac. J. Trop. Biomed. 2014, 4, S545–S553. [Google Scholar] [CrossRef]
- Daliu, P.; Santini, A.; Novellino, E. A Decade of Nutraceutical Patents: Where Are We Now in 2018? Expert Opin. Ther. Pat. 2018, 28, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A Concise Overview on the Chemistry, Occurrence, and Human Health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef] [PubMed]
- Campos-Vega, R.; Dave Oomah, B.; Vergara-Castañeda, H.A. Food Wastes and by-Products; Wiley: Hoboken, NJ, USA, 2020. [Google Scholar]
- Mahboubi, M.; Kazempour, N. Chemical Composition and Antimicrobial Activity of Satureja Hortensis and Trachyspermum Copticum Essential Oil. Iran. J. Microbiol. 2011, 3, 194. [Google Scholar] [PubMed]
- Il-Kwon, P.; Kim, J.; Lee, S.; Shin, S. Nematicidal Activity of Plant Essential Oils and Components from Ajowan (Trachyspermum Ammi), Allspice (Pimenta Dioica) and Litsea (Litsea Cubeba) Essential Oils against Pine Wood Nematode (Bursaphelenchus Xylophilus). J. Nematol. 2007, 39, 275. [Google Scholar]
- Bahman, N.; Adeli, A.; Nickavar, A. Tlc-Bioautography and Gc-Ms Analyses for Detection and Identification of Antioxidant Constituents of Trachyspermum Copticum Essential Oil. Iran. J. Pharm. Res. 2014, 13, 127. [Google Scholar]
- Gilani, G.R.; Mahmood, Z.; Hussain, M. Preliminary Evaluation of Antimicrobial Activity of Cream Formulated with Essential Oil of Trachyuspermum Ammi. Pak. J. Pharm. Sci. 2013, 26, 893–896. [Google Scholar]
- Goudarzi, G.R.; Saharkhiz, M.J.; Sattari, M.; Zomorodian, K. Antibacterial Activity and Chemical Composition of Ajowan (Carum Copticum Benth. & Hook) Essential Oil. J. Agric. Sci. Technol. 2010, 13, 203–208. [Google Scholar]
- Omidpanah, S.; Vazirian, M.; Hosseinkhani, F.; Hadjiakhondi, A.; Hamedani, M.P.; Manayi, A. Antibacterial Activity of Essential Oil of Trachyspermum Ammi (L.) Sprague Ex Turrill against Isolated and Standard Bacteria. Am. J. Essent. Oils Nat. Prod. 2016, 4, 5–11. [Google Scholar]
- Subramaniyan, P.; Jothi, L.J.; Sundharaiya, K.; Shoba, N.; Murugesan, S. Extraction of Essential Oil and Thymol from Different Ajowan (Trachyspermum Ammi L.) Genotypes Using Gas Chromatography. Pharma Innov. J. 2019, 8, 548–552. [Google Scholar]
- Sonar Pankaj, K.; Singh, R.; Saraf, S.K. Phytochemical, Chromatographic and Spectroscopic Investigation of Carum Copticum Seeds and Their Potential as Immunomodulatory Agents. Pharm. Biol. 2016, 54, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Toru, I.; Sega, Y.; Kitajima, J. Water-Soluble Constituents of Ajowan. Chem. Pharm. Bull. 2001, 49, 840–844. [Google Scholar]
- Mohammad, M.; Saharkhiz, M.J.; Hosseini, A.A. In Vitro Lethal Effect of Ajowan (Trachyspermum Ammi L.) Essential Oil on Hydatid Cyst Protoscoleces. Vet. Parasitol. 2012, 187, 203–208. [Google Scholar]
- Iraj, R.; Fakoor, M.H.; Yadegarinia, D.; Gachkar, L.; Allameh, A.; Rezaei, M.B. Antimycotoxigenic Characteristics of Rosmarinus Officinalis and Trachyspermum Copticum L. Essential Oils. Int. J. Food Microbiol. 2008, 122, 135–139. [Google Scholar]
- Hossein, D.M.; Hejazian, S.H.; Morshedi, A.; Rafati, A. The Analgesic Effect of Carum Copticum Extract and Morphine on Phasic Pain in Mice. J. Ethnopharmacol. 2007, 109, 226–228. [Google Scholar]
- Souren, P.; Dubey, R.C.; Maheswari, D.K.; Kang, S.C. Trachyspermum Ammi (L.) Fruit Essential Oil Influencing on Membrane Permeability and Surface Characteristics in Inhibiting Food-Borne Pathogens. Food Control 2011, 22, 725–731. [Google Scholar]
- Abbas, I.B.; Abdollahi, J.; Akbari, H.; Jafarirad, S.; Moharramnejad, S. Anthelmintic Activity of Crude Powder and Crude Aqueous Extract of Trachyspermum Ammi on Gastrointestinal Nematodes in Donkey (Equus Asinus): An in Vivo Study. J. Ethnopharmacol. 2020, 248, 112249. [Google Scholar]
- Gilani, A.H.; Jabeen, Q.; Ghayur, M.N.; Janbaz, K.H.; Akhtar, M.S. Studies on the Antihypertensive, Antispasmodic, Bronchodilator and Hepatoprotective Activities of the Carum Copticum Seed Extract. J. Ethnopharmacol. 2005, 98, 127–135. [Google Scholar] [CrossRef]
- Takase Hinako, M.; Nusse, R. Paracrine Wnt/Β-Catenin Signaling Mediates Proliferation of Undifferentiated Spermatogonia in the Adult Mouse Testis. Proc. Natl. Acad. Sci. USA 2016, 15, E1489–E1497. [Google Scholar] [CrossRef]
- Fereshte, A.; Gilani, M.A.S.; Amidi, F.; Baazm, M.; Korouji, M.; Izadyar, F.; Yazdekhasti, H.; Abbasi, M. Improving the Efficacy of Cryopreservation of Spermatogonia Stem Cells by Antioxidant Supplements. Cell. Reprogramming (Former. Cloning Stem Cells) 2016, 18, 87–95. [Google Scholar]
- Mi, Y.; Zhang, C.; Li, C.; Taneda, S.; Watanabe, G.; Suzuki, A.K.; Taya, K. Quercetin Protects Embryonic Chicken Spermatogonial Cells from Oxidative Damage Intoxicated with 3-Methyl-4-Nitrophenol in Primary Culture. Toxicol. Lett. 2009, 190, 61–65. [Google Scholar] [CrossRef]
- Nayak, G.; Honguntikar, S.D.; Kalthur, S.G.; D’Souza, A.S.; Mutalik, S.; Setty, M.M.; Kalyankumar, R.; Krishnamurthy, H.; Kalthur, G.; Adiga, S.K. Ethanolic Extract of Moringa Oleifera Lam. Leaves Protect the Pre-Pubertal Spermatogonial Cells from Cyclophosphamide-Induced Damage. J. Ethnopharmacol. 2016, 182, 101–109. [Google Scholar] [CrossRef]
- Paul, S.; Kang, S.C. In Vitro Determination of the Contraceptive Spermicidal Activity of Essential Oil of Trachyspermum Ammi (L.) Sprague Ex Turrill Fruits. New Biotechnol. 2011, 28, 684–690. [Google Scholar] [CrossRef]
- Paul, S.; Kang, S.C. Studies on the Viability and Membrane Integrity of Human Spermatozoa Treated with Essential Oil of Trachyspermum Ammi (L.) Sprague Ex Turrill Fruit. Andrologia 2012, 44, 117–125. [Google Scholar] [CrossRef]
- Aghili-Alavi, M.H. Storehouse of Medicaments. In Makhzan-Ol-Advieh; Intisharat va Amoozesh Enghelab Islami Press: Tehran, Iran, 1992. [Google Scholar]
- Pellegrini, M.; di Siena, S.; Claps, G.; di Cesare, S.; Dolci, S.; Rossi, P.; Geremia, R.; Grimaldi, P. Microgravity Promotes Differentiation and Meiotic Entry of Postnatal Mouse Male Germ Cells. PLoS ONE 2010, 5, e9064. [Google Scholar] [CrossRef]
- Khanehzad, M.; Abolhasani, F.; Koruji, S.M.; Kashani, I.R.; Aliakbari, F. The Roles of Sertoli Cells in Fate Determinations of Spermatogonial Stem Cells. Tehran Univ. Med. J. 2016, 73, 878–887. [Google Scholar]
- Saraswat, S.; Kharche, S.D.; Jindal, S.K. Impact of Reactive Oxygen Species on Spermatozoa: A Balancing Act between Beneficial and Detrimental Effects. Iran. J. Appl. Anim. Sci. 2014, 4, 247–255. [Google Scholar]
- Meeran, N.; Fizur, M.; Javed, H.; al Taee, H.; Azimullah, S.; Ojha, S.K. Pharmacological Properties and Molecular Mechanisms of Thymol: Prospects for Its Therapeutic Potential and Pharmaceutical Development. Front. Pharmacol. 2017, 8, 380. [Google Scholar] [CrossRef]
- Baschieri, A.; Ajvazi, M.D.; Tonfack, J.L.F.; Valgimigli, L.; Amorati, R. Explaining the Antioxidant Activity of Some Common Non-Phenolic Components of Essential Oils. Food Chem. 2017, 232, 656–663. [Google Scholar] [CrossRef]
- Kim, Y.S.; Hwang, J.W.; Kang, S.H.; Kim, E.H.; Jeon, Y.J.; Jeong, J.H.; Kim, H.R.; Moon, S.H.; Jeon, B.T.; Park, P.J. Thymol from Thymus Quinquecostatus Celak. Protects against Tert-Butyl Hydroperoxide-Induced Oxidative Stress in Chang Cells. J. Nat. Med. 2014, 68, 154–162. [Google Scholar] [CrossRef]
- Mapelli, M.; Calo, R.; Marabini, L. Thymol and Thymus Vulgaris Extract Protects Human Keratinocyte Cell Line (Hacat) from Uva and Uvb Damage. Oxid. Antioxid. Med. Sci. 2016, 5, 39–48. [Google Scholar] [CrossRef][Green Version]
- LLana-Ruiz-Cabello, M.; Maisanaba, S.; Puerto, M.; Prieto, A.I.; Pichardo, S.; Jos, Á.; Cameán, A.M. Evaluation of the Mutagenicity and Genotoxic Potential of Carvacrol and Thymol Using the Ames Salmonella Test and Alkaline, Endo Iii-and Fpg-Modified Comet Assays with the Human Cell Line Caco-2. Food Chem. Toxicol. 2014, 72, 122–128. [Google Scholar] [CrossRef]
- Hsu, S.S.; Lin, K.L.; Chou, C.T.; Chiang, A.J.; Liang, W.Z.; Chang, H.T.; Tsai, J.Y.; Liao, W.C.; Huang, F.D.; Huang, J.K.; et al. Effect of Thymol on Ca2+ Homeostasis and Viability in Human Glioblastoma Cells. Eur. J. Pharmacol. 2011, 670, 85–91. [Google Scholar] [CrossRef]
- Llana-Ruiz-Cabello, M.; Gutiérrez-Praena, D.; Puerto, M.; Pichardo, S.; Jos, Á.; Cameán, A.M. In Vitro Pro-Oxidant/Antioxidant Role of Carvacrol, Thymol and Their Mixture in the Intestinal Caco-2 Cell Line. Toxicol. In Vitro 2015, 29, 647–656. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).